The Impact of Everolimus and Radiation Therapy on Pulmonary Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
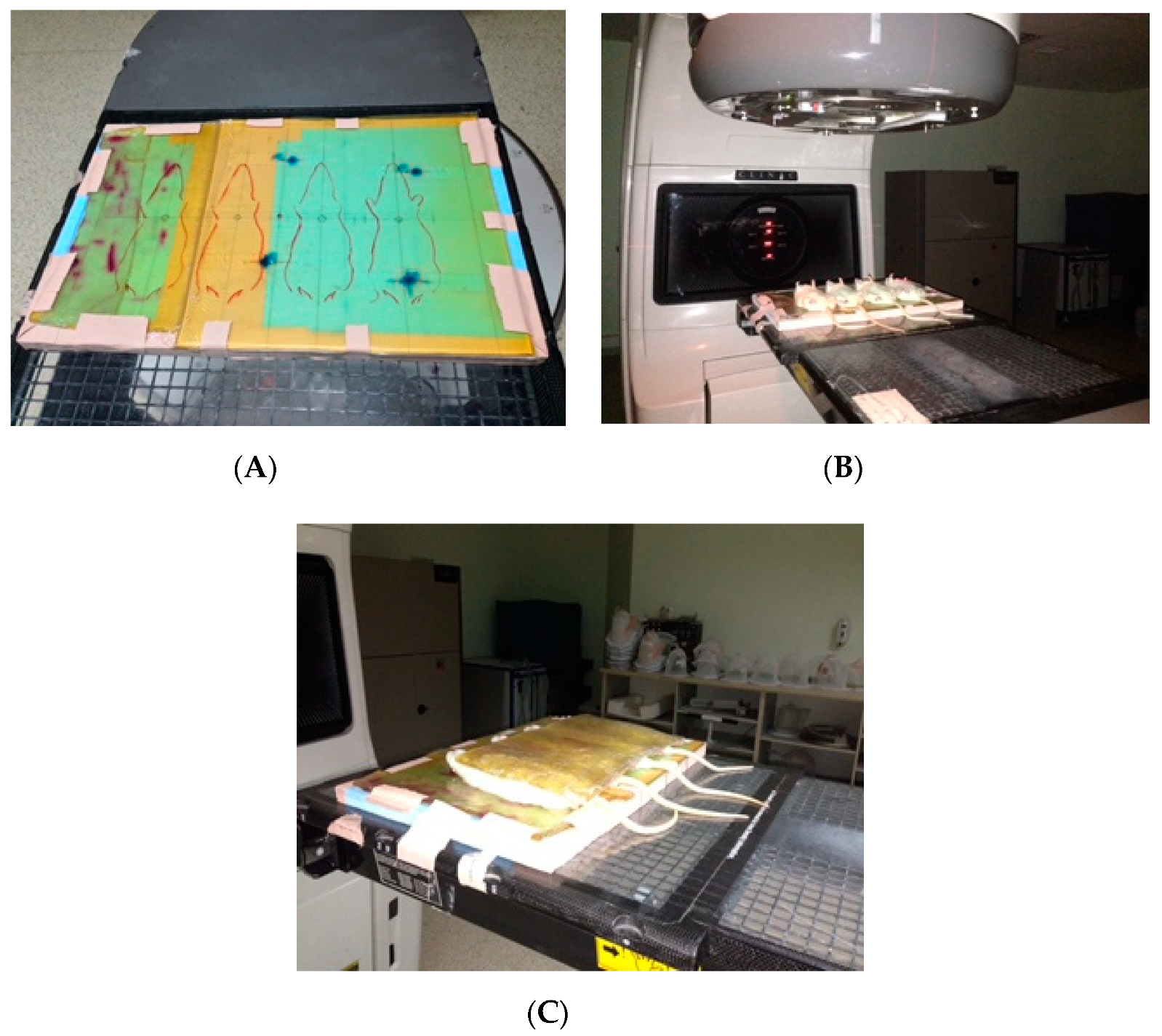
2.2. Administration
2.3. Tissue Preparations
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The therapeutic potential of mTor inhibitors in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, S.; Ciccarese, C.; Cingarlini, S.; Tortora, G.; Massari, F. Suppression of mtor pathway in solid tumors: Lessons learned from clinical experience in renal cell carcinoma and neuroendocrine tumors and new perspectives. Future Oncol. 2015, 11, 1809–1828. [Google Scholar] [CrossRef]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2 dagger. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Baas, M.C.; Struijk, G.H.; Moes, D.J.; Berk, I.A.H.V.D.; Jonkers, R.E.; De Fijter, J.W.; Van Der Heide, J.J.H.; Van Dijk, M.; Berge, I.J.M.T.; Bemelman, F.J. Interstitial pneumonitis caused by everolimus: A case-cohort study in renal transplant recipients. Transpl. Int. 2014, 27, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.E.C.A.B.; Grutters, J.C.; Gerritsen, W.R.; Van Erp, N.P.; Van Herpen, C.M.; Tol, J. mTor inhibitor-induced interstitial lung disease in cancer patients: Comprehensive review and a practical management algorithm. Int. J. Cancer 2016, 138, 2312–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wu, Z.; Ning, W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl. Oncol. 2019, 12, 162–169. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Marks, L.B.; Anscher, M. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin. Radiat. Oncol. 2000, 10, 296–307. [Google Scholar] [CrossRef]
- Bese, N.; Munzuroglu, F.; Uslu, B.; Arbak, S.; Yesiladali, G.; Süt, N.; Altuğ, T.; Ober, A. Vitamin E Protects against the Development of Radiation-induced Pulmonary Fibrosis in Rats. Clin. Oncol. 2007, 19, 260–264. [Google Scholar] [CrossRef]
- Clause, B.T. The Wistar Institute Archives: Rats (not Mice) and History. Mendel Newsl. 1998, 7, 2–7. [Google Scholar] [PubMed]
- The Wistar Institute: History. The Wistar Institute, 2007. Archived from the original on 17 October 2008. Retrieved 9 November 2008. Available online: https://www.google.com.tr/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiJ5sSJxsnqAhX9wcQBHUlZByAQFjAAegQIARAB&url=https%3A%2F%2Fen.wikipedia.org%2Fwiki%2FLaboratory_rat&usg=AOvVaw2744xxjd1PzXE0_Dc1X1pg (accessed on 9 November 2008).
- Hübner, R.-H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–511. [Google Scholar] [CrossRef]
- Bese, N.S.; Umay, C.; Serdengecti, S.; Kepil, N.; Süt, N.; Altuğ, T.; Ober, A. The impact of trastuzumab on radiation-induced pulmonary fibrosis: Results of an experimental study. Med. Oncol. 2009, 27, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.E.; Kemsley, L.; Davies, L.; HoleČek, M.; Berend, N. The Pulmonary Response to Sublethal Thoracic Irradiation in the Rat. Radiat. Res. 1993, 136, 15–21. [Google Scholar] [CrossRef]
- Tamhane, A.C. A Comparison of Procedures for Multiple Comparisons of Means with Unequal Variances. J. Am. Stat. Assoc. 1979, 74, 471–480. [Google Scholar]
- Junpaparp, P.; Sharma, B.; Samiappan, A.; Rhee, J.H.; Young, K.R. Everolimus-induced Severe Pulmonary Toxicity with Diffuse Alveolar Hemorrhage. Ann. Am. Thorac. Soc. 2013, 10, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Fielhaber, J.A.; Carroll, S.F.; Dydensborg, A.B.; Shourian, M.; Triantafillopoulos, A.; Harel, S.; Hussain, S.N.; Bouchard, M.; Qureshi, S.T.; Kristof, A.S. Inhibition of Mammalian Target of Rapamycin Augments Lipopolysaccharide-Induced Lung Injury and Apoptosis. J. Immunol. 2012, 188, 4535–4542. [Google Scholar] [CrossRef] [Green Version]
- Tomei, P.; Masola, V.; Granata, S.; Bellin, G.; Carratu, P.; Ficial, M.; Ventura, V.A.; Onisto, M.; Resta, O.; Gambaro, G.; et al. Everolimus-induced epithelial to mesenchymal transition (EMT) in bronchial/pulmonary cells: When the dosage does matter in transplantation. J. Nephrol. 2016, 29, 881–891. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer 2010, 116, 4256–4265. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.P.; Johnston, C.J.; Finkelstein, J.N. Treatment for radiation-induced pulmonary late effects: Spoiled for choice or looking in the wrong direction? Curr. Drug Targets 2010, 11, 1386–1394. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manegold, P.C.; Paringer, C.; Kulka, U.; Krimmel, K.; Eichhorn, M.; Wilkowski, R.; Jauch, K.-W.; Guba, M.; Bruns, C.J. Antiangiogenic Therapy with Mammalian Target of Rapamycin Inhibitor RAD001 (Everolimus) Increases Radiosensitivity in Solid Cancer. Clin. Cancer Res. 2008, 14, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.W.; Moretti, L.; Mitchell, L.R.; Jung, D.K.; Lu, B. Combined bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin. Cancer Res. 2009, 15, 6096–6105. [Google Scholar] [CrossRef] [Green Version]
- Iacovelli, R.; Palazzo, A.; Mezi, S.; Morano, F.; Naso, G.; Cortesi, E. Incidence and risk of pulmonary toxicity in patients treated with mTOR inhibitors for malignancy. A meta-analysis of published trials. Acta Oncol. 2012, 51, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Beşe, N.; Umay, C.; Yildirim, S.; Ilvan, S.; Dirican, A.; Salar, S.; Altuğ, T.; Ober, A. The effects of tamoxifen on radiation-induced pulmonary fibrosis in Wistar albino rats: Results of an experimental study. Breast 2006, 15, 455–459. [Google Scholar] [CrossRef]
| Group (G) | |
|---|---|
| G1 | Control group |
| G2 | Everolimus only group |
| G3 | RT + sequential everolimus group |
| G4 | RT + concurrent everolimus group |
| G5 | RT only group |
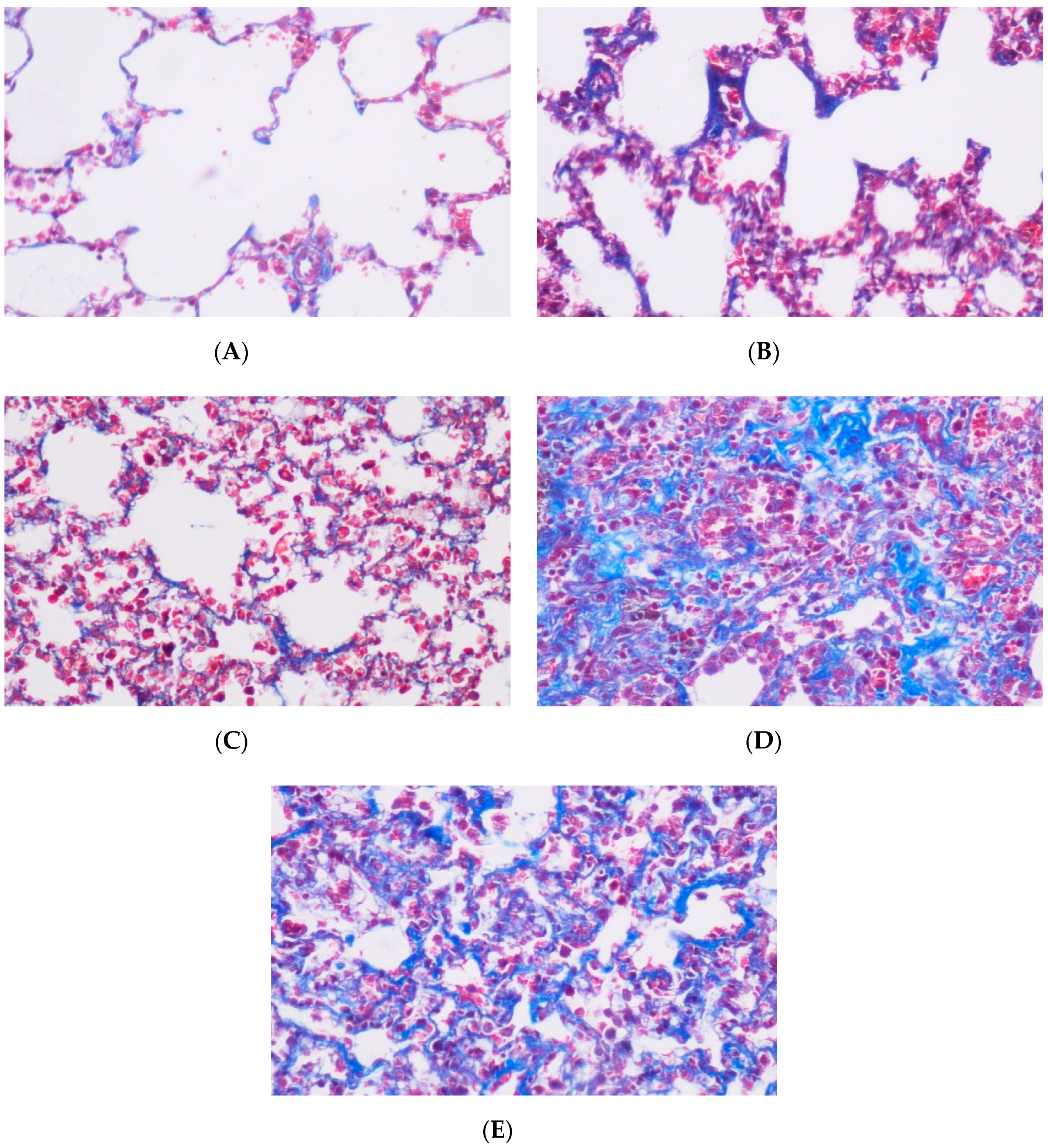
| Grade | Histological Features |
|---|---|
| 0 | Normal lung or minimal fibrous thickening of alveolar or bronchial walls. |
| 1 | Moderate thickening of the wall without obvious damage to lung architecture. |
| 2 | Increased fibrosis with definitive damage to lung structure and formation of fibrous bands or small fibrosis masses. |
| 3 | Severe distortion of the structure and large fibrous areas; ‘‘honeycomb lung’’ is placed in this category. |
| 4 | Total fibrous obliteration of the field. |
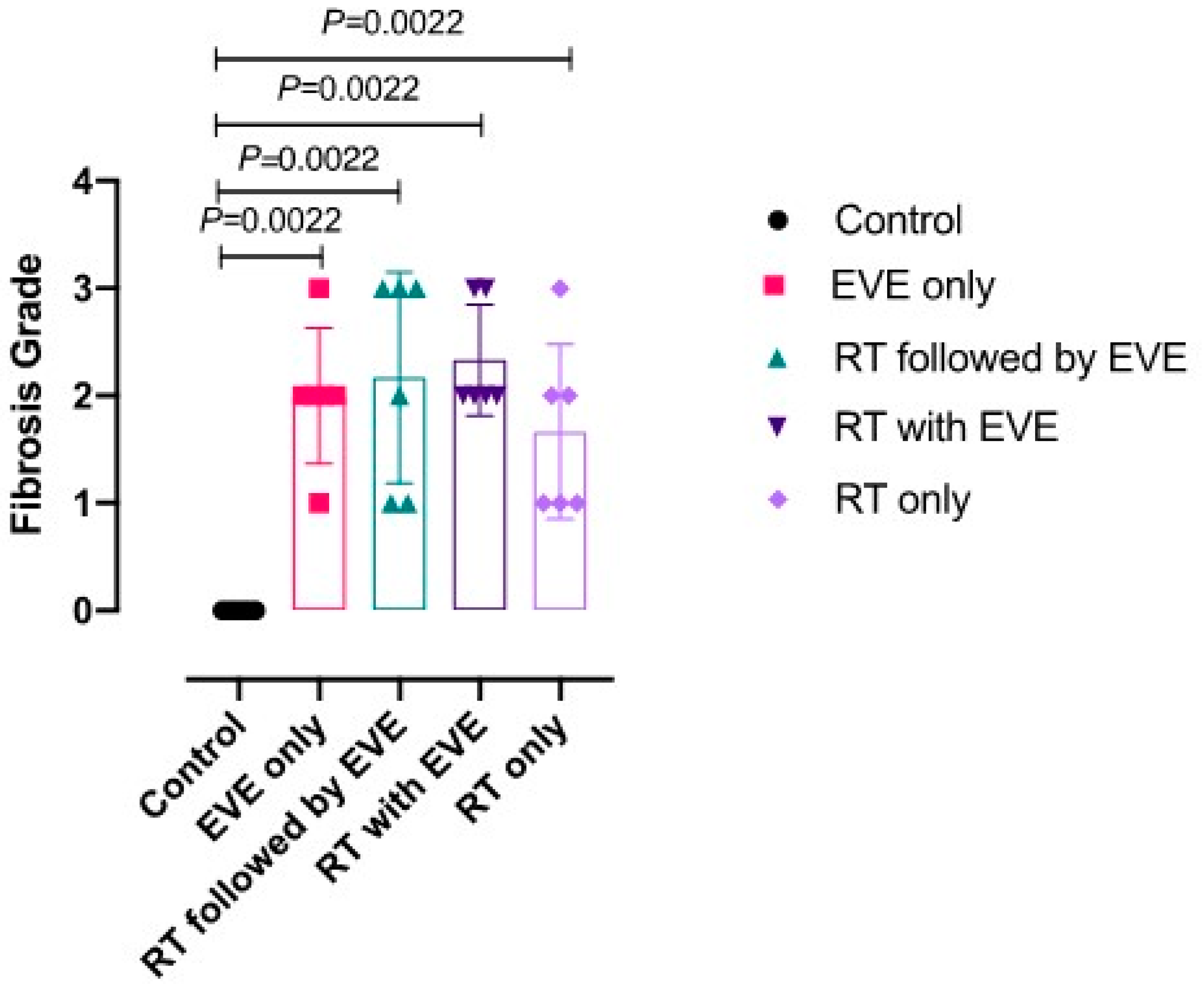
| Study Groups | Median Fibrosis Score (IQR) |
|---|---|
| Group 1, Control (n = 6) | 0,00 (0.0, 0.00) |
| Group 2, EVE only (n = 6) | 2.00 (1.0, 2.0) b |
| Group 3, Sequential RT-EVE (n = 6) | 2.00 (1.75, 3.00) b |
| Group 4, Concurrent EVE (n = 6) | 2.50 (2.00, 3.00) b < 0.05 |
| Group 5, RT only (n = 6) | 2.00 (1.75, 2.25) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eren, M.F.; Ay Eren, A.; Sayan, M.; Yücel, B.; Elagöz, Ş.; Özgüven, Y.; Vergalasova, I.; Altun, A.; Kılıçkap, S.; Daliparty, V.M.; et al. The Impact of Everolimus and Radiation Therapy on Pulmonary Fibrosis. Medicina 2020, 56, 348. https://doi.org/10.3390/medicina56070348
Eren MF, Ay Eren A, Sayan M, Yücel B, Elagöz Ş, Özgüven Y, Vergalasova I, Altun A, Kılıçkap S, Daliparty VM, et al. The Impact of Everolimus and Radiation Therapy on Pulmonary Fibrosis. Medicina. 2020; 56(7):348. https://doi.org/10.3390/medicina56070348
Chicago/Turabian StyleEren, Mehmet Fuat, Ayfer Ay Eren, Mutlay Sayan, Birsen Yücel, Şahende Elagöz, Yıldıray Özgüven, Irina Vergalasova, Ahmet Altun, Saadettin Kılıçkap, Vasudev Malik Daliparty, and et al. 2020. "The Impact of Everolimus and Radiation Therapy on Pulmonary Fibrosis" Medicina 56, no. 7: 348. https://doi.org/10.3390/medicina56070348
APA StyleEren, M. F., Ay Eren, A., Sayan, M., Yücel, B., Elagöz, Ş., Özgüven, Y., Vergalasova, I., Altun, A., Kılıçkap, S., Daliparty, V. M., & Beşe, N. (2020). The Impact of Everolimus and Radiation Therapy on Pulmonary Fibrosis. Medicina, 56(7), 348. https://doi.org/10.3390/medicina56070348





