Postoperative Delirium in Patients with Chronic Obstructive Pulmonary Disease after Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Ethical Statement
2.4. Statistical Analysis
3. Results
3.1. Relationship between COPD and Delirium
3.2. Clinical Characteristics of Patients with COPD
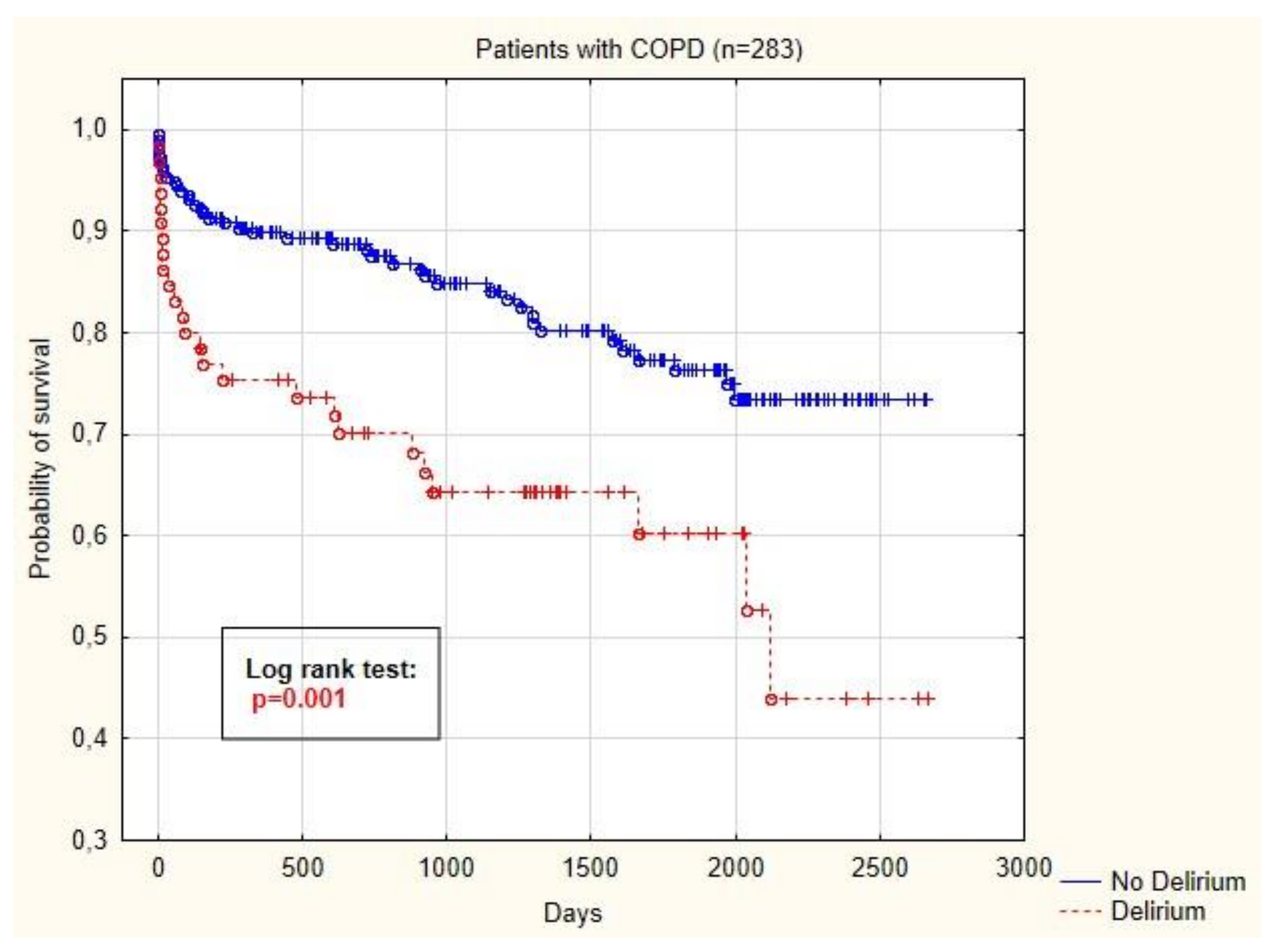
3.3. Survival Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Agostino, R.S.; Jacobs, J.P.; Badhwar, V.; Fernandez, F.G.; Paone, G.; Wormuth, D.W.; Shahian, D.M. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann. Thorac. Surg. 2018, 105, 15–23. [Google Scholar] [CrossRef]
- Licker, M.; Schweizer, A.; Ellenberger, C.; Tschopp, J.M.; Diaper, J.; Clergue, F. Perioperative Medical Management of Patients with COPD. Int. J. COPD 2007, 2, 493–515. [Google Scholar]
- Wada, H.; Miyauchi, K.; Daida, H. Gender Differences in the Clinical Features and Outcomes of Patients with Coronary Artery Disease. Expert Rev. Cardiovasc. Ther. 2019, 17, 127–133. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Perez-Padilla, R.; Jardim, J.R.B.; Muino, A.; Lopez, M.V.; Valdivia, G.; Montes de Oca, M.; Talamo, C.; Hallal, P.C.; Victora, C.G. Chronic Obstructive Pulmonary Disease in Five Latin American Cities (the PLATINO Study): A Prevalence Study. Lancet 2005, 366, 1875–1881. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic Obstructive Pulmonary Disease: Molecular and Cellular Mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- André, S.; Conde, B.; Fragoso, E.; Boléo-Tomé, J.P.; Areias, V.; Cardoso, J. COPD and Cardiovascular Disease. Pulmonology 2019, 25, 168–176. [Google Scholar] [CrossRef]
- American Psychiatric Association. Neurocognitive Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Hollinger, A.; Siegemund, M.; Goettel, N.; Steiner, L.A. Postoperative Delirium in Cardiac Surgery: An Unavoidable Menace? J. Cardiothorac. Vasc. Anesth. 2015, 29, 1677–1687. [Google Scholar] [CrossRef]
- Kotfis, K.; Szylińska, A.; Listewnik, M.; Strzelbicka, M.; Brykczyński, M.; Rotter, I.; Żukowski, M. Early Delirium after Cardiac Surgery: An Analysis of Incidence and Risk Factors in Elderly (≥65 Years) and Very Elderly (≥80 Years) Patients. Clin. Interv. Aging 2018, 13, 1061–1070. [Google Scholar] [CrossRef]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Kotfis, K.; Marra, A.; Ely, E.W. ICU Delirium—A Diagnostic and Therapeutic Challenge in the Intensive Care Unit. Anaesthesiol. Intensive Ther. 2018, 50, 160–167. [Google Scholar] [CrossRef]
- McPherson, J.A.; Wagner, C.E.; Boehm, L.M.; Hall, J.D.; Johnson, D.C.; Miller, L.R.; Burns, K.M.; Thompson, J.L.; Shintani, A.K.; Ely, E.W.; et al. Delirium in the Cardiovascular ICU: Exploring Modifiable Risk Factors. Crit. Care Med. 2013, 41, 405–413. [Google Scholar] [CrossRef]
- Sockalingam, S.; Parekh, N.; Bogoch, I.I.; Sun, J.; Mahtani, R.; Beach, C.; Bollegalla, N.; Turzanski, S.; Seto, E.; Kim, J.; et al. Delirium in the Postoperative Cardiac Patient: A Review. J. Card. Surg. 2005, 20, 560–567. [Google Scholar] [CrossRef]
- Marra, A.; Kotfis, K.; Hosie, A.; MacLullich, A.M.J.; Pandharipande, P.P.; Ely, E.W.; Pun, B.T. Delirium Monitoring: Yes or No? That Is the Question. Am. J. Crit. Care Off. Publ. Am. Assoc. Crit. Nurses 2019, 28, 127–135. [Google Scholar] [CrossRef]
- van Harten, A.E.; Scheeren, T.W.L.; Absalom, A.R. A Review of Postoperative Cognitive Dysfunction and Neuroinflammation Associated with Cardiac Surgery and Anaesthesia. Anaesthesia 2012, 67, 280–293. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Ramlawi, B.; Kuchel, G.A.; McElhaney, J.E.; Xie, D.; Sellke, F.W.; Khabbaz, K.; Levkoff, S.E.; Marcantonio, E.R. Chemokines Are Associated with Delirium after Cardiac Surgery. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 184–189. [Google Scholar] [CrossRef]
- Kotfis, K.; Szylińska, A.; Listewnik, M.; Brykczyński, M.; Ely, E.W.; Rotter, I. Diabetes and Elevated Preoperative Hba1c Level as Risk Factors for Postoperative Delirium after Cardiac Surgery: An Observational Cohort Study. Neuropsychiatr. Dis. Treat. 2019, 15, 511–521. [Google Scholar] [CrossRef]
- Ferrer, M.; Bernadich, O.; Nava, S.; Torres, A. Noninvasive Ventilation after Intubation and Mechanical Ventilation. Eur. Respir. J. 2002, 19, 959–965. [Google Scholar] [CrossRef]
- Corlateanu, A.; Covantev, S.; Mathioudakis, A.G.; Botnaru, V.; Siafakas, N. Prevalence and Burden of Comorbidities in Chronic Obstructive Pulmonary Disease. Respir. Investig. 2016, 54, 387–396. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thoracic Surg. Off. J. Eur. Assoc. Cardio-Thoracic Surg. 2012, 41, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Redfors, B.; Chen, S.; Liu, Y.; Ben-Yehuda, O.; Puskas, J.D.; Kandzari, D.E.; Merkely, B.; Horkay, F.; van Boven, A.J.; et al. Impact of Chronic Obstructive Pulmonary Disease on Prognosis after Percutaneous Coronary Intervention and Bypass Surgery for Left Main Coronary Artery Disease: An Analysis from the EXCEL Trial. Eur. J. Cardio-Thoracic Surg. 2018, 55, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Szylińska, A.; Kotfis, K.; Listewnik, M.; Brykczyński, M.; Marra, A.; Rotter, I. The Burden of Chronic Obstructive Pulmonary Disease in Open Heart Surgery—A Retrospective Cohort Analysis of Postoperative Complications: STROBE Compliant. Medicine 2020, 99, e19675. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chen, C.-W.; Lu, C.-L.; Lai, W.-W.; Huang, M.-H.; Tsai, L.-M.; Li, C.-Y.; Lai, C.-H. The Association between Recent Hospitalized COPD Exacerbations and Adverse Outcomes after Percutaneous Coronary Intervention: A Nationwide Cohort Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 169–179. [Google Scholar] [CrossRef]
- Ried, M.; Unger, P.; Puehler, T.; Haneya, A.; Schmid, C.; Diez, C. Mild-to-Moderate COPD as a Risk Factor for Increased 30-Day Mortality in Cardiac Surgery. Thorac. Cardiovasc. Surg. 2010, 58, 387–391. [Google Scholar] [CrossRef]
- O’Boyle, F.; Mediratta, N.; Chalmers, J.; Al-Rawi, O.; Mohan, K.; Shaw, M.; Poullis, M. Long-Term Survival of Patients with Pulmonary Disease Undergoing Coronary Artery Bypass Surgery. Eur. J. Cardio-Thoracic Surg. 2013, 43, 697–703. [Google Scholar] [CrossRef][Green Version]
- Leavitt, B.J.; Ross, C.S.; Spence, B.; Surgenor, S.D.; Olmstead, E.M.; Clough, R.A.; Charlesworth, D.C.; Kramer, R.S.; O’Connor, G.T. Long-Term Survival of Patients with Chronic Obstructive Pulmonary Disease Undergoing Coronary Artery Bypass Surgery. Circulation 2006, 114 (Suppl. 1), I430–1434. [Google Scholar] [CrossRef]
- McAllister, D.A.; Wild, S.H.; MacLay, J.D.; Robson, A.; Newby, D.E.; MacNee, W.; Innes, J.A.; Zamvar, V.; Mills, N.L. Forced Expiratory Volume in One Second Predicts Length of Stay and In-Hospital Mortality in Patients Undergoing Cardiac Surgery: A Retrospective Cohort Study. PLoS ONE 2013, 8, e64565. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.Z.; Mohan, K.; Shaw, M.; Al-Rawi, O.; Elsayed, H.; Walshaw, M.; Chalmers, J.A.C.; Fabri, B.M. Impact of Chronic Obstructive Pulmonary Disease Severity on Surgical Outcomes in Patients Undergoing Non-Emergent Coronary Artery Bypass Grafting. Eur. J. Cardio-Thoracic Surg. 2012, 42, 108–113, discussion 113. [Google Scholar] [CrossRef]
- Adabag, A.S.; Wassif, H.S.; Rice, K.; Mithani, S.; Johnson, D.; Bonawitz-Conlin, J.; Ward, H.B.; McFalls, E.O.; Kuskowski, M.A.; Kelly, R.F. Preoperative Pulmonary Function and Mortality after Cardiac Surgery. Am. Heart J. 2010, 159, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.S.; Weiner, M.M.; Arora, R.C.; Chung, I.; Deshpande, R.; Varghese, R.; Augoustides, J.; Ramakrishna, H. Current Approach to Diagnosis and Treatment of Delirium after Cardiac Surgery. Ann. Card. Anaesth. 2016, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-P.; Jing, Z.-Z.; Song, J.-F.; Zhang, P. A retrospective study on risk factors associated with postoperative delirium in elderly patients with spinal operation. Zhongguo Gu Shang 2019, 32, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Tilouche, N.; Hassen, M.F.; Ali, H.B.S.; Jaoued, O.; Gharbi, R.; El Atrous, S.S. Delirium in the Intensive Care Unit: Incidence, Risk Factors, and Impact on Outcome. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2018, 22, 144–149. [Google Scholar] [CrossRef]
- Austin, V.; Crack, P.J.; Bozinovski, S.; Miller, A.A.; Vlahos, R. COPD and Stroke: Are Systemic Inflammation and Oxidative Stress the Missing Links? Clin. Sci. 2016, 130, 1039–1050. [Google Scholar] [CrossRef]
- Lahousse, L.; Tiemeier, H.; Ikram, M.A.; Brusselle, G.G. Chronic Obstructive Pulmonary Disease and Cerebrovascular Disease: A Comprehensive Review. Respir. Med. 2015, 109, 1371–1380. [Google Scholar] [CrossRef]
- Yousefshahi, F.; Samadi, E.; Paknejad, O.; Hagh, E.B.; Aminzadeh, S. Effect of Hypoxemia in the Determination of Short-Term Prognosis of Coronary Artery Bypass Graft Patients: A Prospective Study. Anesthesiol. Pain Med. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Kotfis, K.; Biernawska, J.; Zegan-Barańska, M.; Żukowski, M. Peripheral Blood Lymphocyte Subsets (CD4+, CD8+ T Cells, NK Cells) in Patients with Cardiovascular and Neurological Complications after Carotid Endarterectomy. Int. J. Mol. Sci. 2015, 16, 10077–10094. [Google Scholar] [CrossRef]
- Gosselt, A.N.; Slooter, A.J.; Boere, P.R.; Zaal, I.J. Risk Factors for Delirium after On-Pump Cardiac Surgery: A Systematic Review. Crit. Care 2015, 19, 346. [Google Scholar] [CrossRef]
- Saller, T.; Petzold, A.; Zetterberg, H.; Kuhle, J.; Chappell, D.; von Dossow, V.; Klawitter, F.; Schurholz, T.; Hagl, C.; Reuter, D.A.; et al. A Case Series on the Value of Tau and Neurofilament Protein Levels to Predict and Detect Delirium in Cardiac Surgery Patients. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2019, 163, 241–246. [Google Scholar] [CrossRef]
- Alifier, M.; Olsson, B.; Andreasson, U.; Cullen, N.C.; Czyżewska, J.; Jakubów, P.; Sieśkiewicz, A.; Stasiak-Barmuta, A.; Hirnle, T.; Kornhuber, J.; et al. Cardiac Surgery Is Associated with Biomarker Evidence of Neuronal Damage. J. Alzheimers. Dis. 2020, 74, 1211–1220. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Grega, M.A.; Bailey, M.M.; Pham, L.D.; Zeger, S.L.; Baumgartner, W.A.; Selnes, O.A.; McKhann, G.M. Delirium after Coronary Artery Bypass Graft Surgery and Late Mortality. Ann. Neurol. 2010, 67, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Zegan-Barańska, M.; Strzelbicka, M.; Safranow, K.; Żukowski, M.; Ely, E.W. Validation of the Polish Version of the Critical Care Pain Observation Tool (CPOT) to Assess Pain Intensity in Adult, Intubated Intensive Care Unit Patients: The POL-CPOT Study. Arch. Med. Sci. 2018, 14, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Strzelbicka, M.; Zegan-Barańska, M.; Safranow, K.; Brykczyński, M.; Żukowski, M.; Ely, E.W. Validation of the Behavioral Pain Scale to Assess Pain Intensity in Adult, Intubated Postcardiac Surgery Patients: A Cohort Observational Study—POL-BPS. Medicine 2018, 97, e12443. [Google Scholar] [CrossRef] [PubMed]
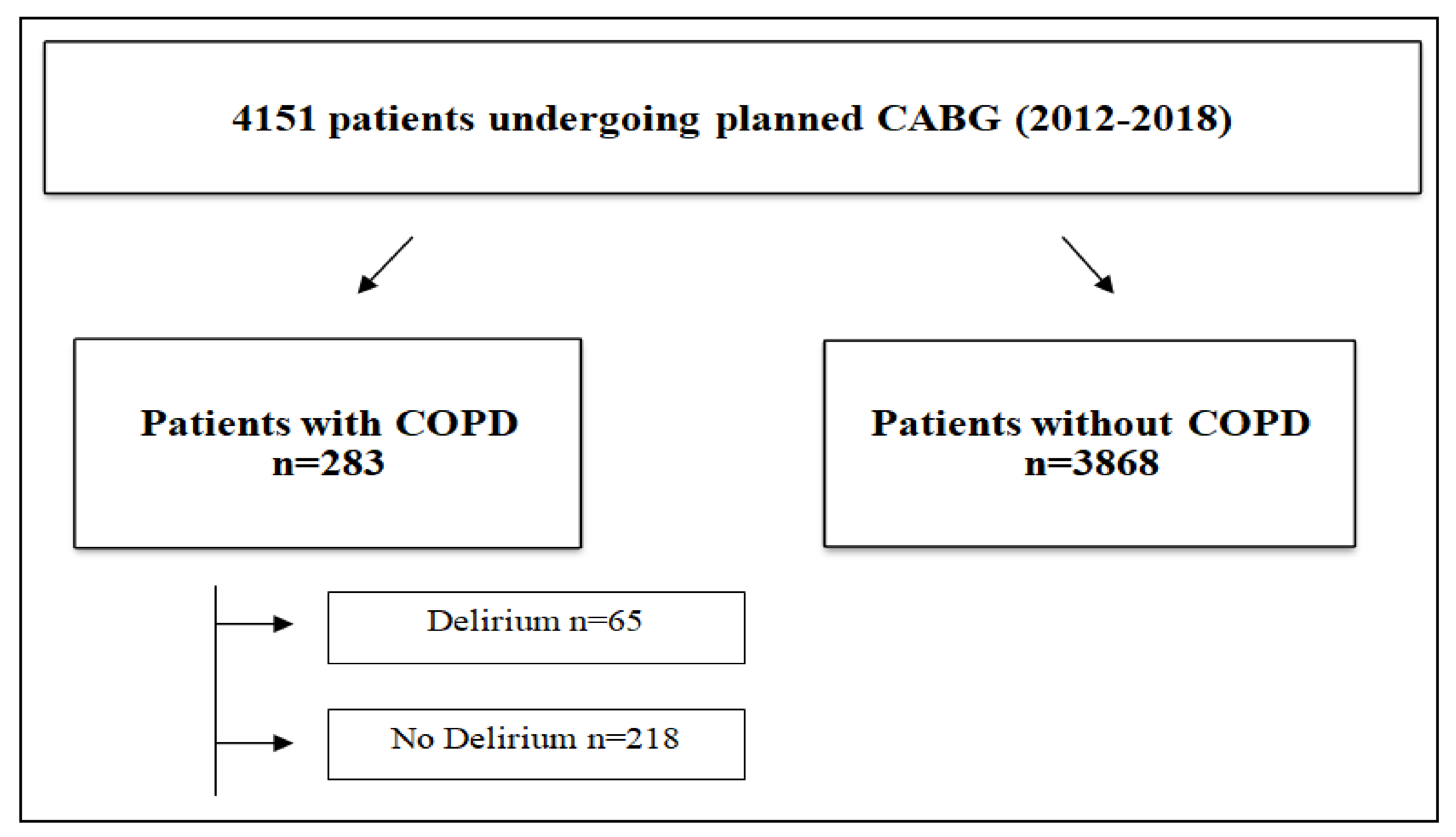
| No COPD (n = 3868) | COPD (n = 283) | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| No Delirium | 3199 | 82.73% | 218 | 77.03% | 0.015 |
| Delirium | 668 | 17.27% | 65 | 22.97% | |
| Variables | COPD Patients (n = 283) | |||
|---|---|---|---|---|
| No Delirium (n = 218) | Delirium (n = 65) | p | ||
| Demographic data | ||||
| Age (years), Me (Q1–Q3) | 66.0 (62.0–72.0) | 68.0 (63.0–73.0) | 0.335 | |
| Female, n (%) | 54 (24.77) | 23 (35.38) | 0.091 | |
| BMI (kg/m2), Me (Q1–Q3) | 29.3 (26.2–32.4) | 29.4 (25.3–31.9) | 0.957 | |
| Smoking, n (%) | never smoked | 44 (20.28) | 8 (12.31) | 0.149 |
| ex–smoker | 141 (64.98) | 42 (64.62) | ||
| current smoker | 32 (14.75) | 15 (23.08) | ||
| Smoking (years), Me (Q1–Q3) | 40.0(30.0–45.0) | 40.0 (30.0–50.0) | 0.368 | |
| Preoperative data | ||||
| EuroScore Logistic II (%), Me (Q1–Q3) | 2.2 (1.3–3.5) | 2.7 (1.6–3.9) | 0.041 | |
| scale CCS, Me (Q1–Q3) | 3.0 (3.0–4.0) | 3.0 (2.0–3.0) | 0.081 | |
| scale NYHA, Me (Q1–Q3) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 0.522 | |
| Ejection Fraction (%), Me (Q1–Q3) | 50.0 (40.0–55.0) | 45.0 (35.0–50.0) | 0.079 | |
| CKMB (µg/L), Me (Q1–Q3) | 21.0 (18.0–26.5) | 22.00 (18.0–27.0) | 0.921 | |
| CRP (mg/L), Me (Q1–Q3) | 2.8 (1.0–5.7) | 2.2 (1.2–4.5) | 0.649 | |
| Glycated hemoglobin (%), Me (Q1–Q3) | 5.8 (5.5–6.3) | 5.9 (5.6–6.6) | 0.199 | |
| Creatinine (mg/dL), Me (Q1–Q3) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.743 | |
| GFR (mL/min/1.73 m2), Me (Q1–Q3) | 80.0 (63.0–92.0) | 76.0 (62.0–91.0) | 0.569 | |
| Co-morbidities | ||||
| Stroke, n (%) | 9 (4.13) | 1 (1.54) | 0.321 | |
| TIA/RIND, n (%) | 4 (1.83) | 2 (3.08) | 0.542 | |
| CCS IV, n (%) | 59 (27.06) | 12 (18.46) | 0.160 | |
| NYHA III and IV, n (%) | 30 (13.76) | 9 (13.85) | 0.851 | |
| ICA stenosis, n (%) | 11 (5.05) | 5 (7.69) | 0.614 | |
| Chronic Renal Failure, n (%) | 11 (7.59) | 8 (17.02) | 0.109 | |
| Glucose intolerance, n (%) | 6 (2.75) | 2 (3.08) | 0.774 | |
| Diabetes, n (%) | 74 (33.94) | 30 (46.15) | 0.077 | |
| Arterial hypertension, n (%) | 169 (77.52) | 53 (81.54) | 0.489 | |
| Thyroid disease, n (%) | 17 (7.80) | 8 (12.31) | 0.381 | |
| AF paroxysmal, n (%) | 27 (12.39) | 5 (7.69) | 0.409 | |
| AF persistent or permanent, n (%) | 9 (4.13) | 1 (1.54) | 0.542 | |
| Hyperlipidemia, n (%) | 73 (33.49) | 26 (40.00) | 0.334 | |
| Peripheral vascular disease, n (%) | 69 (31.65) | 22 (33.85) | 0.739 | |
| Variables | COPD Patients (n = 283) | |||
|---|---|---|---|---|
| No Delirium (n = 218) | Delirium (n = 65) | p | ||
| Intraoperative data | ||||
| CPB time (min), Me (Q1–Q3) | 50.0 (42.0–60.0) | 52.0 (45.0–66.0) | 0.198 | |
| Aortic cross-clamping time, Me (Q1–Q3) | 30.0 (24.0–36.0) | 30.0 (26.0–37.5) | 0.394 | |
| Number of grafts, Me (Q1–Q3) | 3.0 (2.0–4.0) | 3.00 (2.0–3.0) | 0.553 | |
| Hemofiltration on CPB, n (%) | 37 (16.97) | 18 (27.69) | 0.055 | |
| Hemofiltration (mL), Me (Q1–Q3) | 1500.0 (1300.0–1800.0) | 1850.0 (1500.0–2000.0) | 0.123 | |
| Intubation time (min), Me (Q1–Q3) | 645.0 (490.0–850.0) | 775.0 (585.0–1050.0) | 0.007 | |
| Re-intubation, n (%) | 10 (4.59) | 9 (13.85) | 0.019 | |
| Re-intubation time (min), Me (Q1–Q3) | 49.0 (5.0–60.0) | 88.00 (36.0–100.0) | 0.307 | |
| Postoperative data | ||||
| CKMB (µg/L), Me (Q1–Q3) | 42.0 (34.0–57.0) | 48.0 (36.0–100.0) | 0.053 | |
| Max CKMB > 120 | 4 (1.83) | 3 (4.62) | 0.205 | |
| Acute myocardial infarction, n (%) | 4 (1.83) | 2 (3.08) | 0.542 | |
| Low Output syndrome | 4 (1.83) | 3 (4.62) | 0.205 | |
| Intensive Care Unit length of stay (days), Me (Q1–Q3) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 0.946 | |
| Hemofiltration in the ICU, n (%) | 19 (8.72) | 9 (13.85) | 0.221 | |
| RBC (units), Me (Q1–Q3) | 2.0 (2.0–2.0) | 2.0 (2.0–4.0) | 0.094 | |
| RBC (n, %) | 70 (32.11) | 33 (50.77) | 0.006 | |
| Plasma (units), Me (Q1–Q3) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 0.845 | |
| Plasma (n, %) | 35 (16.06) | 12 (18.46) | 0.647 | |
| PLR (mL), Me (Q1–Q3) | 300.0 (250.0–500.0) | 300.0 (250.0–600.0) | 0.869 | |
| PLR (n, %) | 42 (19.27) | 16 (24.62) | 0.446 | |
| Drainage (mL), Me (Q1–Q3) | 400.0 (262.5–540.0) | 420.0 (285.0–625.0) | 0.426 | |
| CRP after surgery (mg/L), Me (Q1–Q3) | Day 1 | 74.2 (44.1–94.6) | 67.0 (51.1–72.0) | 0.497 |
| Day 2 | 242.0 (166.0–305.0) | 254.0 (219.0–288.0) | 0.538 | |
| Day 3 | 141.5 (94.5–235.5) | 226.5 (212.5–278.0) | 0.009 | |
| Day 4 | 111.0 (66.0–158.0) | 156.0 (88.0–180.0) | 0.102 | |
| Day 5 | 106.0 (85.5–122.0) | 120.0 (82.5–176.5) | 0.431 | |
| Highest Creatinine (mg/dL), Me (Q1–Q3) | 1.1 (0.9–1.4) | 1.26 (0.9–1.7) | 0.182 | |
| Lowest GFR (mL/min/1.73m2), Me (Q1–Q3) | 66.5 (43.0–86.0) | 51.0 (34.0–78.0) | 0.144 | |
| Pneumonia, n (%) | 131 (3.83) | 86 (11.73) | <0.001 | |
| Hospital mortality, n (%) | 12 (5.50) | 10 (15.38) | 0.009 | |
| Length of hospital stay (LOS), Me (Q1–Q3) | 8.0 (7.0–9.0) | 7.0 (6.0–9.0) | 0.219 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szylińska, A.; Rotter, I.; Listewnik, M.; Lechowicz, K.; Brykczyński, M.; Dzidek, S.; Żukowski, M.; Kotfis, K. Postoperative Delirium in Patients with Chronic Obstructive Pulmonary Disease after Coronary Artery Bypass Grafting. Medicina 2020, 56, 342. https://doi.org/10.3390/medicina56070342
Szylińska A, Rotter I, Listewnik M, Lechowicz K, Brykczyński M, Dzidek S, Żukowski M, Kotfis K. Postoperative Delirium in Patients with Chronic Obstructive Pulmonary Disease after Coronary Artery Bypass Grafting. Medicina. 2020; 56(7):342. https://doi.org/10.3390/medicina56070342
Chicago/Turabian StyleSzylińska, Aleksandra, Iwona Rotter, Mariusz Listewnik, Kacper Lechowicz, Mirosław Brykczyński, Sylwia Dzidek, Maciej Żukowski, and Katarzyna Kotfis. 2020. "Postoperative Delirium in Patients with Chronic Obstructive Pulmonary Disease after Coronary Artery Bypass Grafting" Medicina 56, no. 7: 342. https://doi.org/10.3390/medicina56070342
APA StyleSzylińska, A., Rotter, I., Listewnik, M., Lechowicz, K., Brykczyński, M., Dzidek, S., Żukowski, M., & Kotfis, K. (2020). Postoperative Delirium in Patients with Chronic Obstructive Pulmonary Disease after Coronary Artery Bypass Grafting. Medicina, 56(7), 342. https://doi.org/10.3390/medicina56070342





