Influence of Sedation Level and Ventilation Status on the Diagnostic Validity of Delirium Screening Tools in the ICU—An International, Prospective, Bi-Center Observational Study (IDeAS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
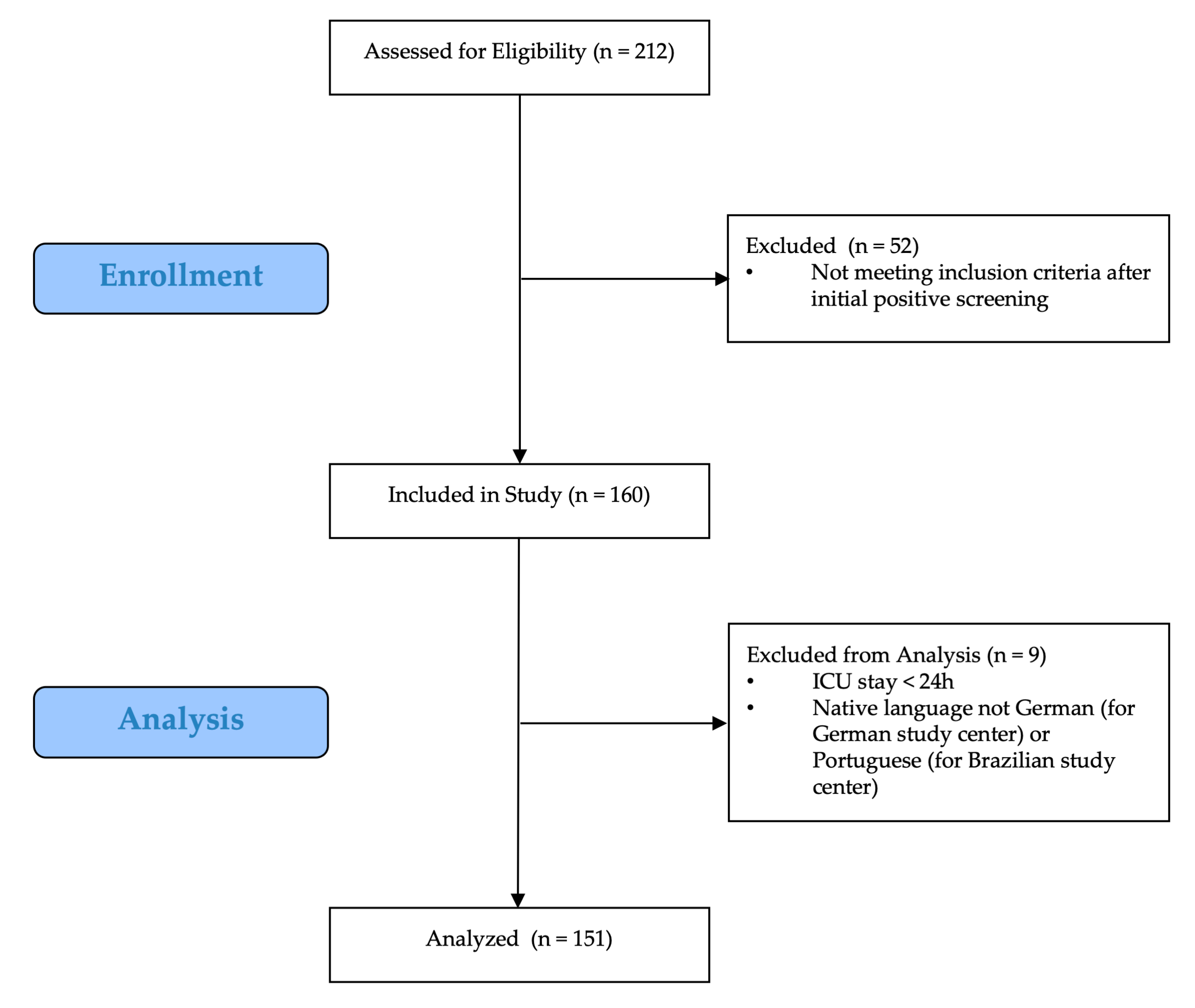
2.2. Study Population
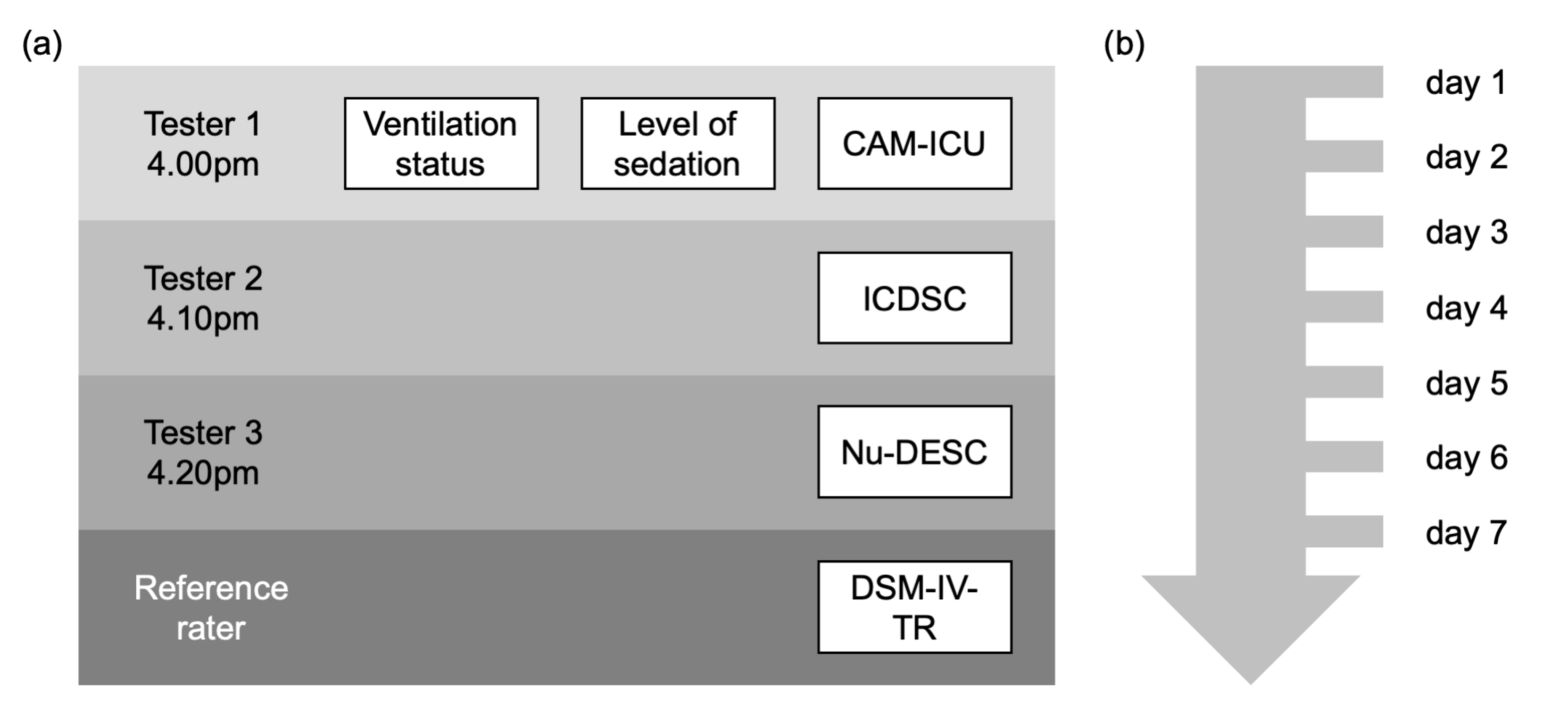
2.3. Delirium Assessment Procedure
2.3.1. Neuropsychiatric Examination According to DSM-IV-TR
2.3.2. Delirium Screening
2.4. Assessment of Covariates and Additional Patient Information
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Study Population and Delirium Incidence Rate
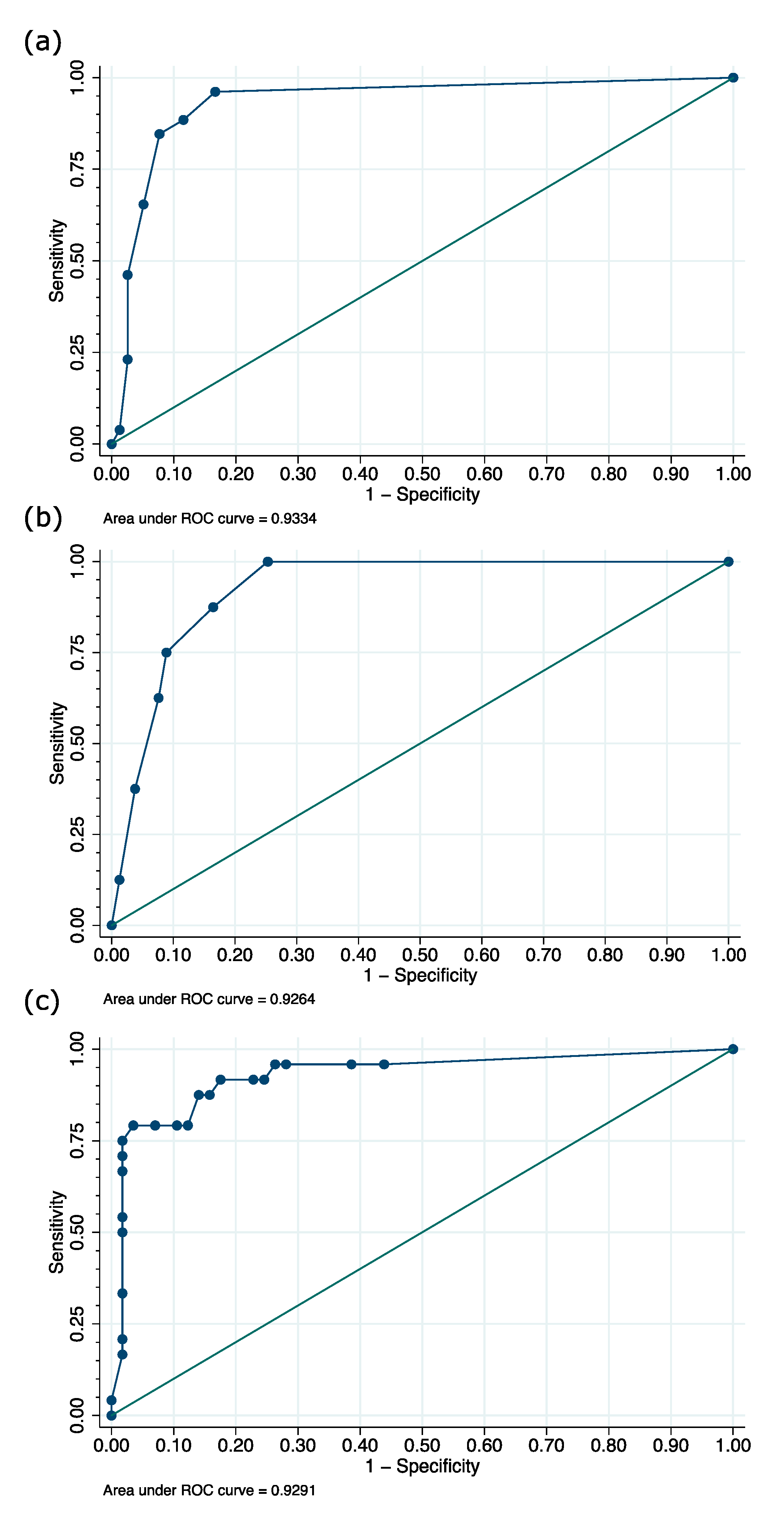
3.2. Validity of Nu-DESC, ICDSC, and CAM-ICU across All Patients
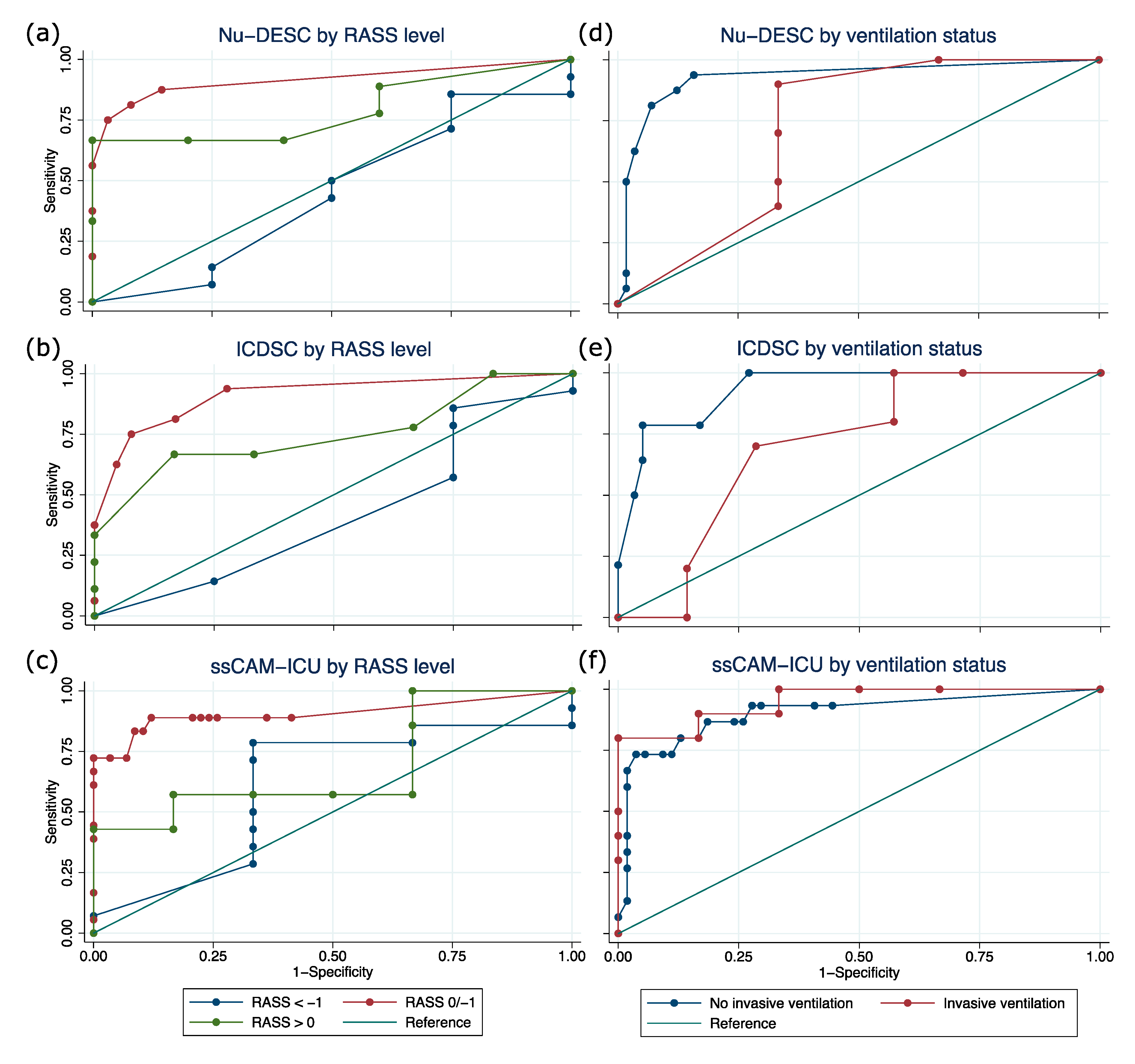
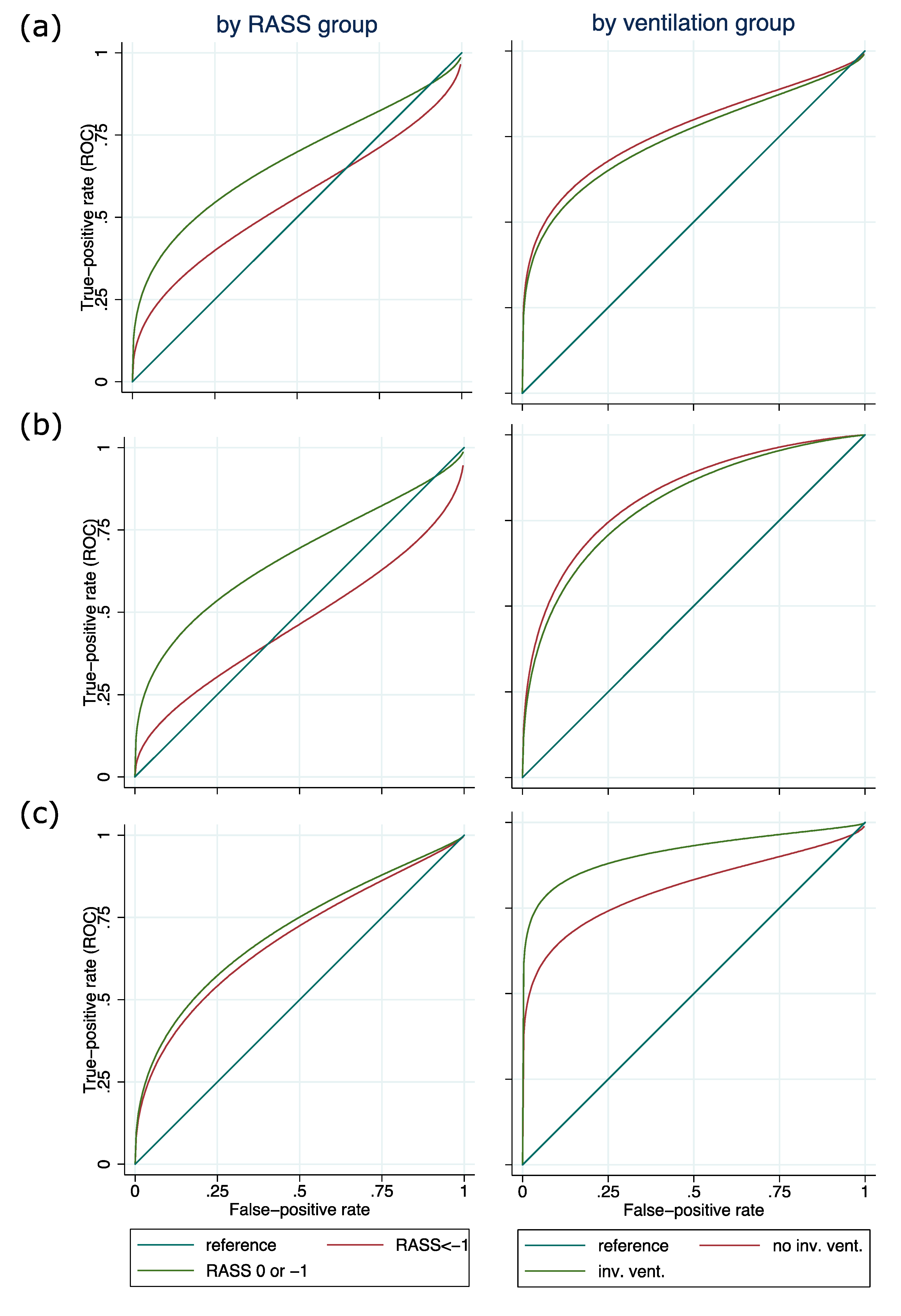
3.3. Validity of Delirium Assessment Using Nu-DESC, ICDSC, and CAM-ICU Depending on Sedation Level and Ventilation Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Delirium | Yes | 22 | 14.6 | 14 | 14.3 | 15 | 22.4 | 15 | 28.3 | 12 | 27.9 | 11 | 30.6 | 5 | 15.6 |
| No | 73 | 48.3 | 39 | 39.8 | 33 | 49.3 | 23 | 43.4 | 20 | 46.5 | 16 | 44.4 | 13 | 40.6 | |
| RASS < –3 | 51 | 33.8 | 30 | 30.6 | 14 | 20.9 | 9 | 17.0 | 7 | 16.3 | 7 | 19.4 | 6 | 18.8 | |
| No data | 5 | 3.3 | 15 | 15.3 | 5 | 7.5 | 6 | 11.3 | 4 | 9.3 | 2 | 5.6 | 8 | 25.0 | |
| RASS group | <–1 | 35 | 23.2 | 19 | 19.4 | 21 | 31.3 | 18 | 34.0 | 15 | 34.9 | 11 | 30.6 | 10 | 31.3 |
| –1/0 | 91 | 60.3 | 60 | 61.2 | 36 | 53.7 | 24 | 45.3 | 20 | 46.5 | 19 | 52.8 | 19 | 59.4 | |
| >0 | 8 | 5.3 | 11 | 11.2 | 4 | 6.0 | 5 | 9.4 | 4 | 9.3 | 3 | 8.3 | 3 | 9.4 | |
| No data | 17 | 11.3 | 8 | 8.2 | 6 | 9.0 | 6 | 11.3 | 4 | 9.3 | 3 | 8.3 | 0 | 0.0 | |
| Invasive ventilation | Yes | 40 | 26.5 | 23 | 23.5 | 24 | 35.8 | 20 | 37.7 | 17 | 39.5 | 15 | 41.7 | 17 | 53.1 |
| No | 92 | 60.9 | 63 | 64.3 | 39 | 58.2 | 27 | 50.9 | 23 | 53.5 | 18 | 50.0 | 13 | 40.6 | |
| No data | 19 | 12.6 | 12 | 12.2 | 4 | 6.0 | 6 | 11.3 | 3 | 7.0 | 3 | 8.3 | 2 | 6.3 | |
| Total | 151 | 100.0 | 98 | 100.0 | 67 | 100.0 | 53 | 100.0 | 43 | 100.0 | 36 | 100.0 | 32 | 100.0 | |
| DSI | Subgroup | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Youden Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Estimate (%) | CI (%) | Estimate (%) | CI (%) | Estimate (%) | CI (%) | Estimate (%) | CI (%) | |||
| Nu-DESC | RASS <−1 | 18 | 85.7 | 57.2–98.2 | 0.0 | 0.0–60.2 | 75.0 | 47.6–92.7 | 0.0 | 0.0–84.2 | −0.14 |
| RASS −1/0 | 78 | 81.2 | 54.4–96.0 | 91.9 | 82.2–97.3 | 72.2 | 46.5–90.3 | 95.0 | 86.1–99.0 | 0.73 | |
| RASS > 0 | 14 | 77.8 | 40.0–97.2 | 40.0 | 5.3–85.3 | 70.0 | 34.8–93.3 | 50.0 | 6.8–93.2 | 0.18 | |
| Inv. Vent. | 16 | 90.0 | 55.5–99.7 | 66.7 | 22.3–95.7 | 81.8 | 48.2–97.7 | 80.0 | 28.4–99.5 | 0.57 | |
| No Inv. Vent. | 73 | 87.5 | 61.7–98.4 | 87.7 | 76.3–94.9 | 66.7 | 43.0–85.4 | 96.2 | 86.8–99.5 | 0.75 | |
| ICDSC | RASS <−1 | 18 | 78.6 | 49.2–95.3 | 25.0 | 0.6–80.6 | 78.6 | 49.2–95.3 | 25.0 | 0.6–80.6 | 0.04 |
| RASS −1/0 | 81 | 62.5 | 35.4–84.8 | 95.4 | 87.1–99.0 | 76.9 | 46.2–95.0 | 91.2 | 81.8–96.7 | 0.58 | |
| RASS > 0 | 15 | 66.7 | 29.9–92.5 | 83.3 | 35.9–99.6 | 85.7 | 42.1–99.6 | 62.5 | 24.5–91.5 | 0.50 | |
| Inv. Vent. | 17 | 70.0 | 34.8–93.3 | 71.4 | 29.0–96.3 | 77.8 | 40.0–97.2 | 62.5 | 24.5–91.5 | 0.41 | |
| No Inv. Vent. | 73 | 64.3 | 35.1–87.2 | 94.9 | 85.9–98.9 | 75.0 | 42.8–94.5 | 91.8 | 81.9–97.3 | 0.59 | |
| CAM-ICU | RASS < −1 | 19 | 86.7 | 59.5–98.3 | 50.0 | 6.8–93.2 | 86.7 | 59.5–98.3 | 50.0 | 6.8–93.2 | 0.37 |
| RASS −1/0 | 76 | 72.2 | 46.5–90.3 | 100.0 | 93.8–100.0 | 100.0 | 75.3–100 | 92.1 | 82.4–97.4 | 0.72 | |
| RASS > 0 | 14 | 62.5 | 24.5–91.5 | 50.0 | 11.8–88.2 | 62.5 | 24.5–91.5 | 50.0 | 11.8–88.2 | 0.13 | |
| Inv. Vent. | 18 | 90.9 | 58.7–99.8 | 71.4 | 29.0–96.3 | 83.3 | 51.6–97.9 | 83.3 | 35.9–99.6 | 0.62 | |
| No Inv. Vent. | 69 | 68.8 | 41.3–89.0 | 96.2 | 87.0–99.5 | 84.6 | 54.6–98.1 | 91.1 | 80.4–97.0 | 0.65 | |
References
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, J.F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Gautam, S.; Margolin, R.; Francis, J.; May, L.; Speroff, T.; Truman, B.; Dittus, R.; Bernard, G.; Inouye, S. The impact of delirium in the intensive care unit on hospital length of stay. Intensiv. Care Med. 2001, 27, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.; Morandi, A.; Thompson, J.; Pun, B.; Brummel, N.; Hughes, C.; Vasilevskis, E.; Shintani, A.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Biniek, R.; Braune, S.; Buerkle, H.; Dall, P.; Demirakca, S.; Eckardt, R.; Eggers, V.; Eichler, I.; et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)—Short version. Ger. Med. Sci. 2015, 13, Doc19. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association DSM-V Task Force. Diagnostic and statistical manual of mental disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Ely, E.W.; Margolin, R.; Francis, J.; May, L.; Truman, B.; Dittus, R.; Speroff, T.; Gautam, S.; Bernard, G.R.; Inouye, S.K. Evaluation of delirium in critically ill patients: Validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit. Care Med. 2001, 29, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Fong, J.J.; Schumaker, G.; O’Connor, H.; Ruthazer, R.; Garpestad, E. Use of a validated delirium assessment tool improves the ability of physicians to identify delirium in medical intensive care unit patients. Crit. Care Med. 2007, 35, 2721–2724. [Google Scholar] [CrossRef]
- Gaudreau, J.-D.; Gagnon, P.; Harel, F.; Tremblay, A.; Roy, M.-A. Fast, systematic, and continuous delirium assessment in hospitalized patients: The nursing delirium screening scale. J. Pain Symptom Manag. 2005, 29, 368–375. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the tntensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Gusmao-Flores, D.; Salluh, J.I.F.; Dal-Pizzol, F.; Ritter, C.; Tomasi, C.D.; De Lima, M.A.S.D.; Santana, L.R.; Lins, R.M.P.; Lemos, P.P.; Serpa, G.V.; et al. The validity and reliability of the Portuguese versions of three tools used to diagnose delirium in critically ill patients. Clinics (Sao Paulo) 2011, 66, 1917–1922. [Google Scholar]
- Ouimet, S.; Riker, R.; Bergeon, N.; Cossette, M.; Kavanagh, B.; Skrobik, Y. Subsyndromal delirium in the ICU: Evidence for a disease spectrum. Intensiv. Care Med. 2007, 33, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Gusmao-Flores, D.; Salluh, J.I.F.; Chalhub, R.Á.; Quarantini, L.C. The confusion assessment method for the intensive care unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the diagnosis of delirium: A systematic review and meta-analysis of clinical studies. Crit. Care 2012, 16, R115. [Google Scholar] [CrossRef] [PubMed]
- Luetz, A.; Heymann, A.; Radtke, F.M.; Chenitir, C.; Neuhaus, U.; Nachtigall, I.; Von Dossow, V.; Marz, S.; Eggers, V.; Heinz, A.; et al. Different assessment tools for intensive care unit delirium: Which score to use? Crit. Care Med. 2010, 38, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Van Velthuijsen, E.L.; Zwakhalen, S.; Warnier, R.M.; Mulder, W.J.; Verhey, F.R.J.; Kempen, G. Psychometric properties and feasibility of instruments for the detection of delirium in older hospitalized patients: A systematic review. Int. J. Geriatr. Psychiatry 2016, 31, 974–989. [Google Scholar] [CrossRef]
- Luetz, A.; Gensel, D.; Müller, J.; Weiss, B.; Martiny, V.; Heinz, A.; Wernecke, K.D.; Spies, C.D. Validity of different delirium assessment tools for critically ill children: Covariates matter. Crit. Care Med. 2016, 44, 2060–2069. [Google Scholar] [CrossRef]
- Khan, B.A.; Perkins, A.J.; Gao, S.; Hui, S.L.; Campbell, N.L.; Farber, M.O.; Chlan, L.L.; Boustani, M.A. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: A novel delirium severity instrument for use in the intensive care unit. Crit. Care Med. 2017, 45, 851–857. [Google Scholar] [CrossRef]
- Nelson, L.S. Teaching staff nurses the CAM-ICU for delirium screening. Crit. Care Nurs. Q. 2009, 32, 137–143. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Devlin, J.W.; Brummel, N.E.; Al-Qadheeb, N.S. Optimising the recognition of delirium in the intensive care unit. Best Pract. Res. Clin. Anaesthesiol. 2012, 26, 385–393. [Google Scholar] [CrossRef]
- McCusker, J.; Cole, M.G.; Voyer, P.; Monette, J.; Champoux, N.; Ciampi, A.; Vu, M.; Belzile, E. Use of nurse-observed symptoms of delirium in long-term care: Effects on prevalence and outcomes of delirium. Int. Psychogeriatr 2011, 23, 602–608. [Google Scholar] [CrossRef]
- Van Eijk, M.M.J.; Van Marum, R.J.; Klijn, I.A.M.; De Wit, N.; Kesecioglu, J.; Slooter, A.J.C. Comparison of delirium assessment tools in a mixed intensive care unit. Crit. Care Med. 2009, 37, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.M.; Boogaard, M.V.D.; Van Marum, R.J.; Benner, P.; Eikelenboom, P.; Honing, M.L.; Van Der Hoven, B.; Horn, J.; Izaks, G.J.; Kalf, A.; et al. Routine use of the confusion assessment method for the intensive care unit: A multicenter study. Am. J. Respir. Crit. Care Med. 2011, 184, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Meagher, D.J.; Morandi, A.; Inouye, S.K.; Ely, E.W.; Adamis, D.; MacLullich, A.M.; Rudolph, J.L.; Neufeld, K.; Leonard, M.; Bellelli, G.; et al. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.; Hayat, M.J.; Coughlin, J.M.; Huberman, A.L.; Leistikow, N.A.; Krumm, S.K.; Needham, D.M. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics 2011, 52, 133–140. [Google Scholar] [CrossRef]
- Radtke, F.M.; Franck, M.; Schust, S.; Boehme, L.; Pascher, A.; Bail, H.J.; Seeling, M.; Luetz, A.; Wernecke, K.-D.; Heinz, A.; et al. A comparison of three scores to screen for delirium on the surgical ward. World J. Surg. 2010, 34, 487–494. [Google Scholar] [CrossRef]
- Boettger, S.; Nuñez, D.G.; Meyer, R.; Richter, A.; Rudiger, A.; Schubert, M.; Jenewein, J. Screening for delirium with the Intensive Care Delirium Screening Checklist (ICDSC): A re-evaluation of the threshold for delirium. Swiss Med. Wkly. 2018, 148, w14597. [Google Scholar] [CrossRef]
- George, C.; Nair, J.S.; Ebenezer, J.A.; Gangadharan, A.; Christudas, A.; Gnanaseelan, L.K.; Jacob, K. Validation of the Intensive Care Delirium Screening Checklist in nonintubated intensive care unit patients in a resource-poor medical intensive care setting in South India. J. Crit. Care 2011, 26, 138–143. [Google Scholar] [CrossRef]
- Patel, S.B.; Poston, J.T.; Pohlman, A.; Hall, J.; Kress, J.P. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am. J. Respir. Crit. Care Med. 2014, 189, 658–665. [Google Scholar] [CrossRef]
- European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014, 12, 141. [Google Scholar] [CrossRef]
- Radtke, F.M.; Franck, M.; Schneider, M.; Luetz, A.; Seeling, M.; Heinz, A.; Wernecke, K.D.; Spies, C.D. Comparison of three scores to screen for delirium in the recovery room. Br. J. Anaesth. 2008, 101, 338–343. [Google Scholar] [CrossRef][Green Version]
- Neufeld, K.; Nelliot, A.; Inouye, S.K.; Ely, E.W.; Bienvenu, O.J.; Lee, H.B.; Needham, D.M. Delirium diagnosis methodology used in research: A survey-based study. Am. J. Geriatr. Psychiatry 2014, 22, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
| No Delirium (n = 116) | Delirium * (n = 35) | p | |
|---|---|---|---|
| Age, yr | 67 (53–75) a | 68 (50–74) a | 0.824 b |
| Height, cm | 170 (163–175) a | 168 (160–177) a | 0.702 b |
| Weight, kg | 75 (65–85) a | 73 (65–80) a | 0.415 b |
| BMI, kg/m2 | 25 (23–29) a | 25 (23–29) a | 0.784 b |
| APACHE II score | 14.5 (11–20) a | 22 (17–28) a | <0.001 b |
| SAPS II score | 30 (23.5–42) a | 45 (37–64) a | <0.001 b |
| SOFA score | 4 (2–7) a | 10 (5–13) a | <0.001 b |
| Male, n | 61 | 17 | 0.703 c |
| Admission mode, n | |||
| Emergency | 7 | 10 | <0.001 d |
| Medical | 45 | 14 | |
| Surgical | 64 | 11 | |
| Diagnose group, n | |||
| Acute respiratory failure | 33 | 13 | <0.001 d |
| Surgical, postoperative | 52 | 3 | |
| Trauma, bleeding | 10 | 10 | |
| Others | 21 | 9 | |
| DSI | n | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (%) | CI (%) | Estimate (%) | CI (%) | Estimate (%) | CI (%) | Estimate (%) | CI (%) | ||
| Nu-DESC 1,2 | 104 | 88.5 | 69.8–97.6 | 88.5 | 79.2–94.6 | 71.9 | 53.3–86.3 | 95.8 | 88.3–99.1 |
| ICDSC 1,3 | 103 | 62.5 | 40.6–81.2 | 92.4 | 84.2–97.2 | 71.4 | 47.8–88.7 | 89.0 | 80.2–94.9 |
| CAM-ICU 2,3 | 81 | 75.0 | 53.3–90.2 | 94.7 | 85.4–98.9 | 85.7 | 63.7–97.0 | 90.0 | 79.5–96.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacul, F.E.; Paul, N.; Spies, C.D.; Sechting, H.; Hecht, T.; Dullinger, J.S.; Piper, S.K.; Luetz, A.; Balzer, F.S.; Wernecke, K.-D.; et al. Influence of Sedation Level and Ventilation Status on the Diagnostic Validity of Delirium Screening Tools in the ICU—An International, Prospective, Bi-Center Observational Study (IDeAS). Medicina 2020, 56, 411. https://doi.org/10.3390/medicina56080411
Nacul FE, Paul N, Spies CD, Sechting H, Hecht T, Dullinger JS, Piper SK, Luetz A, Balzer FS, Wernecke K-D, et al. Influence of Sedation Level and Ventilation Status on the Diagnostic Validity of Delirium Screening Tools in the ICU—An International, Prospective, Bi-Center Observational Study (IDeAS). Medicina. 2020; 56(8):411. https://doi.org/10.3390/medicina56080411
Chicago/Turabian StyleNacul, Flavio E., Nicolas Paul, Claudia D. Spies, Henriette Sechting, Thomas Hecht, Jörn S. Dullinger, Sophie K. Piper, Alawi Luetz, Felix S. Balzer, Klaus-Dieter Wernecke, and et al. 2020. "Influence of Sedation Level and Ventilation Status on the Diagnostic Validity of Delirium Screening Tools in the ICU—An International, Prospective, Bi-Center Observational Study (IDeAS)" Medicina 56, no. 8: 411. https://doi.org/10.3390/medicina56080411
APA StyleNacul, F. E., Paul, N., Spies, C. D., Sechting, H., Hecht, T., Dullinger, J. S., Piper, S. K., Luetz, A., Balzer, F. S., Wernecke, K.-D., Sa, A. K., Barros Ferreira da Costa, C., Eymold, L., Chenitir, C., & Weiss, B. (2020). Influence of Sedation Level and Ventilation Status on the Diagnostic Validity of Delirium Screening Tools in the ICU—An International, Prospective, Bi-Center Observational Study (IDeAS). Medicina, 56(8), 411. https://doi.org/10.3390/medicina56080411





