Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy
Abstract
1. Introduction
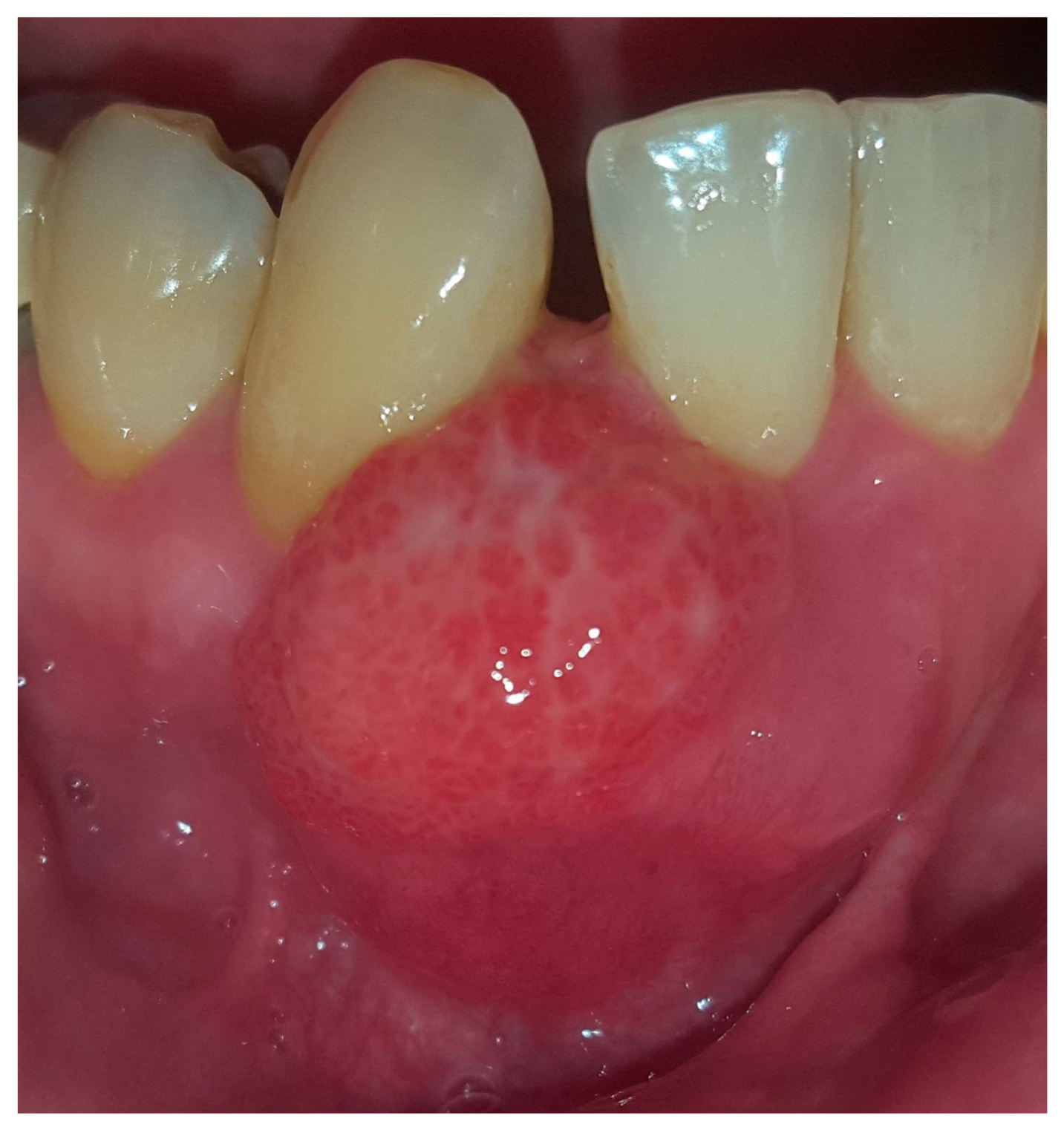
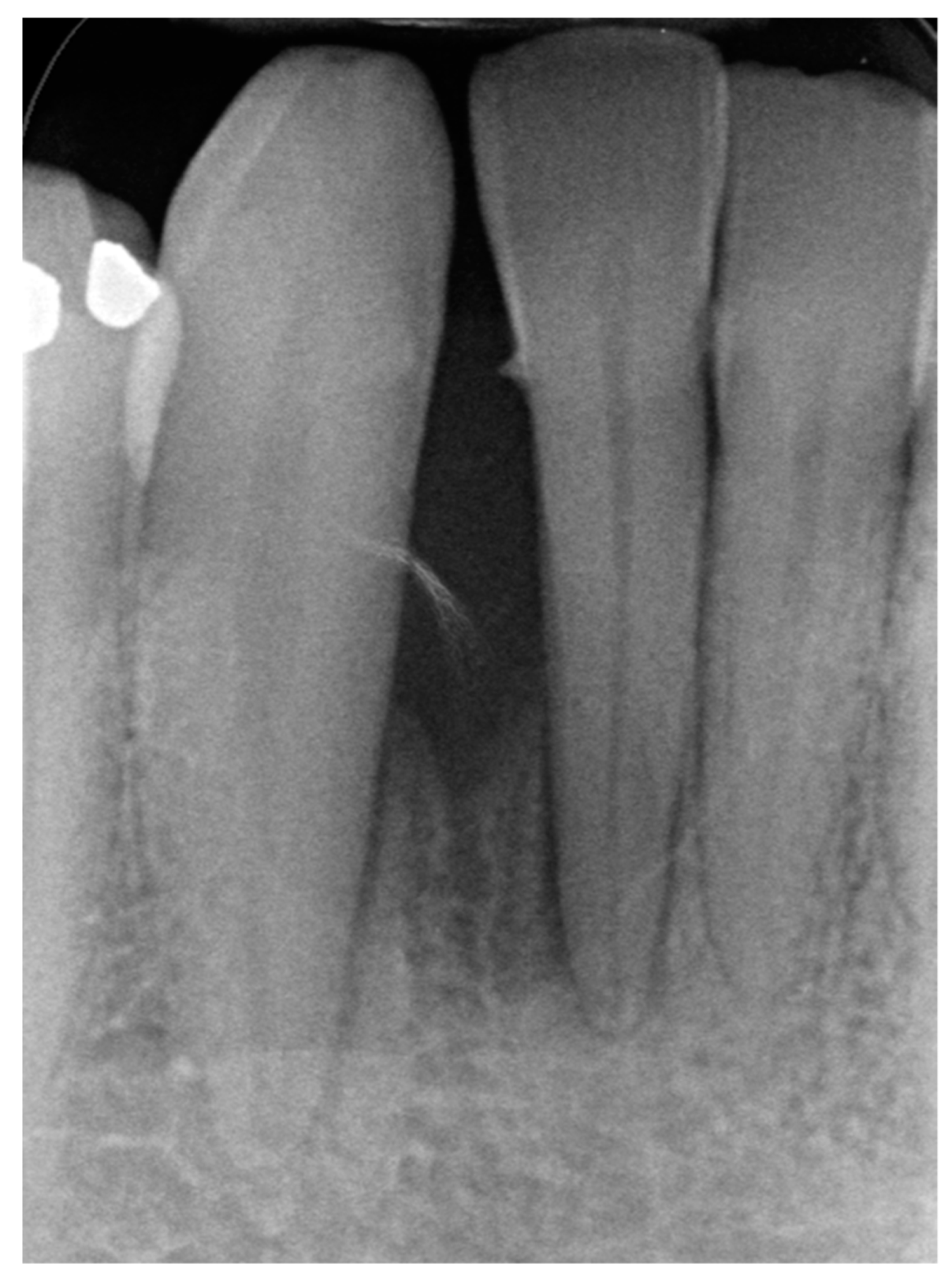
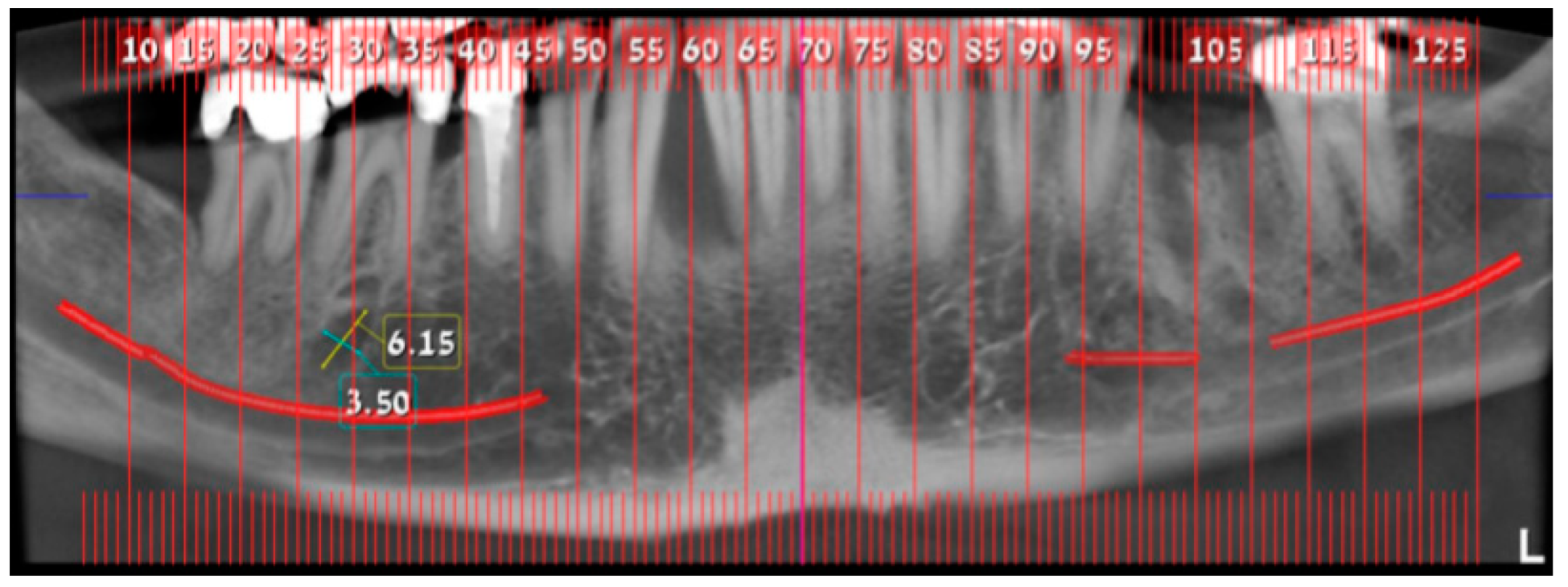
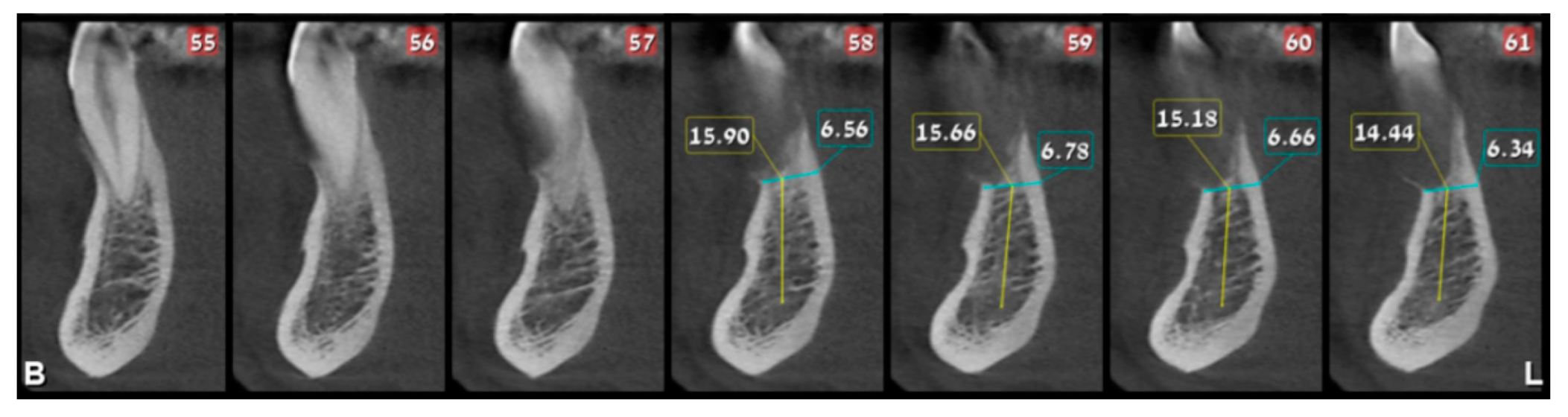
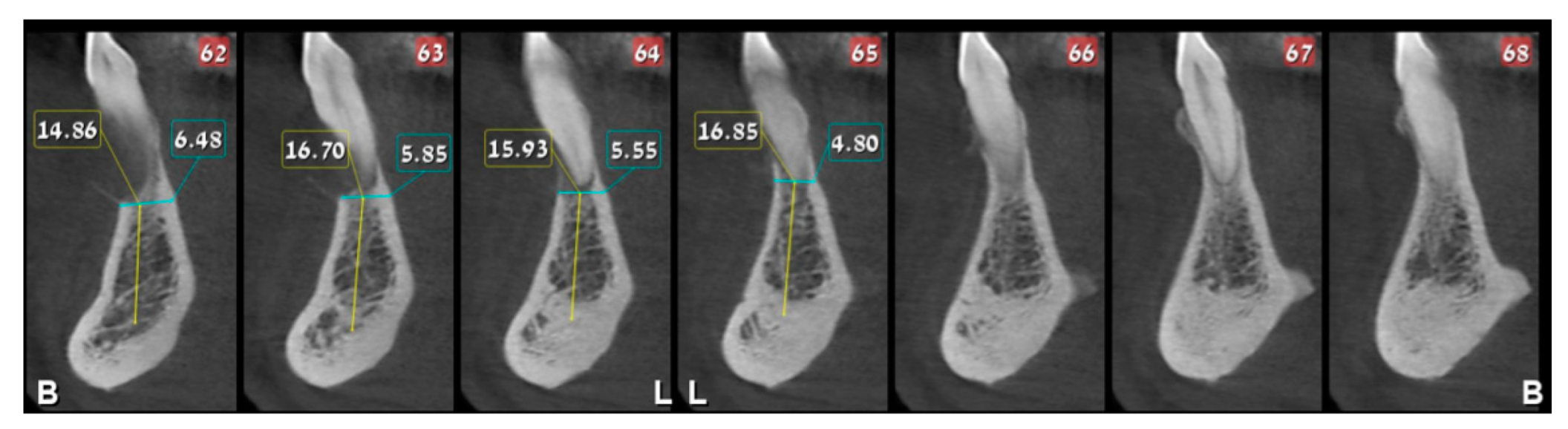
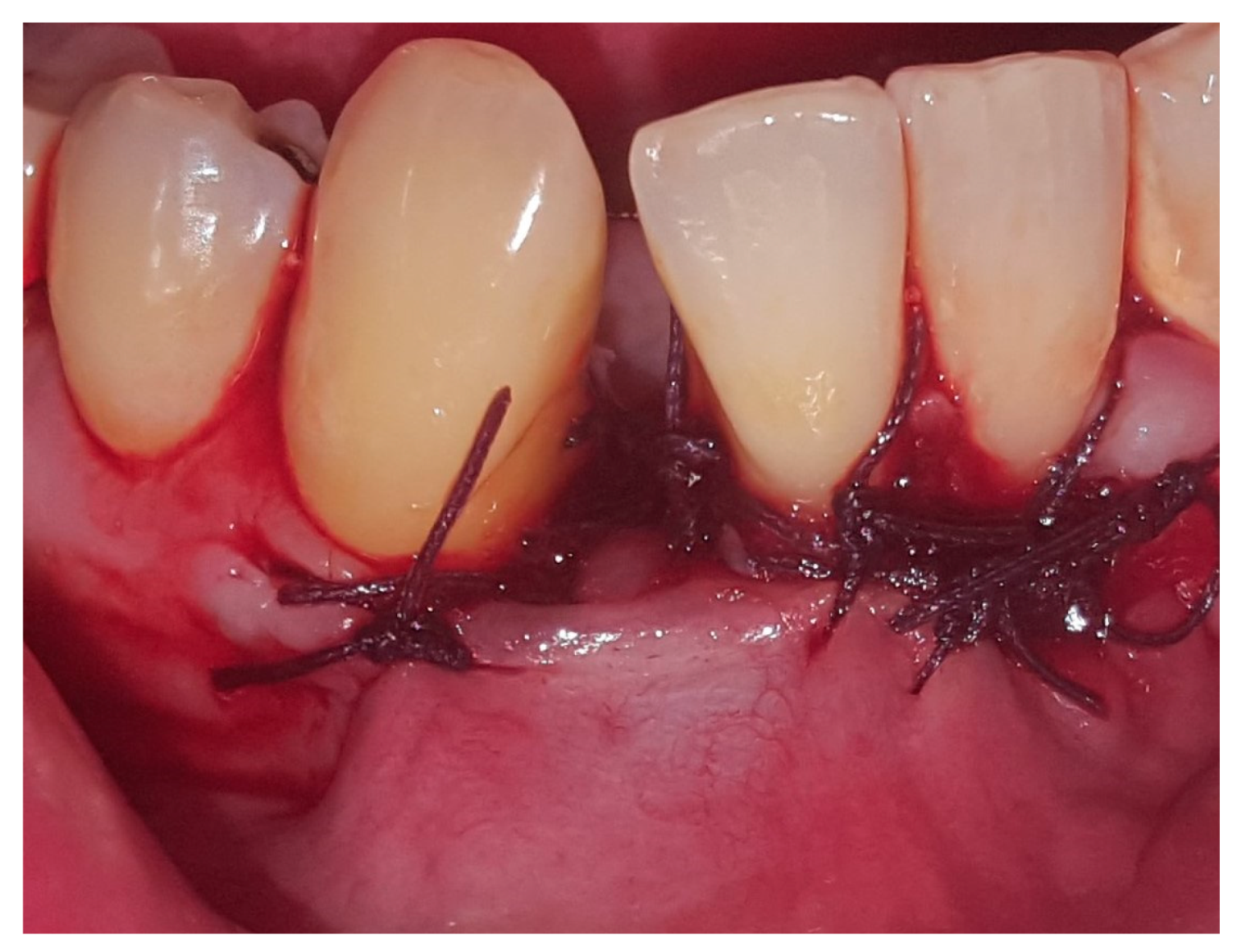
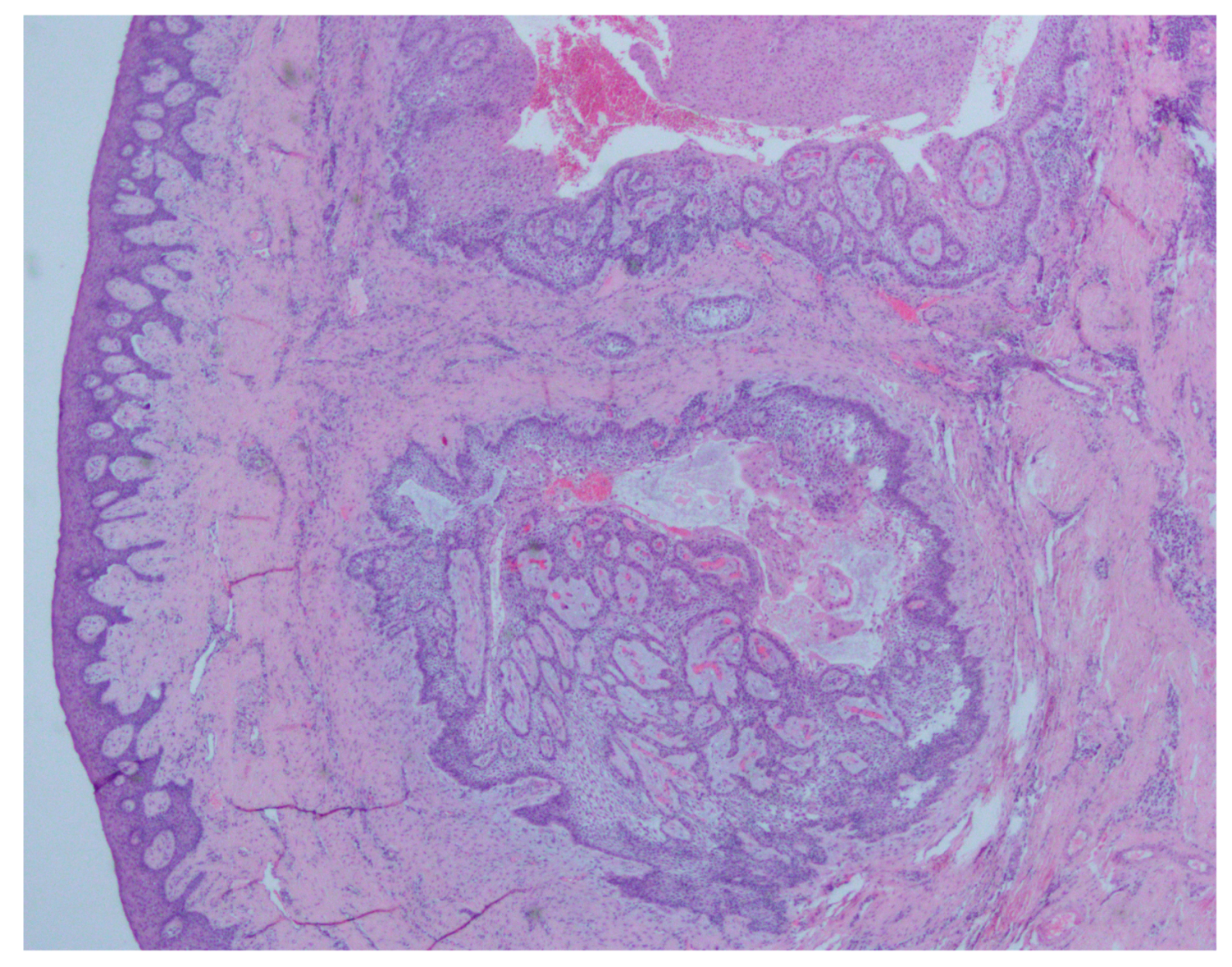
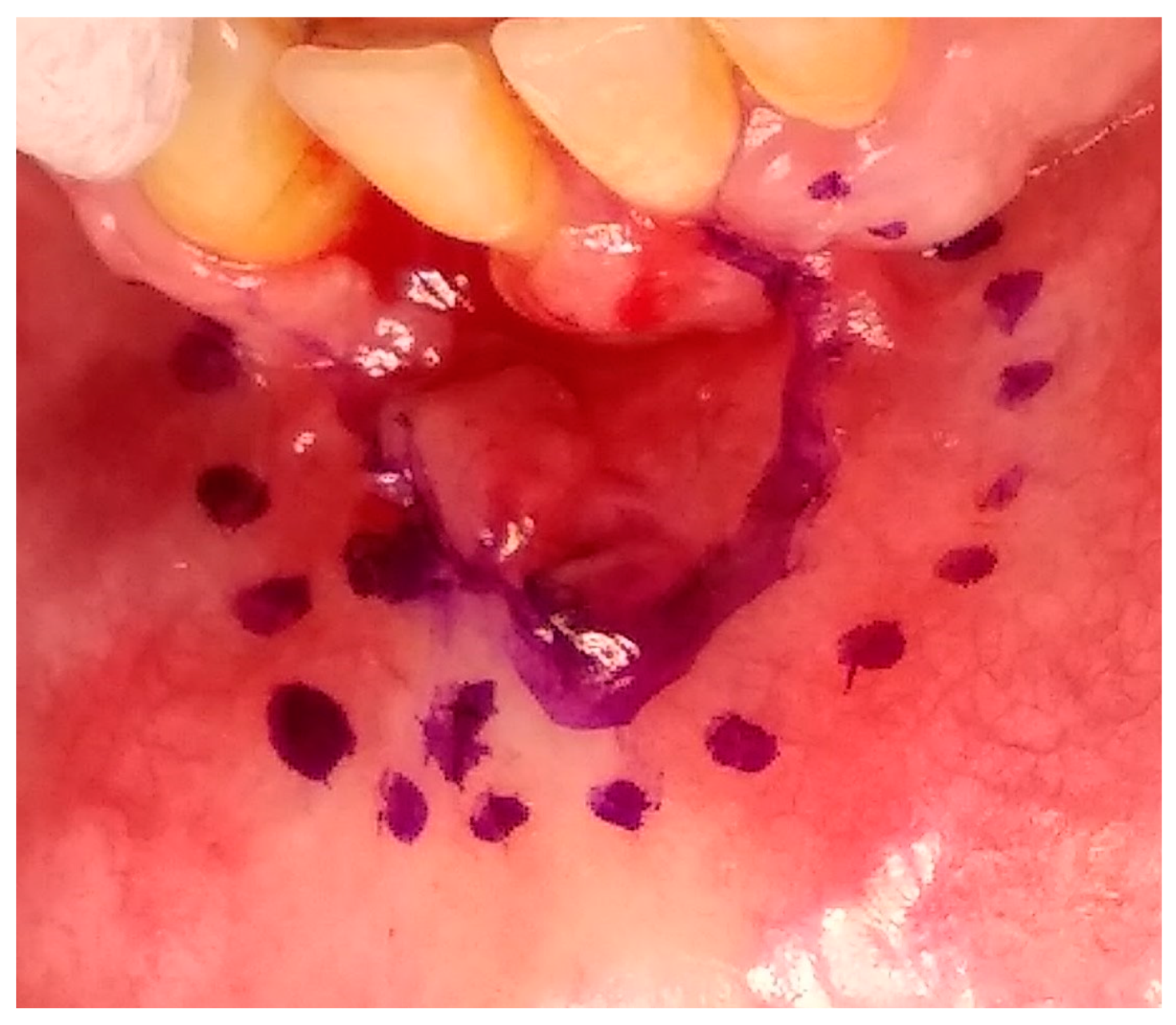
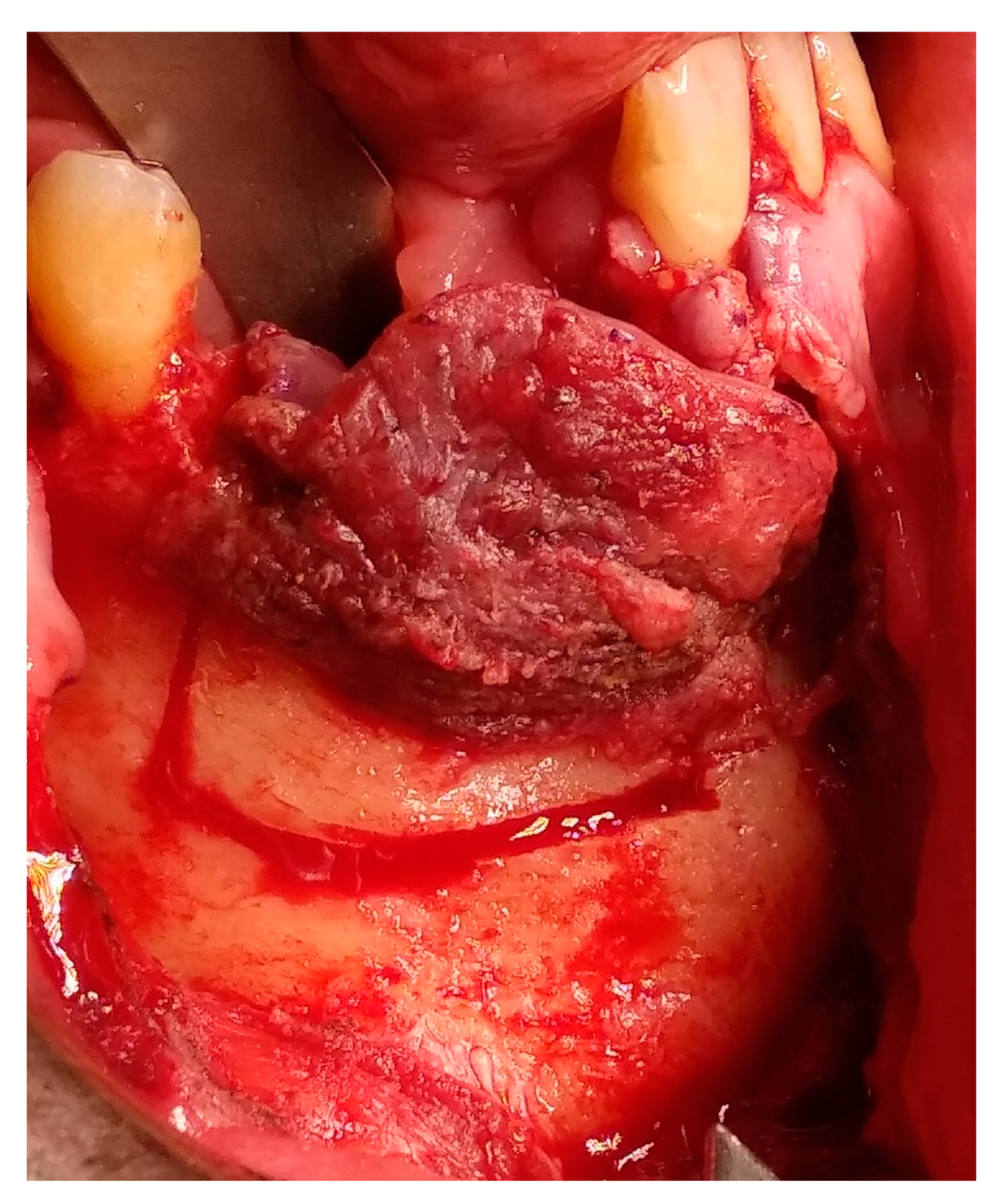
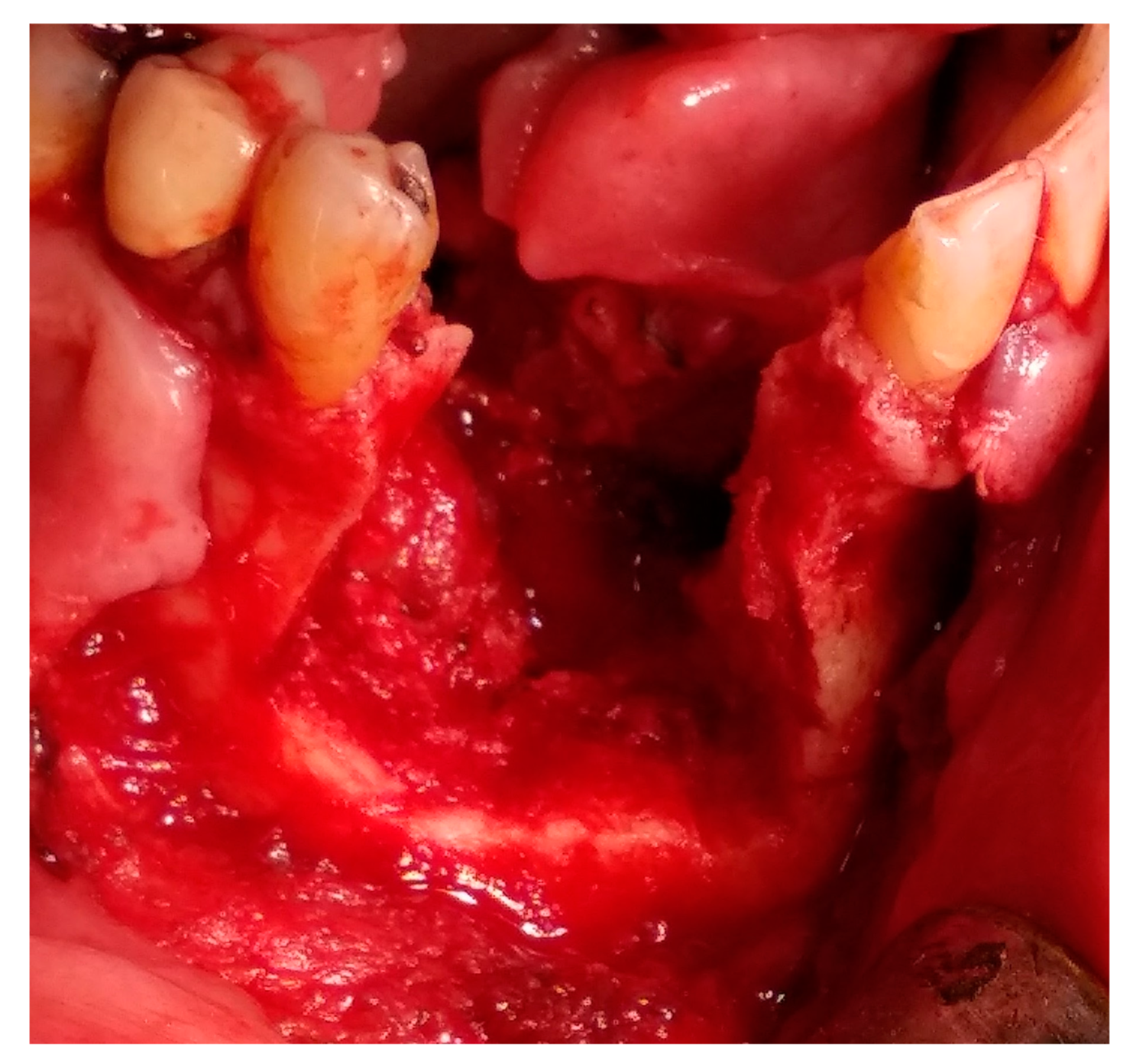
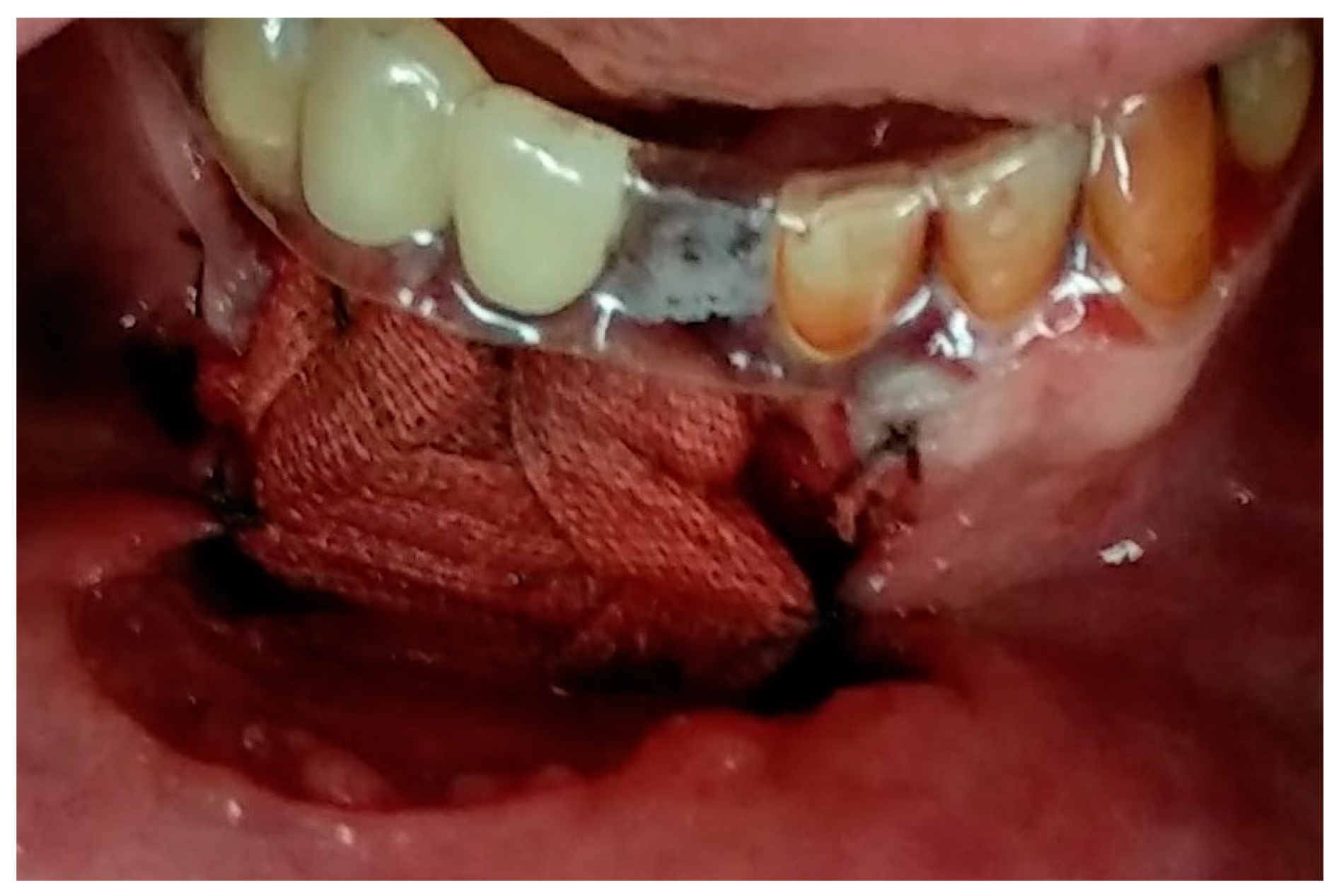
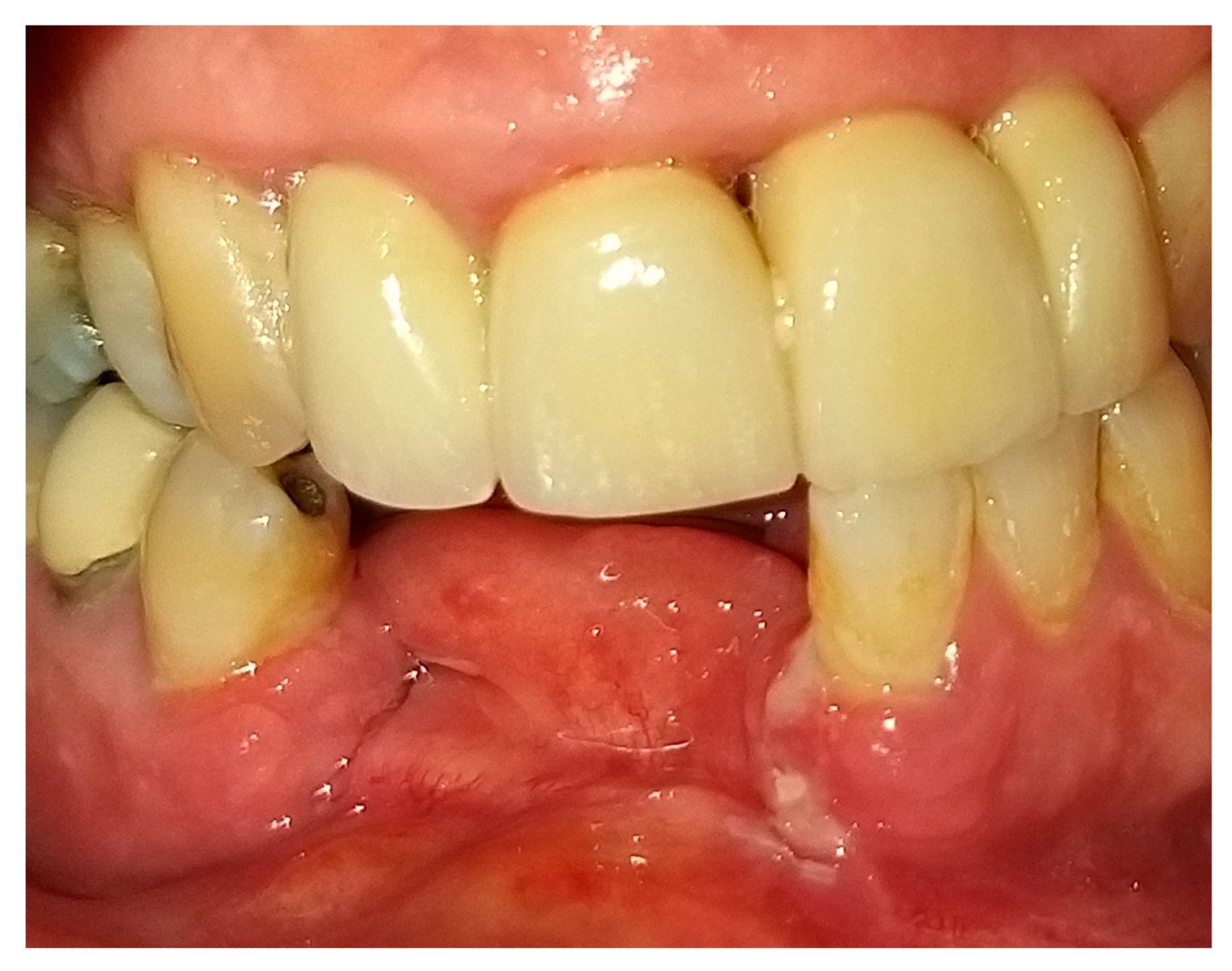
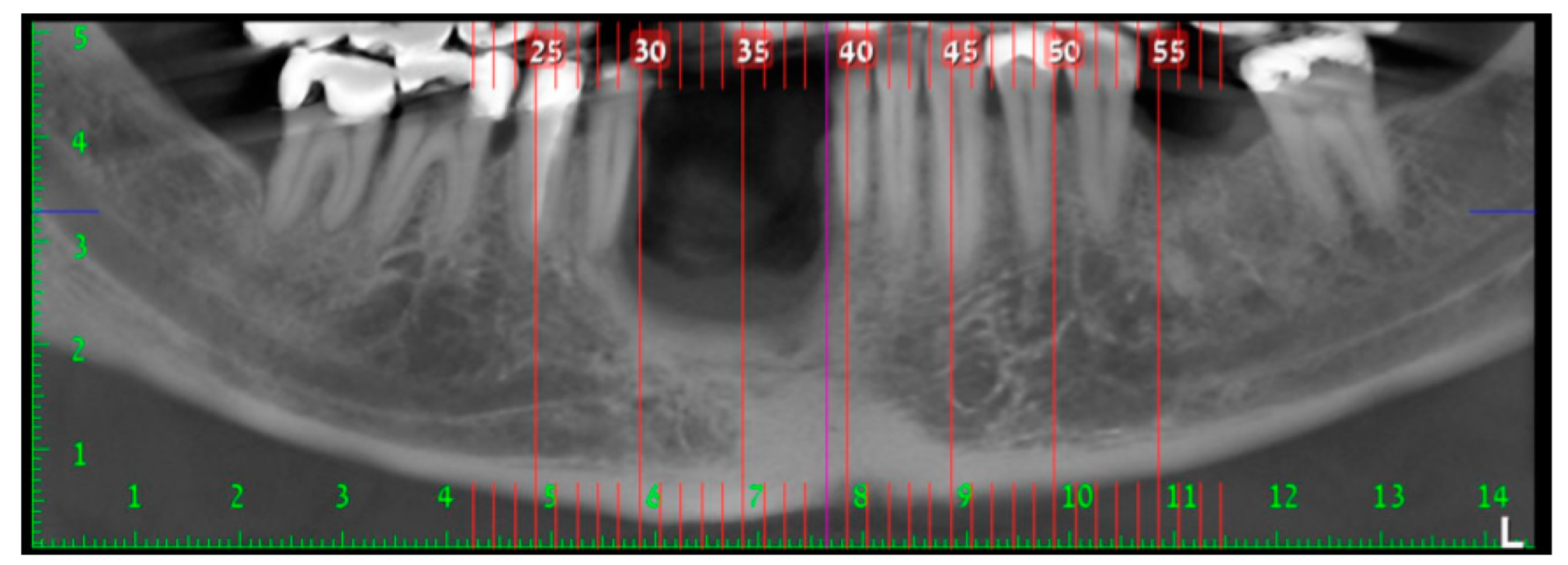
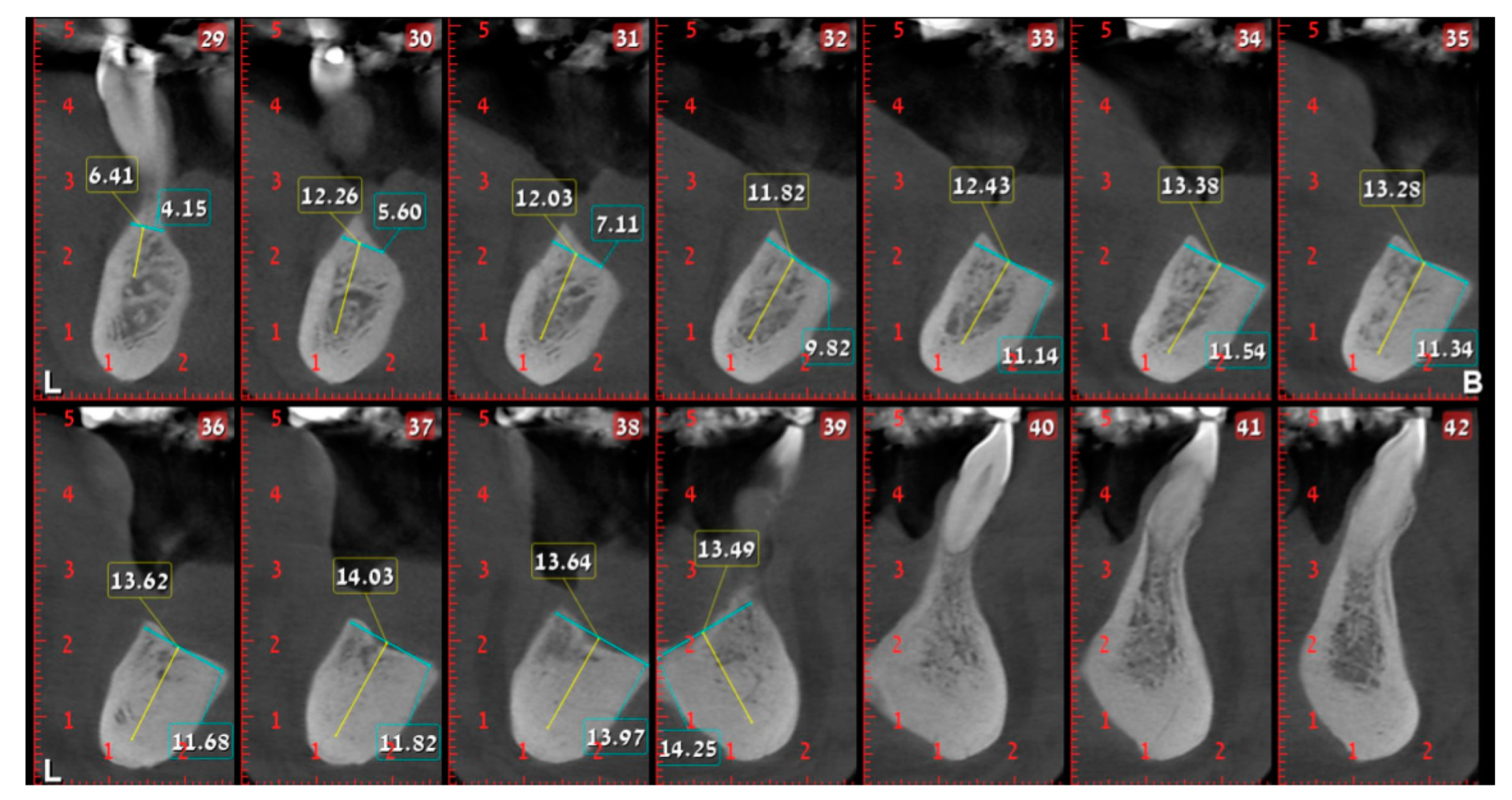
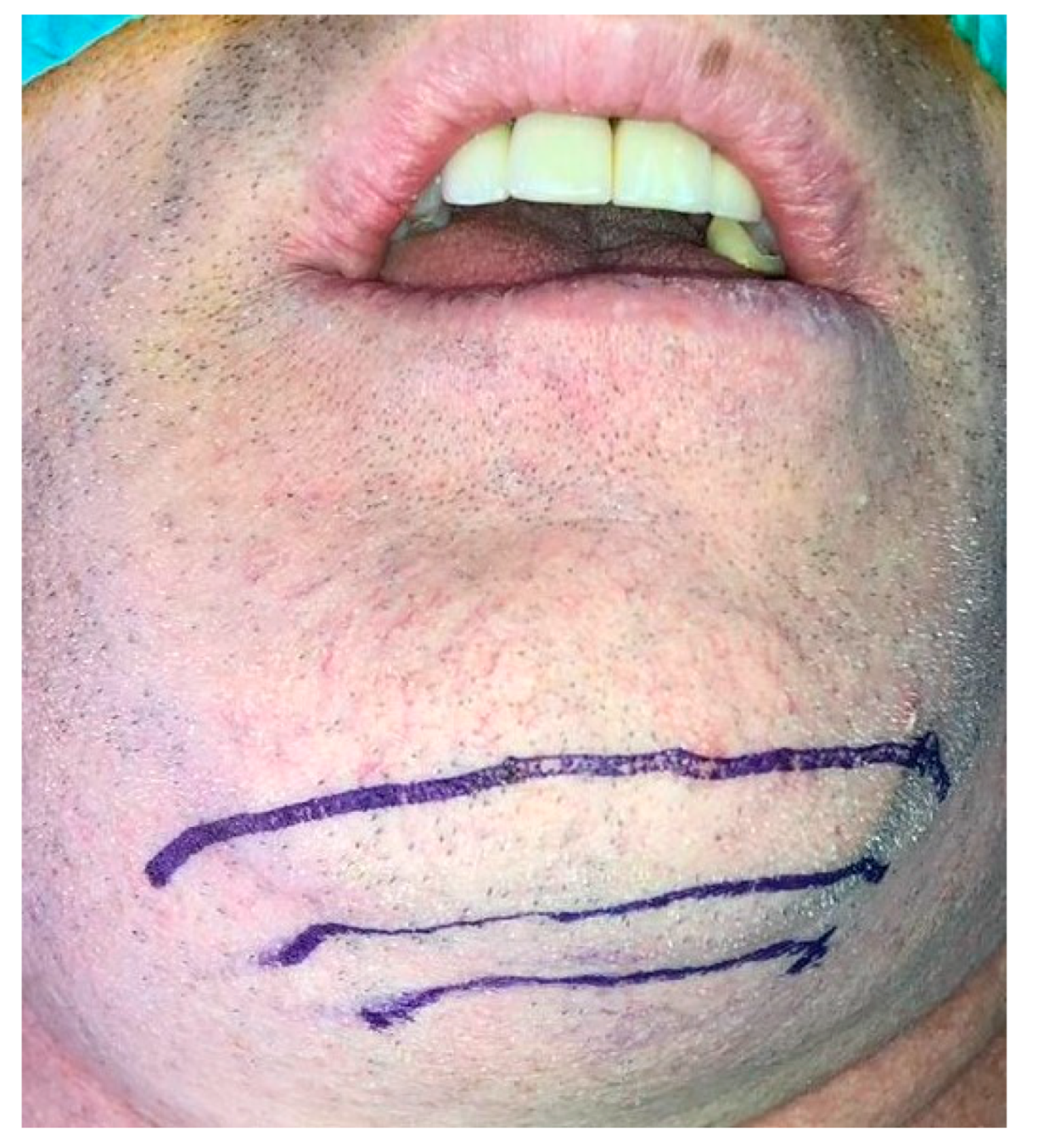
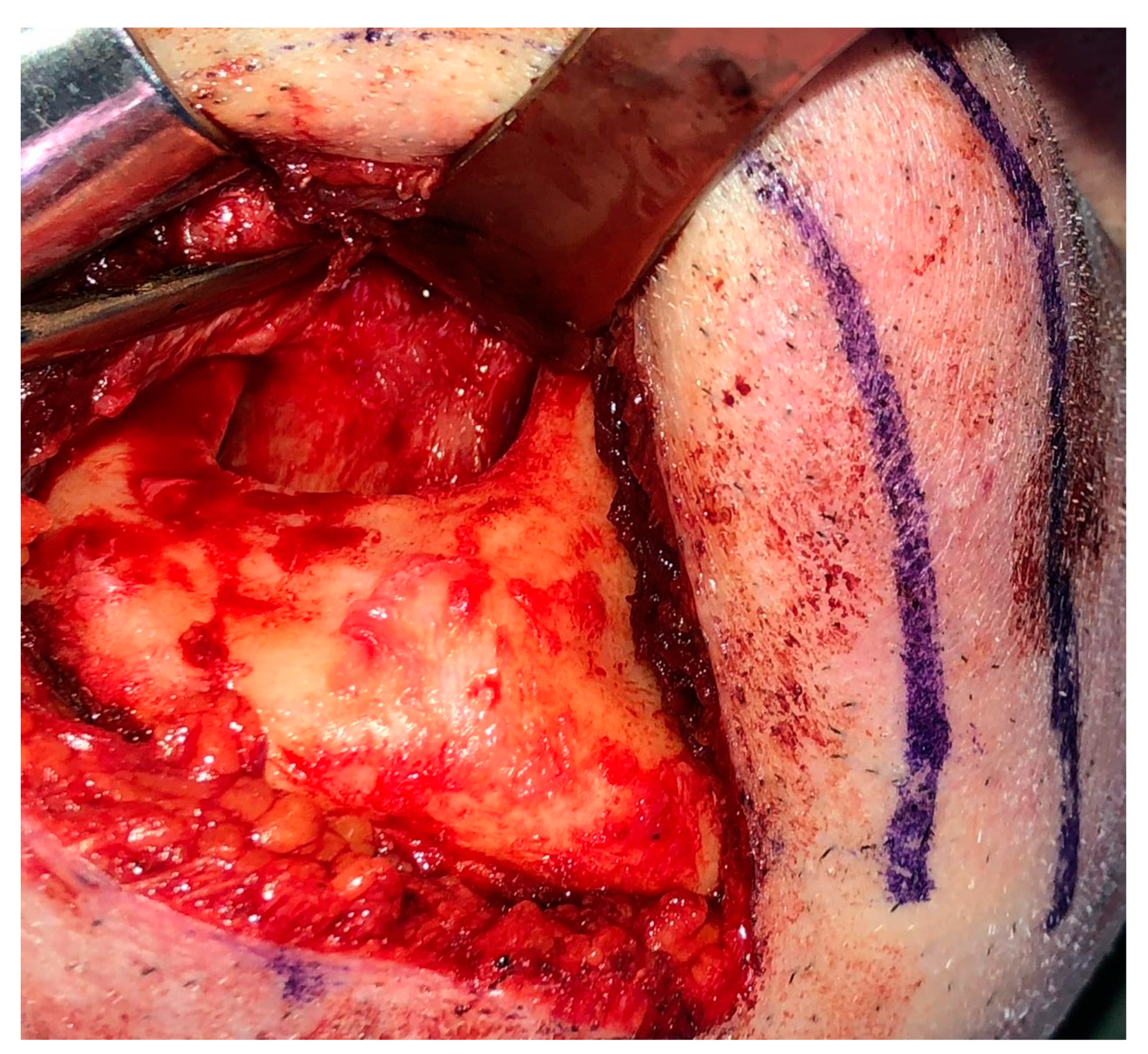
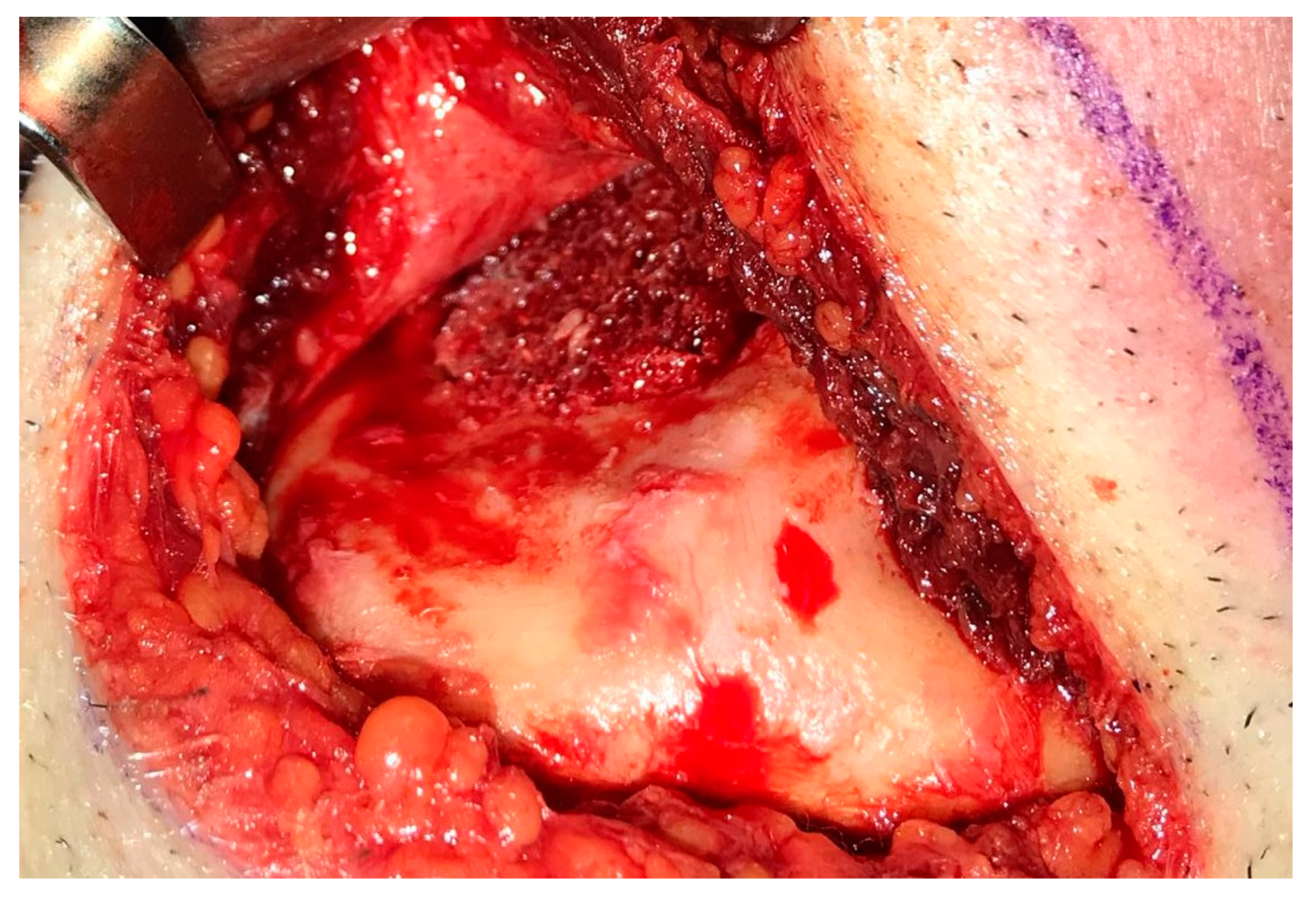
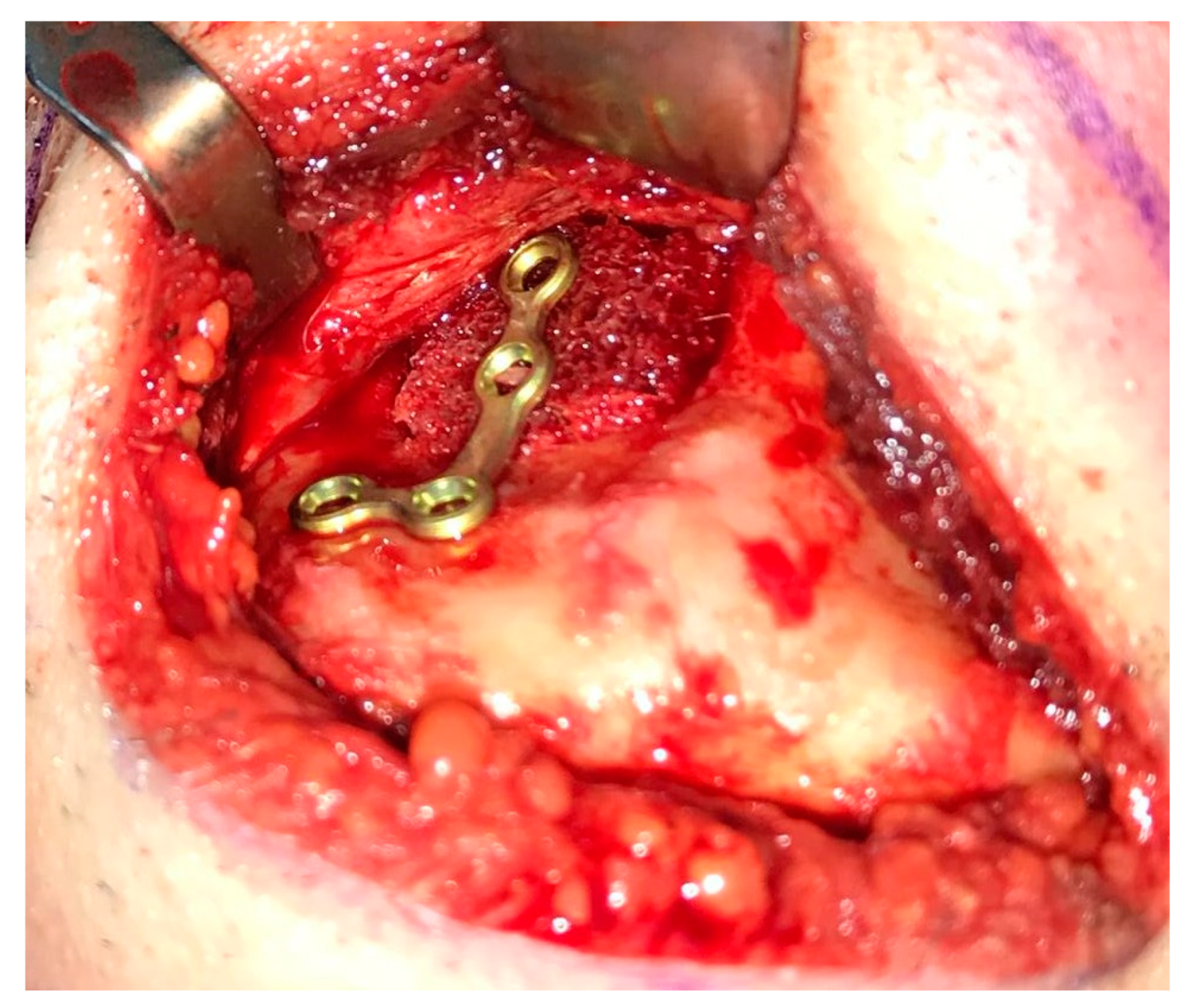
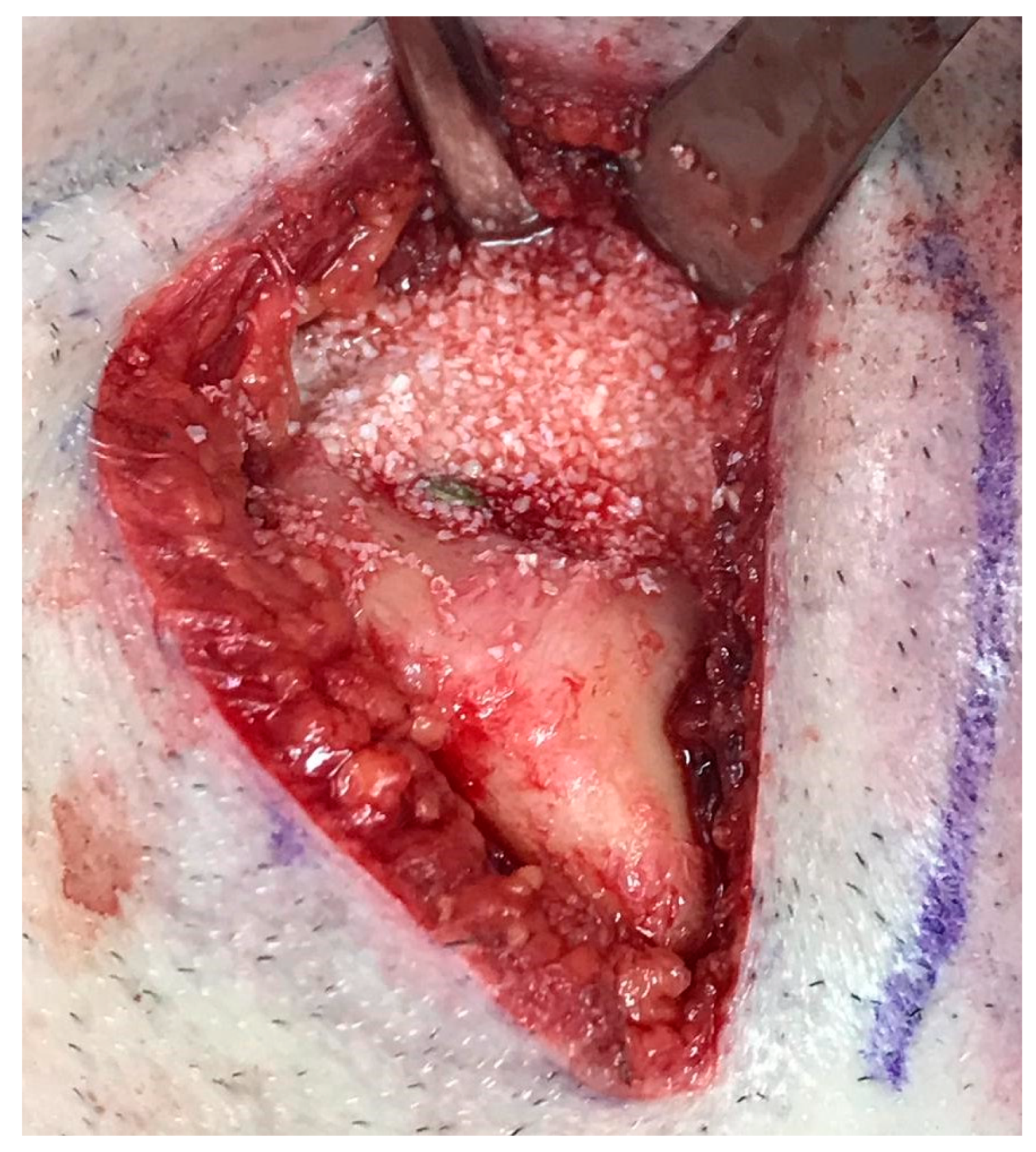
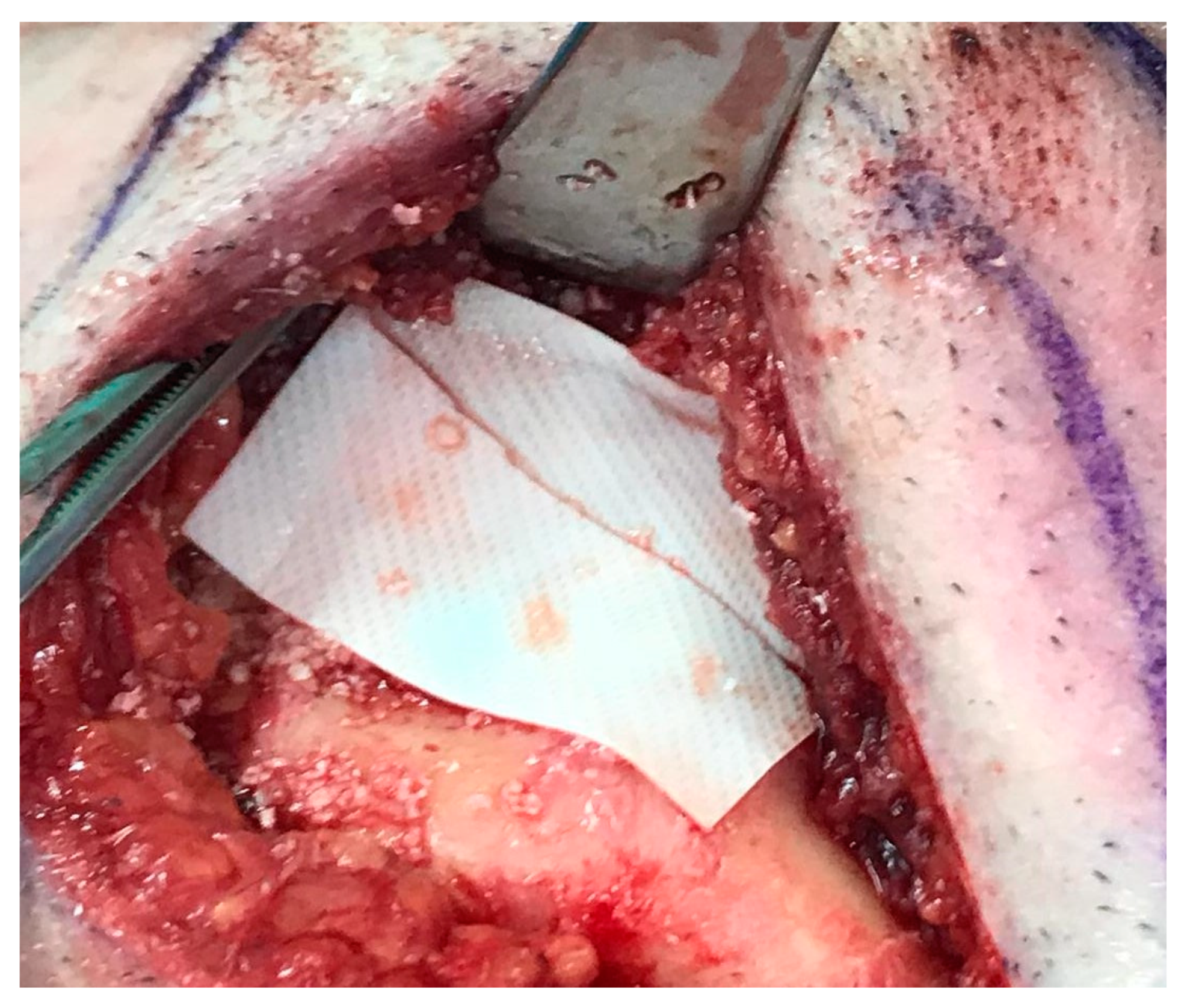
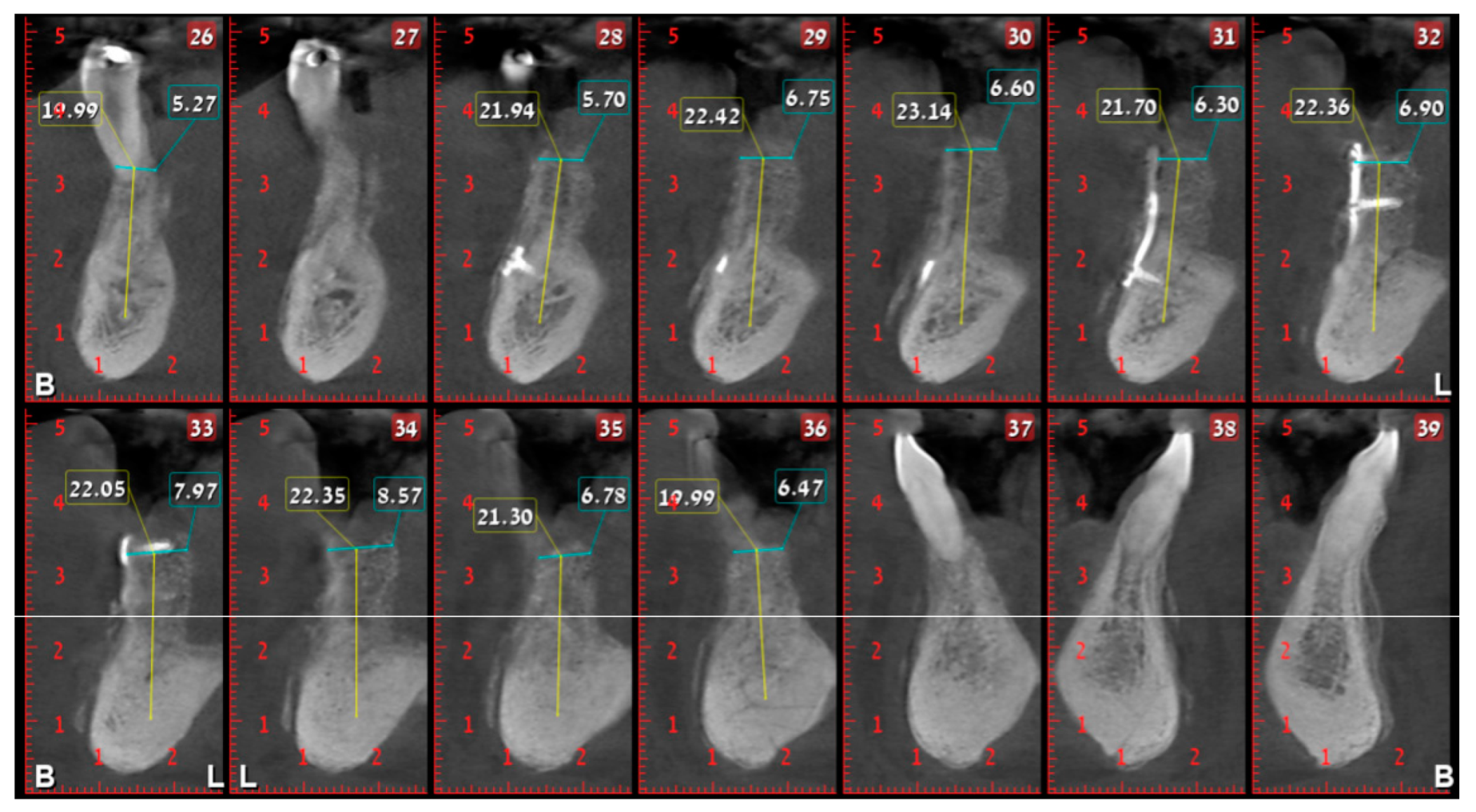
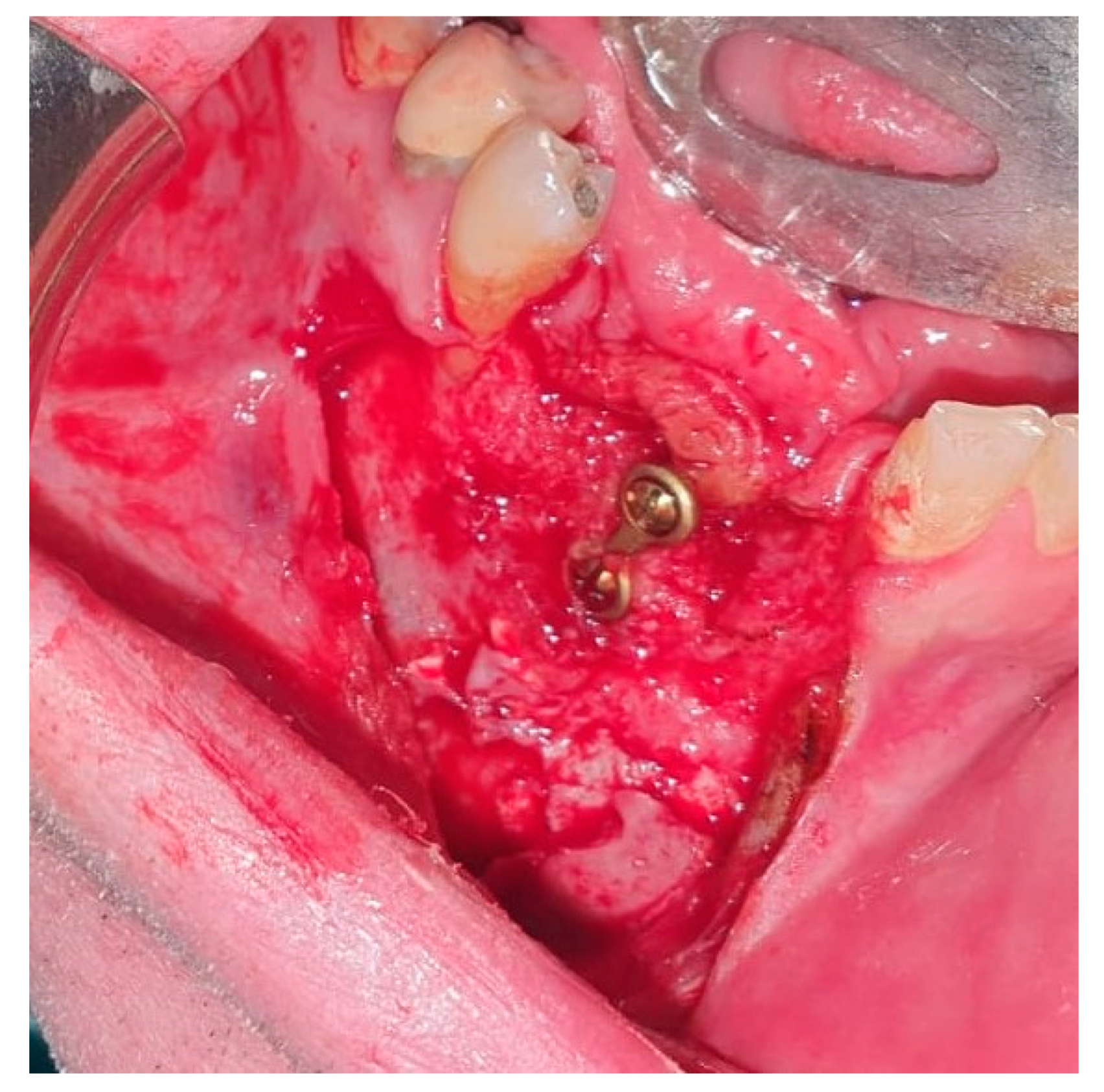
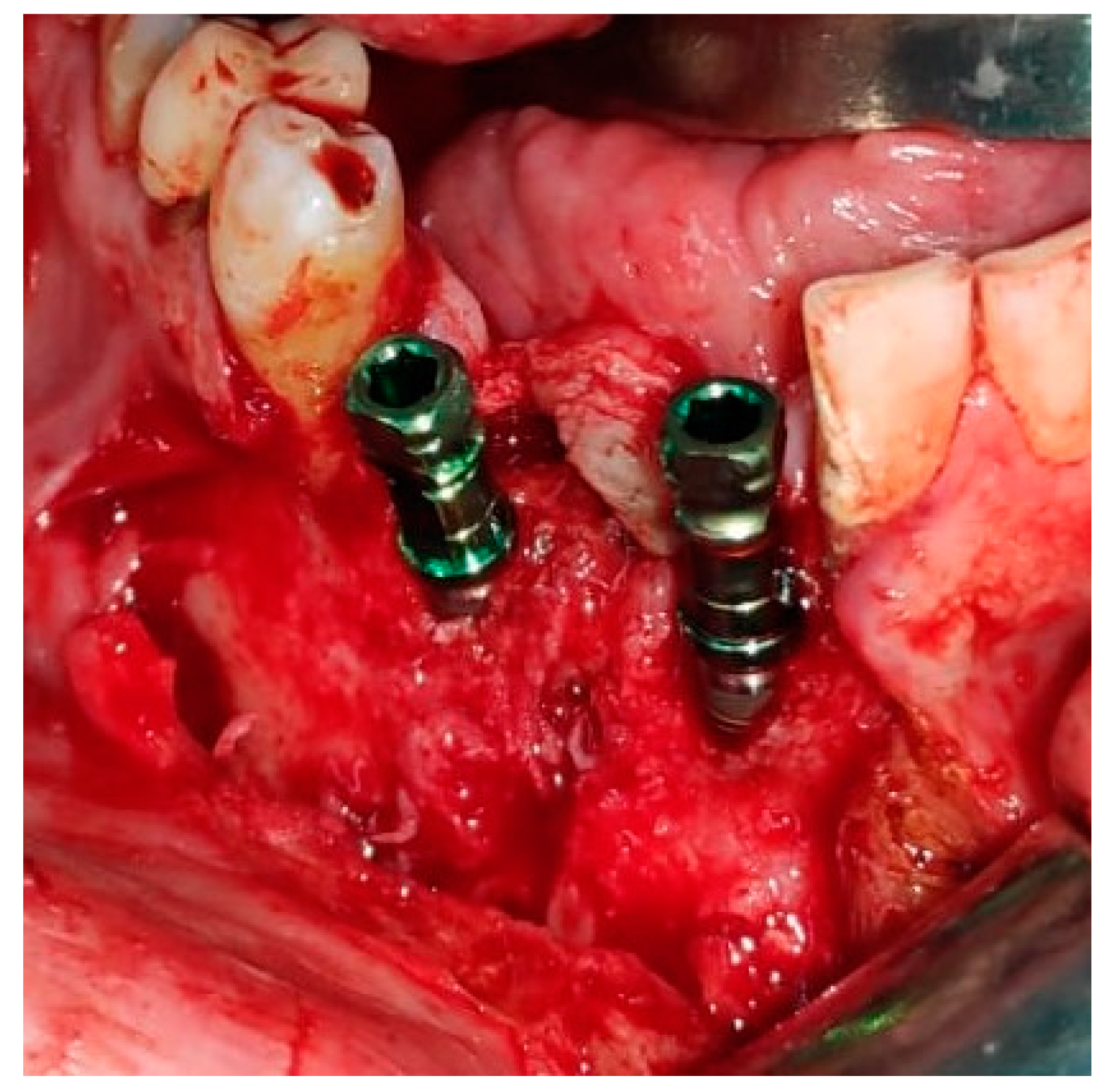
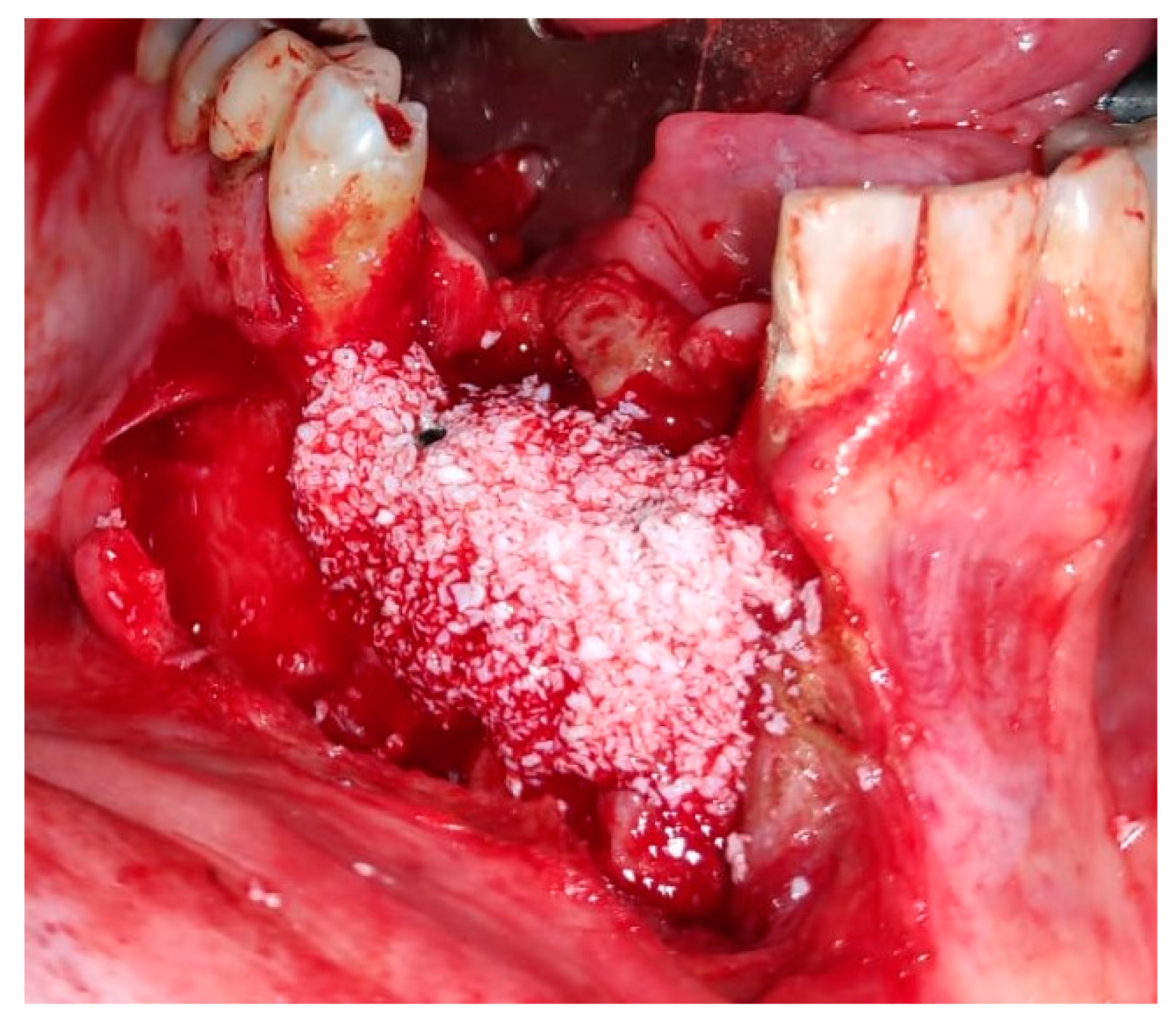
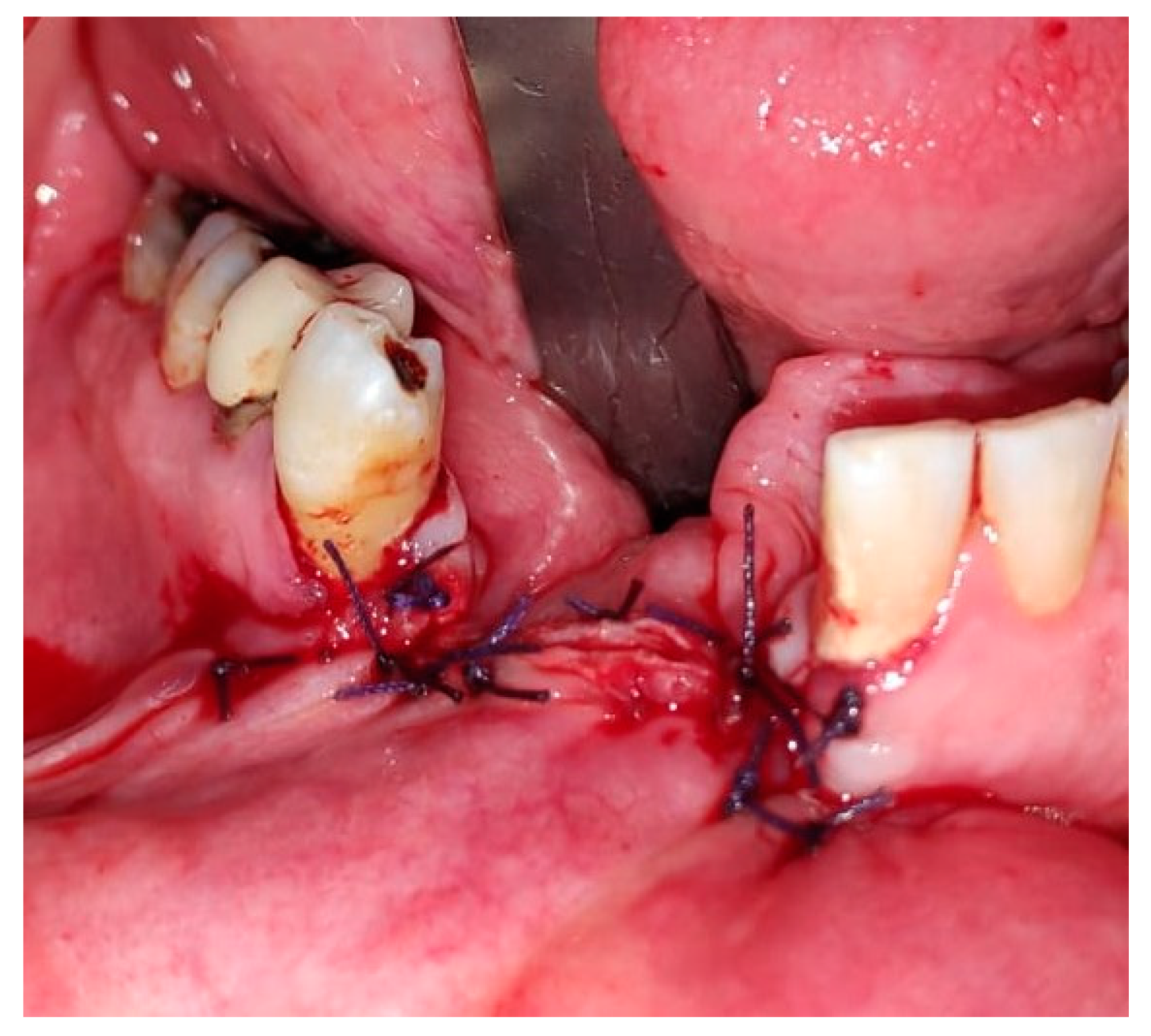
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martins, R.H.; Sobrinho, J.D.A.; Rapoport, A.; Rosa, M.P. Histopathologic features and management of ameloblastoma: Study of 20 cases. Sao Paulo Med. J./Rev. Paul. Med. 1999, 117, 171–174. [Google Scholar] [CrossRef][Green Version]
- Hong, J.; Yun, P.-Y.; Chung, I.-H.; Myoung, H.; Suh, J.-D.; Seo, B.-M.; Lee, J.-H.; Choung, P.-H. Long-term follow up on recurrence of 305 ameloblastoma cases. Int. J. Oral Maxillofac. Surg. 2007, 6, 283–288. [Google Scholar] [CrossRef]
- Miloro, M.; Ghali, G.E.; Larsen, P.; Waite, P. Peterson’s Principles of Oral and Maxillofacial Surgery, 3rd ed.; People’s Medical Publishing House: Montpelier, VT, USA, 2012; Volume 1, pp. 3–4. [Google Scholar]
- Pertovic, I.; Ahmed, Z.U.; Huryn, J.M.; Nelson, J.; Allen, R.J., Jr.; Matros, E.; Rosen, E.B. Oral rehabilitation for patients with marginal and segmental mandibulectomy: A retrospective review of 111 mandibular resection prostheses. J. Prosthet. Dent. 2019, 122, 82–87. [Google Scholar]
- Lundgren, S.; Sjöström, M.; Nyström, E.; Sennerby, L. Strategies in reconstruction of the atrophic maxilla with autogenous bone grafts and endosseous implants. Periodontol 2000 2008, 47, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.M. Bone augmentation using allogeneic Bone blocks with recombinant bone morphogenetic protein-2. Implant Dent. 2017, 26, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Hamer, A.J.; Strachan, J.R.; Black, M.M.; Ibbotson, C.J.; Stockley, I.; Elson, R.A. Biomechanical properties of cortical allograft bone using a new method of bone strength measurement: A comparison of fresh, fresh-frozen, and irradiated bone. J. Bone Jt. Surg. Br. 1996, 78, 363–368. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.S.; Haghighat, K. A bone augment. Tech. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Shellenberger, T.; Wimsatt, J.; Correa, P. Severely resorbed mandible: Predictable reconstruction with soft tissue matrix expansion (tent pole) grafts. J. Maxillofac. Surg. 2002, 60, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Sham, E.; Leong, J.; Maher, R.; Schenberg, M.; Leung, M.; Mansour, A.K. Mandibular ameloblastoma: Clinical experience and literature review. ANZ J. Surg. 2009, 79, 739–744. [Google Scholar] [CrossRef]
- Elnayef, B.; Monje, A.; Gargallo-Albiol, J.; Galindo-Moreno, P.; Wang, H.L.; Hernández-Alfaro, F. Vertical ridge augmentation in the atrophic mandible: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 2017, 32, 291–312. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Podlesh, S.; Anthony, J.P.; Alexander, J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J. Oral Maxillofac. Surg. 1997, 55, 1200–1206. [Google Scholar] [CrossRef]
- Rachmiel, A.; Emodi, O.; Aizenbud, D.; Rachmiel, D.; Shilo, D. Two-stage reconstruction of the severely deficient alveolar ridge: bone graft followed by alveolar distraction osteogenesis. Int. J. Oral Maxillofac. Surg. 2018, 47, 117–124. [Google Scholar] [CrossRef]
- Herford, A.S.; Boyne, P.J. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J. Oral Maxillofac. Surg. 2008, 66, 616–624. [Google Scholar] [CrossRef]
- Hoffmann, A.; Gross, G. BMP signaling pathways in cartilage and bone formation. Crit. Rev.™ Eukaryot. Gene Expr. 2001, 11, 1–3. [Google Scholar] [CrossRef]
- Jäger, M.; Herten, M.; Fochtmann, U.; Fischer, J.; Heringou, P.; Zilkens, C.; Hendrich, C.; Krauspe, R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. 2011, 29, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Falisi, G.; Amantea, M.; Rastelli, C.; Paduano, F.; Marrelli, M. Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: Comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J. Biol. Regul. Homeost. 2015, 29, 713–718. [Google Scholar]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential use of human periapical cyst-mesenchymal stem cells (hpcy-mscs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Uccelli, A.; Prockop, D.J. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr. Opin. Immunol. 2010, 22, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Stem Cells Int. 2018, 1–2. [Google Scholar] [CrossRef]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int. J. Dent. 2013, 1–5. [Google Scholar] [CrossRef]
- Nørholt, S.E.; Jensen, J.; Schou, S.; Pedersen, T.K. Complications after mandibular distraction osteogenesis: A retrospective study of 131 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. End. 2011, 111, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontal and cardiovascular disease: A cross-sectional study. J. Periodontal Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of salivary and serum malondialdehyde and lipid profile of patients with periodontitis and coronary heart disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Ninomiya, T.; Hosoya, A.; Hiraga, T.; Miyazawa, H.; Nakamura, H. 1α,25-Dihydroxyvitamin D3 inhibits osteoblastic differentiation of mouse periodontal fibroblasts. Arch Oral Biol. 2012, 57, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A. Periosteum: A highly underrated tool in dentistry. Int. J. Dent. 2012. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L. Removal of the submandibular gland by a submental approach: A prospective, randomized, controlled study. Oral Oncol. 2008, 44, 295–300. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj Yahya, B.; Rosenfeld, E.; Chaushu, G.; Kaplan, I.; Ben-Zvi, Y.; Hamzani, Y. Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy. Medicina 2020, 56, 326. https://doi.org/10.3390/medicina56070326
Haj Yahya B, Rosenfeld E, Chaushu G, Kaplan I, Ben-Zvi Y, Hamzani Y. Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy. Medicina. 2020; 56(7):326. https://doi.org/10.3390/medicina56070326
Chicago/Turabian StyleHaj Yahya, Bahaa, Eli Rosenfeld, Gavriel Chaushu, Ilana Kaplan, Yehonantan Ben-Zvi, and Yafit Hamzani. 2020. "Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy" Medicina 56, no. 7: 326. https://doi.org/10.3390/medicina56070326
APA StyleHaj Yahya, B., Rosenfeld, E., Chaushu, G., Kaplan, I., Ben-Zvi, Y., & Hamzani, Y. (2020). Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy. Medicina, 56(7), 326. https://doi.org/10.3390/medicina56070326







