Proteinuria and Bilirubinuria as Potential Risk Indicators of Acute Kidney Injury during Running in Outpatient Settings
Abstract
1. Introduction
2. Materials and Methods
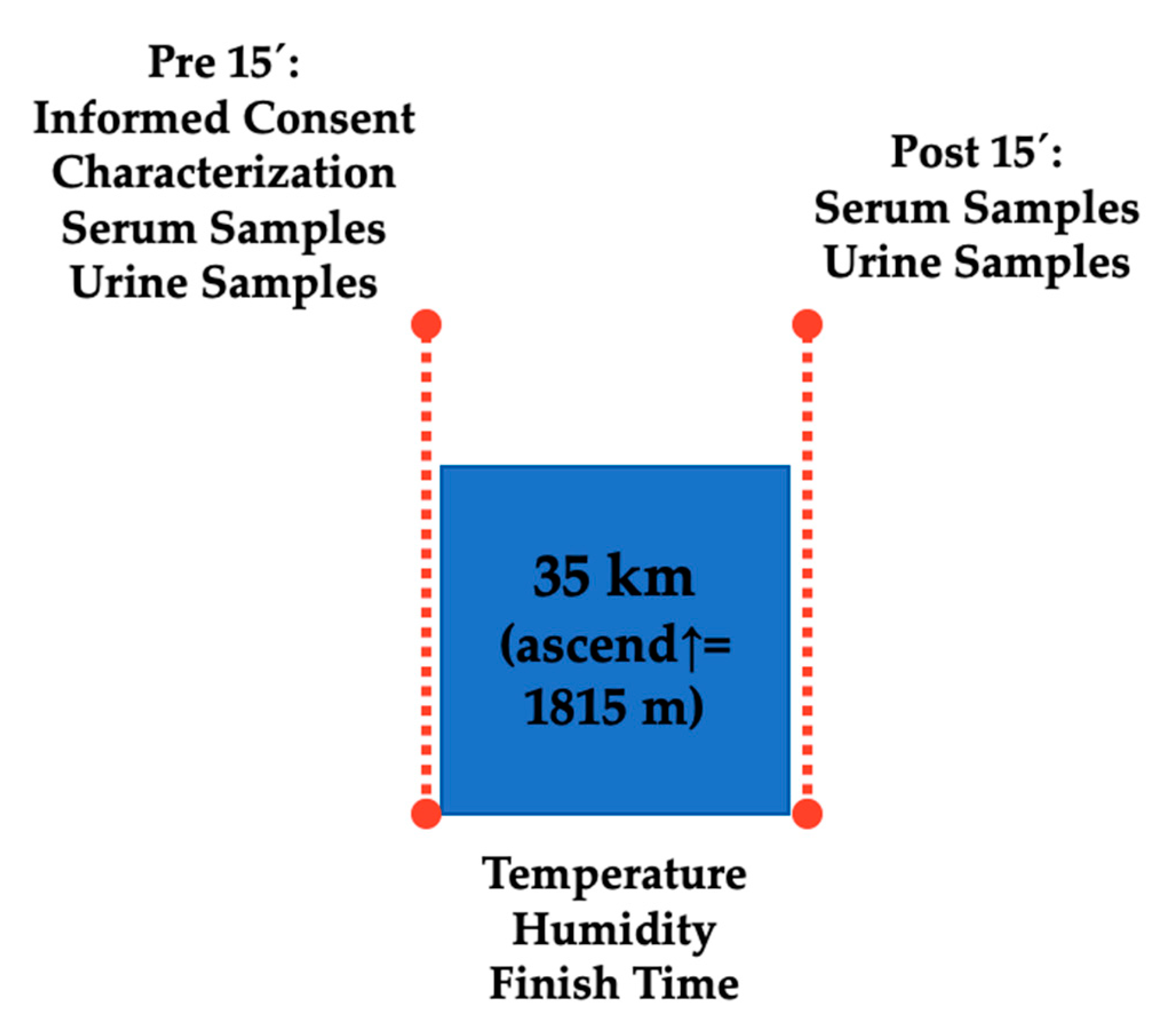
2.1. Design
2.2. Participants
2.3. Materials and Procedures
2.3.1. Serum Test
2.3.2. Urine Test
2.3.3. Urine Specific Gravity
2.4. Statistical Analysis
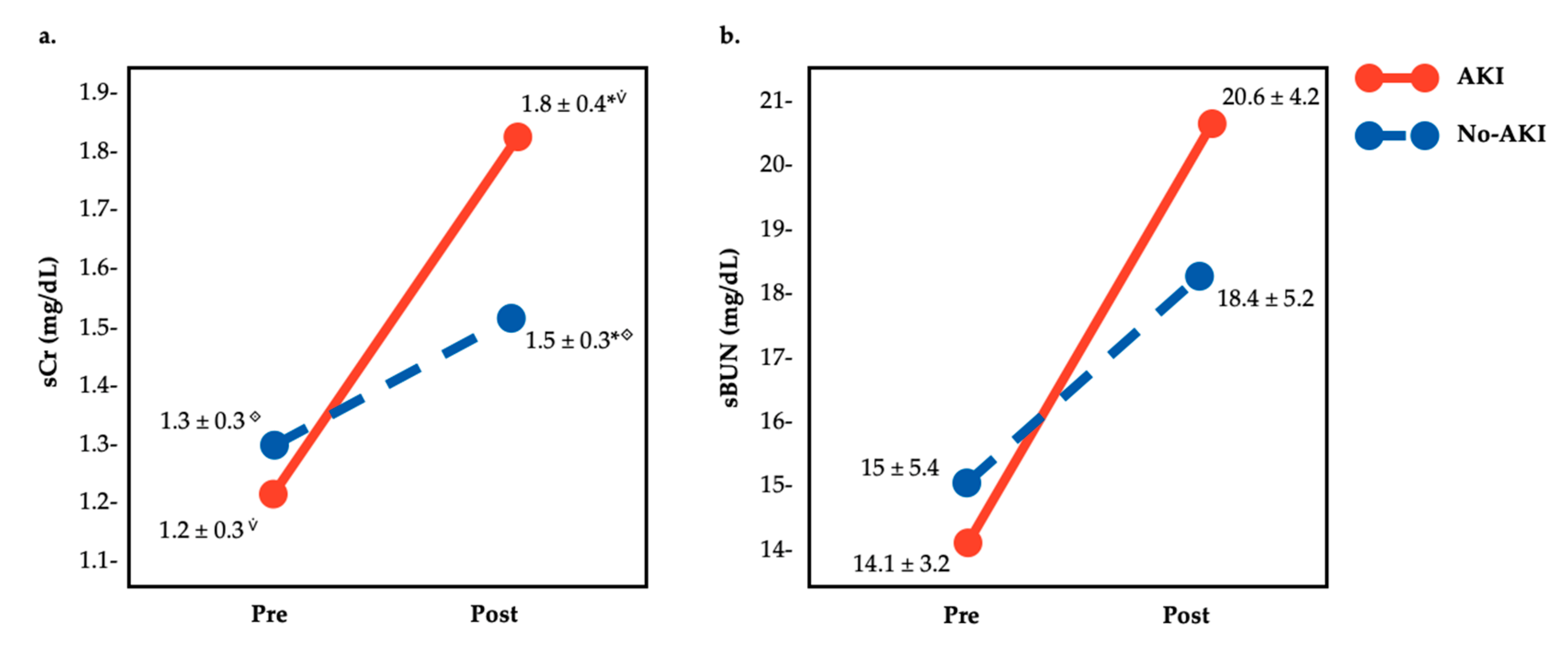
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and Acute Kidney Injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Agapito Fonseca, J.; Jorge, S.; Lopes, J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Kellum, J.A.; Haase, M. Subclinical AKI is still AKI. Crit. Care 2012, 16, 313. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional Rhabdomyolysis and Acute Kidney Injury in Endurance Sports: A Systematic Review. Eur. J. Sport Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Pino-Ortega, J.; Gómez-Carmona, C.; Gutiérrez-Vargas, R.; Timón, R.; Olcina, G. External Workload Indicators of Muscle and Kidney Mechanical Injury in Endurance Trail Running. Int. J. Environ. Res. Public Health 2019, 16, 3909. [Google Scholar] [CrossRef]
- Belli, T.; Macedo, D.V.; de Araújo, G.G.; dos Reis, I.G.M.; Scariot, P.P.M.; Lazarim, F.L.; Nunes, L.A.S.; Brenzikofer, R.; Gobatto, C.A. Mountain Ultramarathon Induces Early Increases of Muscle Damage, Inflammation, and Risk for Acute Renal Injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Ingwerson, J.L.; Rogers, I.R.; Hew-Butler, T.; Stuempfle, K.J. Increasing Creatine Kinase Concentrations at the 161-km Western States Endurance Run. Wilderness Environ. Med. 2012, 23, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.E.; Walter, E.; Venn, R.M.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L.G. Acute kidney injury associated with endurance events—Is it a cause for concern? A systematic review. BMJ Open Sport Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Olcina, G.; Gutiérrez-Vargas, R.; Crowe, J. Heat Strain, External Workload, and Chronic Kidney Disease in Tropical Settings: Are Endurance Athletes Exposed? Front. Physiol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Wołyniec, W.; Kasprowicz, K.; Giebułtowicz, J.; Korytowska, N.; Zorena, K.; Bartoszewicz, M.; Tkachenko, P.R.; Renke, M.; Ratkowski, W. Changes in Water Soluble Uremic Toxins and Urinary Acute Kidney Injury Biomarkers after 10- and 100-km Runs. Int. J. Environ. Res. Public Health 2019, 16, 4153. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin. J. Am. Soc. Nephrol. 2011, 6, 2567–2572. [Google Scholar] [CrossRef]
- Scheer, V. Severe Kidney Injury after a 110-km Trail Race. Cureus 2020, 12, e7814. [Google Scholar]
- McCullough, P.A.; Shaw, A.D.; Haase, M.; Bouchard, J.; Waikar, S.S.; Siew, E.D.; Murray, P.T.; Mehta, R.L.; Ronco, C. Diagnosis of Acute Kidney Injury Using Functional and Injury Biomarkers: Workgroup Statements from the Tenth Acute Dialysis Quality Initiative Consensus Conference. In ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes; McCullough, P.A., Kellum, J.A., Mehta, R.L., Murray, P.T., Ronco, C., Eds.; KARGER: Basel, Switzerland, 2013; pp. 13–29. Available online: https://www.karger.com/Article/FullText/349963 (accessed on 25 April 2020).
- Devarajan, P. Emerging urinary biomarkers in the diagnosis of acute kidney injury. Expert Opin. Med. Diagn. 2008, 2, 387–398. [Google Scholar] [CrossRef]
- Devarajan, P. Biomarkers for the early detection of acute kidney injury. Curr. Opin. Pediatr. 2011, 23, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Lopes, J.A. Complete blood count in acute kidney injury prediction: A narrative review. Ann. Intensive Care 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef]
- Soto, K.; Coelho, S.; Rodrigues, B.; Martins, H.; Frade, F.; Lopes, S.; Luis, C.; Ana Luisa, P.; Prasad, D. Cystatin C as a marker of acute kidney injury in the emergency department. Clin. J. Am. Soc. Nephrol. 2010, 5, 1745–1754. [Google Scholar] [CrossRef]
- Watson, D.; Yang, J.Y.C.; Sarwal, R.D.; Sigdel, T.K.; Liberto, J.; Damm, I.; Louie, V.; Sigdel, S.; Livingstone, D.; Soh, K.; et al. A Novel Multi-Biomarker Assay for Non-Invasive Quantitative Monitoring of Kidney Injury. J. Clin. Med. 2019, 8, 499. [Google Scholar] [CrossRef]
- Shephard, R.J. Exercise proteinuria and hematuria: Current knowledge and future directions. J. Sports Med. Phys. Fit. 2016, 56, 1060–1076. [Google Scholar]
- Wołyniec, W.; Kasprowicz, K.; Rita-Tkachenko, P.; Renke, M.; Ratkowski, W. Biochemical Markers of Renal Hypoperfusion, Hemoconcentration, and Proteinuria after Extreme Physical Exercise. Medicina 2019, 55, 154. [Google Scholar] [CrossRef]
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-distance race. PLoS ONE 2019, 14, e0222544. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Stuempfle, K.J.; Fogard, K.; Hew-Butler, T.; Winger, J.; Weiss, R.H. Urine dipstick analysis for identification of runners susceptible to acute kidney injury following an ultramarathon. J. Sports Sci. 2013, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Vuljanić, D.; Dojder, A.; Špoljarić, V.; Saračević, A.; Dukić, L.; Leniček-Krleža, J.; Tanasković, J.V.; Maradin, I.; Grzunov, A.; Vogrinc, Z.; et al. Analytical verification of 12 most commonly used urine dipsticks in Croatia: Comparability, repeatability and accuracy. Biochem. Med. 2019, 29, 123–132. [Google Scholar] [CrossRef]
- Wołyniec, W.; Ratkowski, W.; Kasprowicz, K.; Małgorzewicz, S.; Aleksandrowicz, E.; Zdrojewski, T.; Wierucki, L.; Puch-Walczak, A.; Żmijewski, P.; Renke, M. Factors influencing post-exercise proteinuria after marathon and ultramarathon races. Biol. Sport 2020, 37, 33–40. [Google Scholar] [CrossRef]
- Sato, Y.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Jensen, T.; Tolan, D.R.; Sanchez-Lozada, L.G.; Newman, L.S.; Butler-Dawson, J.; Sorensen, C.; Glaser, J.; et al. Increase of core temperature affected the progression of kidney injury by repeated heat stress exposure. Am. J. Physiol. Ren. Physiol. 2019, 317, F1111–F1121. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Hostler, D.; Parker, M.D.; Pryor, R.R.; Lohr, J.W.; Johnson, B.D.; Chapman, C.L. The Potential for Renal Injury Elicited by Physical Work in the Heat. Nutrients 2019, 11, 2087. [Google Scholar] [CrossRef]
- Wyness, S.P.; Hunsaker, J.J.H.; Snow, T.M.; Genzen, J.R. Evaluation and analytical validation of a handheld digital refractometer for urine specific gravity measurement. Pract. Lab. Med. 2016, 5, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.E.; Roberts, W.O.; Stone, J.A. National Athletic Trainers’ Association Position Statement: Fluid Replacement for Athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Bongers, C.C.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.; Eijsvogels, T.M.; Deen, P.M.; Hopman, M.T. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol. Rep. 2018, 6, e13734. [Google Scholar] [CrossRef] [PubMed]
- Hansson, E.; Glaser, J.; Weiss, I.; Ekström, U.; Apelqvist, J.; Hogstedt, C.; Peraza, S.; Lucas, R.; Jakobsson, K.; Wesseling, C.; et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup. Environ. Med. 2019, 76, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, N.; Taboga, P.; Rejc, E.; Šimunič, B.; Antonutto, G.; Lazzer, S. Effects of an Uphill Marathon on Running Mechanics and Lower-Limb Muscle Fatigue. Int. J. Sports Physiol. Perform. 2016, 11, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Vernillo, G.; Giandolini, M.; Edwards, W.B.; Morin, J.B.; Samozino, P.; Horvais, N.; Millet, G.Y. Biomechanics and Physiology of Uphill and Downhill Running. Sports Med. 2017, 47, 615–629. [Google Scholar] [CrossRef]
- Carroll, M.F.; Temte, J.L. Proteinuria in adults: A diagnostic approach. Am. Fam. Physician 2000, 62, 1333–1340. [Google Scholar]
- Filha RD, S.; Pinheiro SV, B.; Macedo e Cordeiro, T.; Feracin, V.; Vieira EL, M.; Miranda, A.S.; Simões e Silva, A.C. Evidence for a role of angiotensin converting enzyme 2 in proteinuria of idiopathic nephrotic syndrome. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Akiboye, R.D.; Sharma, D.M. Haematuria in Sport: A Review. Eur. Urol. Focus 2019, 5, 912–916. [Google Scholar] [CrossRef]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- De Paz, J.A.; Villa, J.G.; Lopez, P.; Gonzalez-Gallego, J. Effects of long-distance running on serum bilirubin. Med. Sci. Sports Exerc. 1995, 27, 1590–1594. [Google Scholar] [CrossRef]
- Shin, K.-A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.-J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-distance Running. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F. Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann. Transl. Med. 2019, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vargas, R.; Martín-Rodríguez, S.; Sánchez-Ureña, B.; Rodríguez-Montero, A.; Salas-Cabrera, J.; Gutiérrez-Vargas, J.C. Biochemical and Muscle Mechanical Postmarathon Changes in Hot and Humid Conditions. J. Strength Cond. Res. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Junglee, N.A.; Di Felice, U.; Dolci, A.; Fortes, M.B.; Jibani, M.M.; Lemmey, A.B.; Walsh, N.P.; Macdonald, J.H. Exercising in a hot environment with muscle damage: Effects on acute kidney injury biomarkers and kidney function. Am. J. Physiol. Renal Physiol. 2013, 305, F813–F820. [Google Scholar] [CrossRef] [PubMed]
| AKI | No-AKI | t | p-Value | |
|---|---|---|---|---|
| Age (years) | 39.4 ± 8.8 | 38.1 ± 9.8 | 0.34 | 0.74 |
| Weight (kg) | 69.2 ± 7.3 | 76 ± 14.5 | −0.99 | 0.33 |
| Height (cm) | 171.6 ± 7.6 | 173.1 ± 9.6 | 0.41 | 0.69 |
| Trail running experience (years) | 5 ± 2.6 | 6.1 ± 3.1 | −1.63 | 0.12 |
| Training (hours) | 9.1 ± 2.8 | 8.5 ± 4.5 | −0.45 | 0.66 |
| Variable (Score Criteria) | Pre | Post 0 h | χ2 | p-Value | ||
|---|---|---|---|---|---|---|
| n * | % | n * | % | |||
| Leucocytes (>1) | 0 | 0 | 3 | 17.64 | 4.96 | 0.5 |
| Nitrites (>1) | 0 | 0 | 0 | 0 | - | - |
| Protein (>1) | 0 | 0 | 9 | 52.94 | 0.94 | 0.008 |
| Glucose (>1) | 0 | 0 | 0 | 0 | - | - |
| Ketones (>1) | 0 | 0 | 0 | 0 | - | - |
| Urobilinogen (>1) | 0 | 0 | 3 | 17.64 | 0.23 | 0.625 |
| Bilirubin (>1) | 0 | 0 | 8 | 47.06 | 0.94 | 0.039 |
| Erythrocytes (>1) | 0 | 0 | 5 | 29.41 | 0.58 | 0.125 |
| Variable (Score Criteria) | Pre | Post 0 h | χ2 | p-Value | ||
|---|---|---|---|---|---|---|
| n * | % | n * | % | |||
| Leucocytes (>1) | 0 | 0 | 0 | 0 | - | - |
| Nitrites (>1) | 0 | 0 | 0 | 0 | - | - |
| Protein (>1) | 0 | 0 | 4 | 33.33 | 1.667 | 0.25 |
| Glucose (>1) | 0 | 0 | 0 | 0 | - | - |
| Ketones (>1) | 0 | 0 | 2 | 16.66 | 0.278 | 1 |
| Urobilinogen (>1) | 0 | 0 | 1 | 8.33 | 0.123 | 1 |
| Bilirubin (>1) | 0 | 0 | 5 | 41.66 | 0.741 | 0.063 |
| Erythrocytes (>1) | 0 | 0 | 3 | 33.33 | 2.59 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Valverde, D.; Olcina, G.; Sánchez-Ureña, B.; Pino-Ortega, J.; Martínez-Guardado, I.; Timón, R. Proteinuria and Bilirubinuria as Potential Risk Indicators of Acute Kidney Injury during Running in Outpatient Settings. Medicina 2020, 56, 562. https://doi.org/10.3390/medicina56110562
Rojas-Valverde D, Olcina G, Sánchez-Ureña B, Pino-Ortega J, Martínez-Guardado I, Timón R. Proteinuria and Bilirubinuria as Potential Risk Indicators of Acute Kidney Injury during Running in Outpatient Settings. Medicina. 2020; 56(11):562. https://doi.org/10.3390/medicina56110562
Chicago/Turabian StyleRojas-Valverde, Daniel, Guillermo Olcina, Braulio Sánchez-Ureña, José Pino-Ortega, Ismael Martínez-Guardado, and Rafael Timón. 2020. "Proteinuria and Bilirubinuria as Potential Risk Indicators of Acute Kidney Injury during Running in Outpatient Settings" Medicina 56, no. 11: 562. https://doi.org/10.3390/medicina56110562
APA StyleRojas-Valverde, D., Olcina, G., Sánchez-Ureña, B., Pino-Ortega, J., Martínez-Guardado, I., & Timón, R. (2020). Proteinuria and Bilirubinuria as Potential Risk Indicators of Acute Kidney Injury during Running in Outpatient Settings. Medicina, 56(11), 562. https://doi.org/10.3390/medicina56110562









