Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone
Abstract
1. Introduction
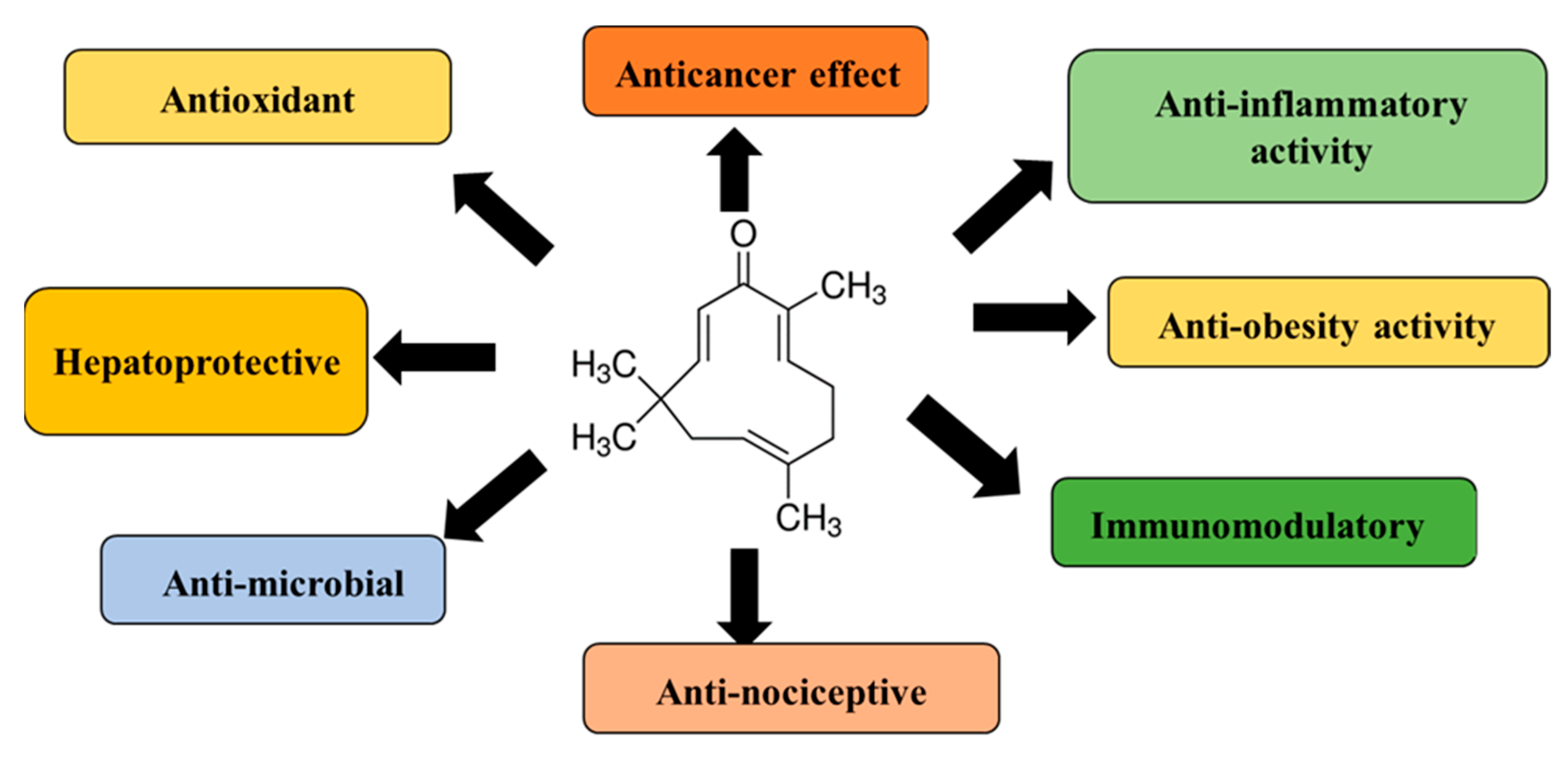
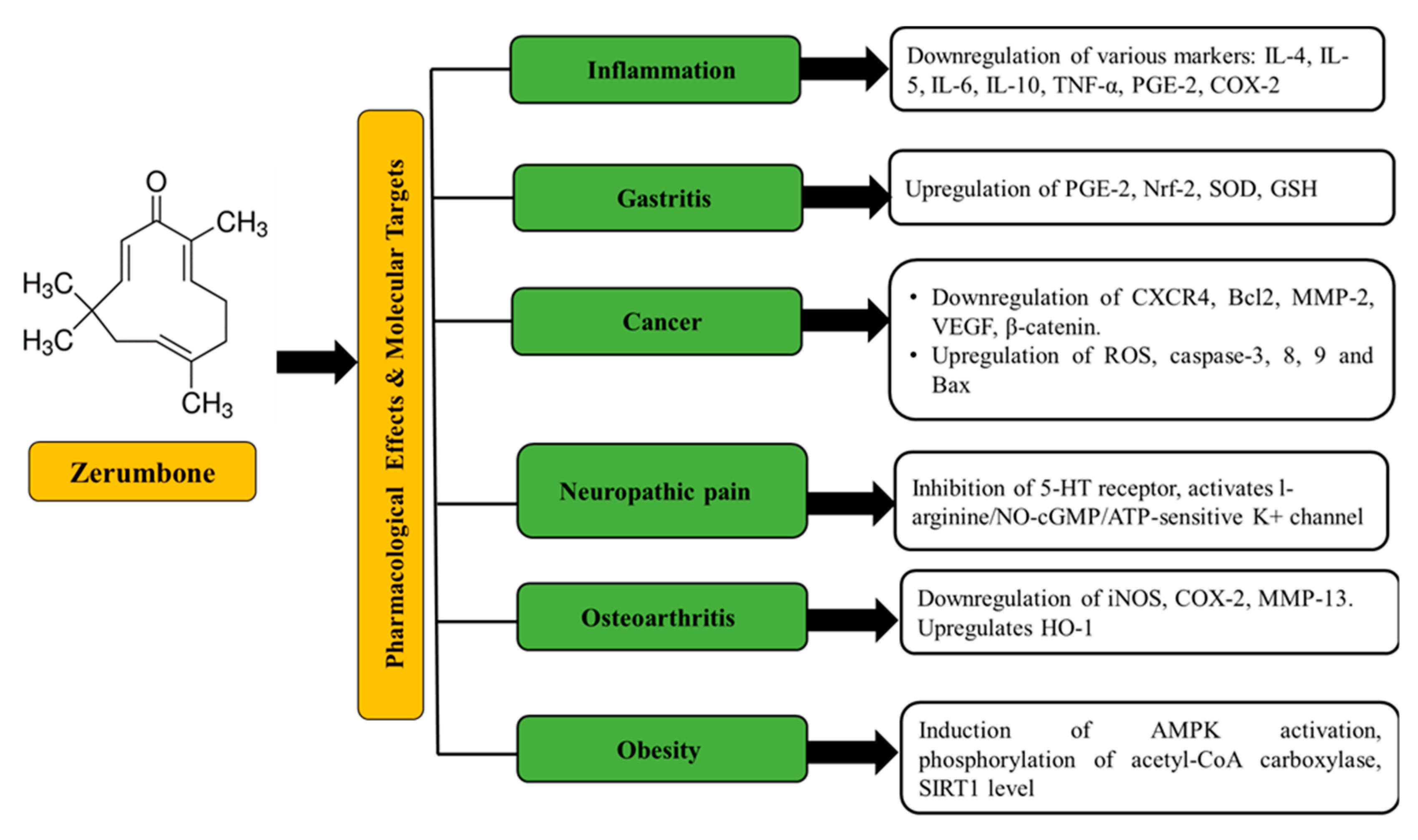
2. Source, Chemistry, Physicochemical Properties, and Pharmacological Effects of Zerumbone
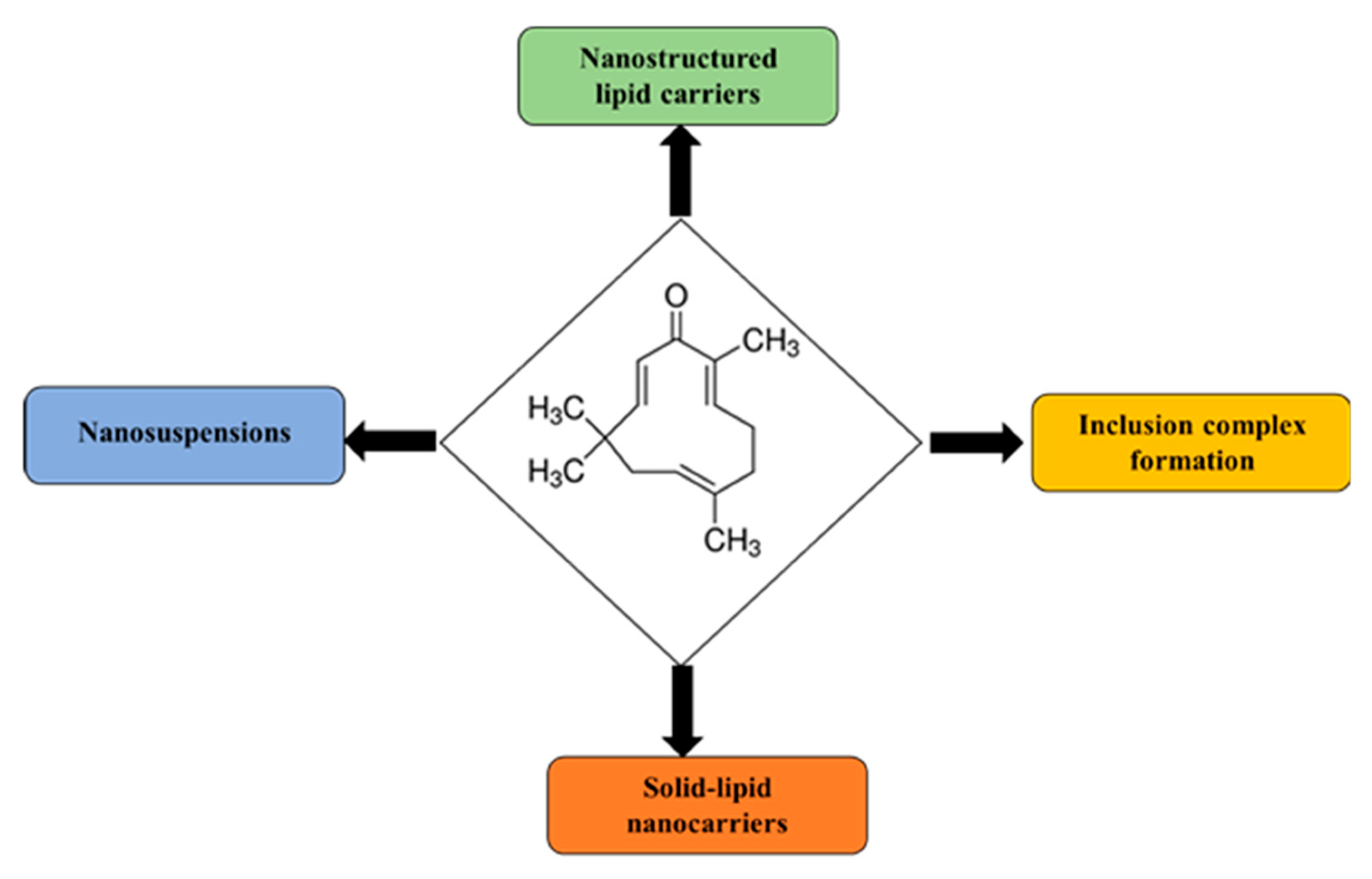
3. Formulation Development and Drug Delivery Strategies of Zerumbone
3.1. Nanostructured Lipid Carriers
3.2. Inclusion Complexes of Cyclodextrins
3.3. Nanosuspensions
4. Pharmacokinetics and Toxicity of Zerumbone and Zerumbone Formulations
5. Patents Related to Zerumbone and Zerumbone Formulations
6. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. The BCS: Where do we go from here? Pharm. Technol. 2001, 25, 68–77. [Google Scholar]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Seenivasan, A.; Panda, T.; Théodore, T. Lovastatin nanoparticle synthesis and characterization for better drug delivery. Open Biotechnol. J. 2011, 5, 28–32. [Google Scholar] [CrossRef]
- Souto, E.; Wissing, S.; Barbosa, C.; Müller, R. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Bartos, C.; Szabó-Révész, P.; Bartos, C.; Katona, G.; Jójárt-Laczkovich, O.; Ambrus, R. The effect of an optimized wet milling technology on the crystallinity, morphology and dissolution properties of micro-and nanonized meloxicam. Molecules 2016, 21, 507. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Paulino, A.; Rauber, G.; Campos, C.; Maurício, M.; De Avillez, R.; Capobianco, G.; Cardoso, S.; Cuffini, S. Dissolution enhancement of Deflazacort using hollow crystals prepared by antisolvent crystallization process. Eur. J. Pharm. Sci. 2013, 49, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesharwani, S.S.; Mathur, H.; Tyagi, M.; Bhat, G.J.; Tummala, H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur. J. Pharm. Sci. 2016, 82, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.K.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Bhat, G.J.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control. Release 2018, 290, 165–179. [Google Scholar] [CrossRef]
- Hassan, M.A.; Suleiman, M.S.; Najib, N.M. Improvement of the in vitro dissolution characteristics of famotidine by inclusion in β-cyclodextrin. Int. J. Pharm. 1990, 58, 19–24. [Google Scholar] [CrossRef]
- Mistry, S.; Patel, P.; Bharadia, P.; Pandya, V.; Modi, D. Novel drug delivery system for lipophilic agents: Solid lipid nanoparticles. J. Pharm. Cosmetol. 2011, 1, 76–89. [Google Scholar]
- Müller, R.H.; MaÈder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Shaji, J.; Jain, V. Solid lipid nanoparticles: A novel carrier for chemotherapy. Int. J. Pharmacol. Pharm. Sci. 2010, 2, 8–17. [Google Scholar]
- Muley, P.; Kumar, S.; El Kourati, F.; Kesharwani, S.S.; Tummala, H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int. J. Pharm. 2016, 500, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Dachineni, R.; Bhat, G.J.; Tummala, H. Hydrophobically modified inulin-based micelles: Transport mechanisms and drug delivery applications for breast cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 101254. [Google Scholar] [CrossRef]
- Júlio, A.; Lima, S.A.C.; Reis, S.; de Almeida, T.S.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2019, 100915. [Google Scholar] [CrossRef]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491s–499s. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.M.; Still, P.C.; Bueno Perez, L.; Grever, M.R.; Douglas Kinghorn, A. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Abdul, A.; Al-Zubairi, A.; Tailan, N.; Wahab, S.; Zain, Z.; Ruslay, S.; Syam, M. Anticancer activity of natural compound (Zerumbone) extracted from Zingiber zerumbet in human HeLa cervical cancer cells. Int. J. Pharmacol. 2008, 4, 160–168. [Google Scholar]
- Foong, J.N.; Selvarajah, G.T.; Rasedee, A.; Rahman, H.S.; How, C.W.; Beh, C.Y.; Teo, G.Y.; Ku, C.L. Zerumbone-Loaded Nanostructured Lipid Carrier Induces Apoptosis of Canine Mammary Adenocarcinoma Cells. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of zerumbone as an anti-cancer agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Arshad, L.; Bukhari, S.N.A. Exploring the immunomodulatory and anticancer properties of zerumbone. Food Funct. 2017, 8, 3410–3431. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Abdul, A.; Ibrahim, T.A.T.; Abdelwahab, S.I.; Elhassan, M.M.; Syam, M. Evaluation of acute toxicity and the effect of single injected doses of zerumbone on the kidney and liver functions in Sprague Dawley rats. Afr. J. Biotechnol. 2010, 9, 4442–4450. [Google Scholar]
- Jin, Y.B.; Seo, W.-D.; Lee, Y.-J.; Lee, Y.-S.; Lee, H.-J. Toxicological evaluation of zerumbone on antitumor effects in mice. Afr. J. Pharm. Pharmacol. 2013, 7, 466–473. [Google Scholar] [CrossRef]
- Murakami, A.; Takahashi, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Terao, J.; Ohigashi, H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The α, β-unsaturated carbonyl group is a prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshida, C.; Murakami, A.; Ohigashi, H.; Osawa, T.; Uchida, K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004, 572, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nathaniel, C.; Elaine-Lee, Y.L.; Yee, B.C.; How, C.W.; Yim, H.S.; Rasadee, A.; Ng, H.S. Zerumbone-loaded nanostructured lipid carrier induces apoptosis in human colorectal adenocarcinoma (Caco-2) cell line. Nanosci. Nanotechnol. Lett. 2016, 8, 294–302. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Yeap, S.K.; Abdul Samad, N.; Andas, R.J.; Muhammad Nadzri, N.; Anasamy, T. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Israf, D.A.; Tham, C.L. Major bioactive compounds in essential oils extracted from the rhizomes of Zingiber zerumbet (L) Smith: A mini-review on the anti-allergic and immunomodulatory properties. Front. Pharmacol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Chakraborty, A.; Coffman, R.; Jorvig, J. Zerumbone, a phytochemical from Asian ginger is a novel inhibitor of Jak2/Stat3 inhibits promigratory gene expression, growth and migration of pancreatic cancer cells. Pancreatology 2013, 2, e18–e19. [Google Scholar] [CrossRef]
- Sidahmed, H.M.A.; Hashim, N.M.; Abdulla, M.A.; Ali, H.M.; Mohan, S.; Abdelwahab, S.I.; Taha, M.M.E.; Fai, L.M.; Vadivelu, J. Antisecretory, gastroprotective, antioxidant and anti-helicobcter pylori activity of zerumbone from Zingiber Zerumbet (L.) Smith. PLoS ONE 2015, 10, e0121060. [Google Scholar] [CrossRef]
- Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Elhassan, M.M.; Murali, S.M. Anticancer and antimicrobial activities of zerumbone from. Int. J. Pharmacol. 2008, 4, 301–304. [Google Scholar] [CrossRef]
- Sulaiman, M.; Perimal, E.; Zakaria, Z.; Mokhtar, F.; Akhtar, M.; Lajis, N.; Israf, D. Preliminary analysis of the antinociceptive activity of zerumbone. Fitoterapia 2009, 80, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Fakurazi, S.; Hairuszah, I.; Lip, J.M.; Shanthi, G.; Nanthini, U.; Shamima, A.; Roslida, H.; Tan, Y. Hepatoprotective action of zerumbone against paracetamol induced hepatotoxicity. J. Med. Sci. 2009, 9, 161–164. [Google Scholar] [CrossRef]
- Keong, Y.S.; Alitheen, N.B.; Mustafa, S.; Aziz, S.A.; Rahman, M.A.; Ali, A.M. Immunomodulatory effects of zerumbone isolated from roots of Zingiber zerumbet. Pak. J. Pharm. Sci. 2010, 23, 75–82. [Google Scholar] [PubMed]
- Murakami, A.; Miyamoto, M.; Ohigashi, H. Zerumbone, an anti-inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors 2004, 21, 95–101. [Google Scholar] [CrossRef]
- Murakami, A.; Hayashi, R.; Takana, T.; Kwon, K.H.; Ohigashi, H.; Safitri, R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: Separately and in combination. Biochem. Pharmacol. 2003, 66, 1253–1261. [Google Scholar] [CrossRef]
- Albaayit, S.F.A.; Rasedee, A.; Abdullah, N. Zerumbone-loaded nanostructured lipid carrier gel facilitates wound healing in rats. Rev. Bras. De Farmacogn. 2020, 30, 272–278. [Google Scholar] [CrossRef]
- Md, S.; Kit, B.; Jagdish, S.; David, D.J.; Pandey, M.; Chatterjee, L.A. Development and in vitro evaluation of a zerumbone loaded nanosuspension drug delivery system. Crystals 2018, 8, 286. [Google Scholar] [CrossRef]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Dev, S. Studies in sesquiterpenes—XVI: Zerumbone, a monocyclic sesquiterpene ketone. Tetrahedron 1960, 8, 171–180. [Google Scholar] [CrossRef]
- Damodaran, N.; Dev, S. Stereochemistry of zerumbone. Tetrahedron Lett. 1965, 6, 1977–1981. [Google Scholar] [CrossRef]
- Dev, S.; Anderson, J.E.; Cormier, V.; Damodaran, N.; Roberts, J.D. Nuclear magnetic resonance spectroscopy. The conformational mobility of humulene and zerumbone. J. Am. Chem. Soc. 1968, 90, 1246–1248. [Google Scholar] [CrossRef]
- Hall, S.; Nimgirawath, S.; Raston, C.; Sittatrakul, A.; Thadaniti, S.; Thirasasana, N.; White, A. Crystal structure of zerumbone [(E, E, E)-2, 6, 9, 9-Tetramethylcycloundeca-2, 6, 10-trien-1-one]. Aust. J. Chem. 1981, 34, 2243–2247. [Google Scholar] [CrossRef]
- Kitayama, T.; Yokoi, T.; Kawai, Y.; Hill, R.K.; Morita, M.; Okamoto, T.; Yamamoto, Y.; Fokin, V.V.; Sharpless, K.B.; Sawada, S. The chemistry of zerumbone. Part 5: Structural transformation of the dimethylamine derivatives. Tetrahedron 2003, 59, 4857–4866. [Google Scholar] [CrossRef]
- Kitayama, T.; Okamoto, T.; Hill, R.K.; Kawai, Y.; Takahashi, S.; Yonemori, S.; Yamamoto, Y.; Ohe, K.; Uemura, S.; Sawada, S. Chemistry of zerumbone. 1. Simplified isolation, conjugate addition reactions, and a unique ring contracting transannular reaction of its dibromide. J. Org. Chem. 1999, 64, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Baby, S.; Dan, M.; Thaha, A.R.; Johnson, A.J.; Kurup, R.; Balakrishnapillai, P.; Lim, C.K. High content of zerumbone in volatile oils of Zingiber zerumbet from southern India and Malaysia. Flavour Fragr. J. 2009, 24, 301–308. [Google Scholar] [CrossRef]
- Kitayama, T. Attractive reactivity of a natural product, zerumbone. Biosci. Biotechnol. Biochem. 2011, 1101072348. [Google Scholar] [CrossRef]
- Al-Zubairi, A.S.; Abdul, A.B.; Yousif, M.; Abdelwahab, S.I.; Elhassan, M.M.; Mohan, S. In vivo and in vitro genotoxic effects of zerumbone. Caryologia 2010, 63, 11–17. [Google Scholar] [CrossRef]
- Eid, E.E.; Abdul, A.B.; Suliman, F.E.O.; Sukari, M.A.; Rasedee, A.; Fatah, S.S. Characterization of the inclusion complex of zerumbone with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2011, 83, 1707–1714. [Google Scholar] [CrossRef]
- Junghanns, J.-U.A.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar]
- Bhatt, D.A.; Pethe, A. Lipid technology—A promising drug delivery system for poorly water soluble drugs. Int. J. Pharma Res. 2010. Available online: https://www.researchgate.net/publication/228467810_Lipid_technology-A_promising_drug_delivery_system_for_poorly_water_soluble_drugs (accessed on 20 June 2020).
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef]
- Atwood, J.L. Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Granero, G.E.; Maitre, M.M.; Garnero, C.; Longhi, M.R. Synthesis, characterization and in vitro release studies of a new acetazolamide–HP-β-CD–TEA inclusion complex. Eur. J. Med. Chem. 2008, 43, 464–470. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, R.; Vasilev, K.; Simovic, S. Nanosuspension technologies for delivery of poorly soluble drugs. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Mu, R.H. Manufacturing of nanoparticles by milling and homogenization techniques. In Nanoparticle Technology for Drug Delivery; CRC Press: Boca Raton, FL, USA, 2006; pp. 45–76. [Google Scholar]
- Eid, E.E.; Bustamam Abdul, A.; Rasedee, A.; Suliman, F.E.O.; Sukari, M.A.; Fatah, S.A. Liquid chromatography–tandem mass spectroscopic method for the determination of zerumbone in human plasma and its application to pharmacokinetics. J. Mass Spectrom. 2011, 46, 772–781. [Google Scholar] [CrossRef] [PubMed]
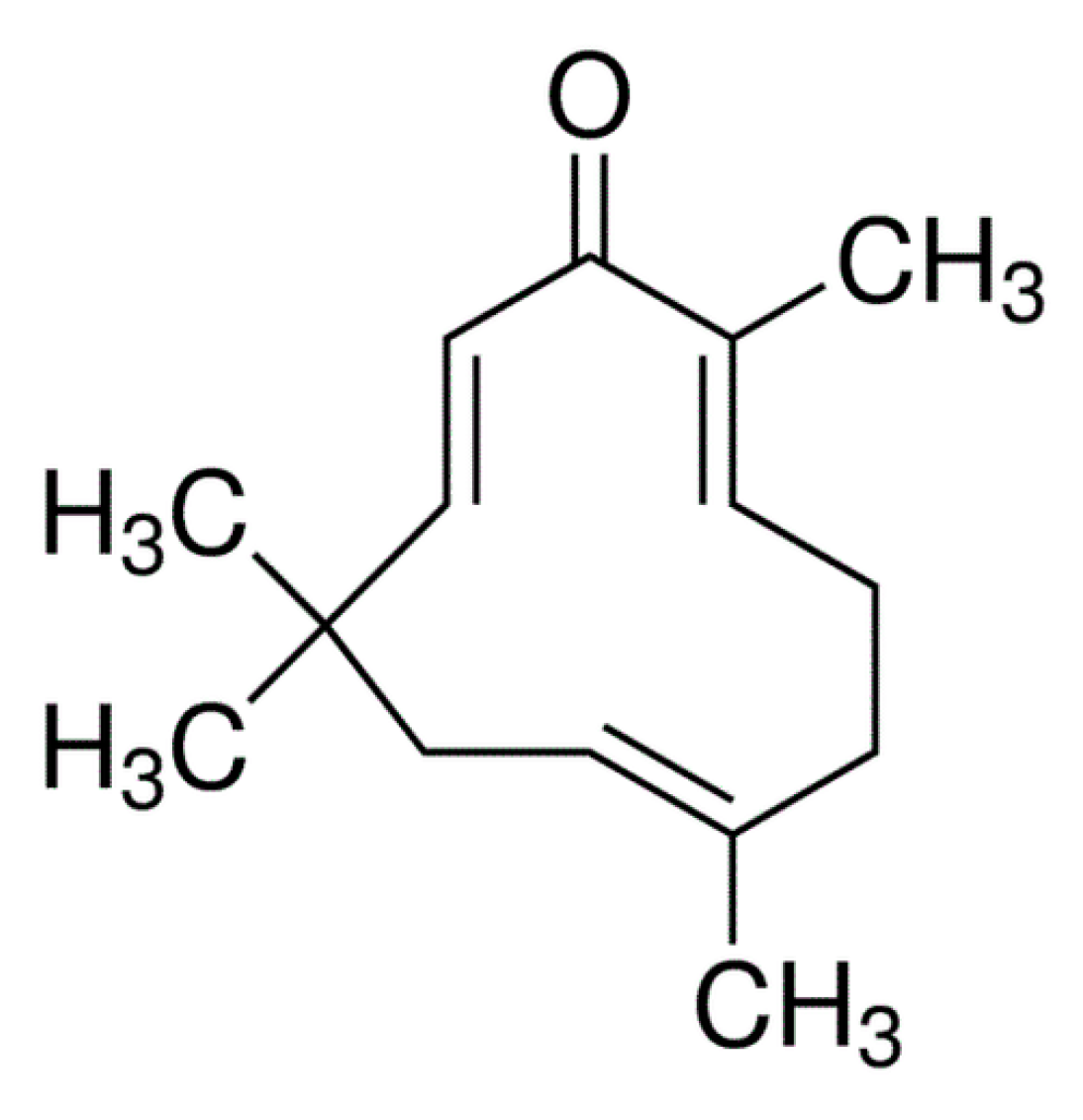
| Characteristics | Description |
|---|---|
| Occurrence | Zingiber species |
| Chemical class | Sesquiterpene |
| IUPAC name | (2E, 6E, 10E)-2,6,9,9-tetramethylcycloundeca-2,6,10-trien-1-one |
| Molecular formula | C15H22O |
| Molecular weight | 218.3 g/mol |
| Lop P | 3.9 |
| Chemical structure | Three-double bond (two conjugated and one isolated), α, β-unsaturated carbonyl group, and a double conjugated carbonyl group in an 11-membered ring structure |
| Flashing point | 272°F |
| Boiling point | 321–322 °C at 760 mmHg |
| Melting point | 65.3 °C |
| Appearance | solid white crystals or powder |
| Stability | Stable for at least 2 years when stored at −20 °C |
| Solubility | Freely soluble in organic solvents such as ethanol and dimethyl sulfoxide (DMSO)Solubility in water ~1–1.5 mg/L at 25 °C |
| Nr | Formulation Strategy | Method of Preparation | Components of Delivery System | Therapeutic Indication | Purpose | Major Results/Conclusions |
|---|---|---|---|---|---|---|
| 1 | Nanostructured lipid carriers [36] | Hot, high-pressure homogenization | Lipid dispersion: hydrogenated palm oil, olive oil, and lipoid S100 Aqueous dispersion: Sorbitol, Tween-80 and thiomersal | Leukemia | Treatment of leukemia | The zerumbone-loaded nanocarriers depict sustained release characteristics and high cytotoxicity in human T-cell acute lymphocytic leukemia. |
| 2 | Nanostructured lipid carriers [37] | Hot, high-pressure homogenization | hydrogenated palm oil, distilled water, olive oil, lipoid- S 100, lecithin, thimerosal, and sorbitol | Antileukemic effect and acute toxicity | Acute toxicity of zerumbone and zerumbone-loaded nanocarriers via the oral route of administration | Both zerumbone and zerumbone-loaded lipid nanocarriers at acute doses do not induce behavioral alterations, toxicological signs, or adverse effects. |
| 3 | Nanostructured lipid nanocarriers [35] | Hot, high-pressure homogenization | Lipid dispersion: hydrogenated palm oil, olive oil, and lipoid S100 Aqueous dispersion: Sorbitol, Tween-80 and thiomersal | Colorectal adenocarcinoma | Increase the potency and efficacy of zerumbone-loaded lipid nanocarriers | Zerumbone lipid nanocarriers depict slow release of the drug without altering the anti-cancer effect. |
| 4 | Nanostructured lipid nanocarriers [28] | Hot, high-pressure homogenization | Lipid dispersion: hydrogenated palm oil, olive oil, and lipoid S100 Aqueous dispersion: Sorbitol, Tween-80 and thiomersal | Canine mammary gland tumor | Antiproliferative effect and the mode of cell death on canine mammary gland tumor adenocarcinoma | Effective in inhibiting the proliferation and inducing apoptosis on the canine mammary gland tumor cells. Inhibition of Bcl-2 and activation of pro-apoptotic Bax gene expressions and activation of caspases of the intrinsic and extrinsic apoptosis pathways were reasons for the effect. |
| 5 | Inclusion complexes [60] | Inclusion complex through freeze-drying | Hydroxypropyl-β-cyclodextrin | Anticancer drug effect | Enhancement of solubility of zerumbone | Important modifications in the solubility and stability of zerumbone |
| 6 | Nanosuspensions [48] | High-pressure homogenization | Hydroxypropyl methylcellulose (HPMC) and sodium dodecyl sulfate (SDS) | Not applicable | Improve solubility and dissolution characteristic’s | Enhanced dissolution and saturation solubility of zerumbone. |
| 7 | Nanostructured lipid carrier gel [47] | Hot, high-pressure homogenization | Carbopol 980 used to prepare the nanostructured lipid carrier gel | Wound healing | Anti-inflammatory activity | The gel decreased inflammatory cell infiltration and degeneration and increased granulation in healing wound tissues. There was an increase of anti-inflammatory IL-10, decreased the pro-inflammatory TNF-α, IL-6 concentrations, and downregulated cyclooxygenase-2 gene expression. |
| Type of Study | Subjects | Dose | Key Results |
|---|---|---|---|
| Acute toxicity [31] | Sprague Dawley rats | 100–3000 mg/kg | The results from this study showed that single injected doses of zerumbone at 100–200 mg/kg had no toxic effects on the renal and liver tissues of rats. The death of all experimental animals was reported at high doses of 2500 and 3000 mg/kg, and 20 and 40% of animals died at doses of 1500 and 2000 mg/kg respectively. In addition, a dose of 500 mg/kg induced nephrocellular and hepatocellular damage leading to renal and hepatic failure. The LD50 value was 1.84 g/kg when injected intraperitoneally. |
| Genotoxicity [59] | Chinese hamster ovary (CHO) cell lines | 2.5 to 80 μM/mL | The results from this study reported that zerumbone at high concentrations had a genotoxic and cytotoxic effect on CHO cells. However, it failed to induced mutagenic effects on Salmonella Typhimurium strain TA100 |
| Year | Patented System | Patent/Publication Number | Clinical Application |
|---|---|---|---|
| 2019 | A gel containing zerumbone from bitter ginger for curative treatment of diabetic ulcers | WO2019173890A1 | A dermatological pharmaceutical composition in the form of a gel for topical use containing zerumbone. |
| 2019 | Method for treating an allergic disease | United States Patent 10688078 | The invention relates to the use of zerumbone for treating an allergic disease. |
| 2018 | New use of zerumbone and compositions comprising zerumbone | EP2802310B1 | The topical use of zerumbone for the treatment of the deficiencies of the capillary network of the skin. It also helps to treat micro-subcutaneous edemas, including bags and/or dark circles under the eyes. |
| 2014 | A composition for treating leukemia | WO2014123406A1 | The composition comprises an effective amount of zerumbone and a pharmaceutically acceptable nanostructured lipid carrier for treating leukemia. |
| 2013 | Inclusion complex of zerumbone with a hydroxypropyl-β-cyclodextrin (HP-β-CD) in aqueous form | MY149711A | The present invention relates to a novel zerumbone inclusion complex having improved solubility properties. |
| 2009 | Immune modulation and anti-allergy activities of Zingiber zerumbet | United States Patent 7588788 | The present invention provides for a method of preparing a nutraceutical formulation comprising zerumbone, and the use of this formulation to regulate the immune system, and more specifically to prevent or to treat an allergic disorder. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesharwani, S.S.; Bhat, G.J. Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone. Medicina 2020, 56, 557. https://doi.org/10.3390/medicina56110557
Kesharwani SS, Bhat GJ. Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone. Medicina. 2020; 56(11):557. https://doi.org/10.3390/medicina56110557
Chicago/Turabian StyleKesharwani, Siddharth S., and G. Jayarama Bhat. 2020. "Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone" Medicina 56, no. 11: 557. https://doi.org/10.3390/medicina56110557
APA StyleKesharwani, S. S., & Bhat, G. J. (2020). Formulation and Nanotechnology-Based Approaches for Solubility and Bioavailability Enhancement of Zerumbone. Medicina, 56(11), 557. https://doi.org/10.3390/medicina56110557