Relationship between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients
Abstract
:1. Introduction
2. Materials and Methods
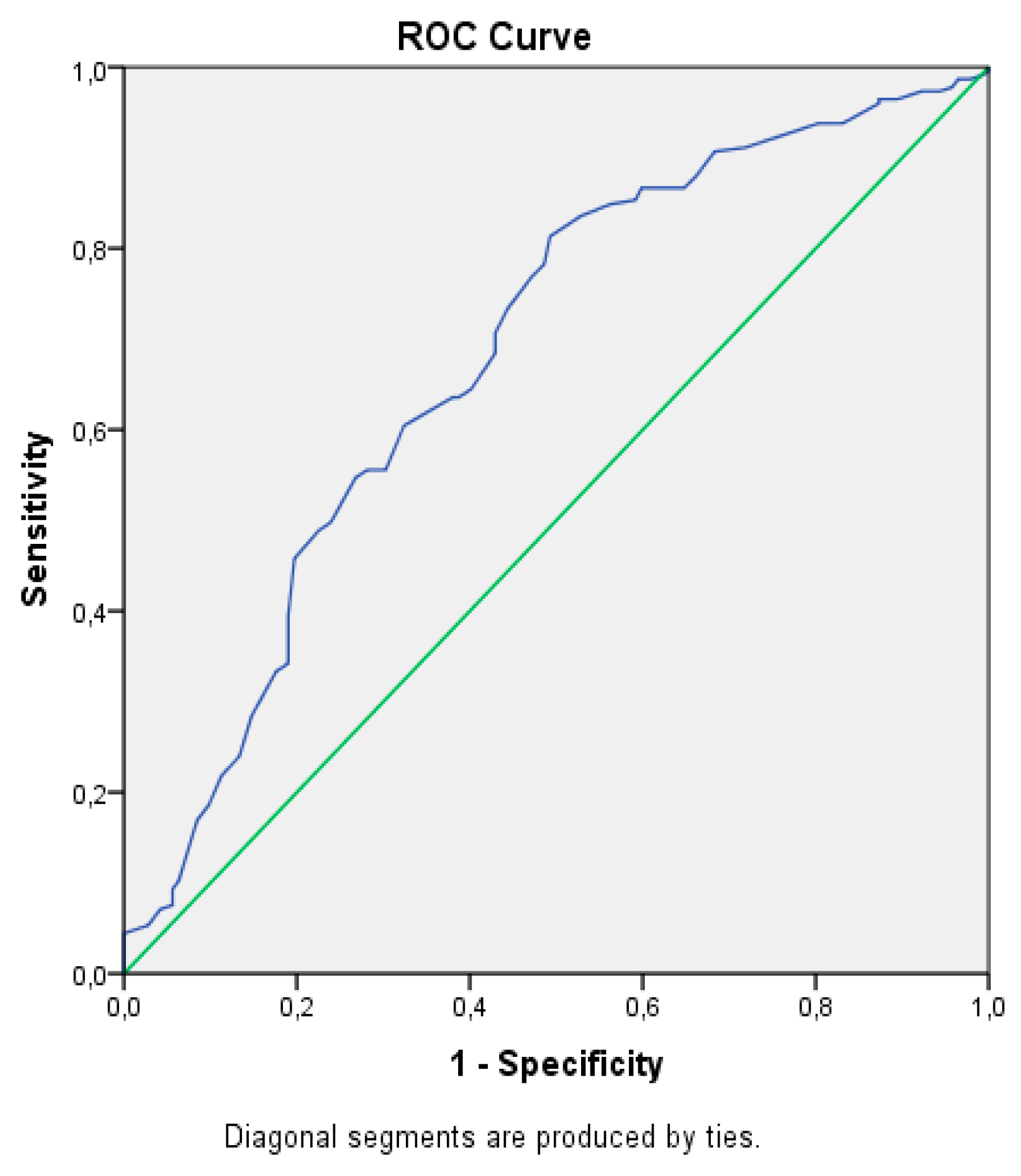
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- LaBrecque, D.R.; Abbas, Z.; Anania, F.; Ferenci, P.; Khan, A.G.; Goh, K.L. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2012, 48, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sookoian, S.; Pirola, C.J. Systematic review with meta-analysis: Risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther. 2017, 46, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef]
- Fan, J.G.; Saibara, T.; Chitturi, S.; Kim, B.I.; Sung, J.J.Y.; Chutaputti, A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J. Gastroenterol. Hepatol. 2007, 22, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Kumagi, T.; Furukawa, S.; Tokumoto, Y.; Hirooka, M.; Abe, M.; Hiasa, Y.; Matsuura, B.; Onji, M. Non-alcoholic fatty liver disease: Factors associated with its presence and onset. J. Gastroenterol. Hepatol. 2013, 28, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.J.; Zhu, G.Q.; Ye, B.Z.; Kong, F.Q.; Zheng, Z.X.; Zou, H.; Shi, K.Q.; Lin, L.; Braddock, M.; Huang, W.J.; et al. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults. Medicine 2015, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Fukatsu, M.; Suzuki, S.; Wada, T.; Joh, T. Elevated serum uric acid predicts impaired fasting glucose and type 2 diabetes only among Japanese women undergoing health checkups. Diabetes Metab. 2011, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.H.; Kozin, F.; O’malley, M.; McCarty, D.J. Release of platelet constituents by monosodium urate crystals. J. Clin. Investig. 1977, 60, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sertoglu, E.; Ercin, C.N.; Celebi, G.; Gurel, H.; Kayadibi, H.; Genc, H.; Kara, M.; Dogru, T. The relationship of serum uric acid with non-alcoholic fatty liver disease. Clin. Biochem. 2014, 47, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Fiorentino, T.V.; Arturi, F.; Perticone, M.; Sciacqua, A.; Perticone, F. Association between noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e88569. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, Y.; Seno, Y.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Katasako, A.; Mori, K.; Matsunaga, Y.; Fukushima, T.; Kanaoka, R.; et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Satta, Y.; Takenaka, O.; Takahata, N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol. Biol. Evol. 2002, 19, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Iribarren, C.; Gross, M.D.; Comstock, G.W.; Cutler, R.G. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis 2000, 148, 131–139. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fattyliver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.J.; Rosado, L.E.F.; Alfenas, R.D.C.G.; Geloneze, B. Critical analysis on theuse of the homeostasis model assessment (HOMA) indexes in the evaluationof the insulin resistance and the pancreatic beta cells functional capacity. Arq. Bras. Endocrinol. Metabol. 2008, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.C.; Stram, D.O.; Porcel, J.P.; Lu, S.C.; Marchand, L.E.; Noureddin, M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The Multiethnic Cohort. Hepatology 2016, 64, 1969–1977. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Järvinen, H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015, 7, 9127–9138. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Yeh, M.L.; Yu, M.L.; Huang, C.F.; Dai, C.Y.; Hsieh, M.Y.; Hsieh, M.H.; Huang, C.I.; Lin, Z.Y.; Chen, S.C.; et al. Hyperuricemia Inversely Correlates with Disease Severity in Taiwanese Nonalcoholic Steatohepatitis Patients. PLoS ONE 2015, 10, e0139796. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, C.M.; Aller, R.; Gutiérrez-García, M.L.; Ampuero, J.; Gómez-Camarero, J.; Martín-Mateos, R.M.; Burgos-Santamaría, D.; Rosales, J.M.; Aspichueta, P.; Buque, X.; et al. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Esp. Enferm. Dig. 2019, 111, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, F.Z.; Lin, K.H.; Chen, Y.H.; Wu, P.C.; Chen, Y.H.; Chen, C.S.; Wang, W.H.; Mar, G.Y.; Yu, H.C. Role of Fatty Liver Index and Metabolic Factors in the Prediction of Nonalcoholic Fatty Liver Disease in a Lean Population Receiving Health Checkup. Clin. Transl. Gastroenterol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Culafic, M.; Vezmar Kovacevic, S.; Dopsaj, V.; Stulic, M.; Vlaisavljevic, Z.; Miljkovic, B.; Culafic, D. A Simple Index for Nonalcoholic Steatohepatitis—HUFA—Based on Routinely Performed Blood Tests. Medicina 2019, 55, 243. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Cammà, C.; Cabibi, D.; Di Marco, V.; Craxì, A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2011, 34, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Colvin, R.; Belt, P.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Schwimmer, J.; Tonascia, J.; Unalp, A.; Lavine, J.E.; et al. Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 90–96. [Google Scholar] [CrossRef]
- Darmawan, G.; Hamijoyo, L.; Hasan, I. Association between serum uric acid and non-alcoholic fatty liver disease: A meta-analysis. Acta Med. Indones. 2017, 49, 136–147. [Google Scholar]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol. 2006, 290, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Suzuki, S.; Fukatsu, M.; Wada, T.; Yoshida, T.; Joh, T. Elevated serum uric acid is an independent risk factor for nonalcoholic fatty liver disease in Japanese undergoing a health checkup. Acta Gastroenterol. Belg. 2010, 73, 12–17. [Google Scholar] [PubMed]
- Xu, C.; Yu, C.; Xu, L.; Miao, M.; Li, Y. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: A prospective observational study. PLoS ONE 2010, 5, e11578. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.L.; Miller, W.G.; Coresh, J.; Fleming, J.; Greenberg, N.; Greene, T.; Hostetter, T.; Levey, A.S.; Panteghini, M.; Welch, M.; et al. Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 2006, 52, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Liang, J. Blood urea nitrogen is elevated in patients with non-alcoholic fatty liver disease. Hepatogastroenterology 2013, 60, 343–345. [Google Scholar] [PubMed]
- Kirvar, A.; Ayaz, T.; Durakoğlugil, T.; Şahin, S.B.; Şahin, O.Z.; Durakoğlugil, E. The Association Between Non-Alcholic Fatty Liver Disease with Carotid Intima Media Thickness. J. Kartal. TR 2015, 26, 13–18. [Google Scholar]
| Characteristics of Patients | NAFLD (n = 225) | Control (n = 142) | P* |
|---|---|---|---|
| Age | 34.24 ± 8.72 | 34.08 ± 9.08 | 0.80 |
| Gender (female/male. n) | 87/138 | 61/81 | 0.41** |
| Body mass index (kg/m2) | 27.25 ± 4.02 | 24.71 ± 3.34 | <0.001 |
| ALT (IU/L) | 22.76 ± 14.13 | 18.47 ± 10.88 | 0.001 |
| AST (IU/L) | 18.48 ± 5.66 | 16.99 ± 4.60 | 0.015 |
| ALP (U/L) | 72.92 ± 24.40 | 66.11 ± 20.06 | 0.009 |
| GGT (U/L) | 20.72 ± 15.76 | 18.23 ± 12.33 | 0.10 |
| Total Biluribin (mg/dl) | 0.59 ± 0.31 | 0.59 ± 0.53 | 0.17 |
| Albumin (g/dll) | 4.68 ± 0.31 | 4.66 ± 0.61 | 0.10 |
| Triglyceride (mg/dL) | 117.74 ± 66.10 | 96.54 ± 43.79 | 0.03 |
| Total cholesterol (mg/dl) | 185.79 ± 41.36 | 178.90 ± 45.13 | 0.03 |
| HOMA-IR | 2.60 ± 1.61 | 1.71 ± 0.77 | <0.001 |
| BUN (mg/dl) | 12.18 ± 3.64 | 12.04 ± 3.13 | 0.32 |
| Creatinin (mg/dl) | 0.79 ± 0.16 | 0.69 ± 0.16 | <0.001 |
| Uric acid (mg/dl) | 4.36 ± 1.36 | 3.48 ± 1.30 | <0.001 |
| Biochemistry Parameters | NAFLD Stage | |
|---|---|---|
| r | P* | |
| Uric Acid | 0.338 | <0.001 |
| Creatinine | 0.243 | <0.001 |
| Blood Urea Nitrogen | 0.069 | 0.185 |
| Independent Factors | Odds Ratio | 95% Cl | P Value |
|---|---|---|---|
| Age | 0.987 | 0.956–1.018 | 0.405 |
| Gender (female/male, %) | 1.602 | 0.848–3.025 | 0.147 |
| Body mass index (kg/m2) | 1.084 | 1.001–1.174 | 0.047** |
| ALT (IU/L) | 1.020 | 0.984–1.057 | 0.289 |
| AST (IU/L) | 1.016 | 0.943–1.094 | 0.678 |
| ALP (U/L) | 1.010 | 0.998–1.022 | 0.114 |
| GGT (U/L) | 0.972 | 0.949–0.994 | 0.015** |
| Total Biluribin (mg/dL) | 1.002 | 0.556–1.809 | 0.994 |
| Albumin (g/dL) | 0.935 | 0.511–1.712 | 0.829 |
| Triglyceride (mg/dL) | 1.002 | 0.997–1.007 | 0.479 |
| Total cholesterol (mg/dl) | 1.001 | 0.994–1.007 | 0.808 |
| HOMA-IR | 1.743 | 1.248–2.434 | 0.001** |
| BUN (mg/dl) | 0.962 | 0.888–1.043 | 0.351 |
| Creatinin (mg/dl) | 38.636 | 5.738–260.146 | 0.000** |
| Uric asit (mg/dl) | 1.592 | 1.299–1.951 | 0.000** |
| Biochemistry Parameters | Group I (n = 201) | Group II (n = 24) | Control (n = 142) |
|---|---|---|---|
| Uric Acid | 4.28 ± 1.32* | 5.07 ± 0.78** | 3.48 ± 1.36 |
| Creatinine | 0.79 ± 0.16* | 0.78 ± 0.16* | 0.69 ± 0.16 |
| Blood Urea Nitrogen | 12.13 ± 3.21 | 12.58 ± 2.43 | 12.04 ± 3.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oral, A.; Sahin, T.; Turker, F.; Kocak, E. Relationship between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients. Medicina 2019, 55, 600. https://doi.org/10.3390/medicina55090600
Oral A, Sahin T, Turker F, Kocak E. Relationship between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients. Medicina. 2019; 55(9):600. https://doi.org/10.3390/medicina55090600
Chicago/Turabian StyleOral, Alihan, Tolga Sahin, Fatih Turker, and Erdem Kocak. 2019. "Relationship between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients" Medicina 55, no. 9: 600. https://doi.org/10.3390/medicina55090600
APA StyleOral, A., Sahin, T., Turker, F., & Kocak, E. (2019). Relationship between Serum Uric Acid Levels and Nonalcoholic Fatty Liver Disease in Non-Obese Patients. Medicina, 55(9), 600. https://doi.org/10.3390/medicina55090600





