Serum and Tissue HIF-2 Alpha Expression in CIN, N-Acetyl Cysteine, and Sildenafil-Treated Rat Models: An Experimental Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Materials
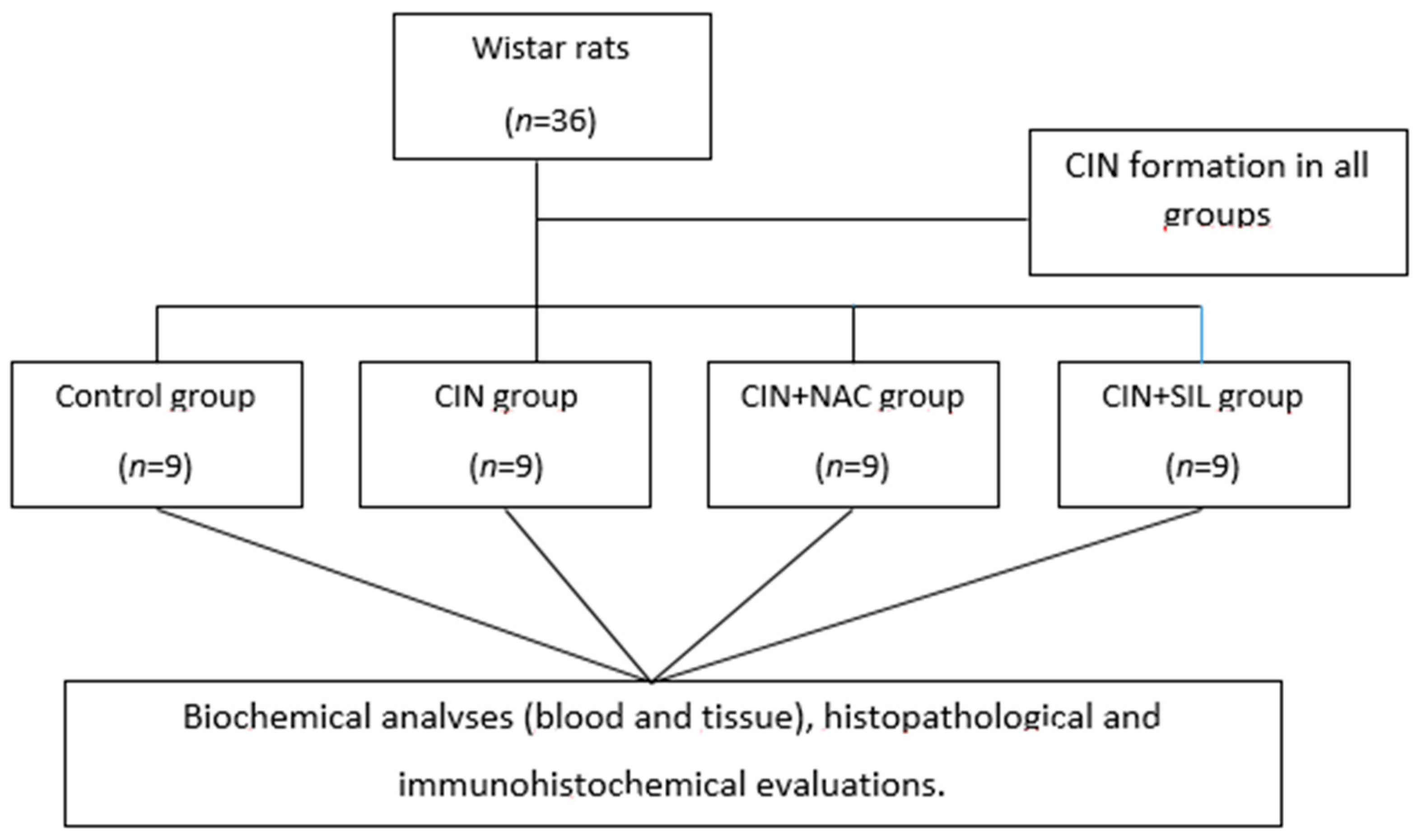
2.2. Model and Grouping
2.3. Biochemical Analyses
2.4. Biochemical Evaluation of Tissue Samples
2.5. Histopathological Evaluation
2.6. Immunohistochemistry
2.7. Quantitative Immunohistochemistry
2.8. Statistical Analyses
3. Results
3.1. Comparison of Renal Function Among the Four Groups
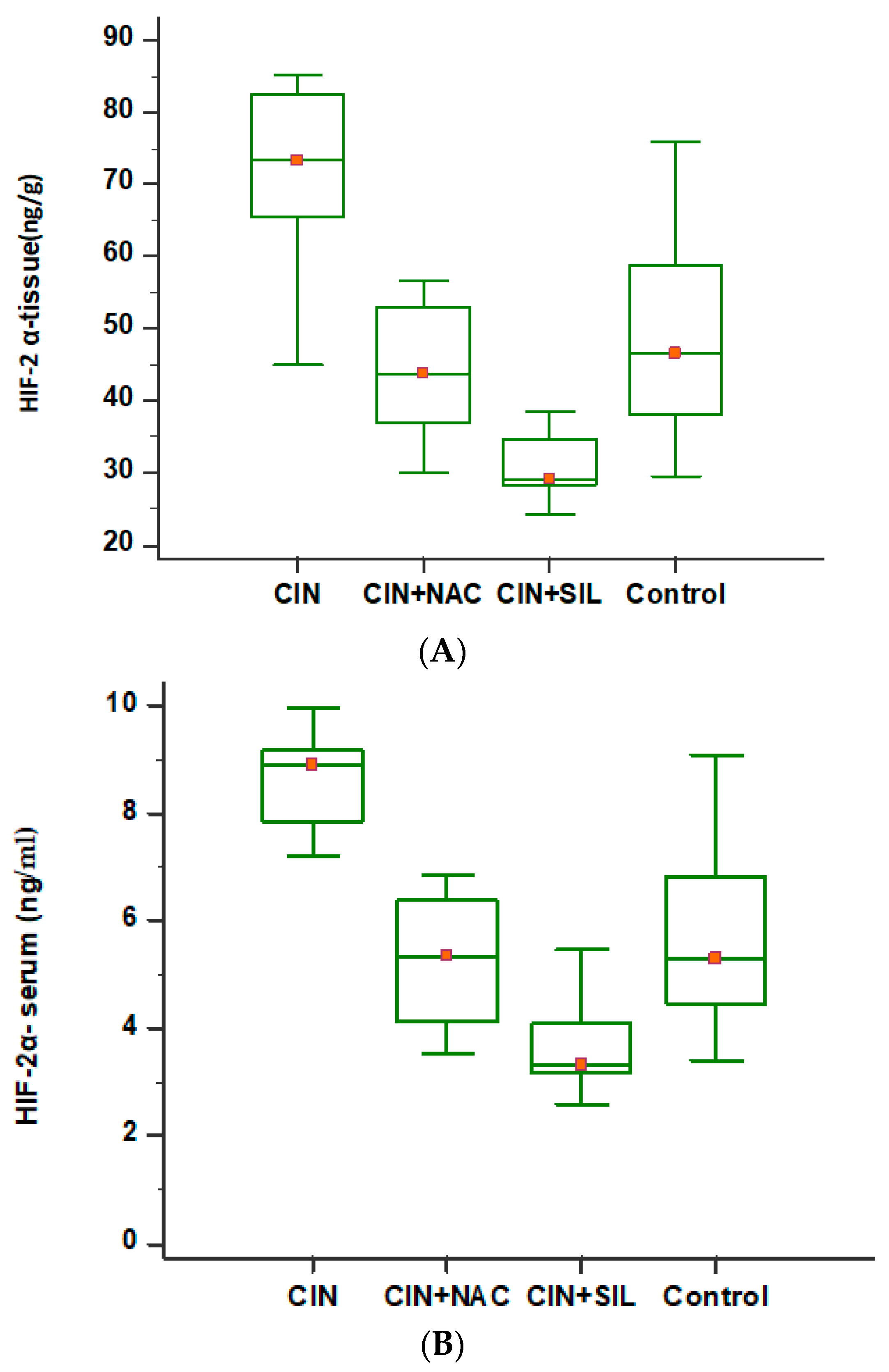
3.2. Comparison of HIF-2α Levels Among the Four Groups
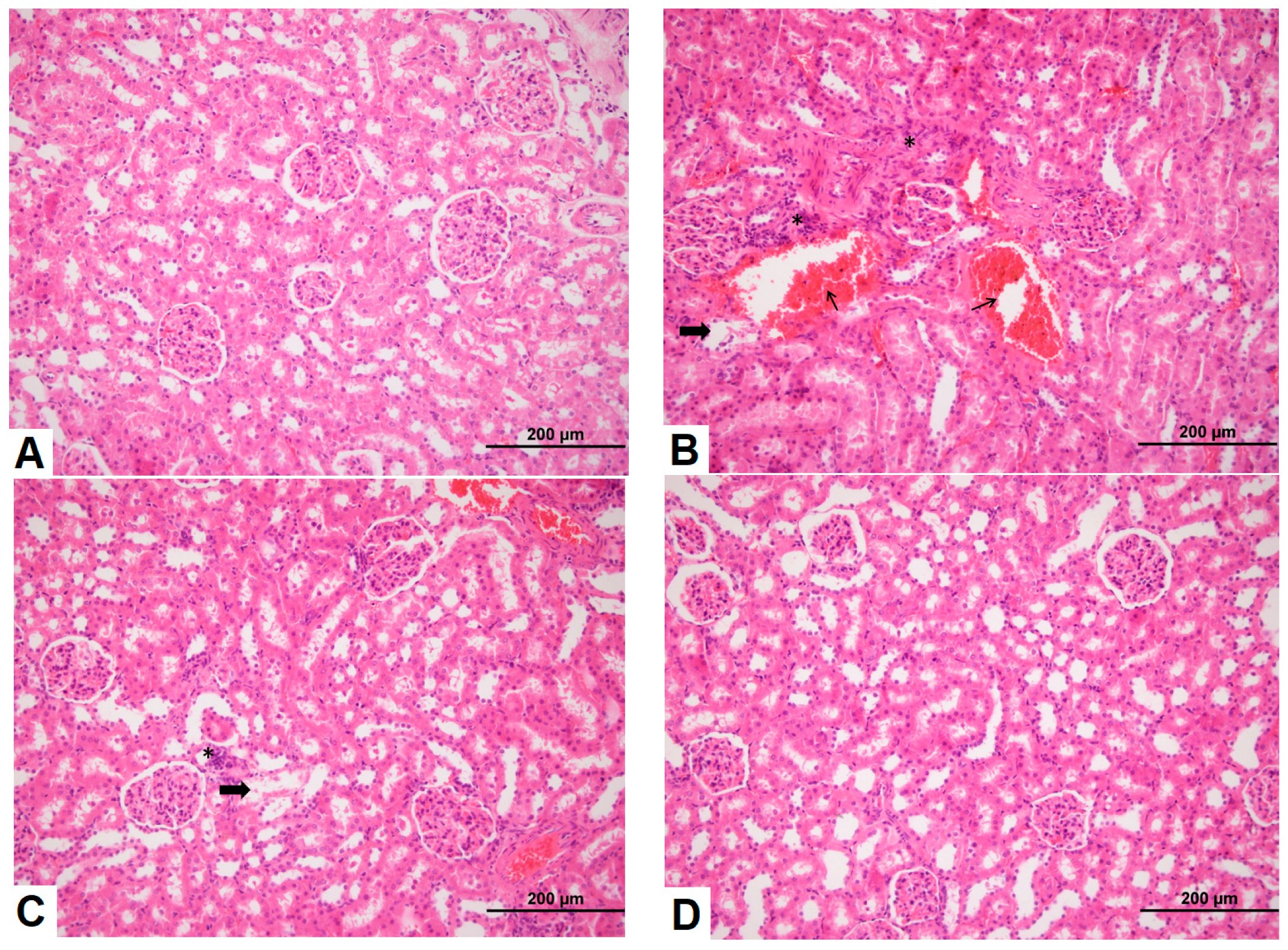
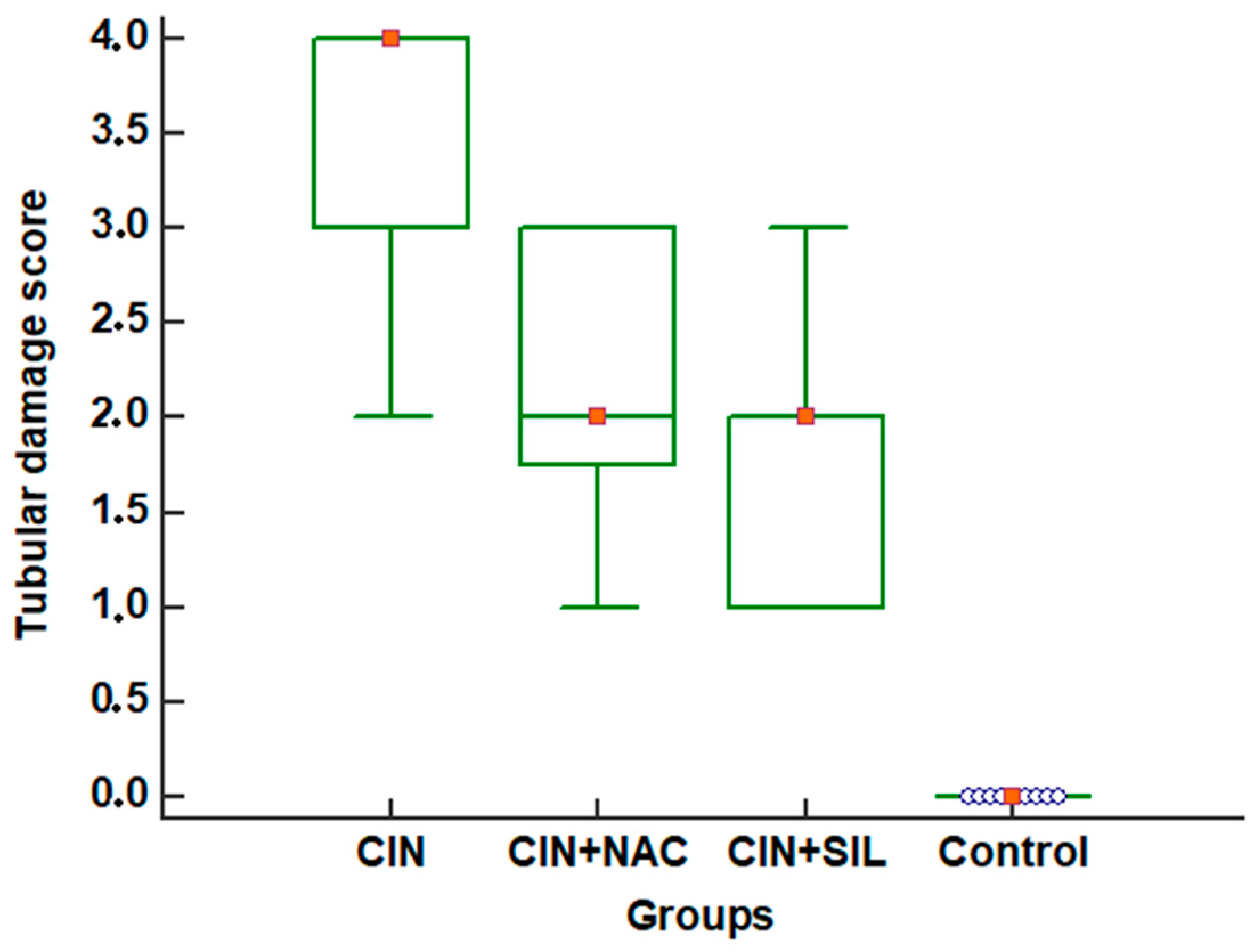
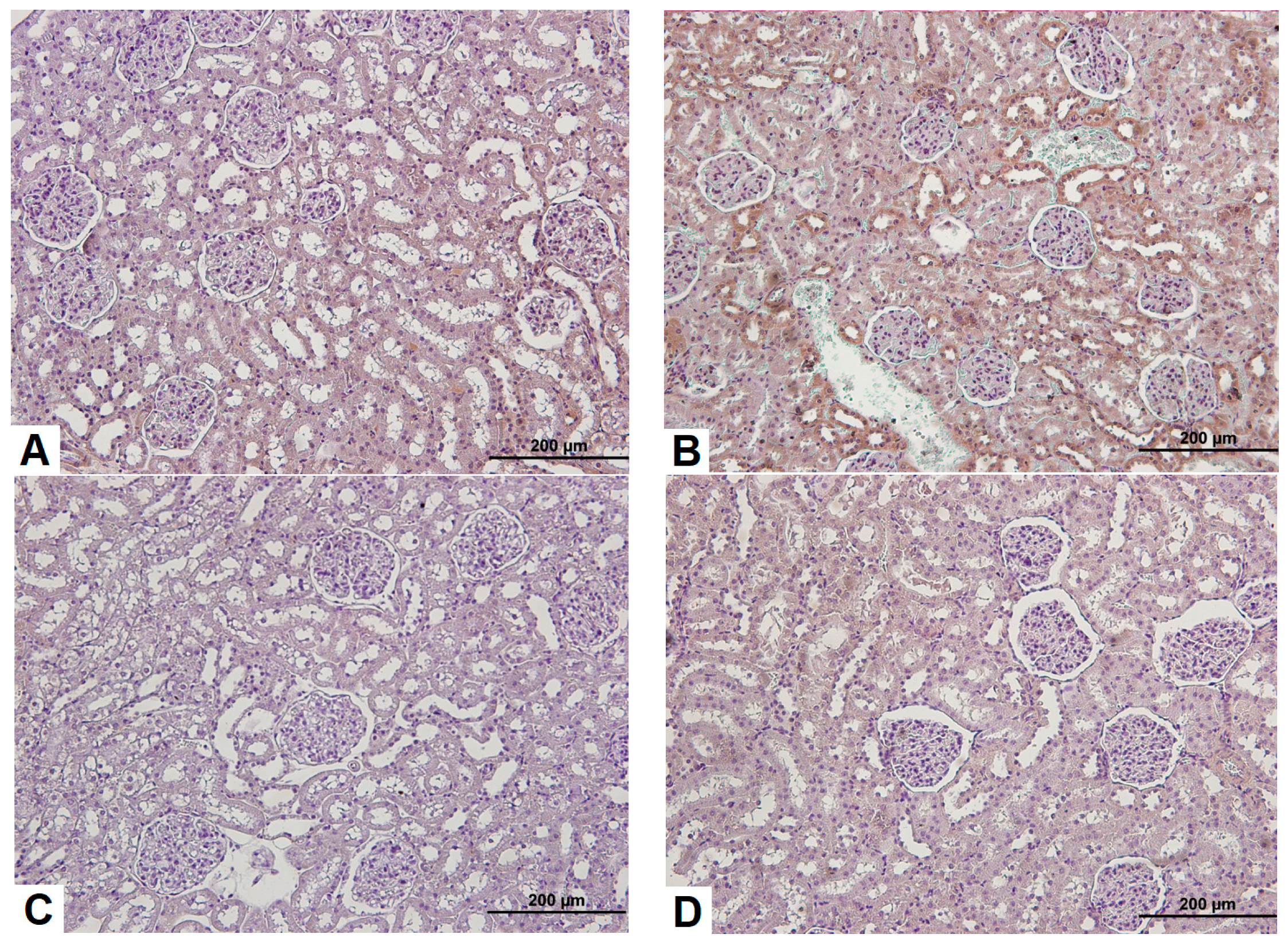
3.3. Effects of Sildenafil on Kidney Histopathological Alterations, Histopathologic Findings in CIN Rats and Treatment Groups
3.4. Observation of Renal Immunohistochemistry for Four Groups
4. Discussion
5. Conclusions
Restrictions of the Study
Author Contributions
Funding
Conflicts of Interest
References
- Xu, R.; Tao, A.; Bai, Y.; Deng, Y.; Chen, G. Effectiveness of N-Acetylcysteine for the Prevention of Contrast-Induced Nephropathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003968. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wei, R.B.; Li, Q.P.; Yang, X.; Li, P.; Huang, M.J.; Wang, R.; Cai, G.Y.; Chen, X.M. Renal Protective Effect of Probucol in Rats with Contrast-Induced Nephropathy and its Underlying Mechanism. Med. Sci. Monit. 2015, 21, 2886–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turedi, S.; Erdem, E.; Karaca, Y.; Tatli, O.; Sahin, A.; Turkmen, S.; Gunduz, A. The High Risk of Contrast-induced Nephropathy in Patients with Suspected Pulmonary Embolism Despite Three Different Prophylaxis: A Randomized Controlled Trial. Acad. Emerg. Med. 2016, 23, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Traub, S.J.; Kellum, J.A.; Tang, A.; Cataldo, L.; Kancharla, A.; Shapiro, N.I. Risk Factors for Radiocontrast Nephropathy After Emergency Department Contrast-enhanced Computerized Tomography. Acad. Emerg. Med. 2013, 20, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaleel, S.A.; Alzokaky, A.A.; Raslan, N.A.; Alwakeel, A.I.; El-Aziz, H.G.A.; Abd-Allah, A.R. Lansoprazole Halts Contrast Induced Nephropathy through Activation of Nrf2 Pathway in Rats; Elsevier Ireland Ltd.: Shannon, Ireland, 2017; Volume 270. [Google Scholar]
- Liu, J.; Wei, Q.; Guo, C.; Dong, G.; Liu, Y.; Tang, C.; Dong, Z. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int. J. Mol. Sci. 2017, 18, 950. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fang, Y.; Liu, H.; Zhu, J.; Zou, J.; Xu, X.; Jiang, S.; Ding, X. The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol. Dial. Transplant. 2012, 27, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thelin, E.P.; Frostell, A.; Mulder, J.; Mitsios, N.; Damberg, P.; Aski, S.N.; Risling, M.; Svensson, M.; Morganti-Kossmann, M.C.; Bellander, B.M. Lesion size is exacerbated in hypoxic rats whereas hypoxia-inducible factor-1 alpha and vascular endothelial growth factor increase in injured normoxic rats: A prospective cohort study of secondary hypoxia in focal traumatic brain injury. Front. Neurol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Bruschetta, G.; Siracusa, R.; Crupi, R.; Di Paola, R.; Esposito, E.; Cuzzocrea, S. A novel protective formulation of Palmitoylethanolamide in experimental model of contrast agent induced nephropathy. Toxicol. Lett. 2016, 240, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.; Heyman, S.N.; Rosen, S.; Shina, A.; Goldfarb, M.; Griethe, W.; Frei, U.; Reinke, P.; Bachmann, S.; Eckardt, K.U. Up-regulation of HIF in experimental acute renal failure: Evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005, 67, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Wiesener, M.S.; Jürgensen, J.S.; Rosenberger, C.; Scholze, C.K.; Hörstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Szade, A.; Grochot-Przeczek, A.; Florczyk, U.; Jozkowicz, A.; Dulak, J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life 2015, 67, 145–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, V.H. A breath of fresh air for Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Kapitsinou, P.P.; Sano, H.; Michael, M.; Kobayashi, H.; Davidoff, O.; Bian, A.; Yao, B.; Zhang, M.Z.; Harris, R.C.; Duffy, K.J.; et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J. Clin. Investig 2014, 124, 2396–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, L.S.; Barboza, J.R.; Freitas, F.P.; Porto, M.L.; Vasquez, E.C.; Meyrelles, S.S.; Gava, A.L.; Pereira, T.M. Sildenafil prevents renal dysfunction in contrast media-induced nephropathy in Wistar rats. Hum. Exp. Toxicol. 2016, 35, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Agmon, Y.; Peleg, H.; Greenfeld, Z.; Rosen, S.; Brezis, M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J. Clin. Investig. 1994, 94, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, T.; Nie, P.; Hu, L.; Yu, Y.; Cui, M.; Cai, Z.; Shen, L.; He, B. A novel rat model of contrast-induced acute kidney injury. Int. J. Cardiol. 2014, 172, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Özbek, K.; Ceyhan, K.; Koç, F.; Söğüt, E.; Altunkaş, F.; Karayakalı, M.; Çelik, A.; Kadı, H.; Köseoğlu, R.D.; Önalan, O. The protective effect of single dose tadalafil in contrast-induced nephropathy: An experimental study. Anatol. J. Cardiol. 2015, 15, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksu, F.; Aksu, B.; Unlu, N.; Karaca, T.; Ayvaz, S.; Erman, H.; Uzun, H.; Keles, N.; Bulur, S.; Unlu, E. Antioxidant and renoprotective effects of sphingosylphosphorylcholine on contrast-induced nephropathy in rats. Ren. Fail. 2016, 38, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ Software; National Institutes of Health: Bethesda, MA, USA, 1997; Volume 2012.
- Hong, S.I.; Ahn, S.; Lee, Y.S.; Kim, W.Y.; Lim, K.S.; Lee, J.H.; Lee, J.L. Contrast-induced nephropathy in patients with active cancer undergoing contrast-enhanced computed tomography. Support. Care Cancer 2016, 24, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Dym, R.J. Solitary Kidney Remains a Risk Factor for Renal Insufficiency, Though Not for Contrast-induced Nephropathy Independently. Radiology 2016, 280, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Ibrahim, S.A.; Amin, E.F.; Kamel, M.Y.; Rifaai, R.A.; Hassan, M.K. Sildenafil ameliorates gentamicin-induced nephrotoxicity in rats: Role of iNOS and eNOS. J. Toxicol. 2014, 2014, 489382. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Lin, H.; Toth, T.; Jones, C.; Ray, P.; Welsh, G.I.; Satchell, S.C.; Sleeman, P.; Angelini, G.D.; Murphy, G.J. Phosphodiesterase-5 inhibition prevents postcardiopulmonary bypass acute kidney injury in swine. Ann. Thorac. Surg. 2011, 92, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yagai, T.; Luo, Y.; Liang, X.; Chen, T.; Wang, Q.; Sun, D.; Zhao, J.; Ramakrishnan, S.K.; Sun, L.; et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 2017, 23, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, U.; Czauderna, S.; Stachurska, A.; Tertil, M.; Nowak, W.; Kozakowska, M.; Poellinger, L.; Jozkowicz, A.; Loboda, A.; Dulak, J. Opposite effects of HIF-1α and HIF-2α on the regulation of IL-8 expression in endothelial cells. Free Radic. Biol. Med. 2011, 51, 1882–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, K.H.; Oh, H.J.; Lim, B.J.; Kim, M.; Han, K.H.; Choi, Y.H.; Kwon, K.; Nam, B.Y.; Park, K.S.; Park, J.T.; et al. Selective tubular activation of hypoxia-inducible factor-2α has dual effects on renal fibrosis. Sci. Rep. 2017, 7, 11351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhan, Q.; Chen, J.; Xu, H.; He, Z. Sevoflurane pretreatment enhance HIF-2alpha expression in mice after renal ischemia/reperfusion injury. Int. J. Clin. Exp. Pathol. 2015, 8, 13114–13119. [Google Scholar] [PubMed]

| Variable | Groups | p | |||
|---|---|---|---|---|---|
| Control | CIN | CIN + SIL | CIN + NAC | ||
| BUN (mg/dL) | 21.440 ± 2.45 | 17.610 ± 1.61 | 22.889 ± 1.965 | 20.111 ± 1.90 | <0.001 |
| sCr (mg/dL) | 0.380 ± 0.34 | 0.344 ± 0.03 | 0.294 ± 0.022 | 0.283 ± 0.025 | <0.001 |
| Urine Urea (mg/dL) | 63.250 ± 122.94 | 170.000 ± 245.85 | 164.111 ± 202.093 | 135.333 ± 224.997 | 0.678 |
| Urine Cr (mg/dL) | 1.220 ± 2.43 | 2.000 ± 4.09 | 2.333 ± 3.000 | 1.000 ± 3.000 | 0.788 |
| HIF-2α-tissue (ng/g) | 49.110 ± 15.74 | 71.082 ± 13.086 | 44.811 ± 9.735 | 31.638 ± 6.448 | <0.001 |
| HIF-2α-serum (ng/mL) | 5.770 ± 2.01 | 8.430 ± 1.330 | 5.252 ± 1.206 | 3.627 ± 0.839 | <0.001 |
| HIF-2α-urine (ng/mL) | 0.024 ± 0.006 | 0.044 ± 0.453 | 0.025 ± 0.007 | 0.0441 ± 0.453 | 0.382 |
| QIRIAR | 82.159 ± 0.437 | 91.864 ± 0.634 | 76.076 ± 0.378 | 79.423 ± 0.366 | <0.001 |
| Variable | Groups | p | |||
|---|---|---|---|---|---|
| Control | CIN | CIN + SIL | CIN + NAC | ||
| Tubular damage score | 0 (0–0) | 4 (2–4) | 2 (1–3) | 2 (1–3) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altintop, I.; Tatli, M.; Karakukcu, C.; Soyer Sarica, Z.; Hanım Yay, A.; Balcioglu, E.; Ozturk, A. Serum and Tissue HIF-2 Alpha Expression in CIN, N-Acetyl Cysteine, and Sildenafil-Treated Rat Models: An Experimental Study. Medicina 2018, 54, 54. https://doi.org/10.3390/medicina54040054
Altintop I, Tatli M, Karakukcu C, Soyer Sarica Z, Hanım Yay A, Balcioglu E, Ozturk A. Serum and Tissue HIF-2 Alpha Expression in CIN, N-Acetyl Cysteine, and Sildenafil-Treated Rat Models: An Experimental Study. Medicina. 2018; 54(4):54. https://doi.org/10.3390/medicina54040054
Chicago/Turabian StyleAltintop, Ismail, Mehmet Tatli, Cigdem Karakukcu, Zeynep Soyer Sarica, Arzu Hanım Yay, Esra Balcioglu, and Ahmet Ozturk. 2018. "Serum and Tissue HIF-2 Alpha Expression in CIN, N-Acetyl Cysteine, and Sildenafil-Treated Rat Models: An Experimental Study" Medicina 54, no. 4: 54. https://doi.org/10.3390/medicina54040054





