Anti-Inflammatory Effects of (3S)-Vestitol on Peritoneal Macrophages
Abstract
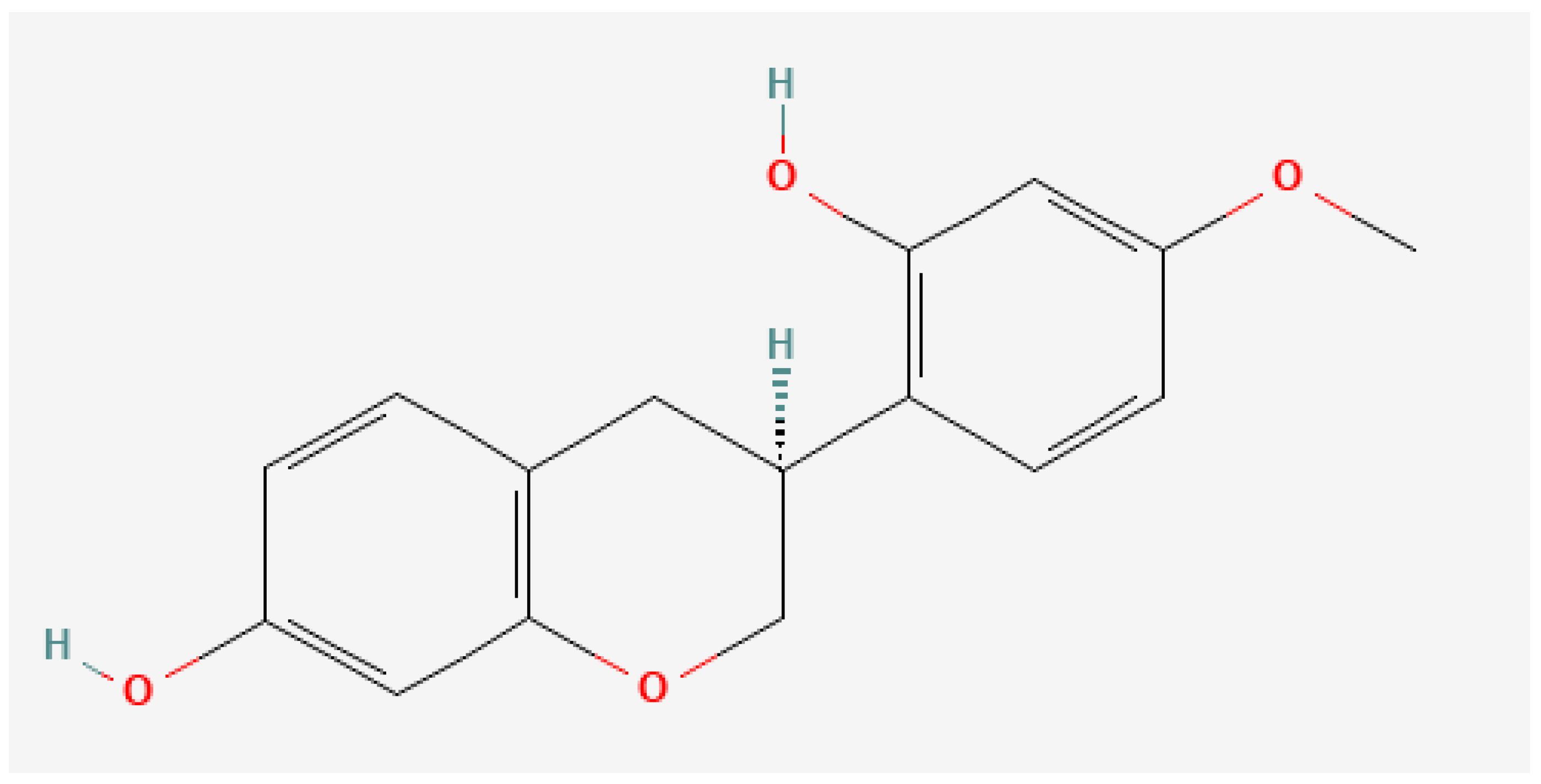
:1. Introduction
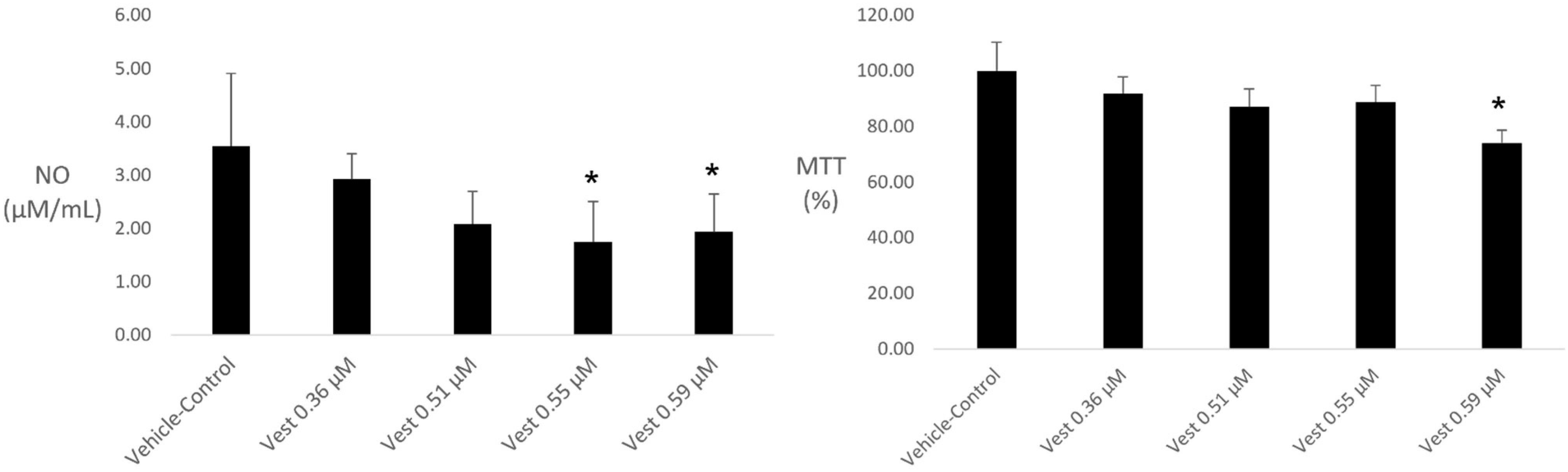
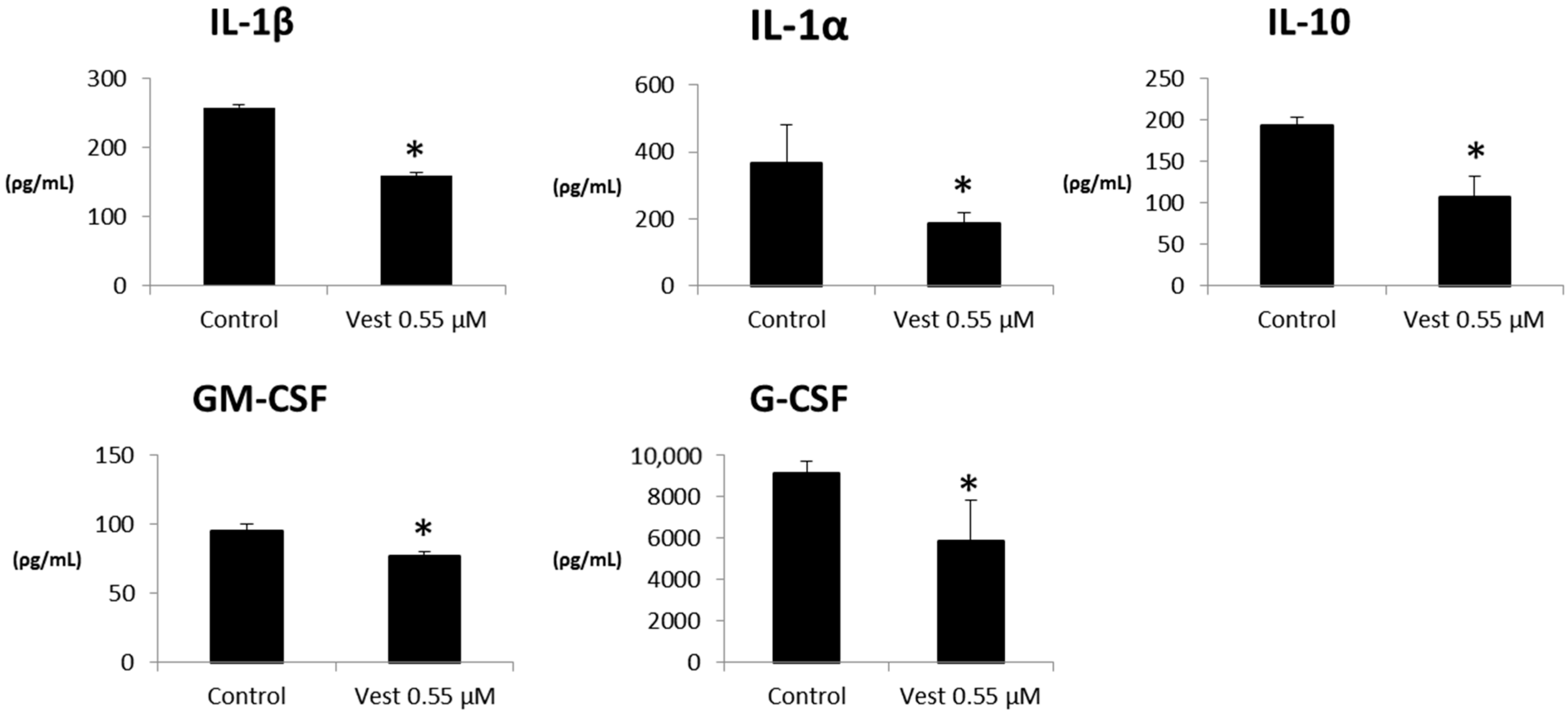
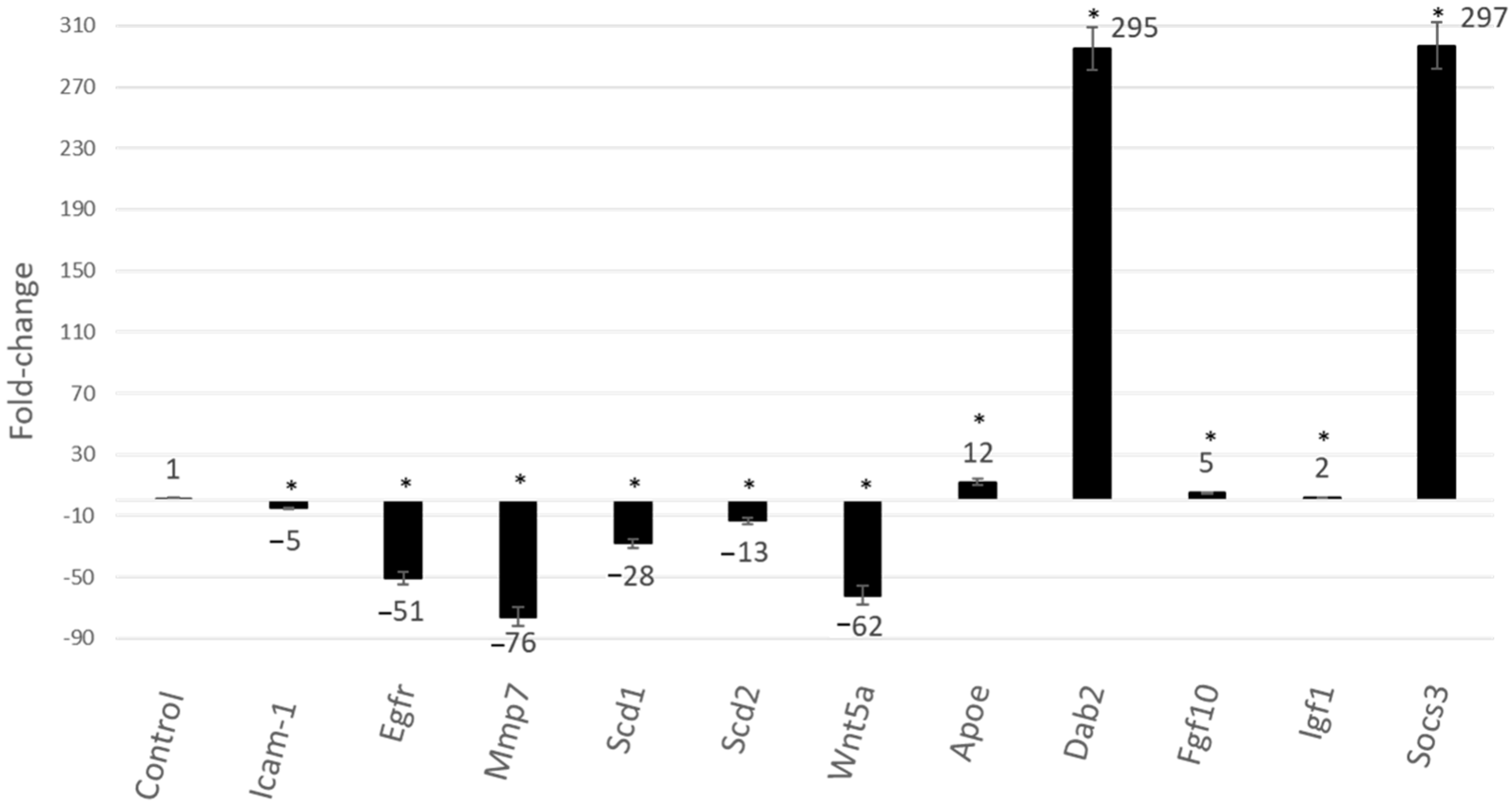
2. Results
2.1. NO Quantification and Cell Viability
2.2. Cytokines Production
2.3. Gene Expression
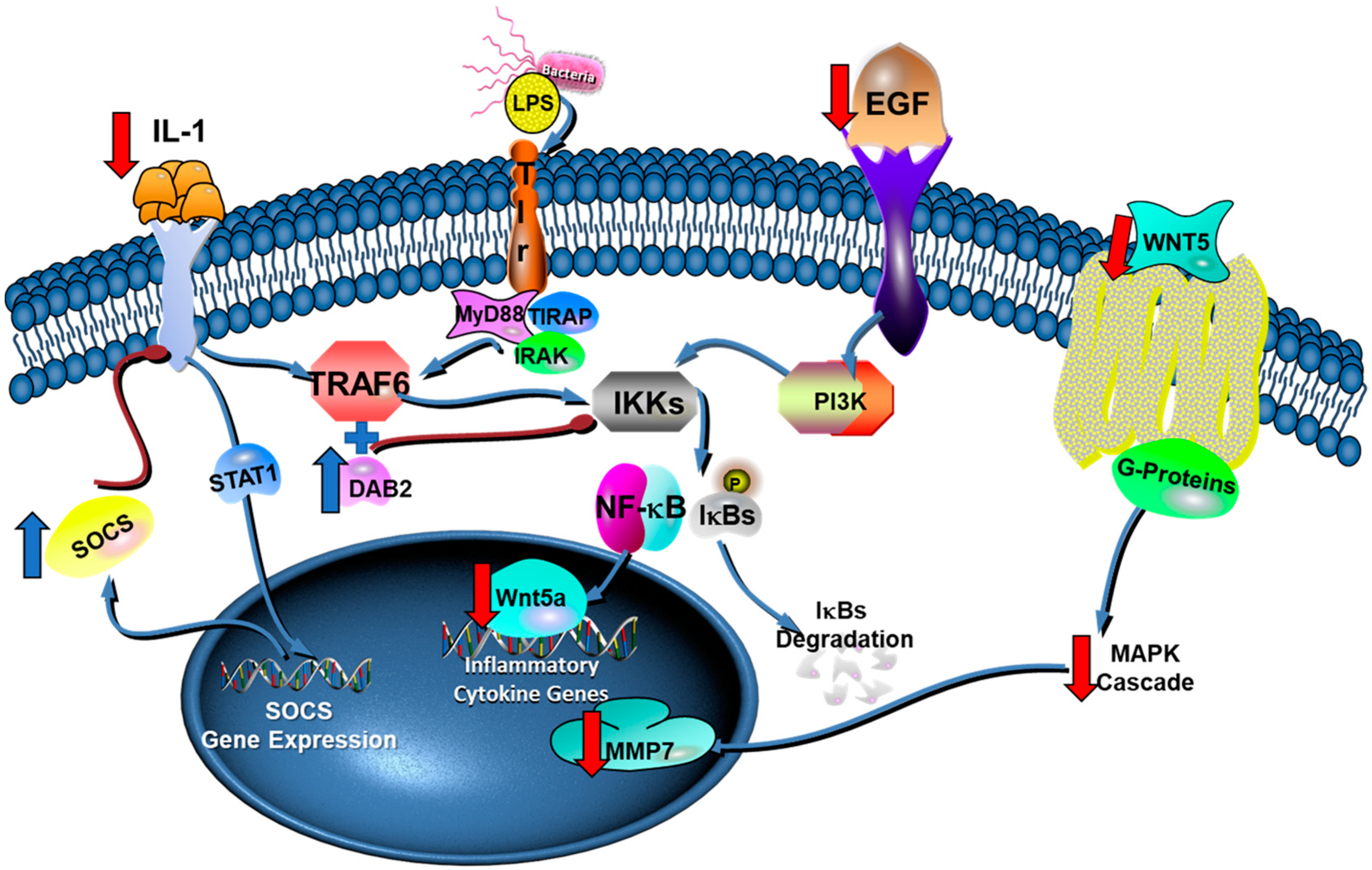
3. Discussion
4. Materials and Methods
4.1. Growing of Eukaryotic Cell
4.2. Macrophages LPS-Activation in the Presence of (3S)-Vestitol
4.3. Nitrite Oxid (NO) Production and CELL Viability
4.4. Cytokines Release
4.5. Analysis of Gene Expression by Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell 2016, 166, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Sun, W.; Zhao, J.; Wu, X.; Lu, J.J.; Chen, X.; Xu, Q.M.; Khan, I.A.; Yang, S. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci. Rep. 2016, 6, 33720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ma, J.; Wang, K.S.; Mi, C.; Wang, Z.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Baicalein inhibits TNF-alpha-induced NF-kappaB activation and expression of NF-kappaB-regulated target gene products. Oncol. Rep. 2016, 36, 2771–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Controlling Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.M.; Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Cocco, L.; Ramazzotti, G.; Ratti, S.; Follo, M.Y.; Martelli, A.M.; et al. Abilities of beta-Estradiol to interact with chemotherapeutic drugs, signal transduction inhibitors and nutraceuticals and alter the proliferation of pancreatic cancer cells. Adv. Biol. Regul. 2020, 75, 100672. [Google Scholar] [CrossRef]
- Brito, C.; Stavroullakis, A.T.; Ferreira, A.C.; Li, K.; Oliveira, T.; Nogueira-Filho, G.; Prakki, A. Extract of acai-berry inhibits osteoclast differentiation and activity. Arch. Oral Biol. 2016, 68, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Ishikawa, K.H.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; Nakamae, A.E.M.; Mayer, M.P.A. Probiotic Bacteria Alter Pattern-Recognition Receptor Expression and Cytokine Profile in a Human Macrophage Model Challenged with Candida albicans and Lipopolysaccharide. Front. Microbiol. 2017, 8, 2280. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Silva, B.; Franchin, M.; Alves, C.F.; Denny, C.; Colon, D.F.; Cunha, T.M.; Alencar, S.M.; Napimoga, M.H.; Rosalen, P.L. Main pathways of action of Brazilian red propolis on the modulation of neutrophils migration in the inflammatory process. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1583–1590. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Casarin, R.C.V.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P.A. Brazilian red propolis effects on peritoneal macrophage activity: Nitric oxide, cell viability, pro-inflammatory cytokines and gene expression. J. Ethnopharmacol. 2017, 207, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.B.; Rosalen, P.L.; Cury, J.A.; Ikegaki, M.; Souza, V.C.; Esteves, A.; Alencar, S.M. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evid.-Based Complement. Altern. Med. 2008, 5, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Rosalen, P.L.; Franchin, M.; Lacerda, R.C.; Bueno-Silva, B.; Benso, B.; Denny, C.; Ikegaki, M.; Alencar, S.M. Chemical Characterization and Antioxidant, Antimicrobial, and Anti-Inflammatory Activities of South Brazilian Organic Propolis. PLoS ONE 2016, 11, e0165588. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Koo, H.; Falsetta, M.L.; Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Effect of neovestitol-vestitol containing Brazilian red propolis on accumulation of biofilm in vitro and development of dental caries in vivo. Biofouling 2013, 29, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Vestitol drives LPS-activated macrophages into M2 phenotype through modulation of NF-kappaB pathway. Int. Immunopharmacol. 2020, 82, 106329. [Google Scholar] [CrossRef]
- Campo Fernandez, M.; Cuesta-Rubio, O.; Rosado Perez, A.; Montes De Oca Porto, R.; Marquez Hernandez, I.; Piccinelli, A.L.; Rastrelli, L. GC-MS determination of isoflavonoids in seven red Cuban propolis samples. J. Agric. Food Chem. 2008, 56, 9927–9932. [Google Scholar] [CrossRef]
- Franchin, M.; Colon, D.F.; Castanheira, F.V.; da Cunha, M.G.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Vestitol Isolated from Brazilian Red Propolis Inhibits Neutrophils Migration in the Inflammatory Process: Elucidation of the Mechanism of Action. J. Nat. Prod. 2016, 79, 954–960. [Google Scholar] [CrossRef]
- de Carvalho, A.e.M. A synopsis of the genus Dalbergia (Fabaceae: Dalbergieae) in Brazil. Brittonia 1997, 49, 87–109. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P. Brazilian Red Propolis Attenuates Inflammatory Signaling Cascade in LPS-Activated Macrophages. PLoS ONE 2015, 10, e0144954. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, M.; Xu, N.; Lv, Q.; Huang, N.; He, J.; Zhang, Y. MiR-181a regulates inflammation responses in monocytes and macrophages. PLoS ONE 2013, 8, e58639. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Reviews. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef]
- Adamson, S.E.; Griffiths, R.; Moravec, R.; Senthivinayagam, S.; Montgomery, G.; Chen, W.; Han, J.; Sharma, P.R.; Mullins, G.R.; Gorski, S.A.; et al. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. J. Clin. Investig. 2016, 126, 1311–1322. [Google Scholar] [CrossRef] [Green Version]
- Geneglobe quiagen. Available online: https://geneglobe.qiagen.com/br/explore/pathway-details/nf-kb-signaling (accessed on 27 April 2022).
- Galic, S.; Sachithanandan, N.; Kay, T.W.; Steinberg, G.R. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem. J. 2014, 461, 177–188. [Google Scholar] [CrossRef]
- Nanbara, H.; Wara-aswapati, N.; Nagasawa, T.; Yoshida, Y.; Yashiro, R.; Bando, Y.; Kobayashi, H.; Khongcharoensuk, J.; Hormdee, D.; Pitiphat, W.; et al. Modulation of Wnt5a expression by periodontopathic bacteria. PLoS ONE 2012, 7, e34434. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Kulwattanaporn, P.; Hosur, K.; Domon, H.; Oda, M.; Terao, Y.; Maeda, T.; Hajishengallis, G. Differential Expression and Roles of Secreted Frizzled-Related Protein 5 and the Wingless Homolog Wnt5a in Periodontitis. J. Dent. Res. 2017, 96, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, D.; Wada, N.; Yoshida, S.; Mitarai, H.; Arima, M.; Tomokiyo, A.; Hamano, S.; Sugii, H.; Maeda, H. Wnt5a suppresses osteoblastic differentiation of human periodontal ligament stem cell-like cells via Ror2/JNK signaling. J. Cell. Physiol. 2018, 233, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Letra, A.; Ghaneh, G.; Zhao, M.; Ray, H., Jr.; Francisconi, C.F.; Garlet, G.P.; Silva, R.M. MMP-7 and TIMP-1, new targets in predicting poor wound healing in apical periodontitis. J. Endod. 2013, 39, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Lundmark, A.; Johannsen, G.; Eriksson, K.; Kats, A.; Jansson, L.; Tervahartiala, T.; Rathnayake, N.; Akerman, S.; Klinge, B.; Sorsa, T.; et al. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J. Clin. Periodontol. 2017, 44, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, M.; O’Connell, M.A.; Pawlinski, R.; Hollis, A.; McGovern, P.; Yan, S.F.; Stern, D.; Mackman, N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 2001, 98, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Zhu, Y.; Chen, W.; Li, L.; Qi, Y.; Wang, X.; Zhao, Y.; Wan, X.; Chen, X. IKKepsilon knockout prevents high fat diet induced arterial atherosclerosis and NF-kappaB signaling in mice. PLoS ONE 2013, 8, e64930. [Google Scholar] [CrossRef] [Green Version]
- Harja, E.; Bucciarelli, L.G.; Lu, Y.; Stern, D.M.; Zou, Y.S.; Schmidt, A.M.; Yan, S.F. Early growth response-1 promotes atherogenesis: Mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ. Res. 2004, 94, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Braesch-Andersen, S.; Paulie, S.; Smedman, C.; Mia, S.; Kumagai-Braesch, M. ApoE production in human monocytes and its regulation by inflammatory cytokines. PLoS ONE 2013, 8, e79908. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Kodvawala, A.; Hui, D.Y. Apolipoprotein E inhibits toll-like receptor (TLR)-3-and TLR-4-mediated macrophage activation through distinct mechanisms. Biochem. J. 2010, 428, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Figueiredo, K.A.; da Silva, H.D.P.; Miranda, S.L.F.; Goncalves, F.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In Vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; Alencar, S.M.A.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian red propolis reduces orange-complex periodontopathogens growing in multispecies biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Cabral, I.S.R.; d’Arce, M.A.B.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/177149#section=2D-Structure (accessed on 27 April 2022).
- Kawamoto, D.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; DiRienzo, J.M.; Mayer, M.P. Alteration of Homeostasis in Pre-osteoclasts Induced by Aggregatibacter actinomycetemcomitans CDT. Front. Cell. Infect. Microbiol. 2016, 6, 33. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Silva, B.; Bueno, M.R.; Kawamoto, D.; Casarin, R.C.; Pingueiro, J.M.S.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P.A. Anti-Inflammatory Effects of (3S)-Vestitol on Peritoneal Macrophages. Pharmaceuticals 2022, 15, 553. https://doi.org/10.3390/ph15050553
Bueno-Silva B, Bueno MR, Kawamoto D, Casarin RC, Pingueiro JMS, Alencar SM, Rosalen PL, Mayer MPA. Anti-Inflammatory Effects of (3S)-Vestitol on Peritoneal Macrophages. Pharmaceuticals. 2022; 15(5):553. https://doi.org/10.3390/ph15050553
Chicago/Turabian StyleBueno-Silva, Bruno, Manuela Rocha Bueno, Dione Kawamoto, Renato C. Casarin, João Marcos Spessoto Pingueiro, Severino Matias Alencar, Pedro Luiz Rosalen, and Marcia Pinto Alves Mayer. 2022. "Anti-Inflammatory Effects of (3S)-Vestitol on Peritoneal Macrophages" Pharmaceuticals 15, no. 5: 553. https://doi.org/10.3390/ph15050553
APA StyleBueno-Silva, B., Bueno, M. R., Kawamoto, D., Casarin, R. C., Pingueiro, J. M. S., Alencar, S. M., Rosalen, P. L., & Mayer, M. P. A. (2022). Anti-Inflammatory Effects of (3S)-Vestitol on Peritoneal Macrophages. Pharmaceuticals, 15(5), 553. https://doi.org/10.3390/ph15050553





