Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation
Abstract
:1. Introduction
2. Results
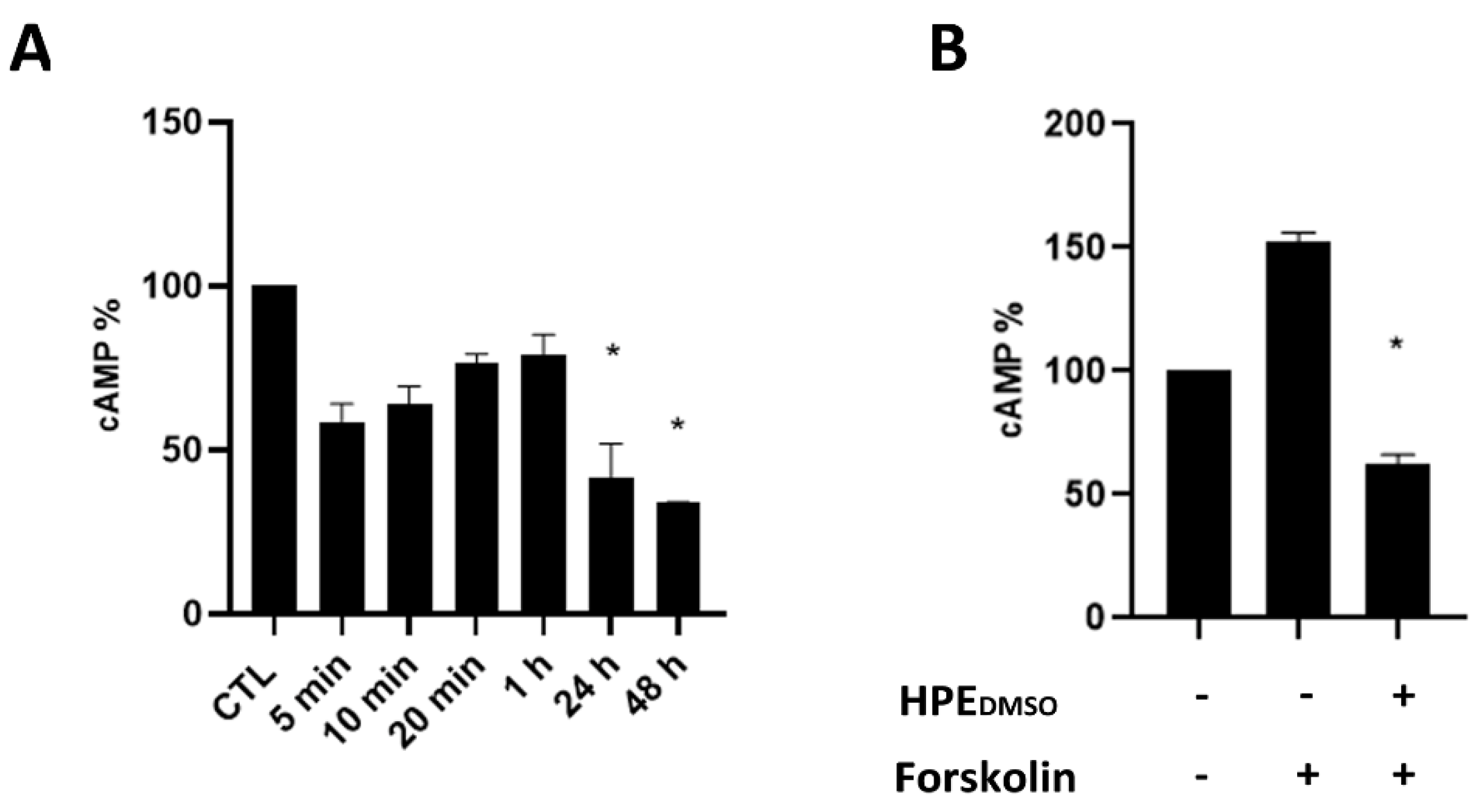
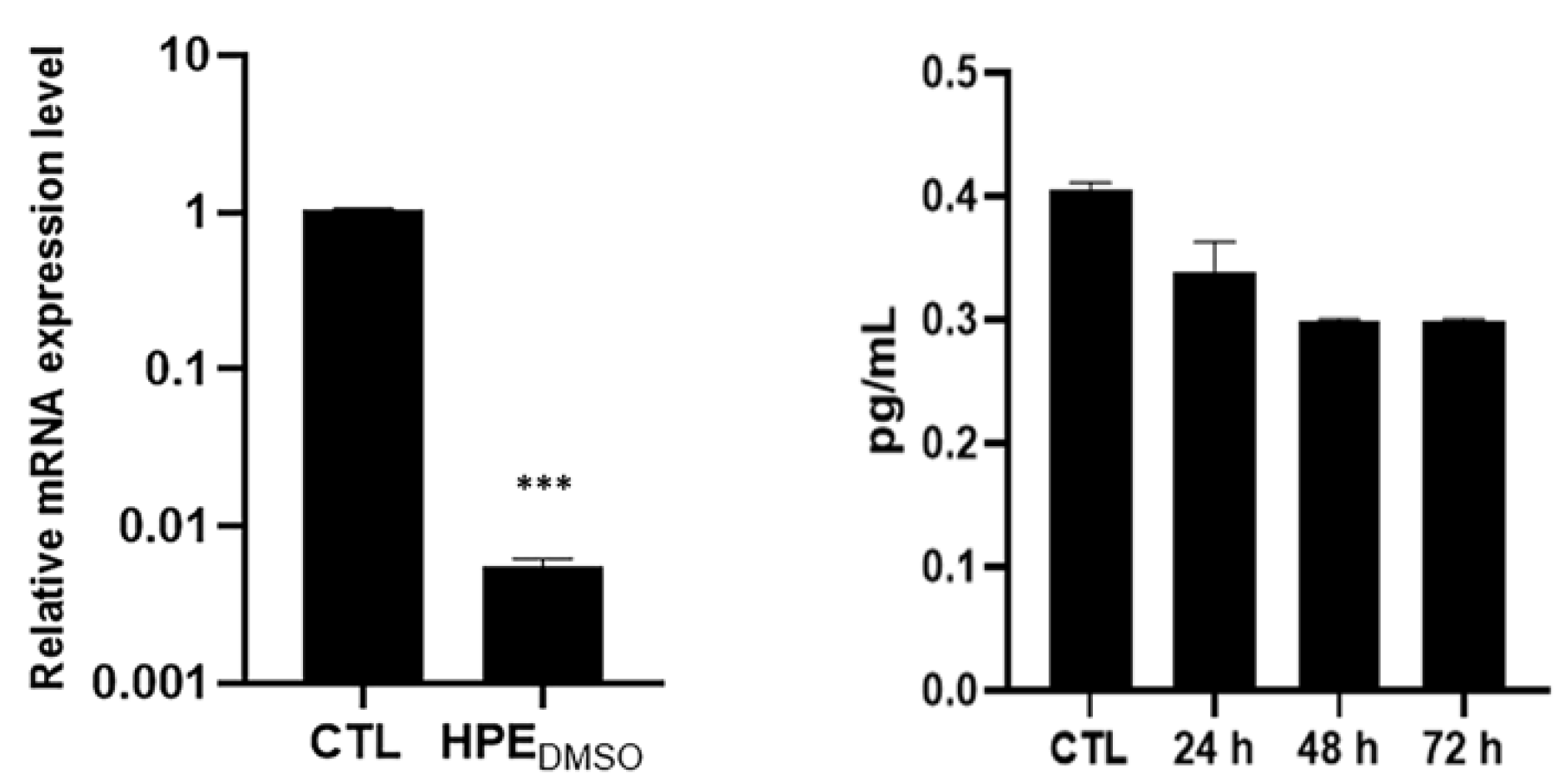
2.1. Effect of HPEDMSO on cAMP Production
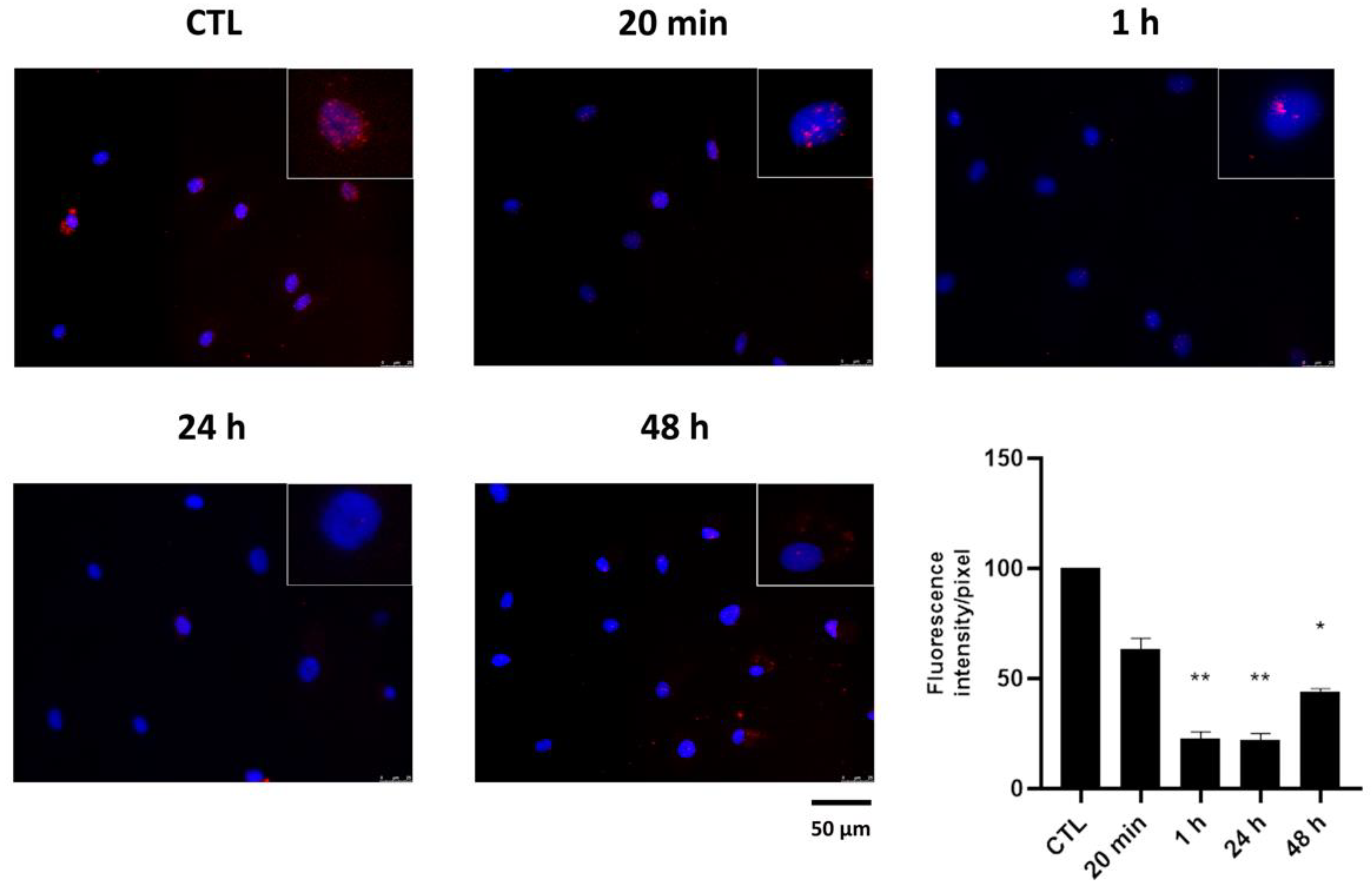
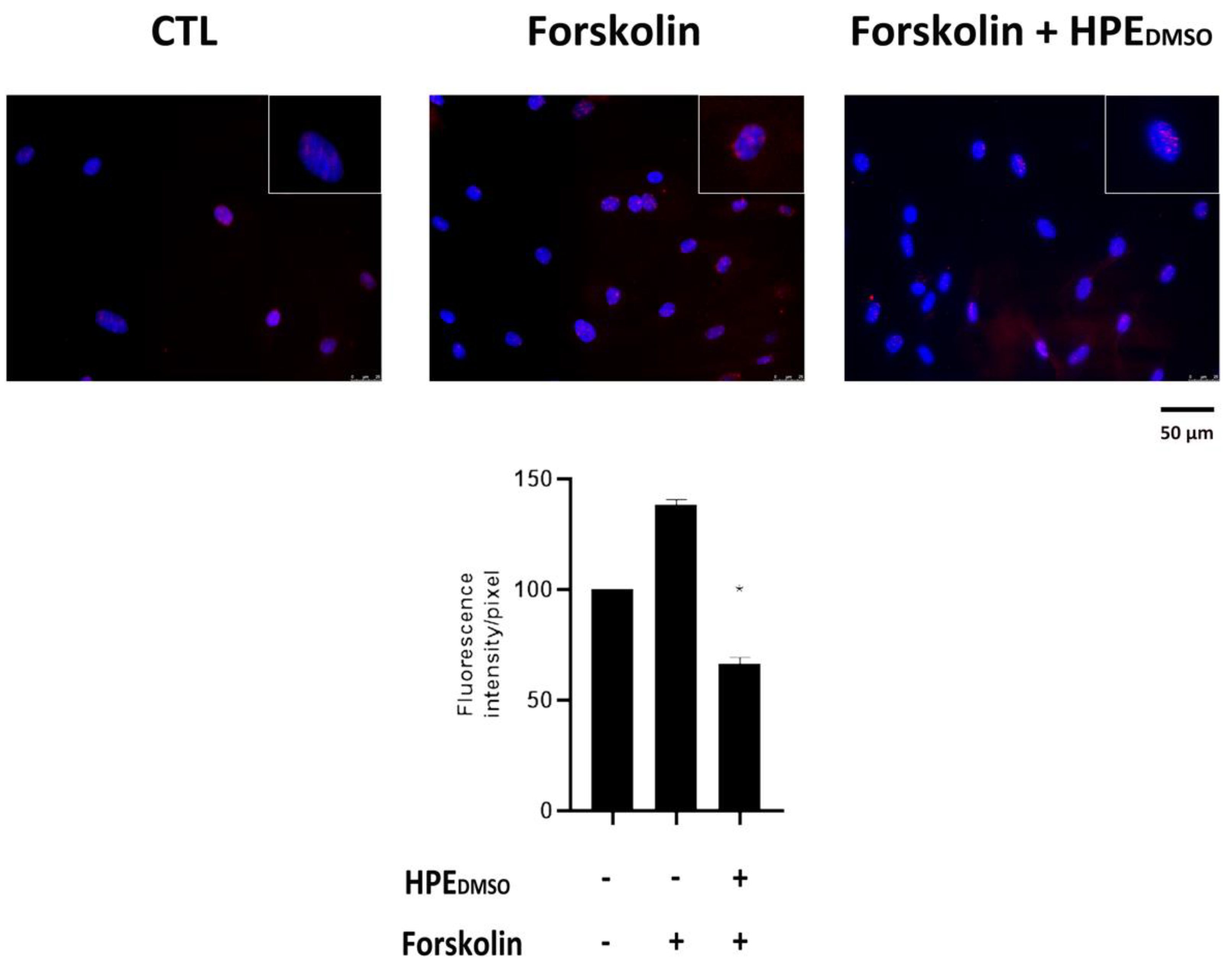
2.2. Effect of HPEDMSO on PKA Activation
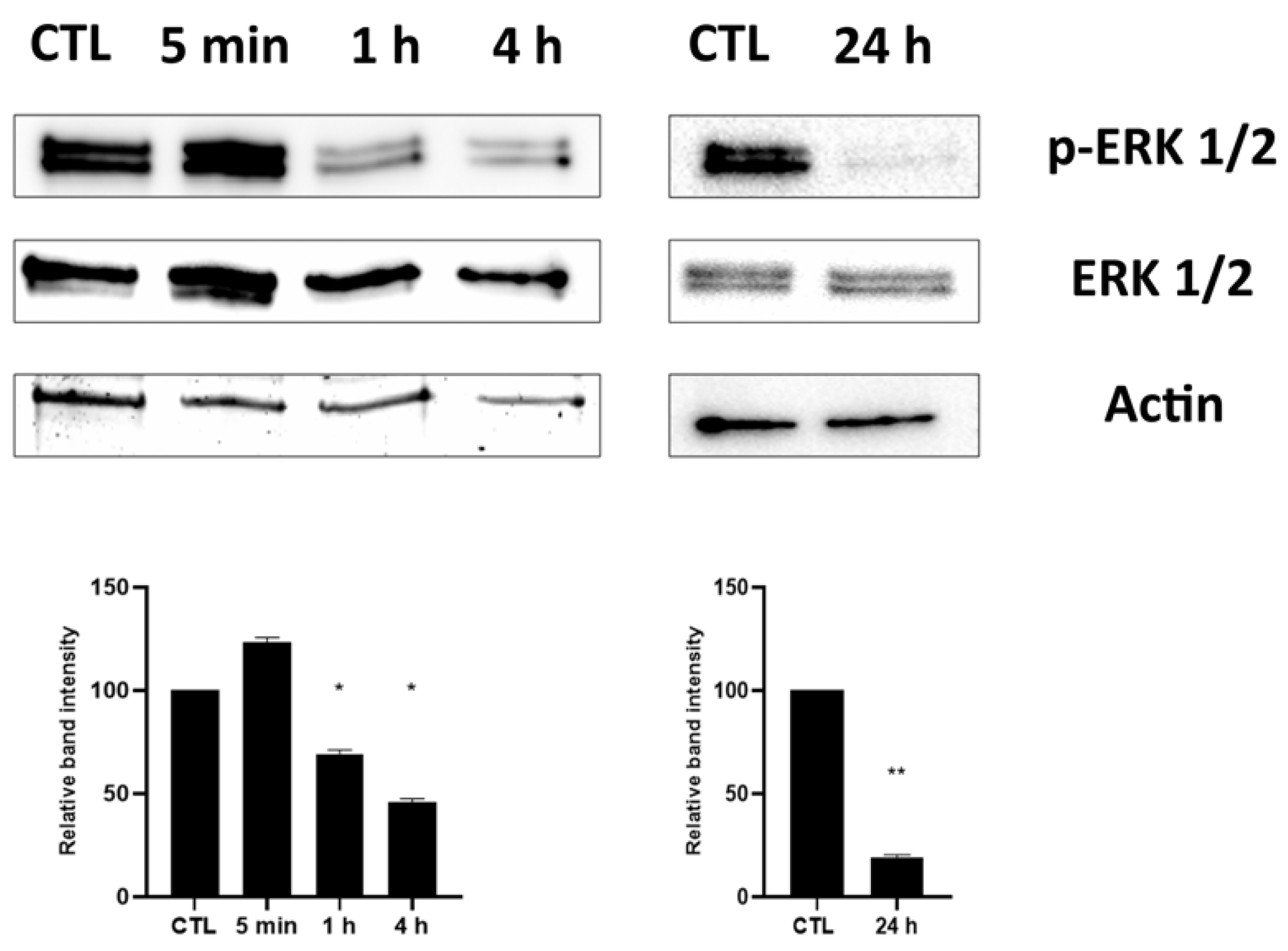
2.3. Effect of HPEDMSO on ERK Phosphorylation
2.4. Effect of HPEDMSO on c-Fos and MMP-13 Expression
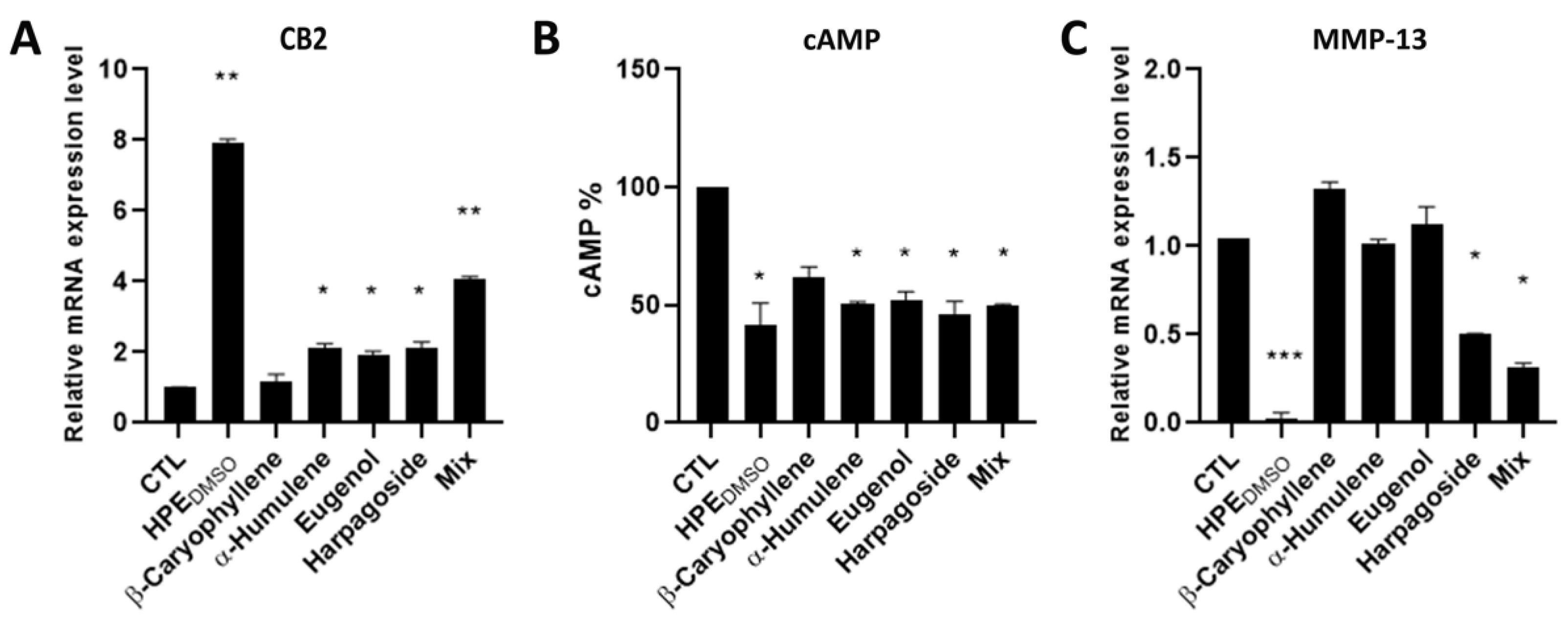
2.5. Activity of Pure Compounds Contained in HPEDMSO Alone and In Combination Compared to HPEDMSO
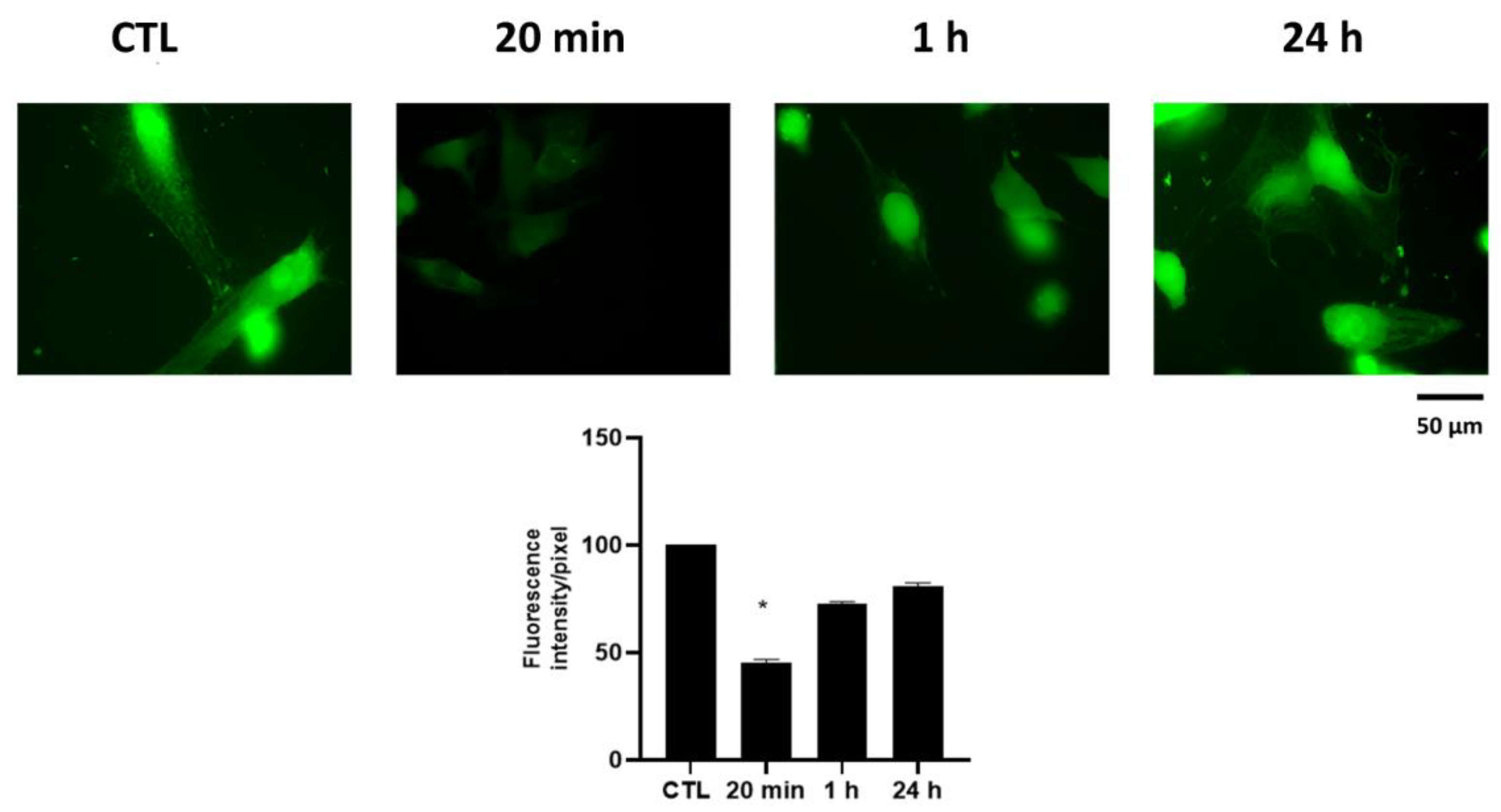
2.6. Effect of HPEDMSO on Ca2+ Intracellular Level
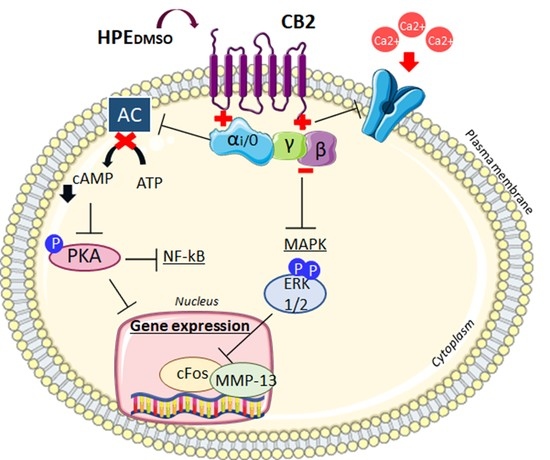
3. Discussion
4. Materials and Methods
4.1. Human Primary Cell Isolation
4.2. Cell Treatment
4.3. Measurement of cAMP
4.4. Immunofluorescence
4.5. Western Blot Analysis
4.6. RNA Extraction and Reverse-Transcription
4.7. Quantitative-Real Time-PCR
4.8. MMP-13 ELISA
4.9. Quantification of Chemical Constituents by GC–FID
4.10. UHPLC-MS Analyses
4.11. Calcium Assay
4.12. Densitometric Analyses
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoppoloni, D.; Politi, L.; Leopizzi, M.; Gaetani, S.; Guazzo, R.; Basciani, S.; Moreschini, O.; De Santi, M.; Scandurra, R.; Scotto d’Abusco, A. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarthr. Cartil. 2015, 23, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzello, C.R.; Loeser, R.F. Editorial: Inflammatory Activity in Symptomatic Knee Osteoarthritis: Not All Inflammation Is Local. Arthritis Rheumatol. 2015, 67, 2797–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, J.; Hong, W.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How Do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Front. Immunol. 2018, 9, 1467. [Google Scholar] [CrossRef]
- Rzeczycki, P.; Rasner, C.; Lammlin, L.; Junginger, L.; Goldman, S.; Bergman, R.; Redding, S.; Knights, A.J.; Elliott, M.; Maerz, T. Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages. Osteoarthr. Cartil. 2021, 29, 1720–1731. [Google Scholar] [CrossRef]
- La Porta, C.; Bura, S.A.; Negrete, R.; Maldonado, R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur. J. Neurosci. 2014, 39, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.D.; Le Maitre, C.L. Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage: Implications for Future Therapies. Cannabis Cannabinoid Res. 2016, 1, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef] [Green Version]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; di Maio, V.; Gullì, M.; Vedova, P.D.; Ammendola, S.; Scotto d’Abusco, A. Antiarthritic Effects of a Root Extract from Harpagophytum procumbens DC: Novel Insights into the Molecular Mechanisms and Possible Bioactive Phytochemicals. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R. Osteoarthritis in 2017: Latest advances in the management of knee OA. Nat. Rev. Rheumatol. 2018, 14, 73–74. [Google Scholar] [CrossRef] [Green Version]
- Scotto d’Abusco, A.; Politi, L.; Giordano, C.; Scandurra, R. A peptidyl-glucosamine derivative affects IKKalpha kinase activity in human chondrocytes. Arthritis Res. Ther. 2010, 12, R18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopreiato, M.; Di Cristofano, S.; Cocchiola, R.; Mariano, A.; Guerrizio, L.; Scandurra, R.; Mosca, L.; Raimondo, D.; Scotto d’Abusco, A. Biochemical and Computational Studies of the Interaction between a Glucosamine Derivative, NAPA, and the IKKα Kinase. Int. J. Mol. Sci. 2021, 22, 1643. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Harpagophytum Procumbens DC. and/or Harpagophytum zeyheri Decne., Radix. (EMA/HMPC/627058/2015); European Medicines Agency (EMA): Amsterdam, The Netherlands, 2015. [Google Scholar]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Chrubasik, S.; Manheimer, E. Harpgophytum procumbens for osteoarthritis and low back pain: A systematic review. BMC Complement. Altern. Med. 2004, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Wegener, T.; Lüpke, N.-P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of Devil’s Claw (Harpagophytum procumbens DC.). Phytotherapy Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef]
- Haseeb, A.; Ansari, M.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Harpagophytum Procumbens Dc. and/or Harpagophytum Zeyheri Decne, Radix; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2008. [Google Scholar]
- Horecka, A.; Hordyjewska, A.; Blicharski, T.; Kurzepa, J. Osteoarthritis of the knee—Biochemical aspect of applied therapies (review). Bosn. J. Basic Med. Sci. 2022. [Google Scholar] [CrossRef]
- Honvo, G.; Reginster, J.-Y.; Rabenda, V.; Geerinck, A.; Mkinsi, O.; Charles, A.; Rizzoli, R.; Cooper, C.; Avouac, B.; Bruyère, O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 65–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiebich, B.; Heinrich, M.; Hiller, K.; Kammerer, N. Inhibition of TNF-α synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine 2001, 8, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.; Gericke, N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.; Erb, M. Root volatiles in plant-plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; Da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Chicca, A.; Caprioglio, D.; Minassi, A.; Petrucci, V.; Appendino, G.; Taglialatela-Scafati, O.; Gertsch, J. Functionalization of β-Caryophyllene Generates Novel Polypharmacology in the Endocannabinoid System. ACS Chem. Biol. 2014, 107, 911499–911507. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Sibin, A.P.A.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marçal-Natali, M.R.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; El-Alim, A.E.-A.F.A.; Galal, A.A.; El-Sayed, R.G.; El-Naseery, N.I. Anti-arthritic effect of β-caryophyllene and its ameliorative role on methotrexate and/or leflunomide-induced side effects in arthritic rats. Life Sci. 2019, 233, 116750. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kohsaka, H.; Takayasu, A.; Yokoyama, W.; Miyabe, C.; Miyabe, Y.; Harigai, M.; Miyasaka, N.; Nanki, T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet. Disord. 2014, 15, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F. Signaling transduction: Target in osteoarthritis. Curr. Opin. Rheumatol. 2004, 16, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ke, T.; Zhang, Y.; Guo, L.; Chen, F.; He, W. Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway. Mol. Med. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428. [Google Scholar] [CrossRef] [Green Version]
- Scotto d’Abusco, A.; Calamia, V.; Cicione, C.; Grigolo, B.; Politi, L.; Scandurra, R. Glucosamine affects intracellular signalling through inhibition of mitogen-activated protein kinase phosphorylation in human chondrocytes. Arthritis Res. Ther. 2007, 9, R104. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Makino, H.; Seki, S.; Yahara, Y.; Shiozawa, S.; Aikawa, Y.; Motomura, H.; Nogami, M.; Watanabe, K.; Sainoh, T.; Ito, H.; et al. A selective inhibition of c-Fos/activator protein-1 as a potential therapeutic target for intervertebral disc degeneration and associated pain. Sci. Rep. 2017, 7, 16983. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of Caryophyllane Sesquiterpenes in the Entourage Effect of Felina 32 Hemp Inflorescence Phytocomplex in Triple Negative MDA-MB-468 Breast Cancer Cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef]
- Charan Raja, M.R.; Srinivasan, V.; Selvaraj, S.; Mahapatra, S.K. Eugenol: A versatile phytomedicine. Int. J. Pharm. Pharm. Sci. 2015, 7, 35–40. [Google Scholar]
- Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers 2020, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Romiti, N.; Tramonti, G.; Corti, A.; Chieli, E. Effects of Devil’s Claw (Harpagophytum procumbens) on the multidrug transporter ABCB1/P-glycoprotein. Phytomedicine 2009, 16, 1095–1100. [Google Scholar] [CrossRef]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The Natural cAMP Elevating Compound Forskolin in Cancer Therapy: Is It Time? J. Cell. Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Petrelli, L.; Guidolin, D.; Fan, C.; de Caro, R.; Stecco, C. Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. Int. J. Mol. Sci. 2020, 21, 2936. [Google Scholar] [CrossRef]

| Compound | HPEDMSO µg ±, SEM in 1 mg Extract |
|---|---|
| β-Caryophyllene | 0.082 ± 0.0002 |
| α-Humulene | 0.024 ± 0.0004 |
| Eugenol | 0.004 ± 0.0002 |
| Harpagoside | 4 ± 0.0049 |
| Gene | Primer Sequences |
|---|---|
| CB2 NM_009924 | 5′-ATGCTGTGCCTCATCAACTC-3′ 5′-CTCACACACTTCTTCCAGTG-3′ |
| MMP-13 NM_002427 | 5′-TTCTTGTTGCTGCGCATGA-3′ 5′-TGCTCCAGGGTCCTTGGA-3′ |
| c-FOS NM_005252 | 5′-CGAGCCCTTTGATGACTTCCT-3′ 5′-GGAGCGGGCTGTCTCAGA-3′ |
| 18S NM_003286.2 | 5′-CGCCGCTAGAGGTGAAATTC-3′ 5′-CATTCTTGGCAAATGCTTTCG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.; Bigioni, I.; Mattioli, R.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Mariani, P.F.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals 2022, 15, 457. https://doi.org/10.3390/ph15040457
Mariano A, Bigioni I, Mattioli R, Di Sotto A, Leopizzi M, Garzoli S, Mariani PF, Dalla Vedova P, Ammendola S, Scotto d’Abusco A. Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals. 2022; 15(4):457. https://doi.org/10.3390/ph15040457
Chicago/Turabian StyleMariano, Alessia, Irene Bigioni, Roberto Mattioli, Antonella Di Sotto, Martina Leopizzi, Stefania Garzoli, Pier Francesco Mariani, Pietro Dalla Vedova, Sergio Ammendola, and Anna Scotto d’Abusco. 2022. "Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation" Pharmaceuticals 15, no. 4: 457. https://doi.org/10.3390/ph15040457
APA StyleMariano, A., Bigioni, I., Mattioli, R., Di Sotto, A., Leopizzi, M., Garzoli, S., Mariani, P. F., Dalla Vedova, P., Ammendola, S., & Scotto d’Abusco, A. (2022). Harpagophytum procumbens Root Extract Mediates Anti-Inflammatory Effects in Osteoarthritis Synoviocytes through CB2 Activation. Pharmaceuticals, 15(4), 457. https://doi.org/10.3390/ph15040457