Hydraulic Response of Deciduous and Evergreen Broadleaved Shrubs, Grown on Olympus Mountain in Greece, to Vapour Pressure Deficit
Abstract
:1. Introduction
2. Results
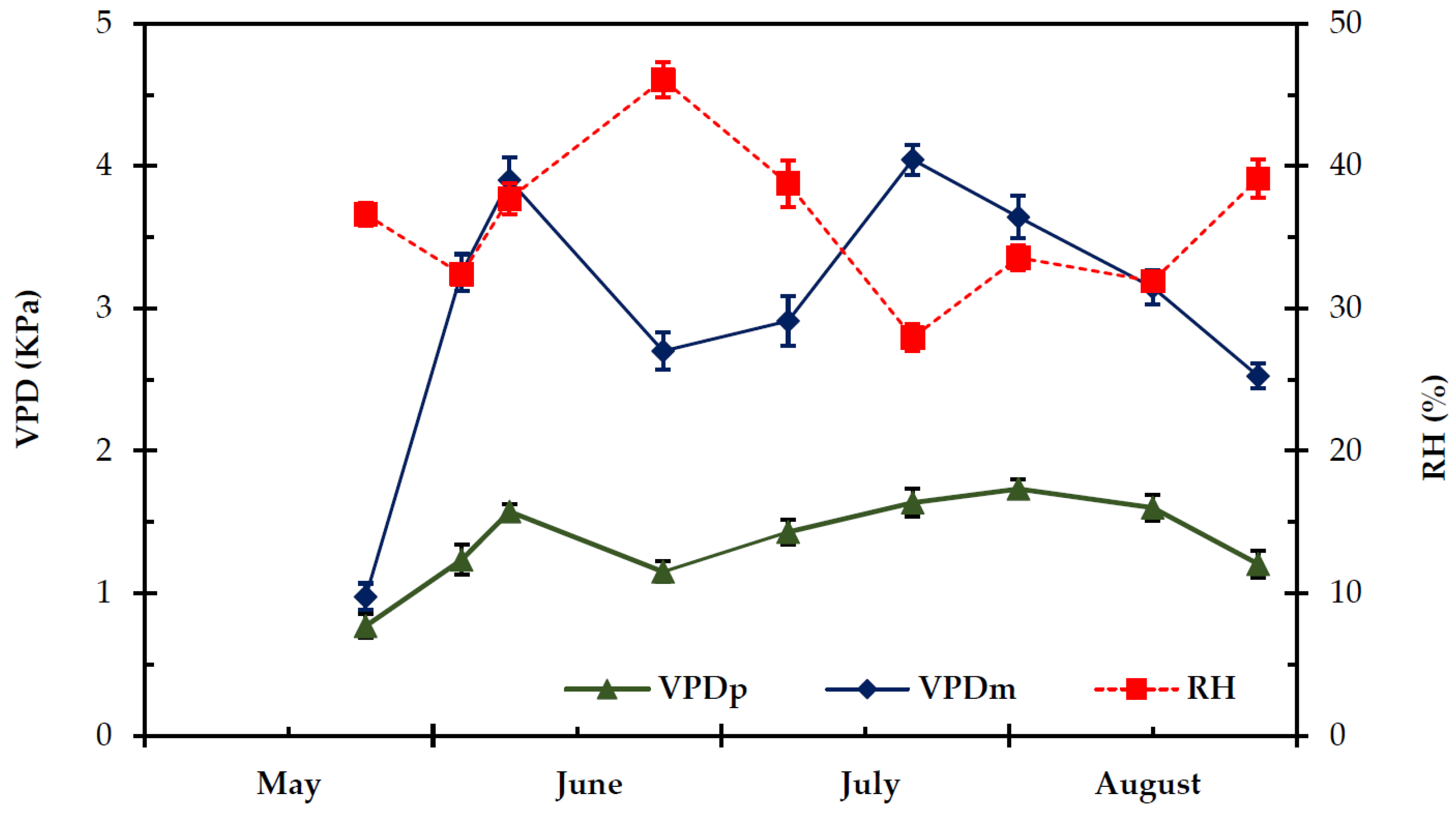
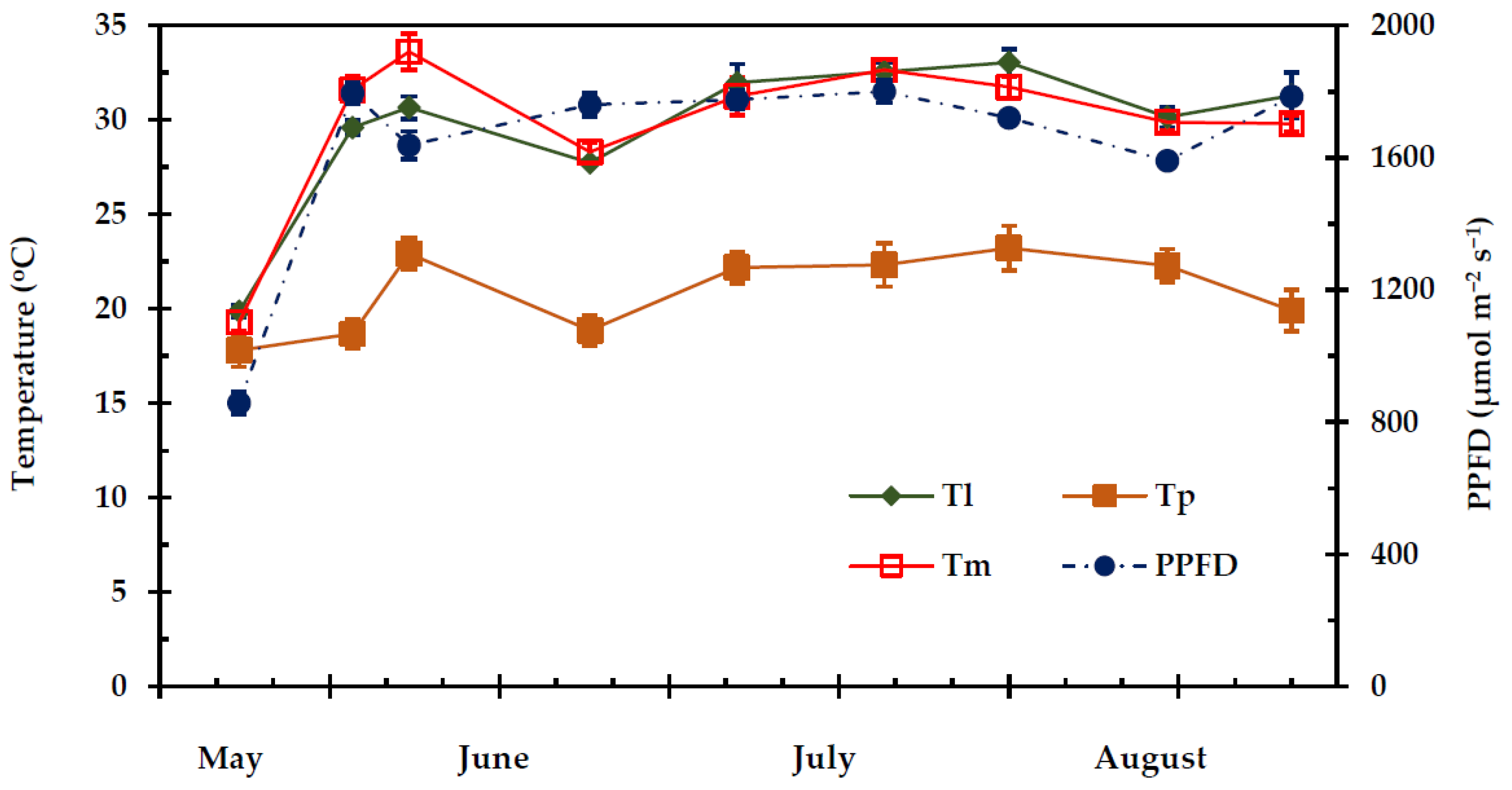
2.1. Climatic Conditions
2.2. Physiological Parameters
2.3. Leaf Water Potential
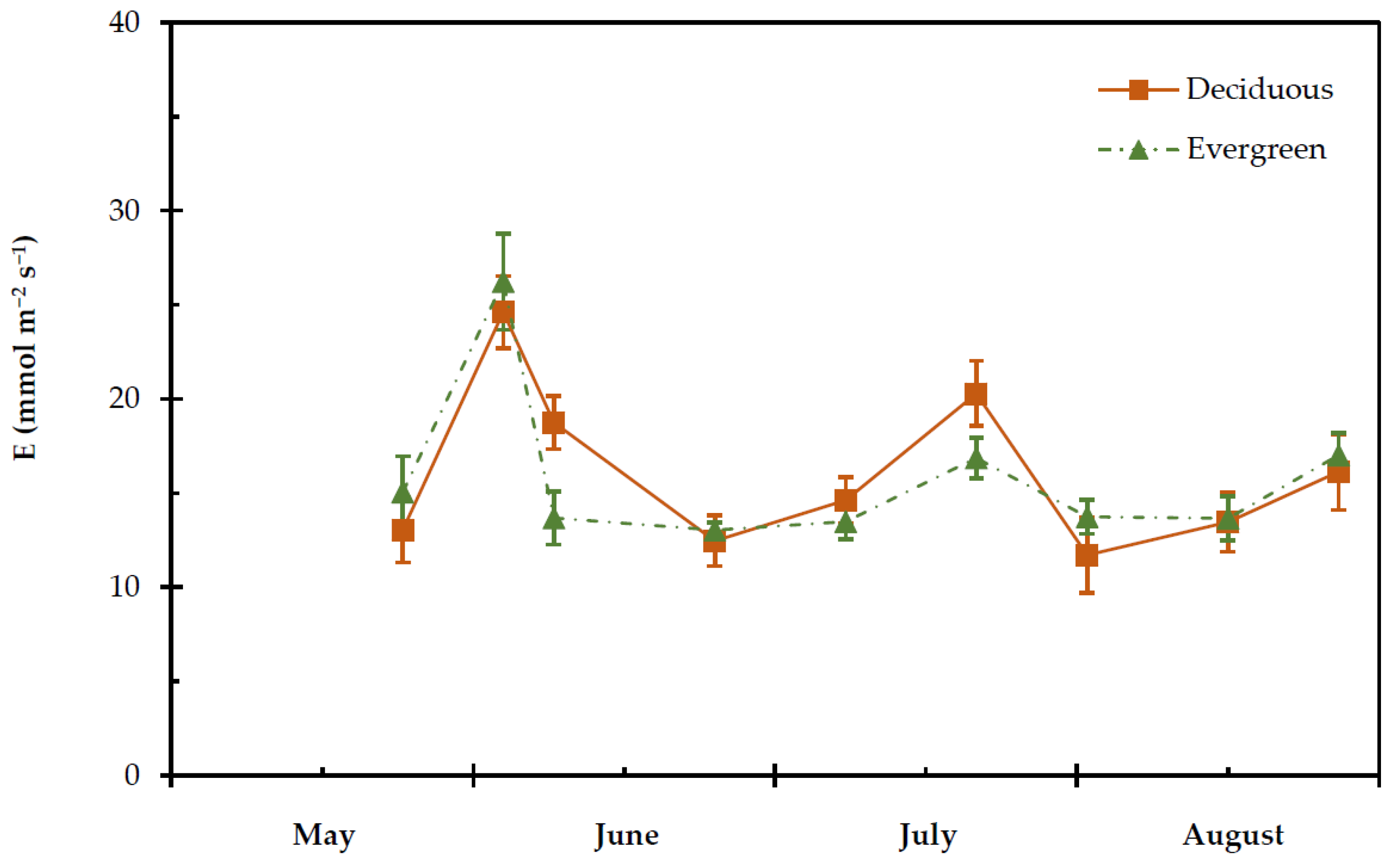
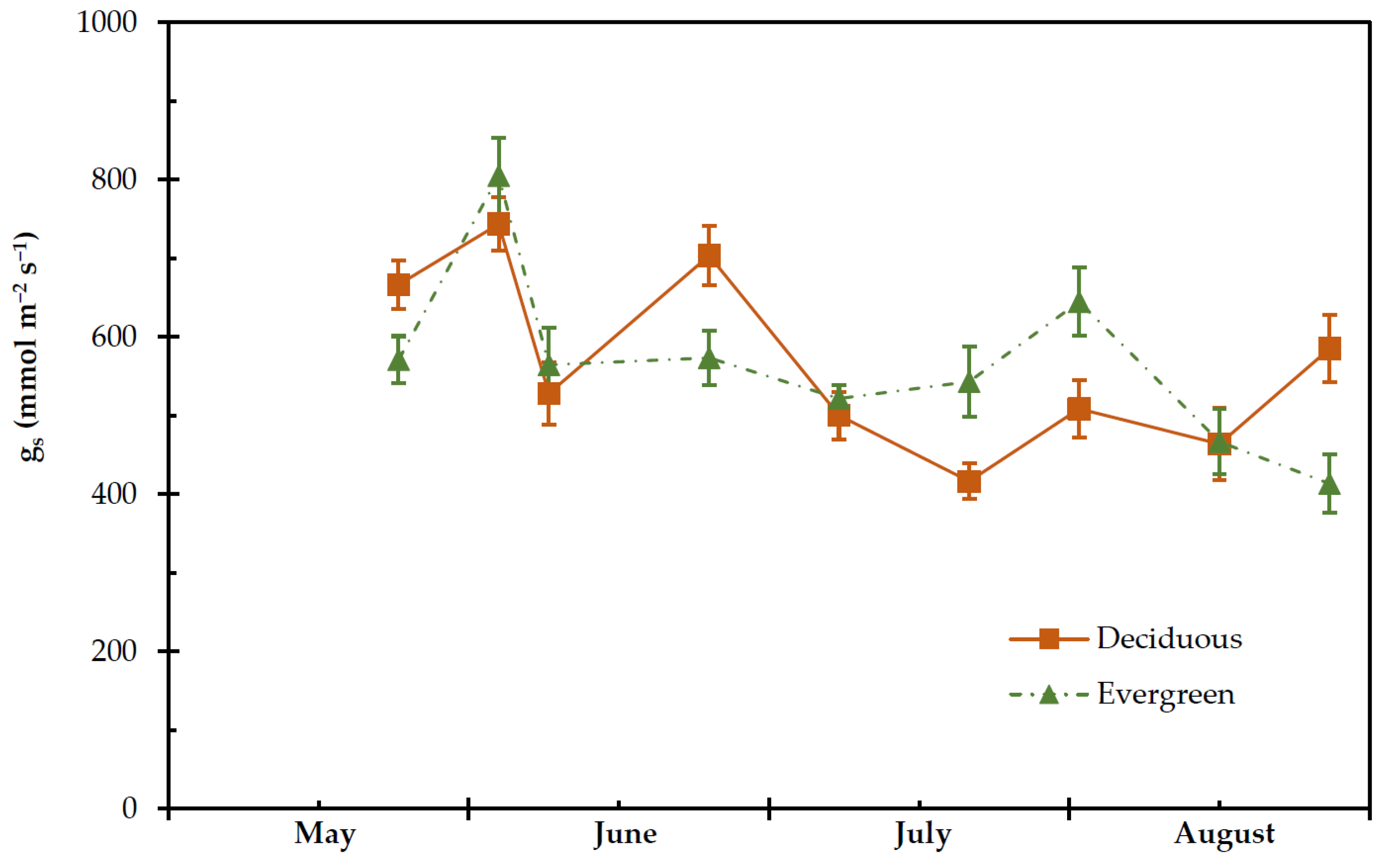
2.4. Transpiration Rate, Stomatal and Leaf Hydraulic Conductance
3. Discussion
4. Materials and Methods
4.1. Study Area and Climate
4.2. Plant Material
4.3. Physiological Parameters
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Moreno, M.; Simioni, G.; Limousin, J.M.; Rodriguez-Calcerrada, J.; Ruffault, J.; Cochard, H.; Torres, J.; Delzon, S.; Tournant, A.; Dumas, P.J. Mediterranean trees vulnerability to climate change will not be minimized through hydraulic safety traits adjustments. In Proceedings of the EGU General Assembly Conference Abstracts, vEGU21: Gather Online, 19–30 April 2021; p. EGU21-3850. [Google Scholar]
- Zindros, A.; Radoglou, K.; Milios, E.; Kitikidou, K. Tree Line Shift in the Olympus Mountain (Greece) and Climate Change. Forests 2020, 11, 985. [Google Scholar] [CrossRef]
- Kramer, P.J. Drought stress and the origin of adaptations. In Adaptation of Plants to Water and High Temperature Stress; Turner, N.C., Kramer, P.J., Eds.; John Wiley & Sons: New York, NY, USA, 1980; pp. 7–21. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, H.A.; Ryan, M.G. A physiological basis for biosphere-atmosphere interactions in the boreal forest: An overview. Tree Physiol. 1997, 17, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, M.; Sinclair, T.R.; Pradhan, D. Transpiration response to vapor pressure deficit and soil drying among quinoa genotypes (Chenopodium quinoa Willd.). J. Crop Improv. 2020, 35, 291–302. [Google Scholar] [CrossRef]
- Pereira, J.S. Plant water deficits in Mediterranean ecosystems. In Water Deficits: Plant Response from Cell to Community; Smith, J.A.C., Griffiths, H., Eds.; Bios Scientific Publishers: Oxford, UK, 1993; pp. 237–251. [Google Scholar]
- Kapsali, E.-I.; Karatassiou, M. Seasonal changes in leaf tissue rehydration of one annual and two perennial grass forage species induced by bioclimate. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Karatassiou, M.; Noitsakis, B. Changes of the photosynthetic behaviour in annual C3 species at late successional stage under environmental drought conditions. Photosynthetica 2010, 48, 377–382. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Fang, Y.L.; Leung, L.R.; Wolfe, B.T.; Detto, M.; Knox, R.G.; McDowell, N.G.; Grossiord, C.; Xu, C.G.; Christoffersen, B.O.; Gentine, P.; et al. Disentangling the effects of Vapor Pressure Deficit and Soil Water Availability on Canopy Conductance in a Seasonal Tropical Forest During the 2015 El Nino Drought. J. Geophys. Res.-Atmos. 2021, 126, e2021JD035004. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J. Seasonal changes in apparent hydraulic conductance and their implications for water use of European beech (Fagus sylvatica L.) and sessile oak [Quercus petraea (Matt.) Liebl] in South Europe. Plant Ecol. 2005, 179, 155–167. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Clearwater, M.J.; Goldstein, G. Water transport in trees: Current perspectives, new insights and some controversies. Environ. Exp. Bot. 2001, 45, 239–262. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Lo Gullo, M.A.; Castro Noval, L.; Salleo, S.; Nardini, A. Hydraulic architecture of plants of Helianthus annuus L. cv. Margot: Evidence for plant segmentation in herbs. J. Exp. Bot. 2004, 55, 1549–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Tyree, M.; Sobrado, M.; Stratton, L.; Becker, P. Diversity of hydraulic conductance in leaves of temperate and tropical species: Possible causes and consequences. J. Trop. For. Sci. 1999, 11, 47–60. [Google Scholar]
- Brodribb, T.J.; Holbrook, N.M. Leaf physiology does not predict leaf habit; examples from tropical dry forest. Trees 2005, 19, 290–295. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T. Leaf Hydraulics and Its Implications in Plant Structure and Function. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2005; pp. 93–114. [Google Scholar]
- Pereira, J.S.; Pallardy, S. Water stress limitations to tree productivity. In Biomass Production by Fast-Growing Trees; Pereira, J.S., Landsberg, J.J., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 37–56. [Google Scholar]
- Jordan, W.R.; Dugas, W.A., Jr.; Shouse, P.J. Strategies for crop improvement for drought-prone regions. Agric. Water Manage. 1983, 7, 281–299. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [Green Version]
- Baert, A.; De Schepper, V.; Steppe, K. Variable hydraulic resistances and their impact on plant drought response modelling. Tree Physiol. 2015, 35, 439–449. [Google Scholar] [CrossRef]
- Martınez-Vilalta, J.; Piñol, J.; Beven, K. A hydraulic model to predict drought-induced mortality in woody plants: An application to climate change in the Mediterranean. Ecol. Modell. 2002, 155, 127–147. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breshears, D.D.; Adams, H.D.; Eamus, D.; McDowell, N.G.; Law, D.J.; Will, R.E.; Williams, A.P.; Zou, C.B. The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant Sci. 2013, 4, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stovall, A.E.L.; Shugart, H.; Yang, X. Tree height explains mortality risk during an intense drought. Nat. Commun. 2019, 10, 4385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, R.; Sperry, J.S.; Katul, G.G.; Pataki, D.E.; Ewers, B.E.; Phillips, N.; Schäfer, K.V.R. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.; Cowan, I.; Farquhar, G. The apparent feedforward response of stomata to air vapour pressure deficit: Information revealed by different experimental procedures with two rainforest trees. Plant Cell Environ. 1997, 20, 142–145. [Google Scholar] [CrossRef]
- Carminati, A.; Javaux, M. Soil Rather than Xylem Vulnerability Controls Stomatal Response to Drought. Trends Plant Sci. 2020, 25, 868–880. [Google Scholar] [CrossRef]
- Meinzer, F.; Hinckley, T.; Ceulemans, R. Apparent responses of stomata to transpiration and humidity in a hybrid poplar canopy. Plant Cell Environ. 1997, 20, 1301–1308. [Google Scholar] [CrossRef]
- Monteith, J. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995, 18, 357–364. [Google Scholar] [CrossRef]
- Addington, R.N.; Mitchell, R.J.; Oren, R.; Donovan, L.A. Stomatal sensitivity to vapor pressure deficit and its relationship to hydraulic conductance in Pinus palustris. Tree Physiol. 2004, 24, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, G.Z.; Li, X.; Wang, Y.; Wang, F.Z.; Li, X.M. Seasonal change in response of stomatal conductance to vapor pressure deficit and three phytohormones in three tree species. Plant Signal Behav. 2019, 14, 1682341. [Google Scholar] [CrossRef] [Green Version]
- Körner, C.; Neumayer, M.; Menendez-Riedl, S.P.; Smeets-Scheel, A. Functional morphology of mountain plants. Flora 1989, 182, 353–383. [Google Scholar] [CrossRef]
- McNaughton, K.; Jarvis, P. Effects of spatial scale on stomatal control of transpiration. Agric. For. Meteorol. 1991, 54, 279–302. [Google Scholar] [CrossRef]
- Cunningham, S.C. Photosynthetic responses to vapour pressure deficit in temperate and tropical evergreen rainforest trees of Australia. Oecologia 2005, 142, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, P.; Shen, W.; Niu, J.; Zhu, L.; Ni, G. Biophysical limits to responses of water flux to vapor pressure deficit in seven tree species with contrasting land use regimes. Agric. For. Meteorol. 2015, 200, 258–269. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Funct. Plant Biol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Raftoyannis, Y.; Radoglou, K. Physiological responses of beech and sessile oak in a natural mixed stand during a dry summer. Ann. Bot. 2002, 89, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Schütz, W.; Milberg, P.; Lamont, B.B. Germination requirements and seedling responses to water availability and soil type in four eucalypt species. Acta Oecol. 2002, 23, 23–30. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Karatassiou, M.; Noitsakis, B.; Koukoura, Z. Drought adaptation ecophysiological mechanisms of two annual legumes on semi-arid Mediterranean grassland. Sci. Res. Essays 2009, 4, 493–500. [Google Scholar] [CrossRef]
- Cochard, H.; Venisse, J.-S.; Barigah, T.S.; Brunel, N.; Herbette, S.; Guilliot, A.; Tyree, M.T.; Sakr, S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 2007, 143, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Guyot, G.; Scoffoni, C.; Sack, L. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: Insights into vulnerability and stomatal control. Plant Cell Environ. 2012, 35, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Sellin, A.; Sack, L.; Ounapuu, E.; Karusion, A. Impact of light quality on leaf and shoot hydraulic properties: A case study in silver birch (Betula pendula). Plant Cell Environ. 2011, 34, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Meinzer, F.C.; Qi, J.H.; Goldstein, G.; Cao, K.F. Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant Cell Environ. 2013, 36, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, G.; Ripullone, F.; Borghetti, M.; Raddi, S.; Magnani, F. Contribution of diffusional and non-diffusional limitations to midday depression of photosynthesis in Arbutus unedo L. Trees 2009, 23, 1149–1161. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Jordan, G.J. Internal coordination between hydraulics and stomatal control in leaves. Plant Cell Environ. 2008, 31, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Comstock, J.P. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J. Exp. Bot. 2002, 53, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, I.; Cano, F.J.; Gasco, A.; Cochard, H.; Nardini, A.; Mancha, J.A.; Lopez, R.; Sanchez-Gomez, D. Variation in photosynthetic performance and hydraulic architecture across European beech (Fagus sylvatica L.) populations supports the case for local adaptation to water stress. Tree Physiol. 2015, 35, 34–46. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.; Llorens, P.; Nikinmaa, E.; Nole, A.; Poyatos, R. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef]
- Kolb, K.J.; Davis, S.D. Drought tolerance and xylem embolism in co-occurring species of coastal sage and chaparral. Ecology 1994, 75, 648–659. [Google Scholar] [CrossRef]
- Iovi, K.; Kolovou, C.; Kyparissis, A. An ecophysiological approach of hydraulic performance for nine Mediterranean species. Tree Physiol. 2009, 29, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A. Are sclerophylls and malacophylls hydraulically different? Biol. Plant. 2001, 44, 239–245. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.D.; Wagner, R.J.; Rollinson, C.R.; Kimball, B.A.; Kaye, M.W.; Kaye, J.P. Microclimate and ecological threshold responses in a warming and wetting experiment following whole tree harvest. Theor. Appl. Climatol. 2014, 116, 287–299. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Dai, A. Recent climatology, variability, and trends in global surface humidity. J. Clim. 2006, 19, 3589–3606. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.P.; O’Gorman, P.A. Trends in continental temperature and humidity directly linked to ocean warming. Proc. Natl. Acad. Sci. USA 2018, 115, 4863–4868. [Google Scholar] [CrossRef] [Green Version]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Mitrakos, K. Water relations of evergreen sclerophylls. I. Seasonal changes in the water relations of eleven species from the same environment. Ann. Bot. 1990, 65, 171–178. [Google Scholar] [CrossRef]
- Sellin, A. Does pre-dawn water potential reflect conditions of equilibrium in plant and soil water status? Acta Oecol. 1999, 20, 51–59. [Google Scholar] [CrossRef]
- Williams, L.E.; Trout, T.J. Relationships among vine-and soil-based measures of water status in a Thompson Seedless vineyard in response to high-frequency drip irrigation. Am. J. Enol. Vitic. 2005, 56, 357–366. [Google Scholar]
- Cai, G.; Ahmed, M.A.; Dippold, M.A.; Zarebanadkouki, M.; Carminati, A. Linear relation between leaf xylem water potential and transpiration in pearl millet during soil drying. Plant Soil 2020, 447, 565–578. [Google Scholar] [CrossRef]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Holbrook, N.M. Stomatal Closure during Leaf Dehydration, Correlation with Other Leaf Physiological Traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinzer, F.C.; Johnson, D.M.; Lachenbruch, B.; McCulloh, K.A.; Woodruff, D.R. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 2009, 23, 922–930. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Heberlein, K.; Kassianou, A. Field water relations of Capparis spinosa L. J. Arid Environ. 1997, 36, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Meletiou-Christou, M.-S.; Rhizopoulou, S. Constraints of photosynthetic performance and water status of four evergreen species co-occurring under field conditions. Bot. Stud. 2012, 53, 325–334. [Google Scholar]
- Meletiou-Christou, M.-S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Rhizopoulou, S.; Kapolas, G. In situ study of deep roots of Capparis spinosa L. during the dry season: Evidence from a natural “rhizotron” in the ancient catacombs of Milos Island (Greece). J. Arid Environ. 2015, 119, 27–30. [Google Scholar] [CrossRef]
- Kosugi, Y.; Matsuo, N. Seasonal fluctuations and temperature dependence of leaf gas exchange parameters of co-occurring evergreen and deciduous trees in a temperate broad-leaved forest. Tree Physiol. 2006, 26, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Auge, R.M.; Green, C.D.; Stodola, A.J.; Saxton, A.M.; Olinick, J.B.; Evans, R.M. Correlations of stomatal conductance with hydraulic and chemical factors in several deciduous tree species in a natural habitat. New Phytol. 2000, 145, 483–500. [Google Scholar] [CrossRef] [Green Version]
- Medrano, H.; Flexas, J.; Galmes, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Koukos, D.; Meletiou-Christou, M.-S.; Rhizopoulou, S. Leaf surface wettability and fatty acid composition of Arbutus unedo and Arbutus andrachne grown under ambient conditions in a natural macchia. Acta Bot. Gall. 2015, 162, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Christodoulakis, N.S.; Lampri, P.-N.; Fasseas, C. Structural and cytochemical investigation of the leaf of silverleaf nightshade (Solanum elaeagnifolium), a drought-resistant alien weed of the Greek flora. Aust. J. Bot. 2009, 57, 432–438. [Google Scholar] [CrossRef]
- Río, S.D.; Álvarez Nogal, R.; Candelas, A.; González-Sierra, S.; Herrero, L.; Penas, A. Preliminary study on taxonomic review using histological sections of some Iberian species from the genus Quercus L. (Fagaceae). Am. J. Plant Sci. 2014, 5, 2773–2784. [Google Scholar] [CrossRef] [Green Version]
- Duff, G.A.; Myers, B.A.; Williams, R.J.; Eamus, D.; OGrady, A.; Fordyce, I.R. Seasonal patterns in soil moisture, vapour pressure deficit, tree canopy cover and pre-dawn water potential in a northern Australian savanna. Aust. J. Bot. 1997, 45, 211–224. [Google Scholar] [CrossRef]
- Prior, L.; Eamus, D.; Duff, G. Seasonal and diurnal patterns of carbon assimilation, stomatal conductance and leaf water potential in Eucalyptus tetrodonta saplings in a wet–dry savanna in northern Australia. Aust. J. Bot. 1997, 45, 241–258. [Google Scholar] [CrossRef]
- Devi, M.J.; Reddy, V.R. Transpiration response of cotton to vapor pressure deficit and its relationship with stomatal traits. Front. Plant Sci. 2018, 9, 1572. [Google Scholar] [CrossRef] [Green Version]
- Ben-Asher, J.; y Garcia, A.G.; Flitcroft, I.; Hoogenboom, G. Effect of atmospheric water vapor on photosynthesis, transpiration and canopy conductance: A case study in corn. Plant Soil Environ. 2013, 59, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, T.; Kano, K.; Endo, R.; Kitaya, Y. Photosynthetic properties and response to drought in cucumber seedlings acclimatized to different vapor-pressure-deficit levels. Hort. J. 2017, 86, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Manzoni, S.; Vico, G.; Katul, G.; Palmroth, S.; Jackson, R.B.; Porporato, A. Hydraulic limits on maximum plant transpiration and the emergence of the safety–efficiency trade-off. New Phytol. 2013, 198, 169–178. [Google Scholar] [CrossRef]
- Hopkins, W.G. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: Devon, UK, 2009. [Google Scholar]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Scholz, F.G.; Franco, A.C.; Bustamante, M. Functional convergence in hydraulic architecture and water relations of tropical savanna trees: From leaf to whole plant. Tree Physiol. 2004, 24, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and life history of tropical dry forest tree species: Coordination of species’ drought and shade tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Ball, M.C.; Luly, J.G.; Holtum, J.A. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 2005, 19, 305–311. [Google Scholar] [CrossRef]
- Blumler, M.A. Winter-Deciduous Versus Evergreen Habit in Mediterranean Regions: A Model; Gen. Tech. Report PSW-126; USDA Forest Service: Berkeley, CA, USA, 1991; pp. 194–197.
- Holbrook, N.M.; Whitebeck, J.; Mooney, H. Drought responses of neotropical dry forests. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 243–276. [Google Scholar]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Filippidis, E.; Mitsopoulos, I. Mapping forest fire risk zones based on historical fire data in Mount Olympus, Greece, using geographical information systems. WIT Trans. Ecol. Environ. 2004, 77, 13. [Google Scholar] [CrossRef]
- Klesse, S.; Ziehmer, M.; Rousakis, G.; Trouet, V.; Frank, D. Synoptic drivers of 400 years of summer temperature and precipitation variability on Mt. Olympus, Greece. Clim. Dyn. 2015, 45, 807–824. [Google Scholar] [CrossRef]
- Strid, A. The botanical exploration of Greece. Plant Syst. Evol. 2020, 306, 27. [Google Scholar] [CrossRef]
- Donovan, L.; Grise, D.; West, J.; Pappert, R.; Alder, N.; Richards, J. Predawn disequilibrium between plant and soil water potentials in two cold-desert shrubs. Oecologia 1999, 120, 209–217. [Google Scholar] [CrossRef]
- Kavanagh, K.; Bond, B.; Aitken, S.; Gartner, B.; Knowe, S. Shoot and root vulnerability to xylem cavitation in four populations of Douglas-fir seedlings. Tree Physiol. 1999, 19, 31–37. [Google Scholar] [CrossRef] [Green Version]


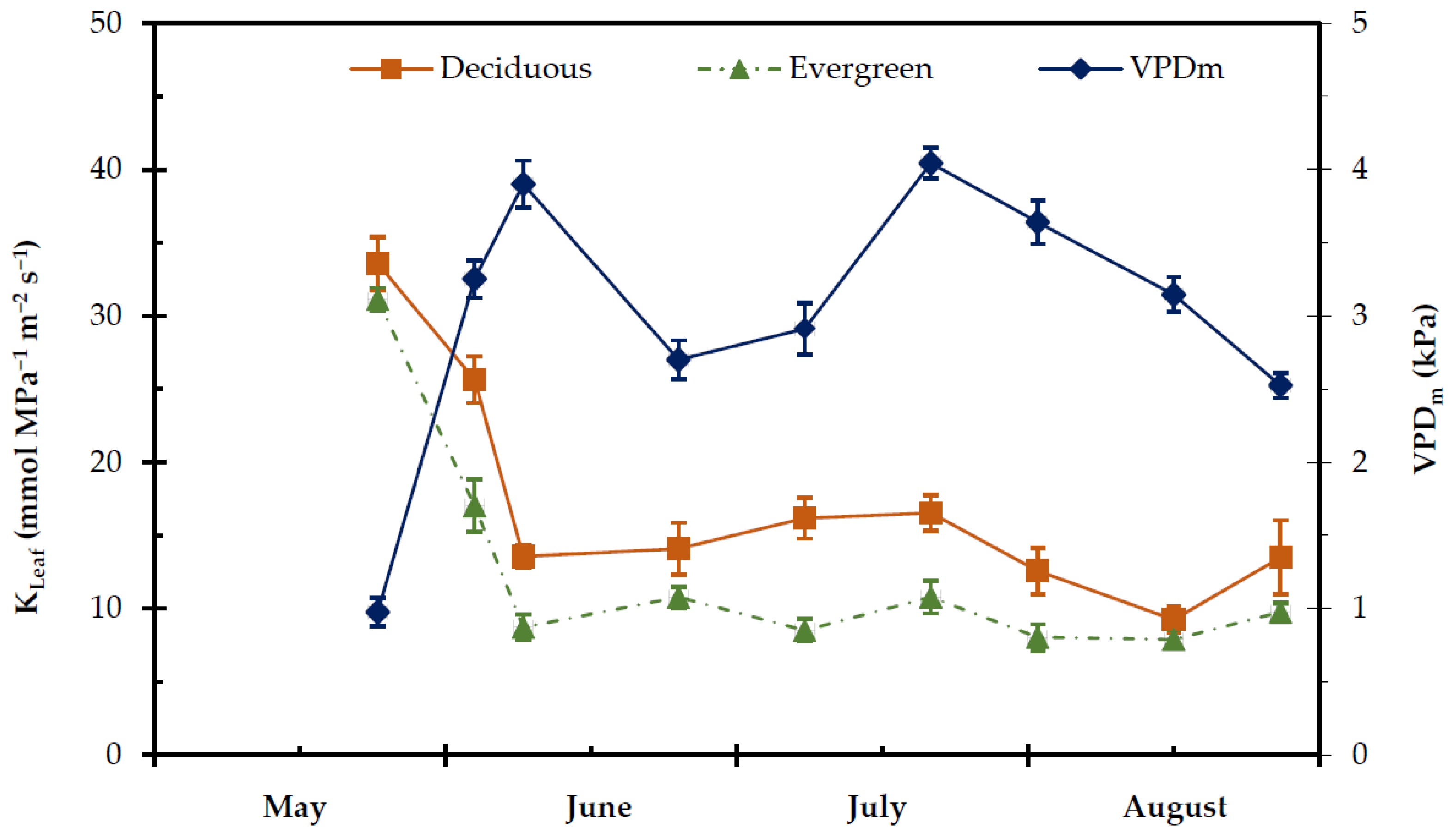
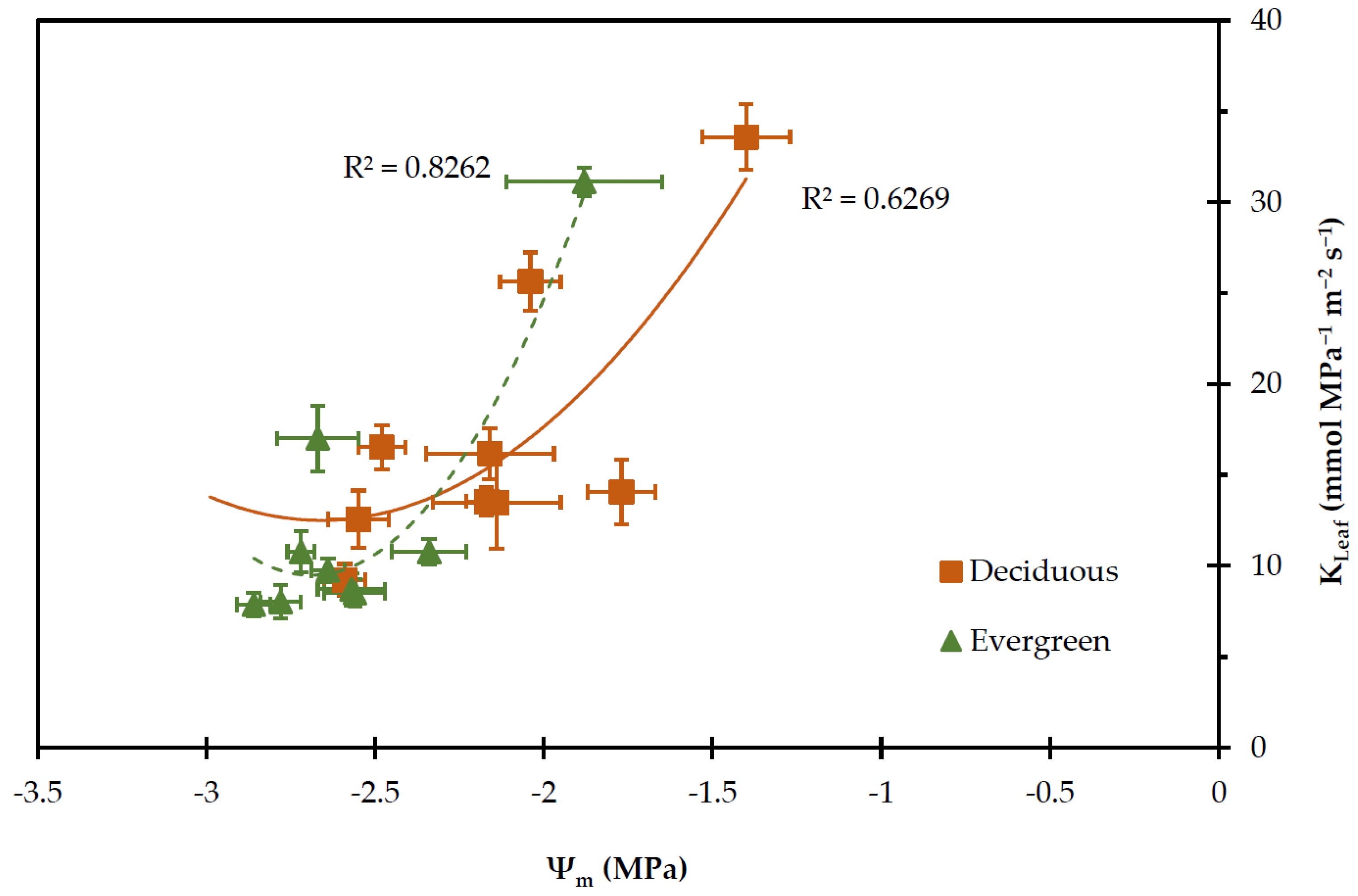

| Parameter | Deciduous | Evergreens | p-Value |
|---|---|---|---|
| Mean ± SE | Mean ± SE | ||
| Ψp (MPa) | −0.94 ± 0.04 | −1.04 ± 0.03 | 0.876 NS |
| Ψm (MPa) | −2.09 ± 0.06 | −2.53 ± 0.05 | 0.001 ** |
| E (mmol m−2 s−1) | 16.12 ± 0.89 | 15.87 ± 0.68 | 0.734 NS |
| gs (mmol m−2 s−1) | 575.97 ± 21.00 | 585.5 ± 27.93 | 0.487 NS |
| KLeaf (mmol MPa−1 m−2 s−1) | 17.18 ± 1.45 | 13.15 ± 1.57 | 0.033 * |
| VPDm (kPa) | 2.79 ± 0.13 | 2.67± 0.11 | 0.149 |
| Deciduous | Ψp | Ψm | E | gs | KLeaf | VPDm | Evergreens |
|---|---|---|---|---|---|---|---|
| Ψp | 1 | 0.387 ** | 0.020 | −0.139 | −0.039 | −0.108 | Ψp |
| Ψm | 0.524 ** | 1 | −0.311 ** | −0.162 | 0.544 ** | −0.451 ** | Ψm |
| E | 0.234 * | −0.013 | 1 | −0.328 ** | 0.135 | 0.147 | E |
| gs | 0.357 ** | 0.620 ** | −0.110 | 1 | −0.037 | 0.157 | gs |
| KLeaf | 0.084 | 0.478 ** | 0.562 ** | 0.209 | 1 | −0.298 ** | KLeaf |
| VPDm | −0.088 | −0.443 ** | 0.271 ** | −0.315 ** | −0.147 | 1 | VPDm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatassiou, M.; Karaiskou, P.; Verykouki, E.; Rhizopoulou, S. Hydraulic Response of Deciduous and Evergreen Broadleaved Shrubs, Grown on Olympus Mountain in Greece, to Vapour Pressure Deficit. Plants 2022, 11, 1013. https://doi.org/10.3390/plants11081013
Karatassiou M, Karaiskou P, Verykouki E, Rhizopoulou S. Hydraulic Response of Deciduous and Evergreen Broadleaved Shrubs, Grown on Olympus Mountain in Greece, to Vapour Pressure Deficit. Plants. 2022; 11(8):1013. https://doi.org/10.3390/plants11081013
Chicago/Turabian StyleKaratassiou, Maria, Panagiota Karaiskou, Eleni Verykouki, and Sophia Rhizopoulou. 2022. "Hydraulic Response of Deciduous and Evergreen Broadleaved Shrubs, Grown on Olympus Mountain in Greece, to Vapour Pressure Deficit" Plants 11, no. 8: 1013. https://doi.org/10.3390/plants11081013






