Abstract
Efficient methodologies for automated seed quality evaluations are important for the seed industry. Advanced seed technology research requires the use of adequate methods to ensure good seed performance under adverse environmental conditions; thus, providing producers with detailed, quick, and accurate information on structural seed integrity and ensuring vigorous production. To address this problem, this study aimed to determine Brachiaria brizantha (Marandu cv., Piatã cv. and Xaraés cv.) seed quality through radiographic imaging analyses associated with vigor tests and anatomical characterizations. Brachiaria seed cultivars displaying different physical and physiological attributes were selected and subjected to the 1000-seed weight test, water content determinations, X-ray analyses, germination tests, and anatomical characterizations. The X-ray analyses made it possible to establish a relationship between the X-ray images and other determined variables. Furthermore, the X-ray images can indicate evidence of internal and external damage that could later compromise germination. The Marandu and Piatã cultivars presented the highest germination percentages, germination speed indices, normal seedling development, and cellular structure preservation compared to the Xaraés cultivar. To summarize, X-ray analyses are efficient methods used for the selection of higher physical quality cultivars and can aid in the decision-making processes of companies and seed producers worldwide.
1. Introduction
Grasslands occupy around 80% of agricultural lands worldwide and represent a wide range of ecosystems [1]. In 2015, total global pasture areas comprised 2.7 billion ha, with Africa encompassing the largest area, of approximately 889 million ha, followed by China (~506 million ha), while Brazil holds about 196 million ha of cultivated pasture areas [2]. Brachiaria spp., an East African species, also known as signal grass, based on the similarity of its flower head structure with a railway signal, is noteworthy in this scenario [3]. These grasses are used in Brazil for biomass production and as animal feed and soil cover in no-till systems, increasing land use efficiency [4] and soil fertility [5].
As cultivation areas increase, so do demands for high quality seeds, which have become essential for the improvement of the global forage production. Therefore, innovative approaches are required to solve never-before-experienced food production and sustainability problems [6]. This has led the seed industry to constantly improve seed lot production and standardization processes, aiming at obtaining and selling seeds with high physiological potential. In this scenario, seeds used for the establishment of production fields are a fundamental part of this problem and their stability and uniformity are strictly related to the yields obtained at the end of the cycles in many agricultural crops [7].
Some features must be assessed to increase seed lot quality. Seeds may undergo physical characteristic alterations, affecting cell anatomy, including enzymatic inactivation, during the harvesting and storage [8]. Changes at the cellular level can be monitored through germination and emergence rates, electrical conductivity tests, and anatomical techniques. These assessments were employed herein as tools to indicate physiological seed quality, which proved promising, and could bring forth new information on the deterioration of stored seeds.
On the other hand, although traditional tests applied independently in seed quality evaluations produce reliable results, Brachiaria seed testing methods are mainly based on the classic tetrazolium test to estimate physiological seed lot potential. This method is, in general, destructive, time-consuming, and influenced by analyst subjectivity. Therefore, new technologies, especially non-destructive high performance, and speedy methods, are required for seed lot assessments.
A high percentage of Brachiaria seeds displayed different fillings, directly linked to seed quality, as seed morphology plays a significant role in the growth of healthy and uniform seedlings [9]. Thus, alterations in Brachiaria seed development and filling may result in abnormal seedlings. Among other optical technologies, automated X-ray imaging analyses comprise a very interesting tool in this regard due to their high precision, allowing for the detection of damaged or malformed seeds.
X-ray imaging analyses may be employed to evaluate internal seed structures, as well as seed coat features, which allow for physiological performance probability predictions, identifying alterations that indicate the production of abnormal organs and their inevitable consequences during field cultivation, enabling pattern recognition, data management, and providing accurate, fast and non-destructive assessments [10,11]. However, the use of radiographic images for the evaluation of agricultural commodities is still at a preliminary stage, and further studies are required to better determine the quality parameters of seed lots [12].
In this context, this study hypothesized that quality parameters in different Brachiaria seed cultivars through imaging analyses are correlated to physiological and anatomical tests results. Thus, the aim of this study was to evaluate the physiological quality of Brachiaria seeds employing automated X-ray imaging analyses and compare the findings to germination and anatomical characterizations, in order to quickly and effectively select seed lots.
2. Results
2.1. Water Content and 1000-Seed Weight
Similar water contents were noted among the three studied cultivars, ranging from 9.1 to 9.8%, with the Marandu cultivar presenting the highest values and Xaraés, the lowest. The 1000-seed weight test results indicated a higher density for the Xaraés cultivar when compared to the other investigated cultivars (damage not shown).
2.2. Internal Seed Morphology Assessment Employing the X-ray Technique
Seed classifications according to the X-ray technique indicated that the highest number of damaged seeds with endosperm spaces was found for the Xaraés cultivar (Figure 1I–L) compared to the Marandu (Figure 1A–D), and Piatã (Figure 1E–H) cultivars.

Figure 1.
Differences in internal Brachiaria brizantha seed structures demonstrated by X-ray assessments. (A–D) Marandu cv. (E–H) Piatã cv. (I–L), Xaraés cv. Yellow arrows indicate damaged seeds.
The X ray images allow analysts to highlight and select areas presenting physical integrity through grey pixel levels (Figure 2). Figure 2 displays the original X-ray image and its 3D representation with color tone variations according to tissue integrity and density (Figure 2). The 3D histogram technique highlights vigorous regions with warm colors (Figure 2A,C,E), which develop into seedlings, presenting a better development index, while regions with lower physiological quality, which can trigger abnormal seedling development and low germination rates, are indicated in cold colors (Figure 2B,D,F).
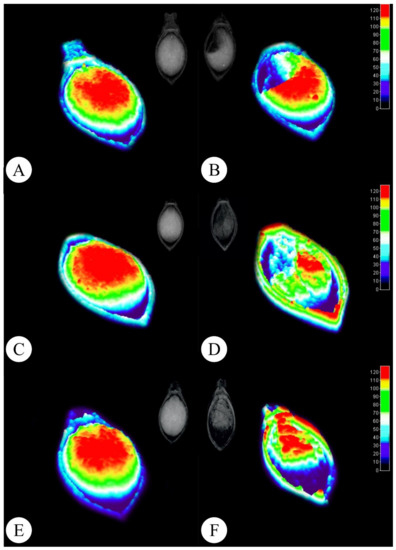
Figure 2.
Radiographic Brachiaria brizantha seed images and their representations in 3D. (A,B) Marandu cv. (C,D) Piatã cv. (E,F) Xaraés cv. Left column: higher tissue density seeds. Right column: lower tissue density seeds.
Physical seed qualities according to the X-ray images are presented in Table 1. The Marandu cultivar exhibited a greater area and percentage of filling compared to the Piatã and Xaraés cultivars. The Piatã cultivar displayed a similar relative density compared to the Marandu cultivar. The Xaraés cultivar presented the smallest area and lowest relative density and filling percentage compared to the Marandu and Piatã cultivars.

Table 1.
Physical parameters evaluated from X-ray images of Brachiaria seeds, Marandu, Piatã, and Xaraés cultivars, using the ImageJ program.
2.3. Germination Test
Similar speed index, germination percentage, normal and non-germinated seedling values were observed between the Marandu and Piatã cultivars, with no significant differences (Table 2). However, the Xaraés cultivar exhibited a significant reduction in all analyzed variables compared to the Marandu and Piatã cultivars.

Table 2.
Germination speed index (GSI), germination percentage (%), normal, abnormal, and non-germinated seedlings of the three investigated Brachiaria cultivars after 13 days of germination.
2.4. Anatomical Seed Characterization
Differences in seed filling led to Brachiaria endosperm region alterations (Figure 3B,D,F) compared to seeds displaying no physical changes (Figure 3A,C,E).
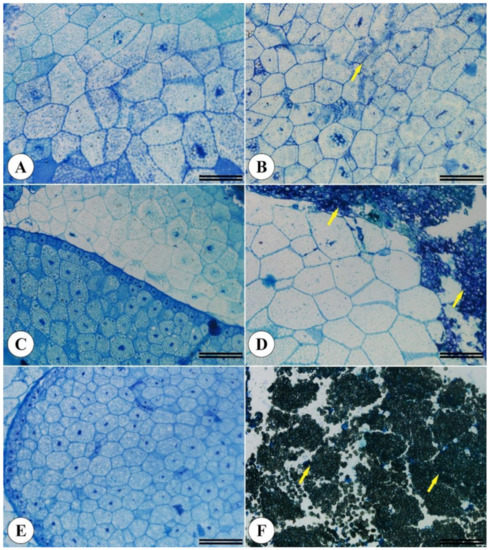
Figure 3.
Morphoanatomical Brachiaria brizantha seed structures. Seeds previously selected by the X-ray test displaying no filling alterations (A,C,E) and filling alterations (B,D,F). (A,B) Marandu cv. (C,D) Piatã vc. (E,F) Xaraés cv. A total of 200 seeds from each cultivar were analyzed. Yellow arrows indicate cellular changes.
The PCA concerning physiological quality variables and quantitative data extracted from the Brachiaria seed X-ray images indicate total data variances of 75.40%, 60.20%, and 15.20% for PC1 and PC2, respectively (Figure 4). Three groups of characteristics were detected, namely group I, comprising amy, b-amy, relative density, filling, TP, GSI, area, and G%, positively correlated; group II—PA% and circularity, also positively correlated; and group III—composed of NG%, positively correlated with group II and negatively correlated with group I. Regarding the first component (PC1), seed characteristics NG%, PA%, and circularity exhibited a negative correlation, with NG% as the strongest. The other evaluated characteristics were positively correlated with PC1. Concerning PC2, G% and area were negatively correlated. In general, all characteristics were important for both PC, except for relative density and GSI. Different behaviors are noted regarding the three Brachiaria brizantha cultivars (groups), with the Xaraés cultivar antagonistic to the other cultivars and the Piatã and Marandu cultivars exhibiting similar behaviors.
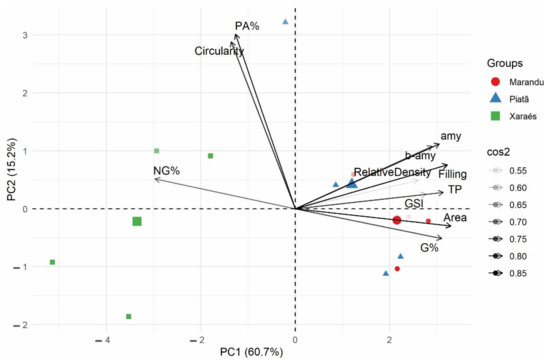
Figure 4.
Biplot of the principal components comprising the following variables: area, roundness, density, filling percentage, germination speed index, germination percentage, PT—total proteins, amy = α-amy alpha amylase, b-amy = β-amy beta amylase, normal and abnormal, and non-germinated Brachiaria brizantha cultivar seedlings. cos2—importance of the variable in defining the principal component (PC); PC1—first principal component; PC2—second principal component.
3. Discussion
The X-ray image analysis technique can adequately indicate physical seed structure and, as a non-destructive and non-subjective methodology, is highly desired by the seed industry [13]. This methodology allowed for the verification of morphological seed changes caused by several factors, such as physiological changes during the maturation process, pest attacks and mechanical damage during the harvesting and storage processes. The endosperm plays a vital role in supporting embryonic growth, providing nutrients, and protecting and controlling embryo growth, acting as a mechanical barrier during seed development and germination [14]. Therefore, quick, and accurate identification of physical endosperm conditions is important to assess the quality of different seed cultivars.
Brachiaria seeds displaying higher density, filling percentages, and cellular integrity exhibited higher physiological quality. These data are paramount for the seed sector, since the quick identification of good and bad quality seeds contributes to optimizing seed production processing and quality control [15,16]. Previous studies on broccoli [17] and leucaena [18] indicate that the determination of seed tissue density employing X-ray images is promising and strongly correlated to physiological seed quality, as higher density seeds are correlated with higher germination percentages and normal seedling development.
Seeds presenting physiological quality changes can undergo important biochemical changes, such as carbohydrate content exudation [19] and oxidation, leading to membrane wall loosening and, as a result, loss of internal protein elasticity, increasing cellular tissue fragility, resulting in cotyledon cracks and irregular development [20]. Furthermore, as evidenced herein, changes in physiological seed quality may be directly linked to seed formation, especially regarding aspects related to cellular disruption in low density seeds with low filling percentages.
Recent research has presented data on internal physical seed parameters evaluated by X-ray images, effectively correlating these data with germination and vigor attributes [17]. In this regard, while studying machine learning to classify Brachiaria brizantha seeds, [15] demonstrated that the IJCropSeed tool developed by the researchers is highly efficient in the selection of higher quality seed lots. In the present study, we verified that this relationship may possibly extend to seeds displaying similar physical characteristics when assessing different cultivars of the same species.
The application of the X-ray test on different Brachiaria cultivar seeds also indicated an association between internal seed morphology and their physiological quality. This technique thus allows for internal seed morphology characterization, such as identification of full and/or malformed seeds, damage by insect predation, mechanical injuries during harvesting, transportation, and/or storage and percentage of empty internal areas [21,22]).
The use of the X-ray technique is advantageous in reducing seed storage costs by permitting seed separation, contributing to more vigorous seed lots [23]. It also comprises a valuable technique for fast, accurate, and non-destructive assessments related to seed performance. The PCA results corroborate these facts, as seed quality and vigor characteristics were closely associated to parameters determined via the X-ray technique, i.e., area, TP, filling, amy activity, and G%. Furthermore, an opposite NG% behavior compared to these parameters was noted.
Alpha and beta amylase enzymatic activities are directly linked to seed quality, as they can be associated with rapid starch hydrolysis, resulting in greater energy available for initial seedling growth [24,25]. Therefore, amylase analyses also display the potential to assist in the selection of higher quality Brachiaria seed lots. In this sense, our findings indicate that seeds presenting higher density, filling, TP, and amy activities form normal seedlings. In addition, differential cultivar behaviors (Xaraés in relation to Piatã and Marandu) also evidence the potential of using the X-ray technique to select seed and cultivar lots.
4. Material and Methods
4.1. Assay Implementation
All experiments were conducted at the Seed Laboratory, IF Goiano—Rio Verde Campus, GO, Brazil, with uncoated commercial Brachiaria brizantha seeds (Marandu cv., Piatã cv. and Xaraés cv.) from the 2020 crop.
4.2. The 1000-Seed Weight Test
The weight of 1000 seeds was obtained from eight 1000-seed replicates for each studied Brachiaria variety and weighed on a precision scale according to established seed analysis rules [13].
4.3. Water Content
Water content was determined by drying the seeds in an oven at 105 ± 3 °C for 24 h, adapted according to [26], using four 50-seed replications, corresponding to 4.5 g for each analyzed Brachiaria cultivar. Seeds were weighed on an analytical balance (0.001 g precision) and calculations were performed according to the following equation:
where Pf: final sample mass (g); Pi: initial sample mass (g); TAi: initial seed water content (%wet base); TAf: desired water content (% wet base).
4.4. Internal Seed Morphology Assessments Employing the X-ray Technique
The X-ray test was performed with 200 Brachiaria seeds, comprising four repetitions of 50 seeds for each cultivar. Briefly, seeds were placed on transparent acrylic plates on double-sided transparent adhesive tape, and subjected to radiation employing a Faxitron HP 43855A X-ray equipment set at 30 Kv for 10 s. The digital images were analyzed using the IJCropSeed plugin developed for the ImageJ® software [7] (Table 3).

Table 3.
X-ray image variables automatically analyzed using the ImageJ software.
After the X-ray seed evaluations, the characterization of the 3D images of the three investigated cultivars was performed using the ImageJ® software. The images were processed in the 3D plugins in the interactive 3D surface plot option, an option that highlights colors in relation to the gray fabric density per pixel. After performing the evaluations, the images were extracted from the software and saved in JPEG format.
4.5. Germination Test
The germination test was carried out on sheets of blotting paper moistened with distilled water at 2.5 times the mass of the dry substrate. Four replications comprising 50 seeds each were conditioned in transparent plastic boxes previously washed and dried in a greenhouse. The plastic boxes were maintained in a germination chamber regulated with a photoperiod of 8 h with lighting and 16 h without lighting at a temperature of 35 °C [26].
The percentage of normal germinated seedlings (represented by seedlings displaying all essential structures developed, i.e., root system, shoots, and coleoptile) was computed in the first germination count on the 7th day (G7) and on the final count on the 21st day (G21). The final counts included normal seedlings, abnormal seedlings (those that do not display the potential to continue their development and give rise to normal plants, even growing under favorable conditions), hard seeds (seeds that do not absorb water for a longer period than normal and, thus appear as seeds that have just been placed on the substrate, unswollen at the end of the test), and dead seeds (seeds that are softened, do not exhibit any sign of germination, and do not germinate at the end of the test) [26].
4.6. Morphoanatomical Characterizations
Morphoanatomical characterizations were performed by fixing 10 seeds of each Brachiaria cultivar according to Karnovsky [27], for 24 h, followed by prewashing in phosphate buffer and dehydration in an increasing ethyl series and, finally, historesin pre-infiltration and infiltration (Leica, Wetzlar, Germany), according to the manufacturer’s recommendations.
For the structural evaluations, samples were cross sectioned at 7 μm thickness on a tabletop rotary microtome (1508R, Logen Scientific, Shanghai, China) and stained with toluidine blue [28]. Observations were performed in the seed reserve region and images were photographed using an Olympus microscope (BX61, Tokyo, Japan) coupled with a DP-72 camera using the brightfield option.
4.7. Biochemical Analyses
Enzymatic activities were assessed in 30 whole Brachiaria seeds from each cultivar. The seeds were first individually stored in aluminum foil in liquid nitrogen (N2) and then in an ultra-freezer at −80 °C prior to further analyses.
The enzyme extracts used to determine α-amylase (α-amy) and β-amylase (β-amy) enzymatic activities were obtained from 0.200 to 0.250 g of frozen seeds homogenized in 2 mL of potassium phosphate buffer (100 mM) (pH 6.8), containing 0.1 mM ethylenediaminetetraacetic acid (EDTA), 5% polyvinylpyrrolidone (PVPP) (m/v), and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenates were then kept overnight for 14 h at 10 °C and centrifuged at 12,000× g for 15 min at 4 °C. The final supernatants were used as extracts for enzymatic determinations.
Enzymatic activities were determined by the 1% 3,5-dinitrosalicylic acid (DNS) method, as described by Bernfeld [29], Tárrago and Nicolás [30], and Kishorekumar [31]. The reducing sugars formed by the actions of alpha and beta amylase were quantified by determining sample absorbances at 540 nm and calculations were performed using a standard maltose curve at 0.5 mg mL−1 2%.
Total protein concentrations were determined by mixing 10 µL of the crude extracts used to determine the enzymatic activities to 1190 µL of Bradford’s solution and determining sample absorbances at 595 nm Bradford [32], expressed as mg.g−1.
4.8. Statistical Analyses
The quantitative data were first submitted to homogeneity analysis (Levene’s test) and error normality (Shapiro-Wilk test) evaluations. As data normality was confirmed, an ANOVA test followed by the Scott-Knott test were applied. * indicates p < 0.05 and **, p < 0.01. Statistical analyses concerning the media test were performed using the software Assistat®.
Correlation assessments and a principal component analysis (PCA) were performed employing preliminary database consistency excluding outlier values through a boxplot analysis. The data were then normalized, where the means were equal to 0 and variances, 1. A variance and covariance matrix was then plotted for the PCA analysis [33]. The accumulated total variation was established as a criterion for the selection of the principal components (PC), maintaining the K first PC capable of explaining at least 70% of the total data variation [34].
5. Conclusions
X-ray image analyses are associated with physiological and biochemical Brachiaria seed quality. Thus, this technique is efficient in the selection of higher quality cultivars and can aid in the decision-making processes of companies and seed producers worldwide. However, despite associations with physiological seed quality, the imaging X-ray technique does not indicate chemical damage or other non-physical damage types, such as genetic effects.
Author Contributions
L.V.C., A.A.R., D.A.R., C.L.R. and J.d.F.S. designed the research. L.V.C., A.A.R., D.A.R., S.T.d.C. and C.L.R. conducted the experiments, collected the samples and performed physiological measurements. L.V.C. performed the biochemical analysis. A.A.R., D.A.R., C.L.R. and S.C.V.F. performed the anatomical and seed analyses. A.A.R., D.A.V. and A.R.N. performed the statistical analyses. All authors analyzed and discussed the data. L.V.C., A.A.R., D.A.R. and C.L.R. wrote the manuscript with contributions from all other authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Goiano Federal Institute of Education, Science, and Technology—Campus Rio Verde for providing financial support. AA Rodrigues is grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the PDCTR scholarship, process number 202110267000866.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Bosi, C.; Sentelhas, P.C.; Huth, N.I.; Pezzopane, J.R.M.; Andreucci, M.P.; Santos, P.M. APSIM-Tropical Pasture: A model for simulating perennial tropical grass growth and its parameterisation for palisade grass (Brachiaria brizantha). Agric. Syst. 2020, 184, 102917. [Google Scholar] [CrossRef]
- Goldewijk, K.K.; Beusen, A.; Doelman, J.; Stehfest, E. New anthropogenic land use estimates for the Holocene: HYDE 3.2. Earth Syst. Sci. Data 2017, 9, 927–953. [Google Scholar] [CrossRef]
- Low, S. Signal grass (Brachiaria decumbens) toxicity in grazing ruminants. Agriculture 2015, 5, 971–990. [Google Scholar] [CrossRef]
- Mateus, G.P.; Crusciol, C.A.C.; Pariz, C.M.; Borghi, E.; Costa, C.; Martello, J.M.; Franzluebbers, A.J.; Castilhos, A.M. Sidedress nitrogen application rates to sorghum intercropped with tropical perennial grasses. Agron. J. 2016, 108, 433–447. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Nascente, A.S.; Borghi, E.; Soratto, R.P.; Martins, P.O. Improving soil fertility and crop yield in a tropical region with palisadegrass cover crops. Agron. J. 2015, 107, 2271–2280. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Jhonston, M.; Muelller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Silva, L.J.; Silva, J.M.; Dias, D.C.F.S.; Pereira, M.D. IJCropSeed: An open-access tool for high-throughput analysis of crop seed radiographs. Comput. Electron. Agric. 2020, 175, 105555. [Google Scholar] [CrossRef]
- Vilela, L.; Martha Junior, G.B.; Macedo, M.C.M.; Marchao, R.L.; Guimaraes Júnior, R.; Pulrolink, K.; Maciel, G.A. Integrated crop-livestock systems in the Cerrado region. Pesq. Agropec. Bras. 2011, 46, 1127–1138. [Google Scholar] [CrossRef]
- Meng, L.S.; Wang, Y.B.; Loake, G.J.; Jiang, J.H. Seed Embryo Development Is Regulated via an AN3-MINI3 Gene Cascade. Front. Plant. Sci 2016, 7, 1645. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric. 2019, 1, 35–47. [Google Scholar] [CrossRef]
- Gargiulo, L.; Grimberg, A.; Valencia, R.R.C.; Carlsson, A.S.; Mele, G. Morpho-densitometric traits for quinoa (Chenopodium quinoa Willd.) seed phenotyping by two X-ray micro-CT scanning approaches. J. Cereal Sci. 2019, 90, 102829. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Yasmin, J.; Collins, W.; Cho, B.K. X-ray CT image analysis for morphology of muskmelon seed in relation to germination. Biosyst. Eng. 2018, 175, 183–193. [Google Scholar] [CrossRef]
- Mahajan, S.; Mittal, S.K.; Das, A. Machine vision based alternative testing approach for physical purity, viability and vigour testing of soybean seeds (Glycine max). J. Food Sci. Technol. 2018, 55, 3949–3959. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm During Seed Germination. Plant. Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef]
- Medeiros, A.D.; Silva, L.J.; Ribeiro, J.P.O.R.; Ferreira, K.C.; Rosas, J.T.F.; Santos, A.A.; Silva, C.B. Machine Learning for Seed Quality Classification: An Advanced Approach Using Merger Data from FT-NIR Spectroscopy and X-ray Imaging. Sensors 2020, 20, 4319. [Google Scholar] [CrossRef]
- Rahman, A.; Cho, B.K. Assessment of seed quality using non-destructive measurement techniques: A review. Seed Sci. Res. 2016, 26, 285–305. [Google Scholar] [CrossRef]
- Abud, H.F.; Cicero, S.M.; Gomes Junior, F.G. Imagens radiográficas e relação da morfologia interna e potencial fisiológico de sementes de brócolis. Acta Sci. Agron. 2018, 40, 1–9. [Google Scholar]
- Medeiros, A.D.; Araújo, J.O.; Zavala-León, M.J.; Silva, L.J.; Dias, D.C.F.S. Parameters based on x-ray images to assess the physical and physiological quality of Leucaena leucocephala seeds. Ciênc. Agrotec. 2018, 42, 643–652. [Google Scholar] [CrossRef]
- He, X.; Feng, X.; Sun, D.; Liu, F.; Bao, Y.; He, Y. Rapid and nondestructive measurement of rice seed vitality of different years using near-infrared hyperspectral imaging. Molecules 2019, 24, 2227. [Google Scholar] [CrossRef]
- Jyoti; Malik, C.P. Seed deterioration: A review. Int. J. Life Sci. Bt Pharm. Res. 2013, 2, 374–385. [Google Scholar]
- Arruda, N.; Cicero, S.M.; Gomes-Junior, F.G. Análise radiográfica para avaliar a estrutura da semente de Crotalaria juncea L. J. Seed Sci. 2016, 38, 161–168. [Google Scholar] [CrossRef][Green Version]
- Al-Turki, T.A.; Baskin, C.C. Determination of seed viability of eight wild Saudi Arabian species by germination and X-ray tests. Saudi J. Biol. Sci. 2017, 24, 822–829. [Google Scholar] [CrossRef][Green Version]
- Medeiros, A.D.; Martins, M.S.; Silvia, L.J.; Pereira, M.D.; León, M.J.Z.; Dias, D.C.F.S. X-ray imaging and digital processing application in non-destructive assessing of melon seed quality. J. Seed Sci. 2020, 42, e202042005. [Google Scholar] [CrossRef]
- Oliveira, G.E.; Von, P.R.G.; de Andrade, T.; Pinho, E.V.R.; Santos, C.D.; Veiga, A.D. Physiological quality and amylase enzyme expression in maize seeds. Ciênc Agrotec. 2013, 37, 40–48. [Google Scholar] [CrossRef]
- Medeiros, J.C.; Sales, J.F.; Zuchi, J.; Nascimento, K.J.T.; Silva, F.H.L.; Castro, S.T.; Costa, A.C.; Rodrigues, A.A. A multivariate approach to the physical and physiological quality of hybrid corn seeds affected by Molicutes and MRFV. Euphytica 2021, 217, 96. [Google Scholar] [CrossRef]
- Regras Para Análise de Sementes; Ministério da Agricultura e Reforma Agrária, Coordenação de Laboratório Vegetal: Brasília, Brasil, 2009; ISBN 978-85-99851-70-8.
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- O’Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Bernfeld, P. [17] Amylases α and β. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 1, pp. 149–152. [Google Scholar]
- Tárrago, J.F.; Nicolás, G. Starch Degradation in the Cotyledons of Germinating Lentils. Plant. Physiol 1976, 58, 618–621. [Google Scholar] [CrossRef]
- Kishorekumar, A.; Jaleel, C.A.; Manivannan, P.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Comparative effects of different triazole compounds on growth, photosynthetic pigments and carbohydrate metabolism of Solenostemon rotundifolius. Colloids Surf. B Biointerfaces 2007, 60, 207–212. [Google Scholar] [CrossRef]
- Bradford, M.N. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Ferreira, D.F. Estatística Multivariada; Editora Ufla: Lavras, Brazil, 2008; 662p. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).