Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program
Abstract
1. Introduction
2. Methodology
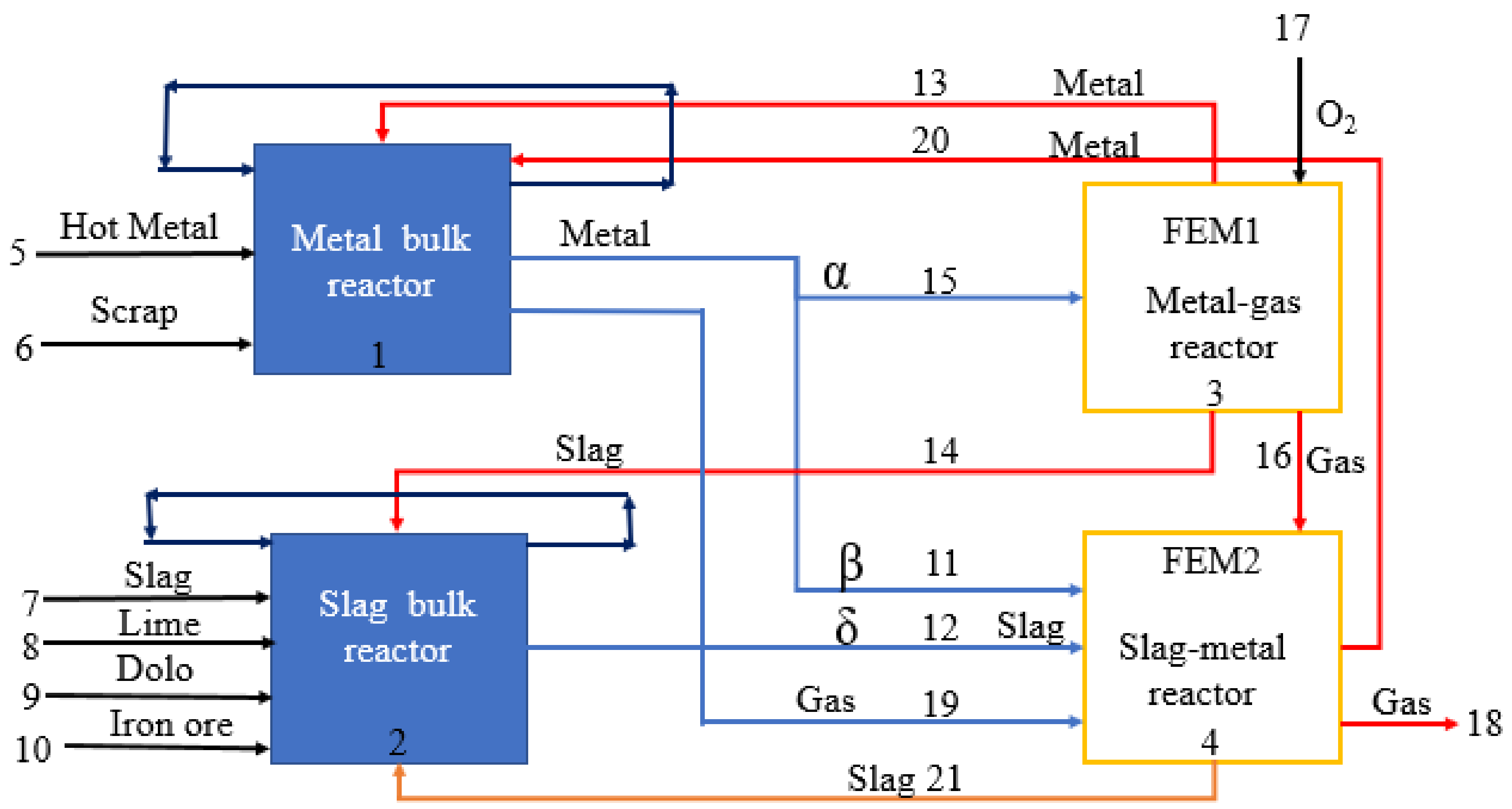
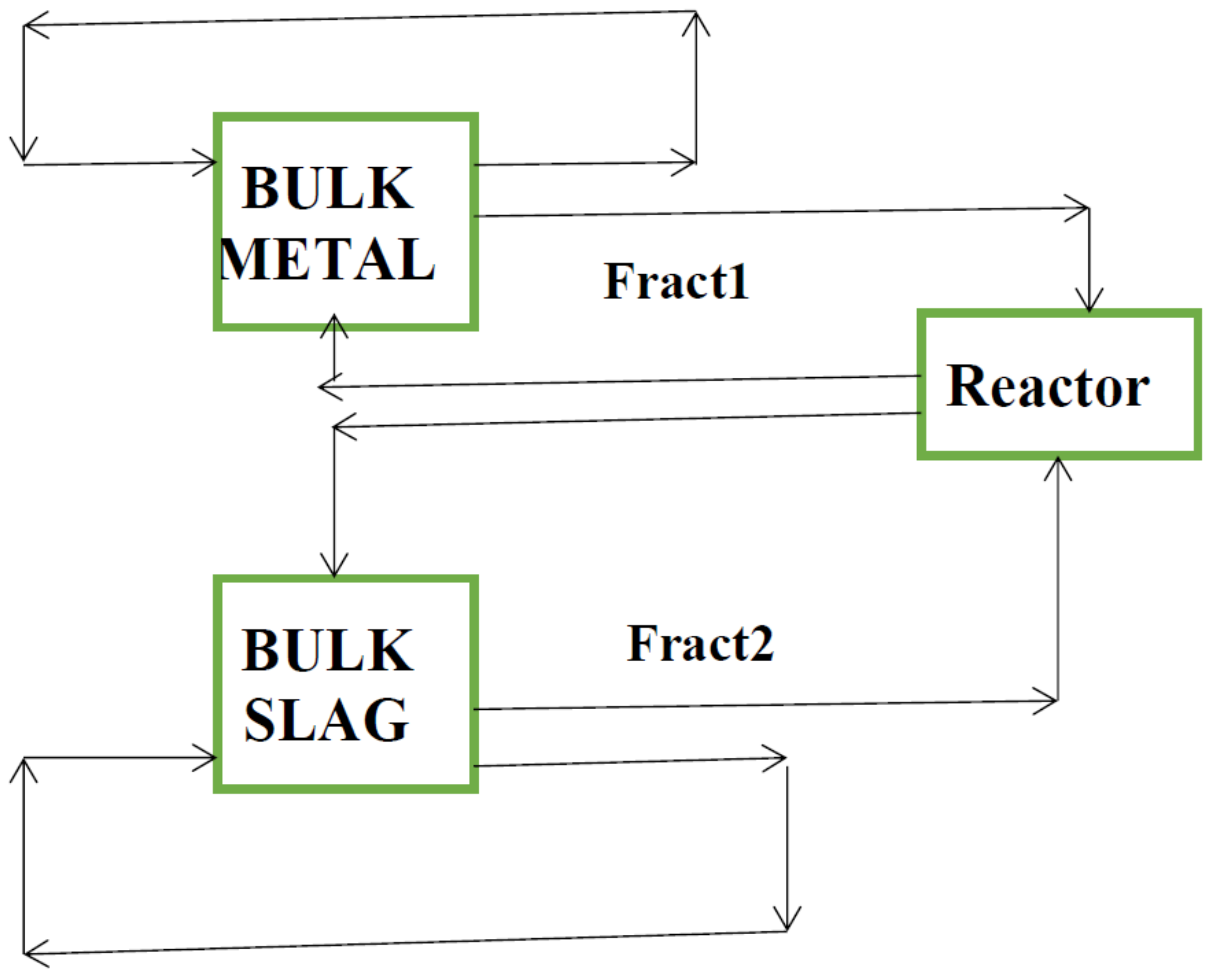
2.1. Model Description
2.2. Mass Transfer Coefficient and Decarburization Calculation
2.3. Model assumptions
- We entered 5,488,000 kcal heat into the model for thermal heat balance. It was assumed that 274,400 kcal/min heat was lost through conduction via refractory lining and lance system, and radiation via converter mouth [51].
- Hot metal = 145,000 kg, with charging complete in 1 min (Stream 5); scrap = 12,000 kg, charged incessantly in 20 min at a constant rate (Stream 6); lime = 10,000 kg, charged incessantly in 20 min at a constant rate (Stream 8); dolomite = 3000 kg, charged incessantly in 20 min at a constant rate (stream 9); oxygen = 9000 nm3, charged incessantly in 20 min at a constant rate (stream 17). Total blowing time taken for modelling is around 20 min with a time step of 1 min.
- All reactions are at equilibrium at the gas–metal slag–metal interfaces, and reactions both in metal and slag phases are mass-transfer-controlled.
- Flux and scrap dissolution take place linearly with blowing time.
- Alpha (α), beta (β), and delta (δ) values were chosen so that there was reasonable agreement between model results and actual plant observations.
3. Results
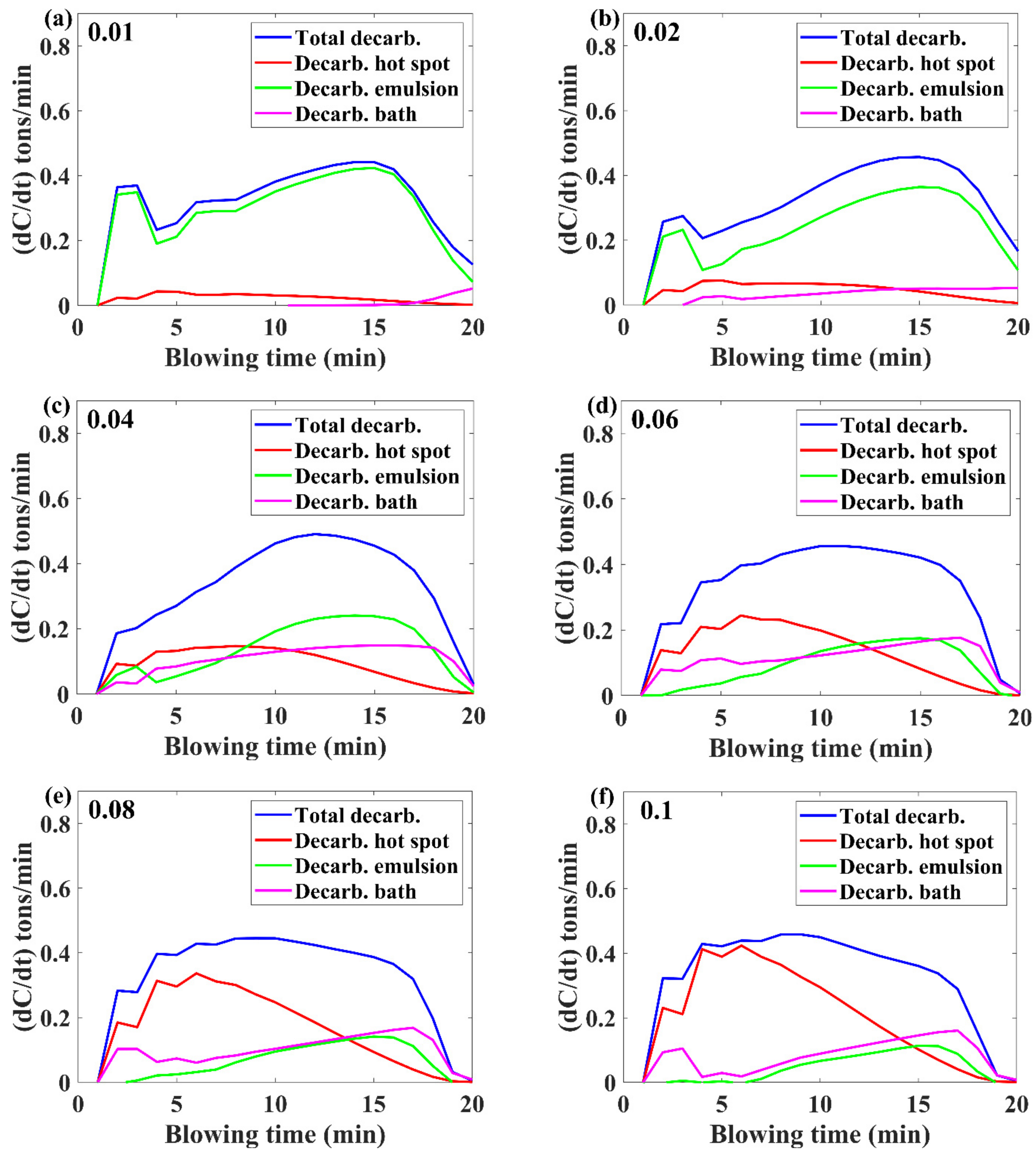
3.1. Decarburization Rate Evolution
| Input Materials | Elements | wt.% |
|---|---|---|
| Hot metal 145 tons | Carbon | 4.44 |
| Manganese | 0.4 | |
| Silicon | 0.50 | |
| Sulfur | 0.0028 | |
| Phosphorous | 0.12 | |
| Scrap 12 tons | Carbon | 0.2 |
| Manganese | 0.36 | |
| Silicon | 0.021 | |
| Sulfur | 0.0022 | |
| Dolomite 3 tons | MgO | 40.5 |
| CaO | 58 | |
| SiO2 | 0.85 | |
| Sulfur | 0.25 | |
| Lime 10 tons | CaO | 93.5 |
| SiO2 | 6.5 | |
| Sulfur | 0 | |
| Initial temperature | 1317 °C | |
| Blowing time (Min) | 20 | |
| O2 flow rate (Nm3/min) | 450 | |
| Iron ore | Mass in tons | 2 |
| Retained slag weight | 3 tons | 19% SiO2, 39% CaO, 12% MgO, 8% FeO, MnO 1% |
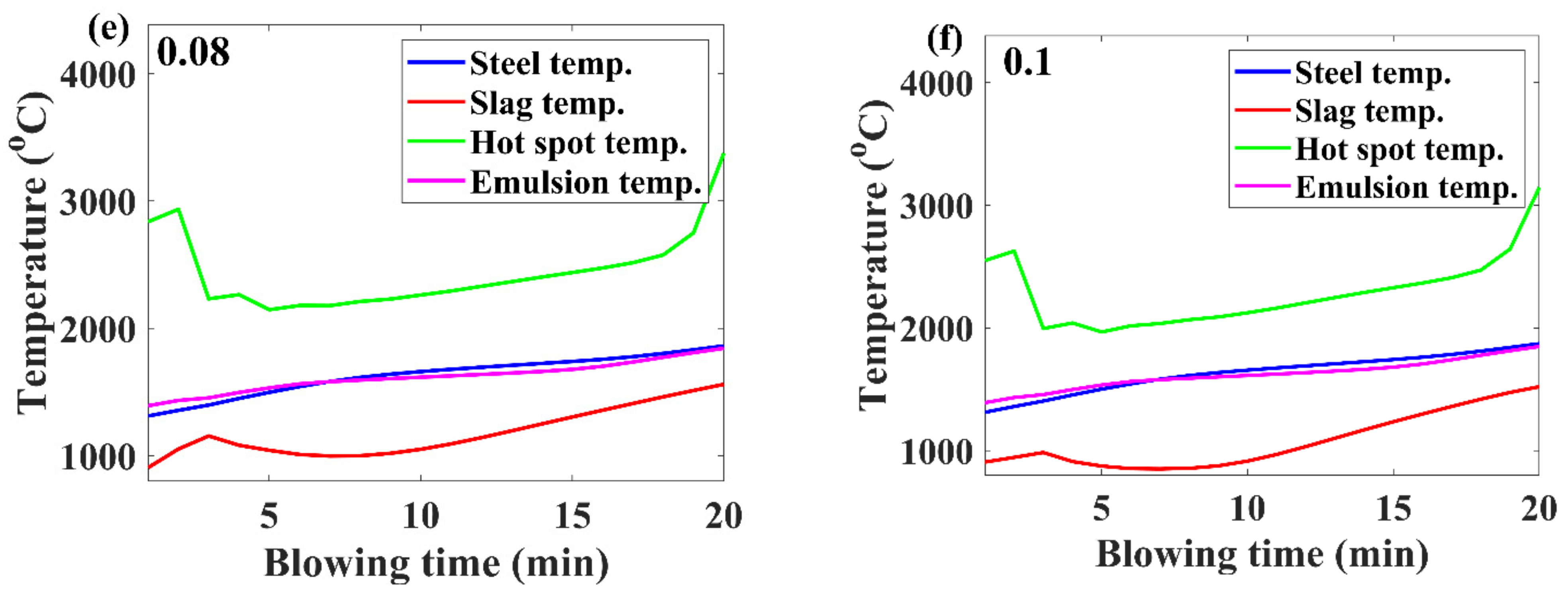
3.2. Temperature Evolution
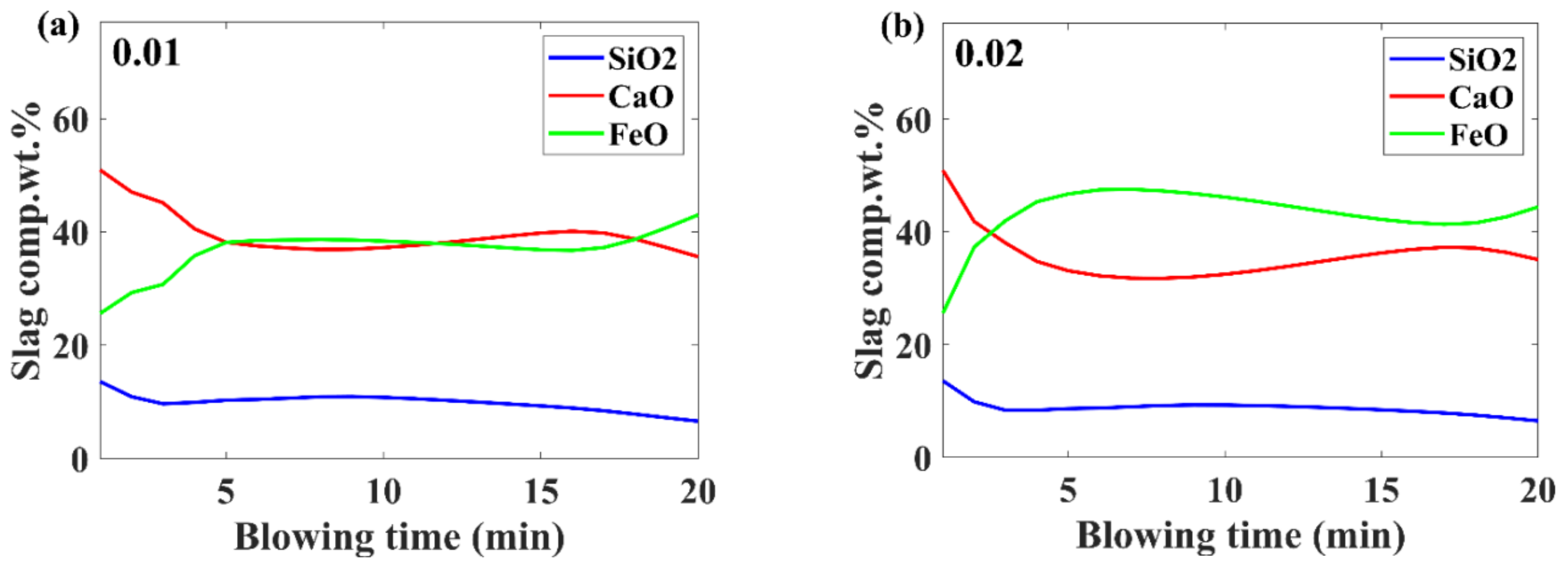
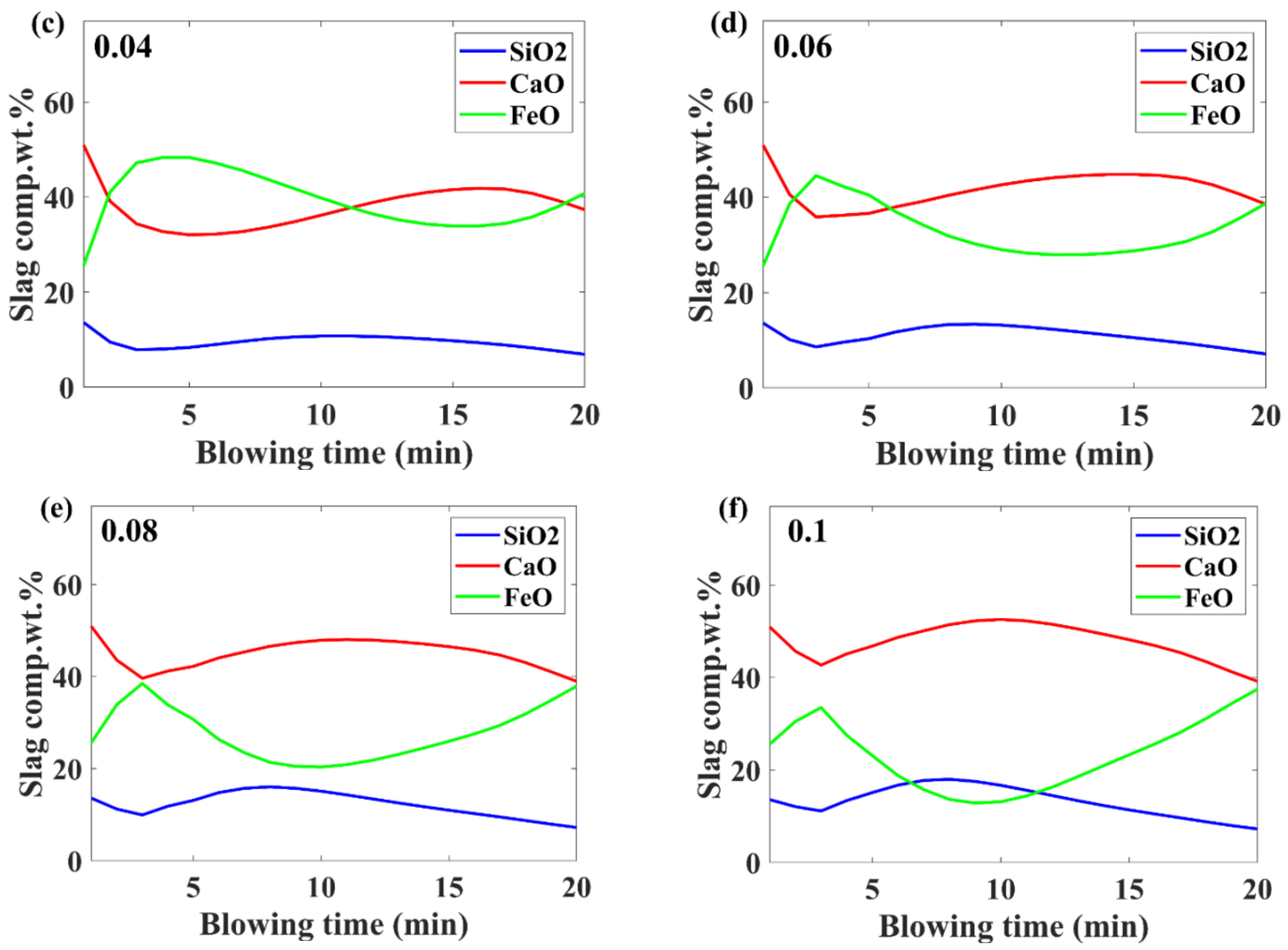
3.3. Slag Composition Evolution
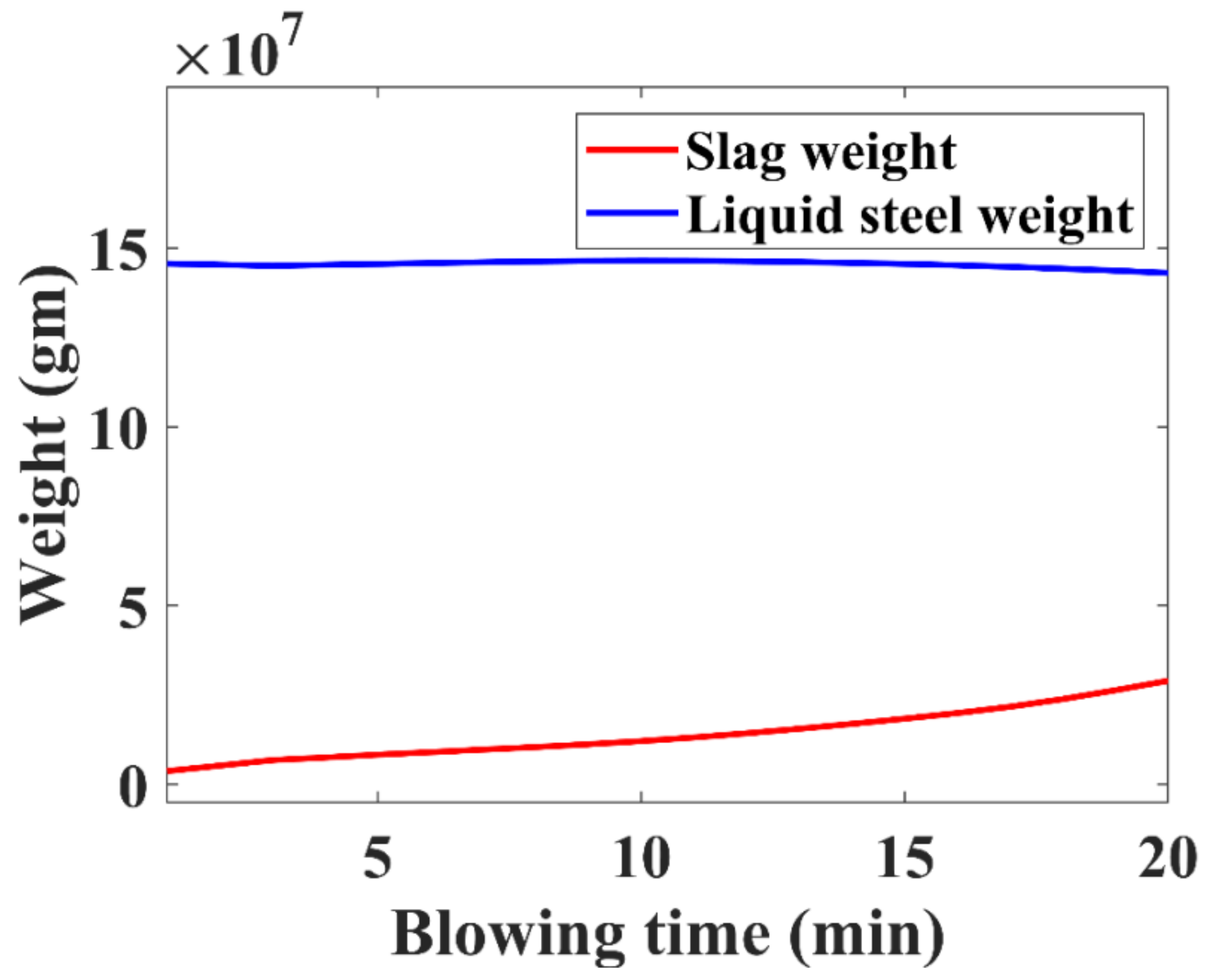
3.4. Liquid Steel and Slag Weight
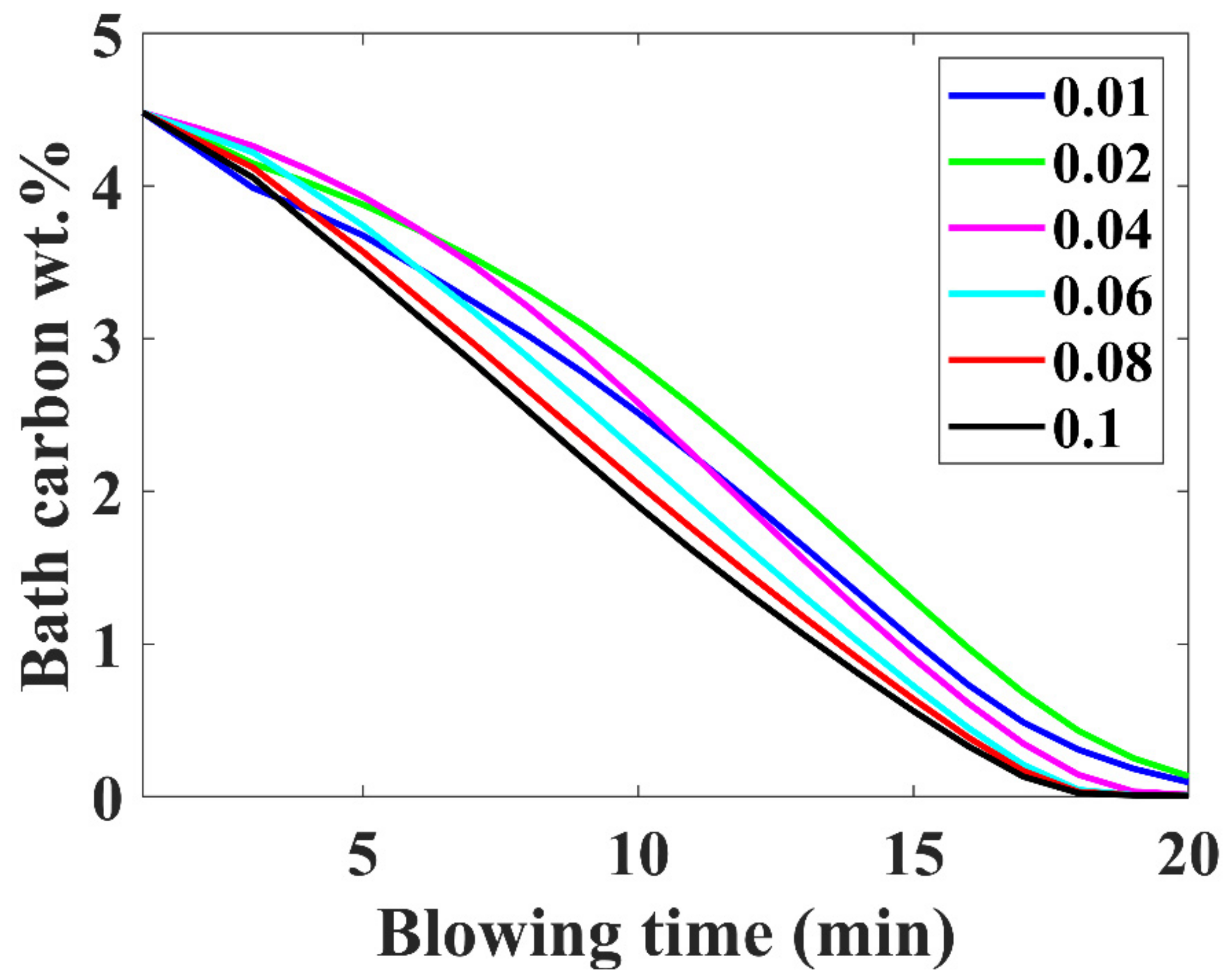
3.5. Carbon Removal Trajectory
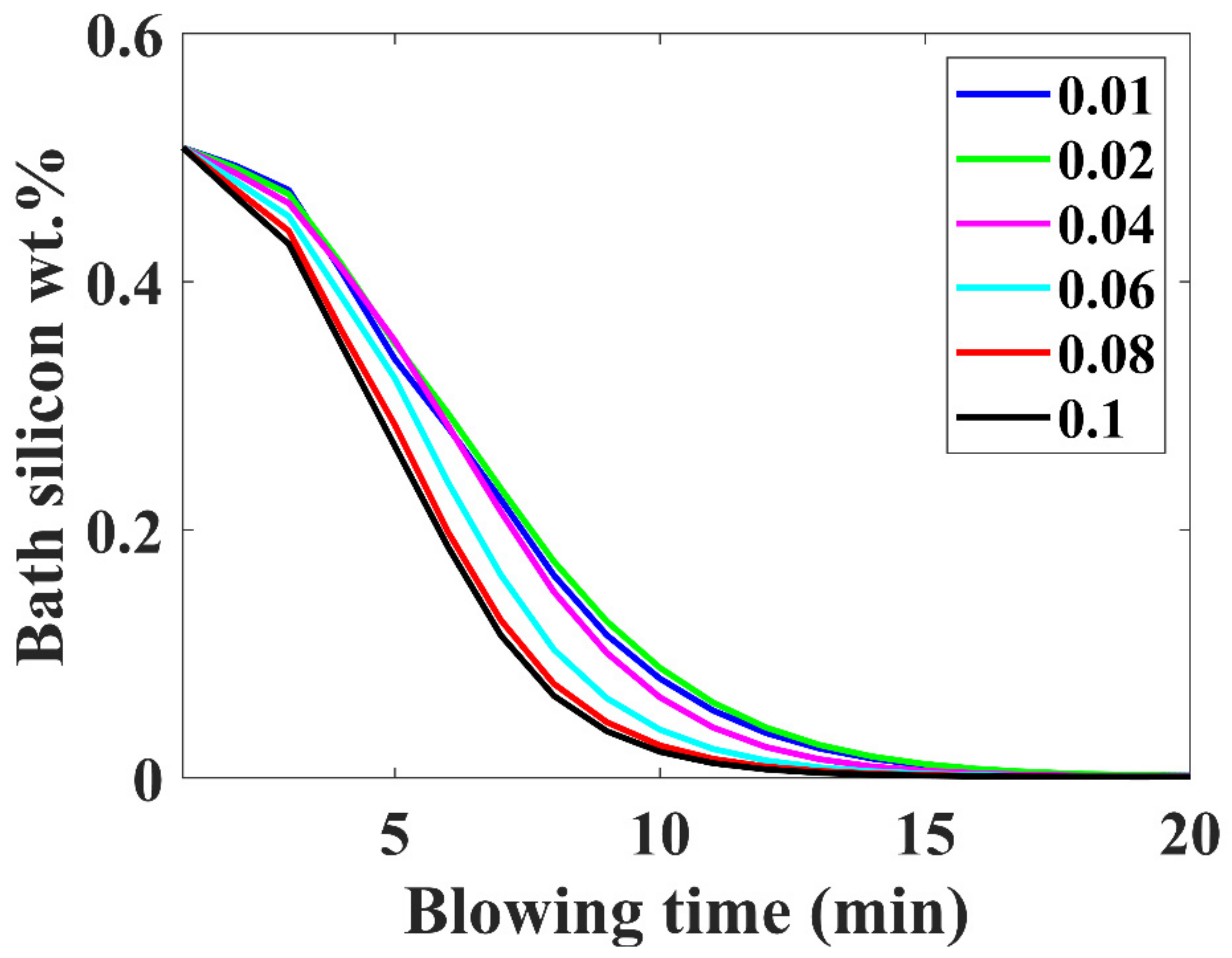
3.6. Silicon Removal Trajectory
3.7. Verification with Plant Data
4. Discussion
5. Conclusions
- Higher inialpha values mean higher mass transfer coefficient in the metal bath resulting in a higher contribution by hot-spot decarburization, lower levels of FeO formation and emulsion contribution in decarburization, and vice versa.
- The observed practical phenomena in converters match with the calculated results for inialpha values greater than 0.06.
- If the benchmark of the hot-spot temperature was in the range from 2000 to 3000 ℃, then as per the model results, the major contribution of decarburization came from hot-spot reactions in most of the blow; however, by the end of the process, it was governed by emulsion.
- The model can be used as a guiding tool to control the BOF steel-making process and for obtaining better insights into the process. The model is still under development to incorporate other submodels such as scarp, iron ore, flux dissolution, and heat loss. The purpose of the current study was to understand decarburization phenomena in BOF and their various contributions, which it could successfully achieve.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Deo, B. Fundamentals of Steelmaking Metallurgy Chapter 3. Available online: https://www.researchgate.net/publication/28265101 (accessed on 6 June 2021).
- Barron, M.A.; Medina, D.Y.; Hilerio, I. Numerical simulation of decarburization in a top-blown basic oxygen furnace. Model. Simul. Mat. Sci. Eng. 2014, 4, 94–100. [Google Scholar] [CrossRef]
- Jalkanen, H. Experiences in physicochemical modelling of oxygen converter process (BOF). Adv. Process. Met. Mater. 2006, 2, 541–554. [Google Scholar]
- Sarkar, R.; Gupta, P.; Basu, S.; Ballal, N.B. Dynamic modelling of LD. Converter steelmaking: Reaction modelling using Gibbs free energy minimization. Metall. Mater. Trans. B 2015, 46, 961–976. [Google Scholar] [CrossRef]
- Kruskopf, A.; Visuri, V.V. A Gibbs Energy Minimization Approach for Modelling of Chemical Reactions in a Basic Oxygen Furnace. Metall. Mater. Trans. B 2017, 48, 3281–3300. [Google Scholar] [CrossRef]
- Knoop, W.; Deo, B.; Snoeijer, A.; Unen, G.; Boom, R. A Dynamic slag-droplet model for the steelmaking process. In Proceedings of the 4th International Conference on Molten Slags and Fluxes, Tokyo, Japan, 8–11 June 1992; pp. 302–307. [Google Scholar]
- Lytvynyuk, Y.; Schenk, J.; Hiebler, M.; Sormann, A. Thermodynamic and Kinetic Model of the Converter Steelmaking Process. Part 1: The Description of the BOF Model. Steel Res. Int. 2014, 85, 537–543. [Google Scholar] [CrossRef]
- Kattenbelt, C.; Roffel, B. Dynamic modelling of the main blow in basic oxygen steelmaking using measured step responses. Metall. Mater. Trans. B 2008, 39, 764–769. [Google Scholar] [CrossRef]
- Urquhart, R.C.; Davenport, W.G. Foams and emulsions in oxygen steelmaking. Can. Metall. Q. 1973, 12, 507–516. [Google Scholar] [CrossRef]
- Kootz, T.; Behrens, K.; Maas, H.; Baumgarten, P. Dephosphorization of steel. Stahl Eisen 1965, 85, 857–865. [Google Scholar]
- Koch, K.; Fix, W.; Valentin, P. Einfluss von Sauerstoffangebot und Kohlenstoffausgangsgehalt Sowie von Badgeometrie und Feuerfestmaterial auf den Ablauf der Entwicklung von Fe-C-Schmelzen in einem 50-kg-Aufblastkonverter. Arch. Eisenhüttenwesen 1978, 49, 109–114. [Google Scholar] [CrossRef]
- Rout, B.K.; Brooks, G.A.; Rhamdhani, M.A.; Li, Z.; Frank, N.H.; Schrama, F.N.H.; Sun, J. Dynamic model of basic oxygen steelmaking process based on multizone reaction kinetics: Model derivation and validation. Metall. Mater. Trans. B 2018, 49, 537–557. [Google Scholar] [CrossRef]
- Rout, B.K.; Brooks, G.A.; Rhamdhani, M.A. Transient behavior of dephosphorization kinetics in oxygen steelmaking. In Proceedings of the AISTech 2015 Iron and Steel Technology Conference, Cleveland, OH, USA, 4–7 May 2015; Volume 3, pp. 3225–3237. [Google Scholar]
- Rout, B.K.; Brooks, G.A.; Rhamdhani, M.A.; Li, Z.; Schrama, F.N.H.; Overbosch, A. Dynamic model of basic oxygen steelmaking process based on multizone reaction kinetics: Modelling of decarburization. Metall. Mater. Trans. B 2018, 49, 1022–1033. [Google Scholar] [CrossRef]
- Fruehan, R.J.; Goldstein, D.; Sarma, B.; Story, S.R.; Glaws, P.C.; Pasewicz, H.U. Recent advances in the fundamentals of the kinetics of steelmaking reactions. Metall. Mater. Trans. B 2000, 31, 891–898. [Google Scholar] [CrossRef]
- Fruehan, R.J.; Martonik, L.J. The rate of decarburization of liquid iron by CO2 and H2. Metall. Mater. Trans. B 1974, 5, 1027–1032. [Google Scholar] [CrossRef]
- Simento, N.J.; Hayes, P.C.; Peter, H.C. Decarburisation of liquid Fe-C-S drops using multiple oxidants of O2, CO2, and H2O. ISIJ Int. 1999, 39, 1217–1223. [Google Scholar] [CrossRef]
- Widlund, D.; Sarma, D.S.; Jonsson, P.G. Studies on decarburization and desiliconization of levitated Fe-C-Si alloy droplets. ISIJ Int. 2006, 46, 1149–1157. [Google Scholar] [CrossRef][Green Version]
- Sun, H.; Gao, K.; Sahajwalla, V.; Mori, K.; Pehlke, R.D. Kinetics of gas oxidation of liquid Fe-C-S alloys and carbon boil phenomenon. ISIJ Int. 1999, 39, 1125–1133. [Google Scholar] [CrossRef]
- Sun, H.; Pehlke, R.D. Kinetics of oxidation of multicomponent liquid iron alloys by oxidizing gases using levitation melting. Trans. Am. Foundry Soc. 1992, 100, 371–376. [Google Scholar]
- Zughbi, H.D. Decarburization of Fe/C melts in a crucible: Effects of gas flow rate and Composition. Scand. J. Metall. 2003, 32, 194–202. [Google Scholar] [CrossRef]
- Zughbi, H.D. Decarburization of Fe/C melts in a crucible: Effects of bath sulfur level and bath surface area. Scand. J. Metall. 2004, 33, 242–250. [Google Scholar] [CrossRef]
- Wu, P.; Yang, Y.; Barati, M.; McLean, A. Decarburization of levitated Fe-Cr-C droplets by carbon dioxide. Metall. Mater. Trans. B 2014, 45, 2211–2221. [Google Scholar] [CrossRef]
- Nagasaka, T.; Fruehan, R.J. Kinetics of the reaction of H2O gas with liquid iron. Metall. Mater. Trans. B 1994, 25, 245–253. [Google Scholar] [CrossRef]
- Baker, L.A.; Warner, N.A.; Jenkins, A.E. Kinetics of decarburization of liquid iron in an oxidizing atmosphere using the levitation technique. Trans. Metall. Soc. AIME 1964, 230, 1228–1235. [Google Scholar]
- El-Kaddah, N.H.; Robertson, D.G.C. The kinetics of gas-liquid metal reactions involving levitated drops. Carburization and decarburization of molten iron in CO-CO2 gas mixtures at high pressures. Metall. Mater. Trans. B 1978, 9, 191–199. [Google Scholar] [CrossRef]
- Sun, H.; Pehlke, R.D. Kinetics of oxidation of carbon in liquid iron-carbon-silicon manganese-sulfur alloys by carbon dioxide in nitrogen. Metall. Mater. Trans. B 1995, 26, 335–344. [Google Scholar] [CrossRef]
- Distin, P.A.; Hallett, G.D.; Richardson, F.D. Some reactions between drops of iron and flowing gases. J. Iron Steel Inst. 1968, 206, 821–833. [Google Scholar]
- Modigel, M.; Traebert, A.; Monheim, P.; Petersen, S.; Pickartz, U. A new tool for process modelling of metallurgical processes. Comput. Chem. Eng. 2001, 25, 723–727. [Google Scholar] [CrossRef]
- Graveland-Gisolf, E.; Mink, P.; Overbosch, A.; Boom, R.; Gendt, G.D.; Deo, B. Slag droplet model, a dynamic tool to simulate and optimize the refining conditions in BOF. Steel Res. Int. 2003, 74, 125–130. [Google Scholar] [CrossRef]
- Chigwedu, C.; Kempken, J.; Pluschkell, W. A new approach for the dynamic simulation of the BOF process. Stahl Essen 2006, 126, 25–30. [Google Scholar]
- Traebert, A.; Modigell, M.; Monheim, P.; Hack, K. Development of a modelling technique for non-equilibrium metallurgical process. Scand. J. Metall. 1999, 28, 285–290. [Google Scholar]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking part 1: Model development and validation. ISIJ Int. 2011, 51, 1086–1092. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking part 2: Application of bloated droplet theory for decarburization in emulsion zone. ISIJ Int. 2011, 51, 1093–1101. [Google Scholar] [CrossRef]
- Dogan, N.; Brooks, G.A.; Rhamdhani, M.A. Comprehensive model of oxygen steelmaking Part 3: Decarburization in Impact Zone. ISIJ Int. 2011, 51, 1102–1109. [Google Scholar] [CrossRef]
- Biswas, J.; Gu, K.; Coley, K.S. A decarburization Model for a Fe-C Droplet Reacting in Oxidizing Slag. Metall. Mater. Trans. B 2021, 52, 3888–3906. [Google Scholar] [CrossRef]
- Kozakevitch, P. Study of Basic Phosphate Slag Foams. In International Congress of Oxygen Steelmaking; Le Touquet: Paris, France, 1963. [Google Scholar]
- Meyer, H.W.; Porter, W.F.; Smith, G.C.; Szekely, J. Slag-metal emulsions and their importance in BOF steelmaking. JOM 1968, 20, 35–42. [Google Scholar] [CrossRef]
- Okano, S.; Matsuno, J.; Ooi, H.; Tsuruoka, K.; Koshikawa, T.; Okazaki, A. Model analysis in three different reaction zones in basic oxygen converter. In International Conference on Science and Technology of Iron and Steel; Iron and Steel Institute: Tokyo, Japan, 1971; pp. 227–231. [Google Scholar]
- Price, D.J. LD Steelmaking: Significance of the emulsion in carbon removal. In Process Engineering of Pyrometallurgy Symposium; IMM: London, UK, 1974. [Google Scholar]
- Chatterjee, A.; Lindfors, N.O.; Weste, J.A. Process metallurgy of LD. Steelmaking. Ironmak. Steelmak. 1976, 3, 21–32. [Google Scholar]
- Koch, K.; Fix, W.; Valentin, P. Investigation of the decarburization of Fe-C melts in a 50 kg top-blown converter. Steel Res. Int. 1976, 47, 659–663. [Google Scholar]
- Nakamura, M.; Tate, M. Study of decarburization process of inductively stirred Fe-C Melts. ISIJ Int. 1977, 63, 236–245. [Google Scholar]
- Kawakami, K. Kinetics of blowing reaction in a basic oxygen furnace. J. Met. 1966, 18, 836–844. [Google Scholar] [CrossRef]
- FactSage. Center for Research in Chemical Thermodynamics, Polytechnique de Montreal, Canada. Available online: www.factsage.com (accessed on 1 February 2021).
- Shukla, A.K.; Deo, B.; Millman, S.; Snoeijer, B.; Overbosch, A.; Kapilashrami, A. An insight into the mechanism and kinetics of reactions in BOF Steelmaking process: Theory vs. practice. Steel Res. Int. 2010, 81, 940–948. [Google Scholar] [CrossRef]
- Shukla, A.K.; Deo, B.; Robertson, D.G.C. Scrap dissolution in molten iron containing Carbon for the case of coupled heat and mass transfer control. Metall. Mater. Trans. B 2013, 44, 1407–1427. [Google Scholar] [CrossRef]
- Ghosh, A.; Chatterjee, A. Iron Making and Steel Making; PHI Learning Private Limited: New Delhi, India, 2012; pp. 50–55. [Google Scholar]
- Pal, J.; Ghorai, S.; Bandyopadhyay, D.; Ghosh, S. Performance assessment of partially pre-fused synthetic flux in basic oxygen steelmaking. J. Iron Steel Res. Int. 2015, 22, 916–923. [Google Scholar] [CrossRef]
- Robertson, D.G.C.; Deo, B.; Ohguchi, S. Multicomponent Mixed-Transport-Control Theory for Kinetics of Coupled Slag/Metal and Slag/Metal/Gas Reactions: Application to Desulphurization of Molten Iron. Ironmak. Steelmak. 1984, 11, 41–56. [Google Scholar]
- Shukla, A.K. Thermodynamics based modelling of iron and steelmaking processes using flowsheet-based approach employing METSIM. Trans. Indian Inst. Met. 2019, 72, 767–775. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singha, P.; Shukla, A.K. Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program. Metals 2022, 12, 638. https://doi.org/10.3390/met12040638
Singha P, Shukla AK. Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program. Metals. 2022; 12(4):638. https://doi.org/10.3390/met12040638
Chicago/Turabian StyleSingha, Prasenjit, and Ajay Kumar Shukla. 2022. "Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program" Metals 12, no. 4: 638. https://doi.org/10.3390/met12040638
APA StyleSingha, P., & Shukla, A. K. (2022). Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program. Metals, 12(4), 638. https://doi.org/10.3390/met12040638