Study on Reductive Smelting of High-Iron Red Mud for Iron Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
3. Results and Discussion
3.1. Thermodynamic Analysis
3.2. Effect of Experimental Parameters on the Reduction of Calcified Slag of High-Iron Red Mud
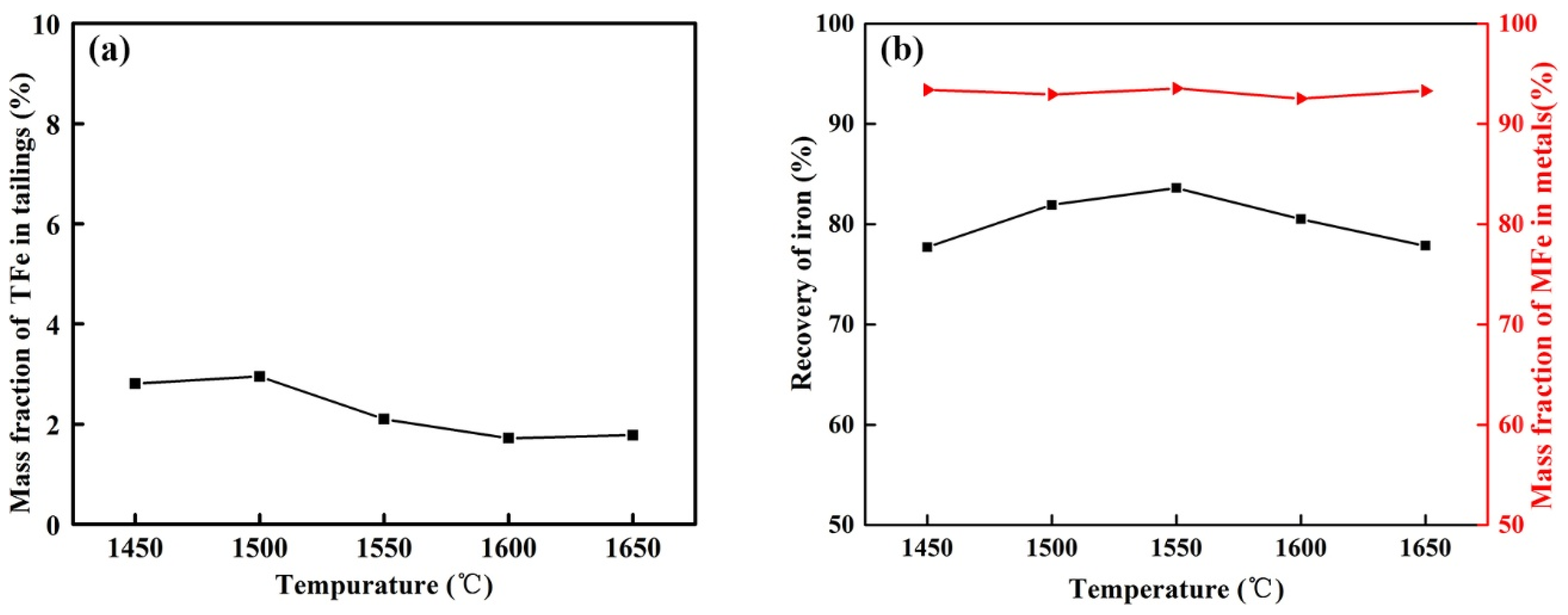
3.2.1. Effect of Temperature on the Reduction of Calcified Slag of High-Iron Red Mud
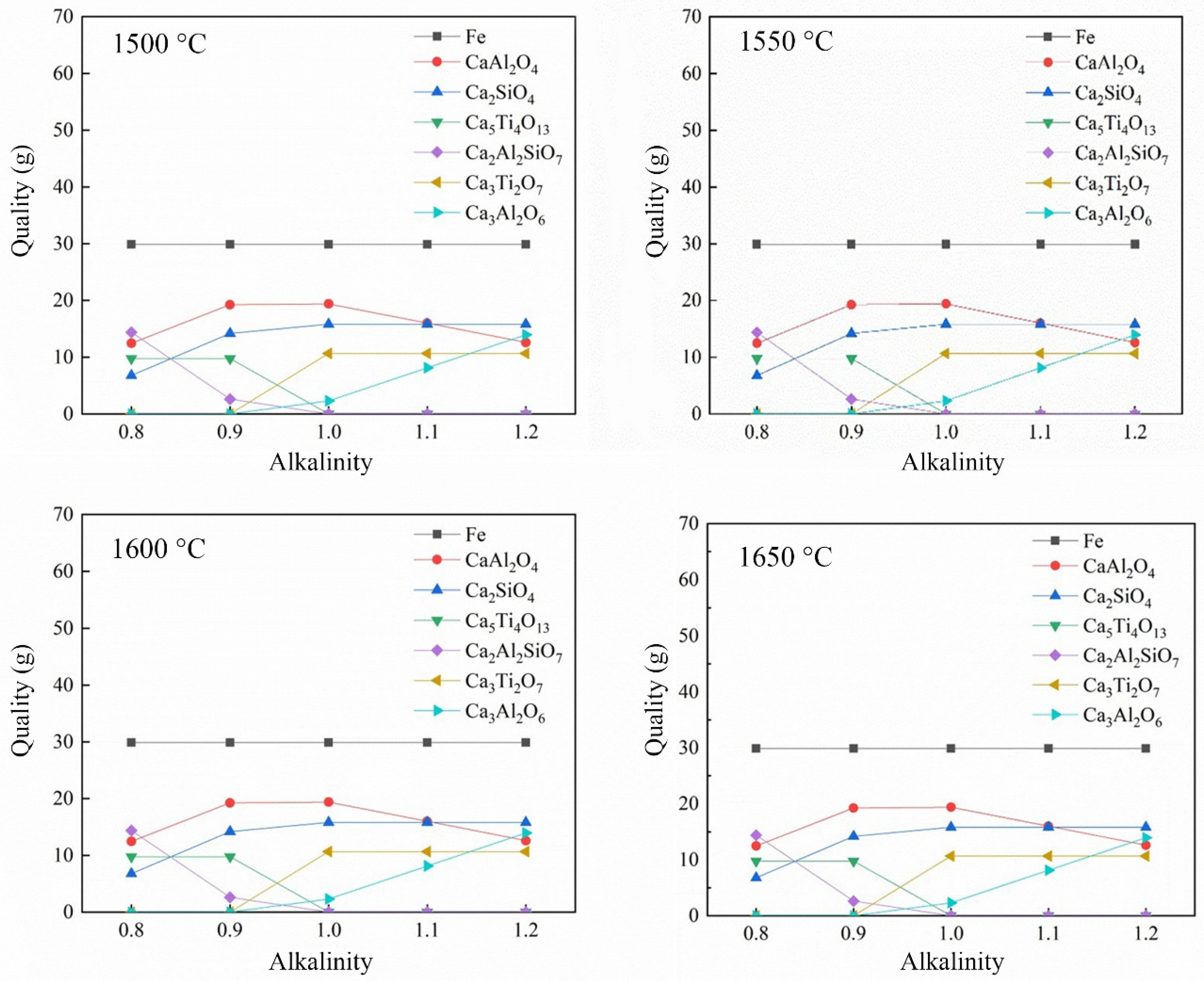
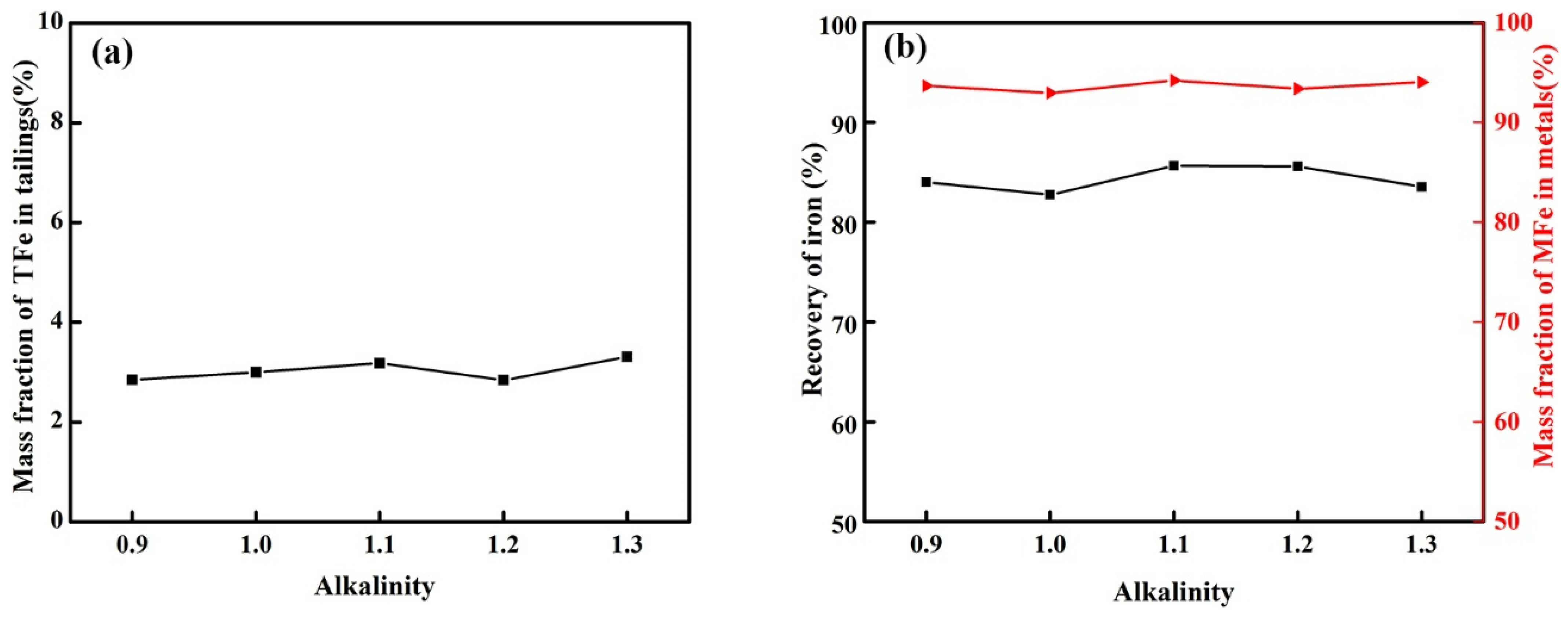
3.2.2. Effect of Alkalinity on the Reduction of Calcified Slag of High-Iron Red Mud
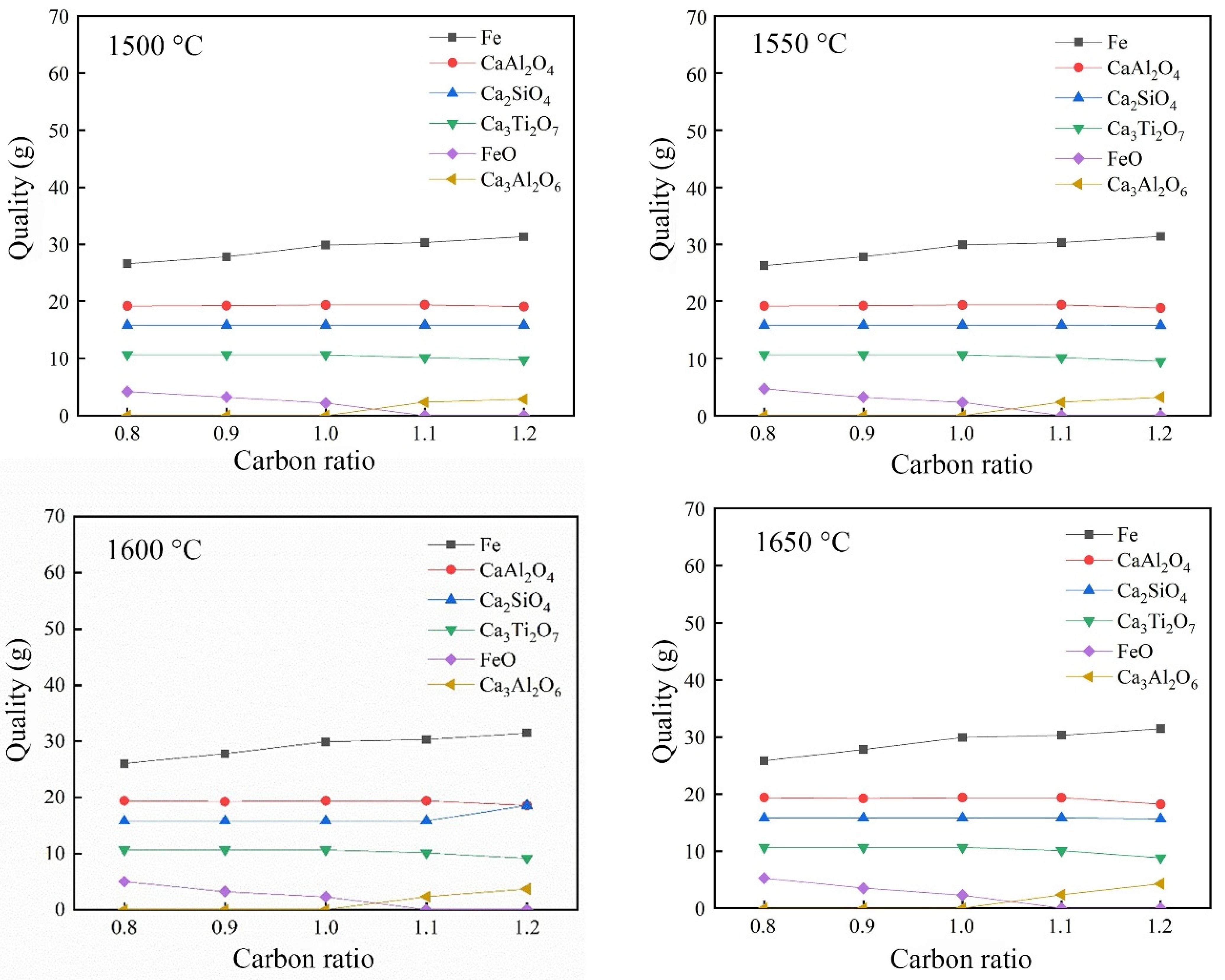
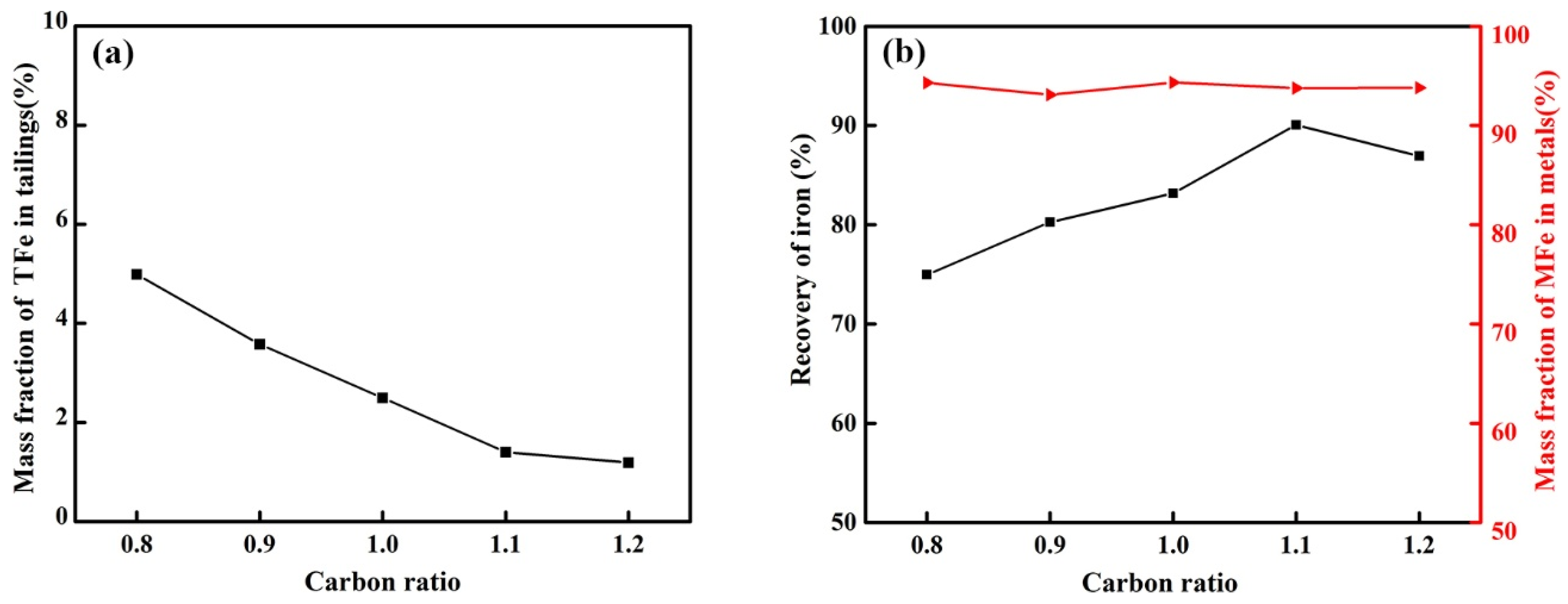
3.2.3. Effect of Carbon Ratio on the Reduction of Calcified Slag of High-Iron Red Mud
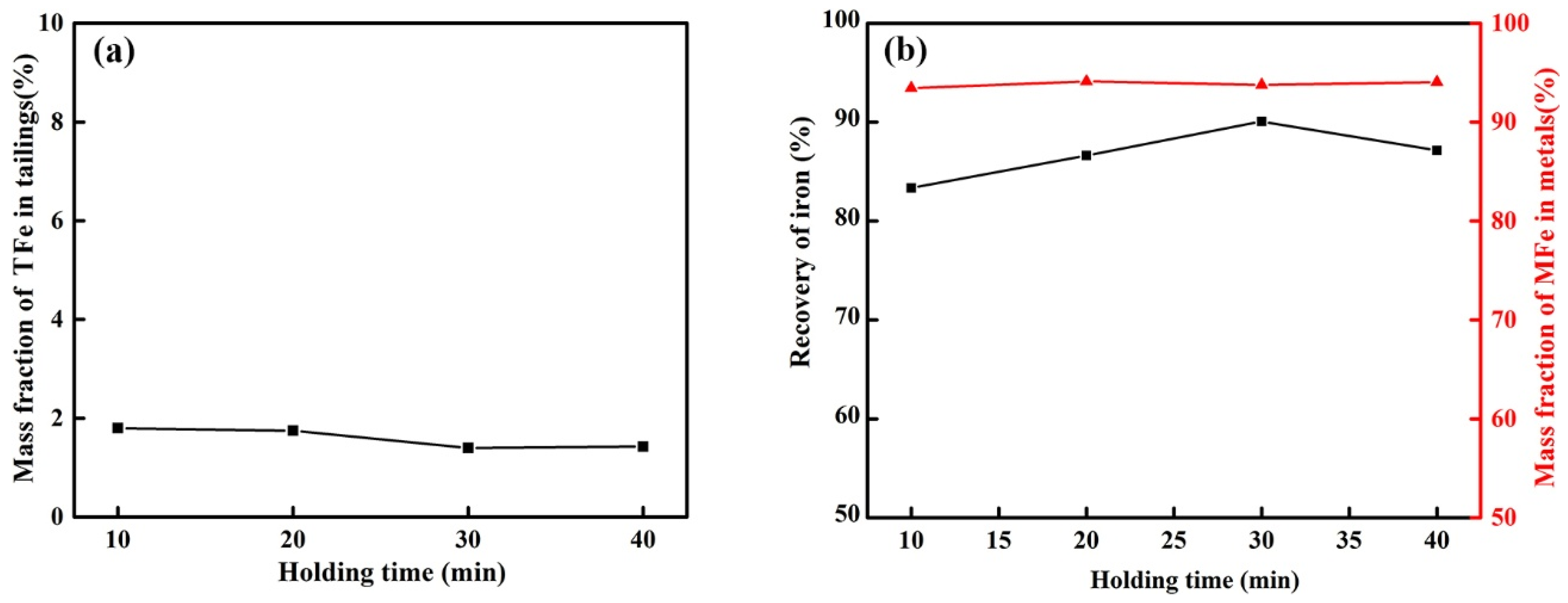
3.2.4. Effect of Holding Time on Reduction of Calcified Slag of High-Iron Red Mud
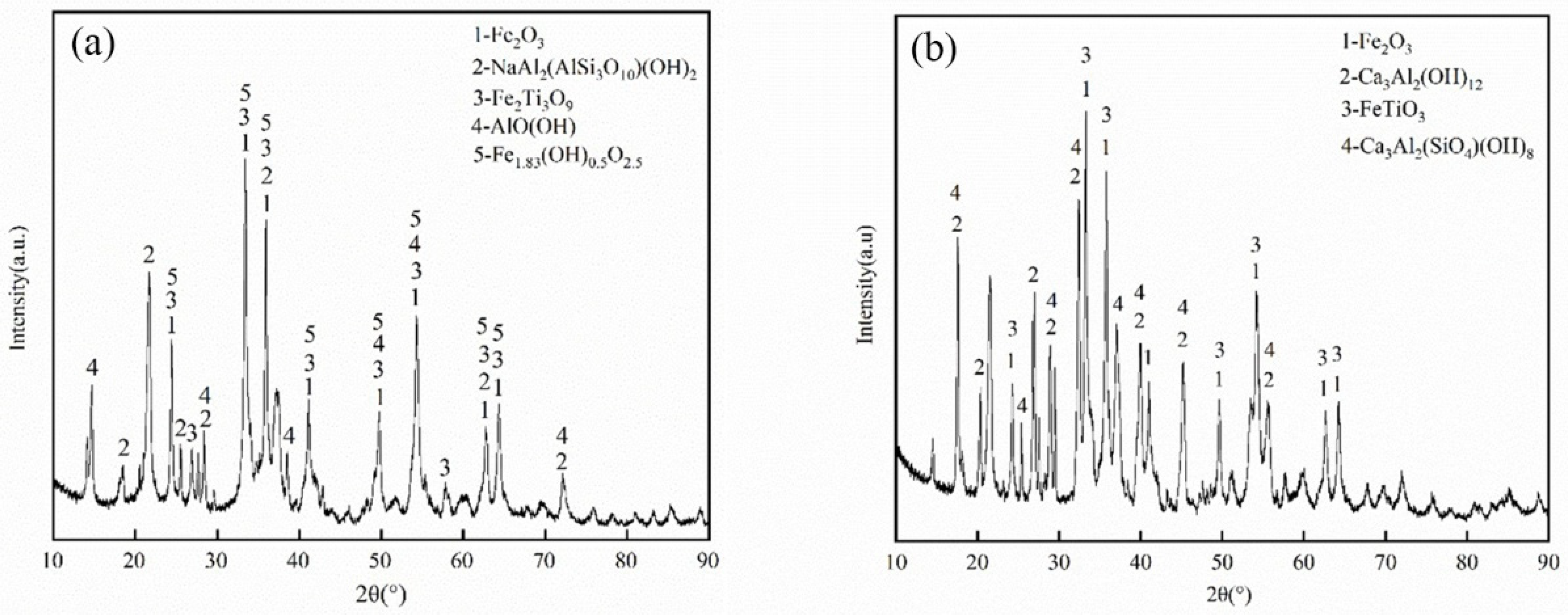
3.3. Analysis and Characterization of Reduction Products
3.3.1. Chemical Composition Analysis and Characterization of Metals
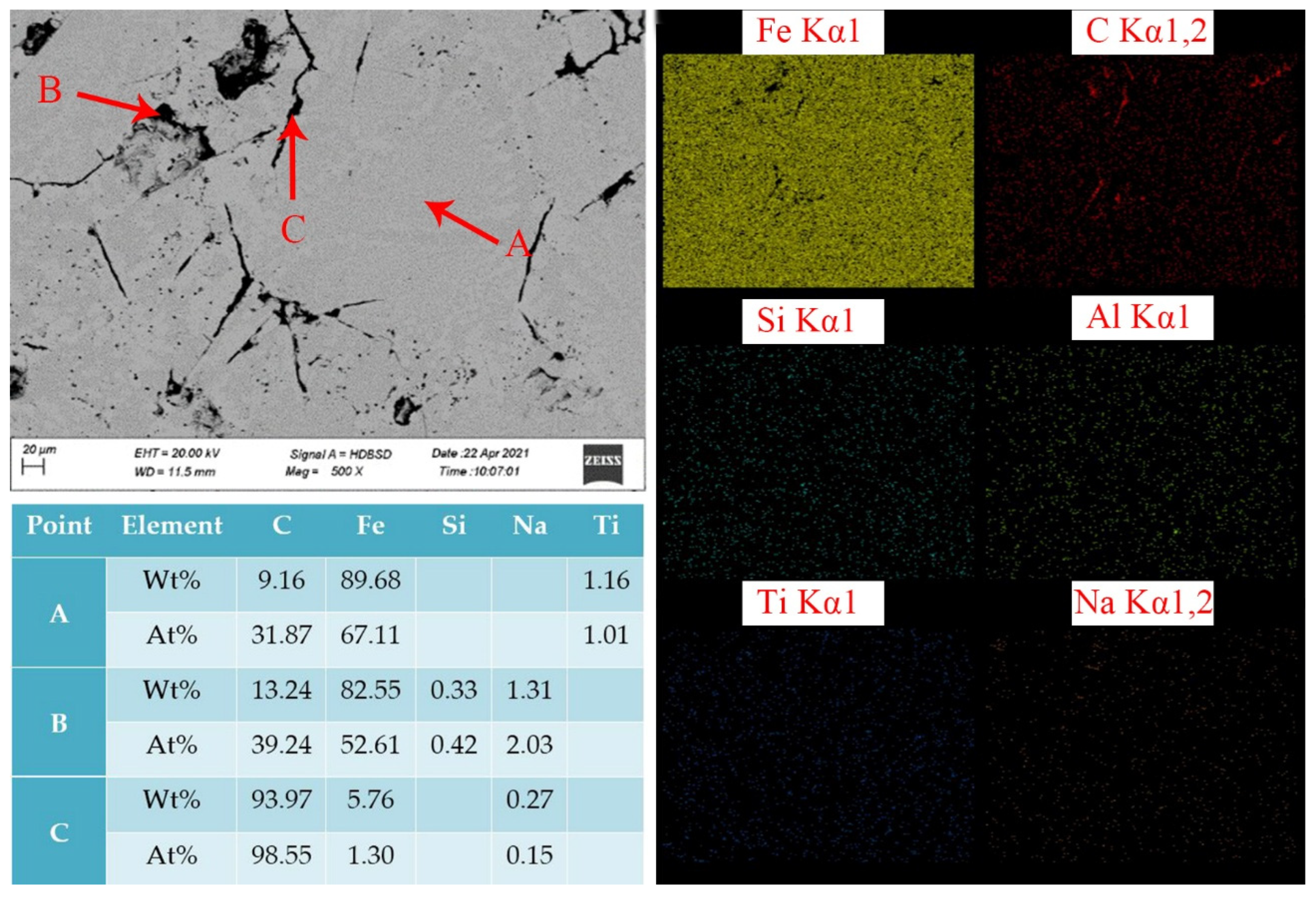
3.3.2. Chemical Composition Analysis and Characterization of Calcified Iron Extraction Tailings
4. Conclusions
- (1)
- Through the calculation of the Gibbs free energy and equilibrium phase, it was inferred that the direct reduction of carbon was dominant in the reduction of high-iron red mud calcified slag, and Fe, Si, Ti, Na and other elements were reduced in this process. The results of the equilibrium phase calculation showed that with increasing alkalinity, 2CaO·Al2O3·SiO2 gradually decreased, 3CaO·Al2O3 gradually increased, and the increase in the carbon ratio was beneficial to the reduction reaction.
- (2)
- The optimum reduction conditions for iron extraction by calcified slag reduction were obtained by single-factor investigations. The optimum reduction conditions were 1550 °C, alkalinity 1.1, carbon ratio 1.1, holding time 30 min, and CaF2 content 3% of the calcified slag mass fraction. The recovery of iron was 90.06%, and the mass fraction of MFe in the metal was 93.76%.
- (3)
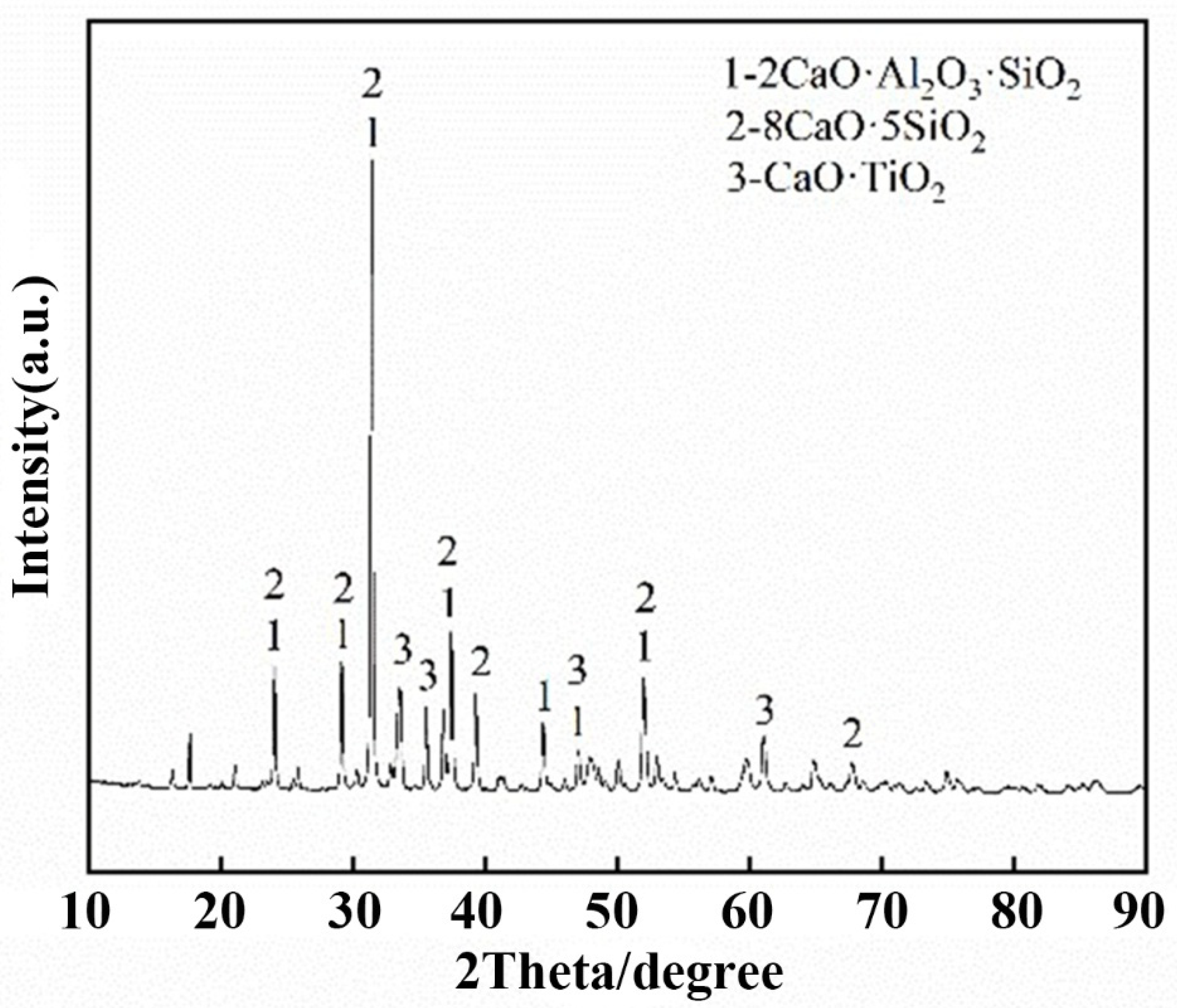
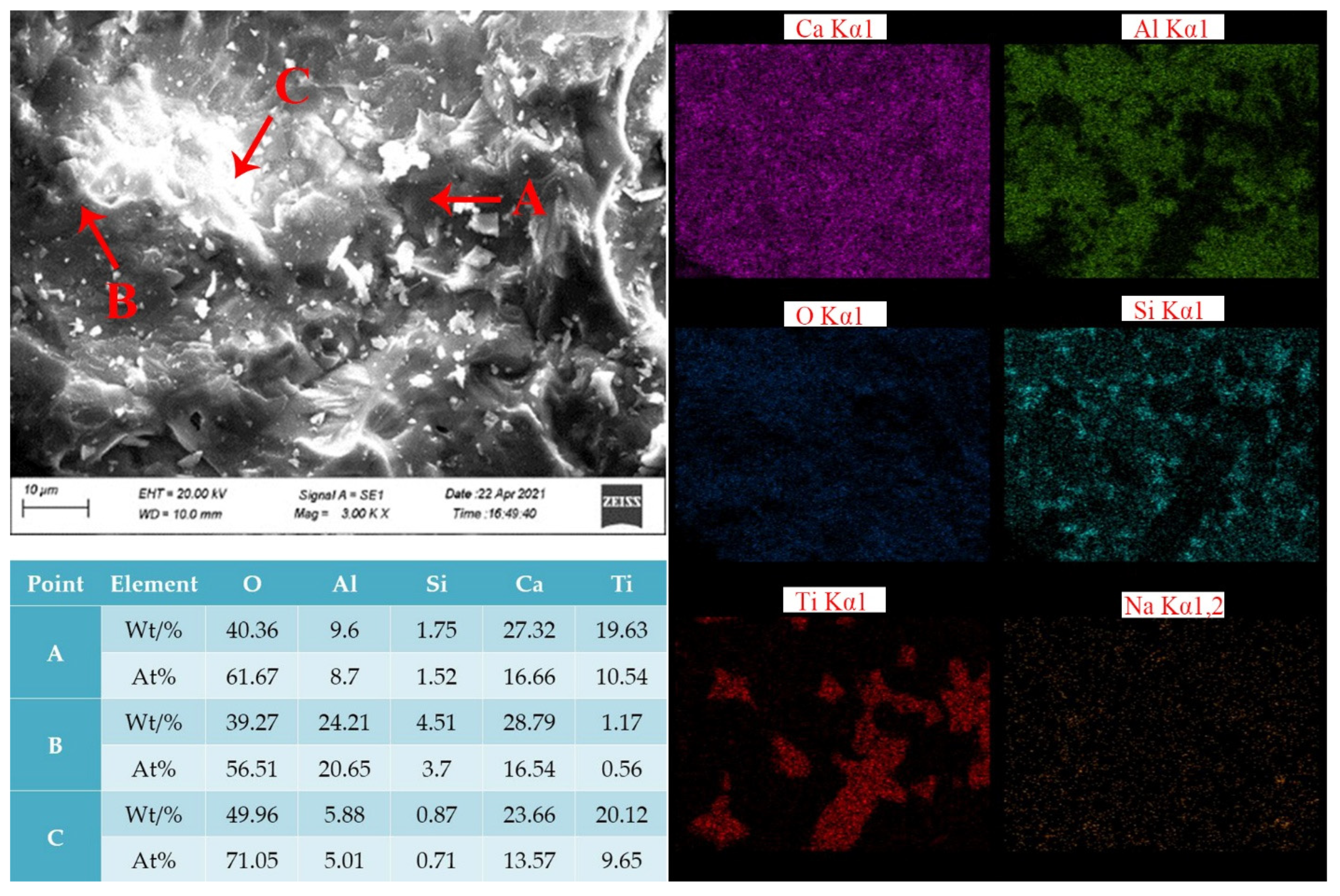
- The levels of CaO, Al2O3 and Na2O were 37.07, 37.67 and 0.48%, respectively, according to the analysis of the chemical composition and mineralogy of calcified iron tailings extracted from red mud with high iron content, which met the expected composition standard of calcified extracted iron tailings for preparing aluminate cement directly from tailings. Through XRD and SEM analyses, the main crystalline phases in calcified extracted iron tailings were identified as 2CaO·Al2O3·SiO2 and CaO·TiO2, which were distributed in aggregates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2020, 289, 125–136. [Google Scholar] [CrossRef]
- Bogatyrev, B.A.; Zhukov, V.V.; Tsekhovsky, Y.G. Formation conditions and regularities of the distribution of large and superlarge bauxite deposits. Lithol. Miner. Resour. 2009, 44, 135–151. [Google Scholar] [CrossRef]
- Li, X.; Xiao, W.; Liu, W.; Liu, G.; Peng, Z.; Zhou, Q.; Qi, T. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferr. Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Nan, X.L.; Zhang, T.A.; Liu, Y.; Dou, Z.H. Analysis of comprehensive utilization of red mud in China. Chin. J. Process Eng. 2010, 10, 264–270. [Google Scholar]
- Jones BE, H.; Haynes, R.J.; Phillips, I.R. Influence of amendments on acidification and leaching of Na from bauxite processing sand. Ecol. Eng. 2015, 84, 435–442. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilization of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Kaußen, F.; Friedrich, B. Reductive Smelting of Red Mud for Iron Recovery. Chem. Ing. Tech. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-scale consumption and zero-waste recycling method of red mud in steel making proess. Minerals 2018, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Sadler Leon, Y.; Venkataraman Chandra, A. Process for enhanced removal of iron from bauxite ores. Int. J. Miner. Process. 1991, 31, 233–246. [Google Scholar] [CrossRef]
- Rai, S.; Nimje, M.T.; Chaddha, M.J.; Modak, S.; Rao, K.R.; Agnihotri, A. Recovery of iron from bauxite residue using advanced separation techniques. Miner. Eng. 2019, 134, 222–231. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Bhoi, B.; Mishra, C.R.; Dash, R.R.; Satapathy, B.K.; Jayasankar, K. Production of pig iron from NALCO redmud by application of plasma smelting technology. In Light Metals; Springer: Cham, Switzerland, 2012; pp. 99–103. [Google Scholar]
- Gu, H.; Hargreaves JS, J.; Jiang, J.Q.; Rico, J.L. Potential routes to obtain value-added iron-containing compounds from red mud. J. Sustain. Metall. 2017, 3, 561–569. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lv, W.; Qi, Y.H.; Zou, Z.S. Recovery of iron and calcium aluminate slag from high-ferrous bauxite by high-temperature reduction and smelting process. Int. J. Miner. Metall. Mater. 2016, 23, 881–890. [Google Scholar] [CrossRef]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Pickles, C.A.; Lu, T.; Chambers, B.; Forster, J. A study of reduction and magnetic separation of iron from high iron bauxite ore. Can. Metall. Q. 2012, 51, 424–433. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; He, Z. Recovery of Iron From High-Iron Red Mud by Reduction Roasting With Adding Sodium Salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Liu, N.; Qi, T.; Wang, Y.; Zhou, Q.; Peng, Z.; Liu, G. Hydrothermal reduction of iron oxide to magnetite in Bayer digestion process. Trans. Nonferr. Met. Soc. China 2015, 25, 3467–3474. [Google Scholar] [CrossRef]
- Rao, R.B.; Besra, L.; Reddy, B.R.; Banerjee, G.N. The Effect of Pretreatment on Magnetic Separation of Ferruginous Minerals in Bauxite. Magn. Electr. Sep. 1996, 8, 115–123. [Google Scholar] [CrossRef]
- Wei, J.; Geng, Y.; Shen, L.; Cen, Q. Analysis of cement production and carbon emission in China. Environ. Sci. Technol. 2015, 38, 80–86. [Google Scholar]
- Rao, D.B.; Das, B. Characterization and Beneficiation Studies of a Low Grade Bauxite Ore. J. Inst. Eng. 2014, 95, 81–93. [Google Scholar] [CrossRef]
- Xue, S.; Wu, Y.; Li, Y.; Kong, X.; Zhu, F.; William, H.; Li, X.; Ye, Y. Industrial wastes applications for alkalinity regulation in bauxite residue A comprehensive review. J. Cent. South Univ. 2019, 26, 268–288. [Google Scholar] [CrossRef]
- Li, L.Y. A study of iron mineral transformation to reduce red mud tailings. Waste Manag. 2001, 21, 525–534. [Google Scholar] [CrossRef]
- Harvey, J.-P.; Lebreux-Desilets, F.; Marchand, J.; Oishi, K.; Bouarab, A.-F.; Robelin, C.; Gheribi, A.E.; Pelton, A.D. On the Application of the FactSage Thermochemical Software and Databases in Materials Science and Pyrometallurgy. Processes 2020, 8, 1156. [Google Scholar] [CrossRef]
- Bale, C.W.; Belisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melancon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Zhang, T.; Li, X.; Chen, X. Investigation of the smelting reduction mechanism and of iron extraction from high-iron red mud. Mater. Res. Express 2020, 7, 126514. [Google Scholar] [CrossRef]
- Liu, X.; Gao, P.; Yuan, S.; Lv, Y.; Han, Y. Clean utilization of high-iron red mud by suspension magnetization roasting. Miner. Eng. 2020, 157, 106553. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Wan, J.; Chen, T.; Zhou, X.; Luo, Y.; Liu, W.; Lu, Q. Efficient improvement for the direct reduction of high-iron red mud by coreduction with high-manganese iron ore. Miner. Eng. 2021, 174, 107024. [Google Scholar] [CrossRef]
- Tian, H.; Sun, S.; Hao, W.; Jiang, Y. Development Status and Existing Problems of Non-Blast Furnace Ironmaking. In Proceedings of the 10th Pacific Rim International Conference on Advanced Materials and Processing (PRICM 10), Xi’An, China, 18 August 2019. [Google Scholar] [CrossRef]


| Composition | NaO2 | Al2O3 | SiO2 | CaO | TiO2 | TFe | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| Red mud content/% | 3.10 | 17.01 | 6.11 | 0.67 | 6.49 | 39.19 | 6.67 |
| Calcified slag content/% | 0.54 | 13.39 | 5.52 | 15.91 | 5.19 | 29.89 | 15.32 |
| Number | Reaction Equation | Number | Reaction Equation |
|---|---|---|---|
| Reaction (1) | Fe3O4 + C = 3FeO + CO | Reaction (11) | Fe3O4 + CO = 3FeO + CO2 |
| Reaction (2) | Fe3O4 + 4C = 3Fe + 4CO | Reaction (12) | Fe3O4 + 4CO = 3Fe + 4CO2 |
| Reaction (3) | Fe2O3 + 3C = 2Fe + 3CO | Reaction (13) | Fe2O3 + 3CO = 2Fe + 3CO2 |
| Reaction (4) | FeO + C = Fe + CO | Reaction (14) | FeO + CO = Fe + CO2 |
| Reaction (5) | MnO + C = Mn + CO | Reaction (15) | MnO + CO = Mn + CO2 |
| Reaction (6) | SiO2 + 2C = Si + 2CO | Reaction (16) | TiO2 + 2CO = Ti + 2CO2 |
| Reaction (7) | TiO2 + 2C = Ti + 2CO | Reaction (17) | SiO2 + 2CO = Si + 2CO2 |
| Reaction (8) | MgO + C = Mg + CO | Reaction (18) | MgO + CO = Mg + CO2 |
| Reaction (9) | Al2O3 + C = 2Al + 3CO | Reaction (19) | Al2O3 + 3CO = 2Al + 3CO2 |
| Reaction (10) | Na2O + C = 2Na + CO | Reaction (20) | Na2O + CO = 2Na + CO2 |
| Element | Fe | C | Si | Mn | P |
|---|---|---|---|---|---|
| Content/% | 93.76 | 3.84 | 0.39 | <0.010 | 0.227 |
| Composition | CaO | Al2O3 | SiO2 | TiO2 | Na2O | Fe2O3 | LOI |
|---|---|---|---|---|---|---|---|
| Content/% | 37.07 | 37.67 | 7.49 | 7.43 | 0.4848 | 1.51 | 7.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, X.; Zhang, T.; Ye, J.; Lv, G.; Zhang, J. Study on Reductive Smelting of High-Iron Red Mud for Iron Recovery. Metals 2022, 12, 639. https://doi.org/10.3390/met12040639
Yang X, Chen X, Zhang T, Ye J, Lv G, Zhang J. Study on Reductive Smelting of High-Iron Red Mud for Iron Recovery. Metals. 2022; 12(4):639. https://doi.org/10.3390/met12040639
Chicago/Turabian StyleYang, Xuewei, Xin Chen, Tingan Zhang, Jiayuan Ye, Guozhi Lv, and Jinshan Zhang. 2022. "Study on Reductive Smelting of High-Iron Red Mud for Iron Recovery" Metals 12, no. 4: 639. https://doi.org/10.3390/met12040639
APA StyleYang, X., Chen, X., Zhang, T., Ye, J., Lv, G., & Zhang, J. (2022). Study on Reductive Smelting of High-Iron Red Mud for Iron Recovery. Metals, 12(4), 639. https://doi.org/10.3390/met12040639






