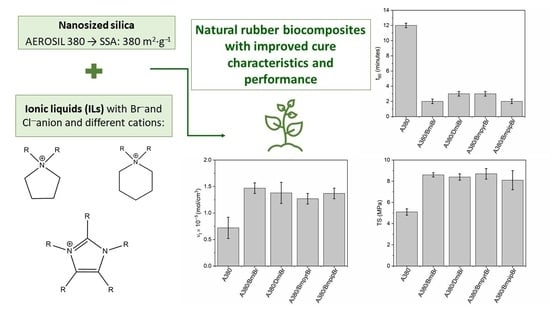
Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of NR Compounds Filled with Nanosized Silica
3. Results and Discussion
3.1. Effect of Ionic Liquids on Cure Characteristics and Crosslink Density of NR Composites Filled with Nanosized Silica
3.2. Effect of Ionic Liquids on the Tensile Properties and Hardness of NR Composites Filled with Nanosized Silica
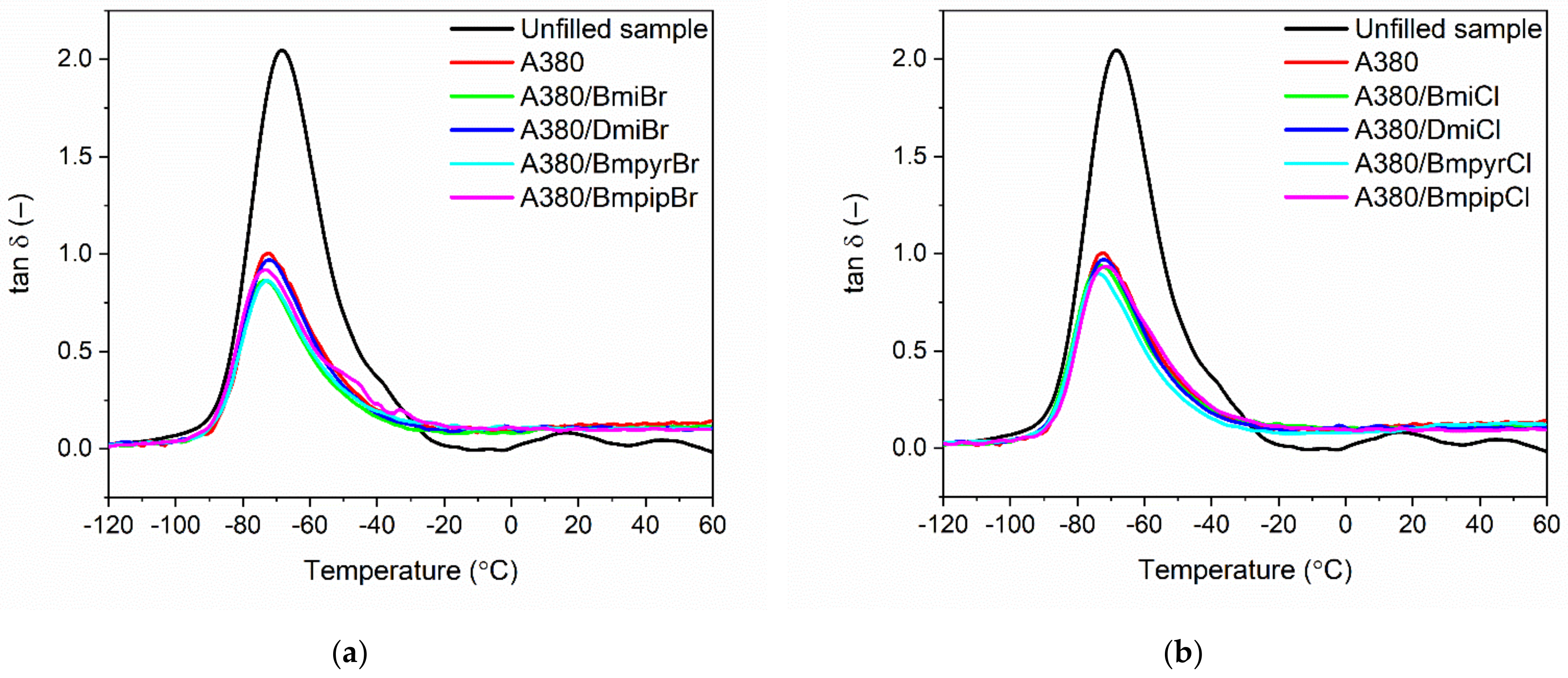
3.3. Effect of Ionic Liquids on the Dynamic Mechanical Properties of NR Composites Filled with Nanosized Silica
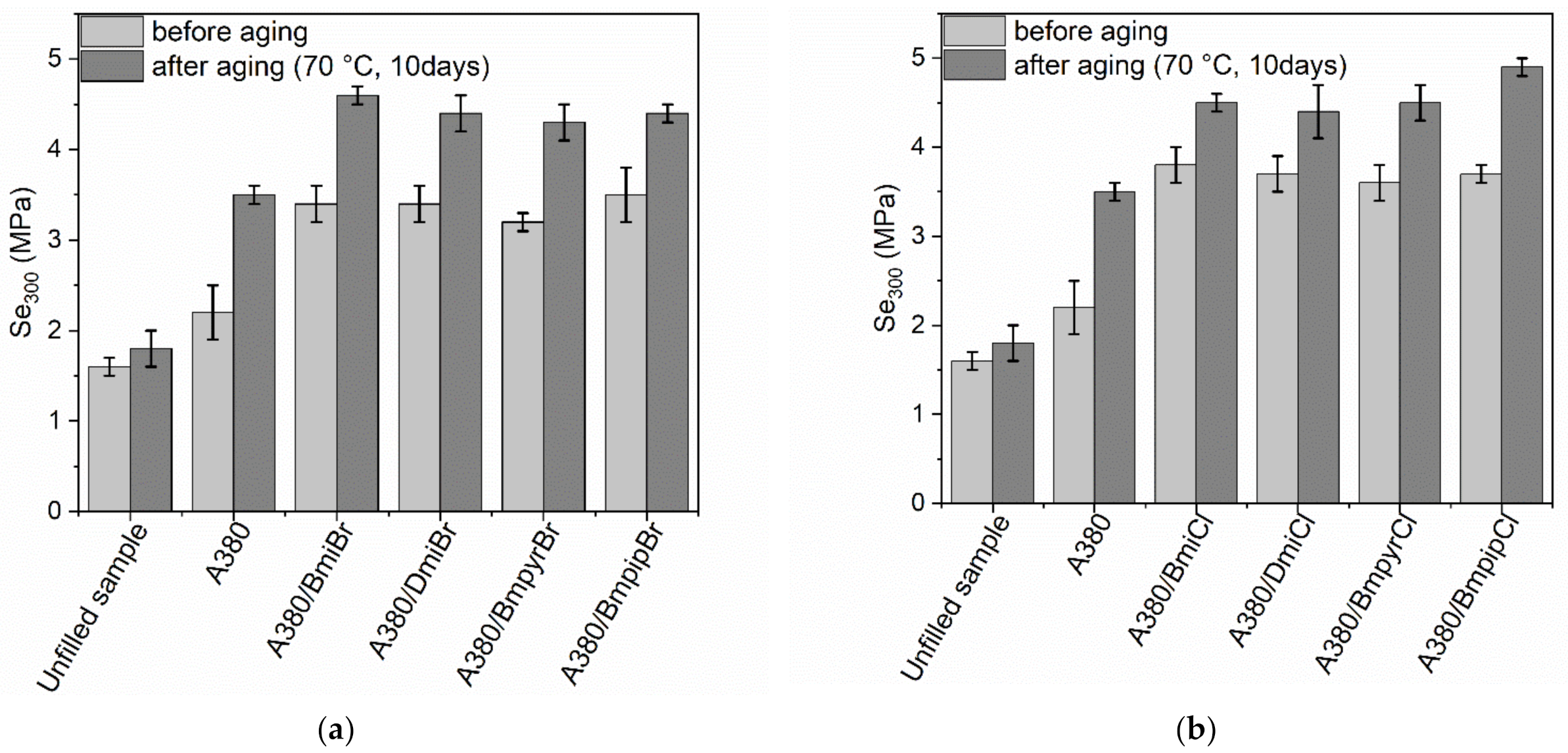
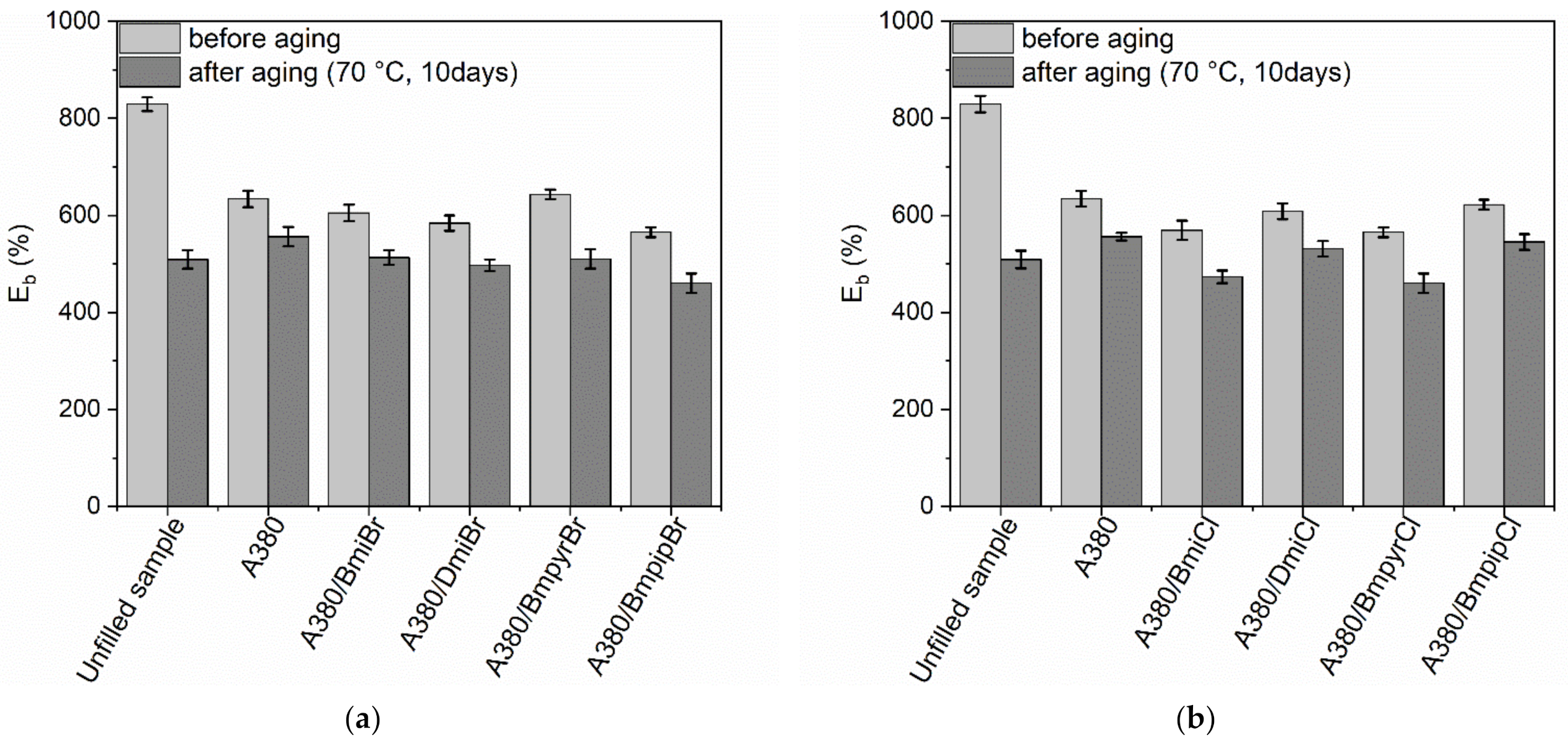
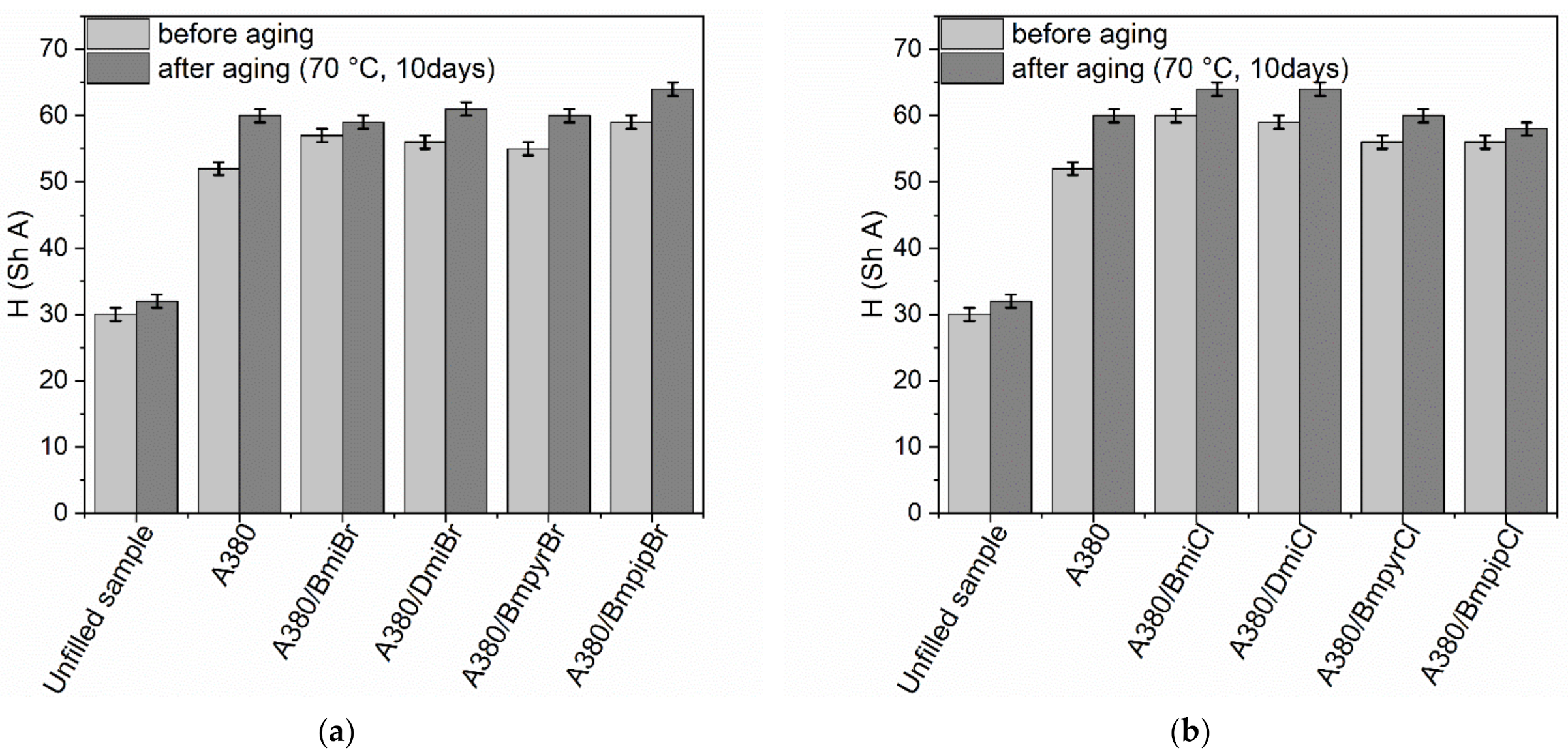
3.4. Thermo-Oxidative Aging Resistance of NR Composites Filled with Nanosized Silica
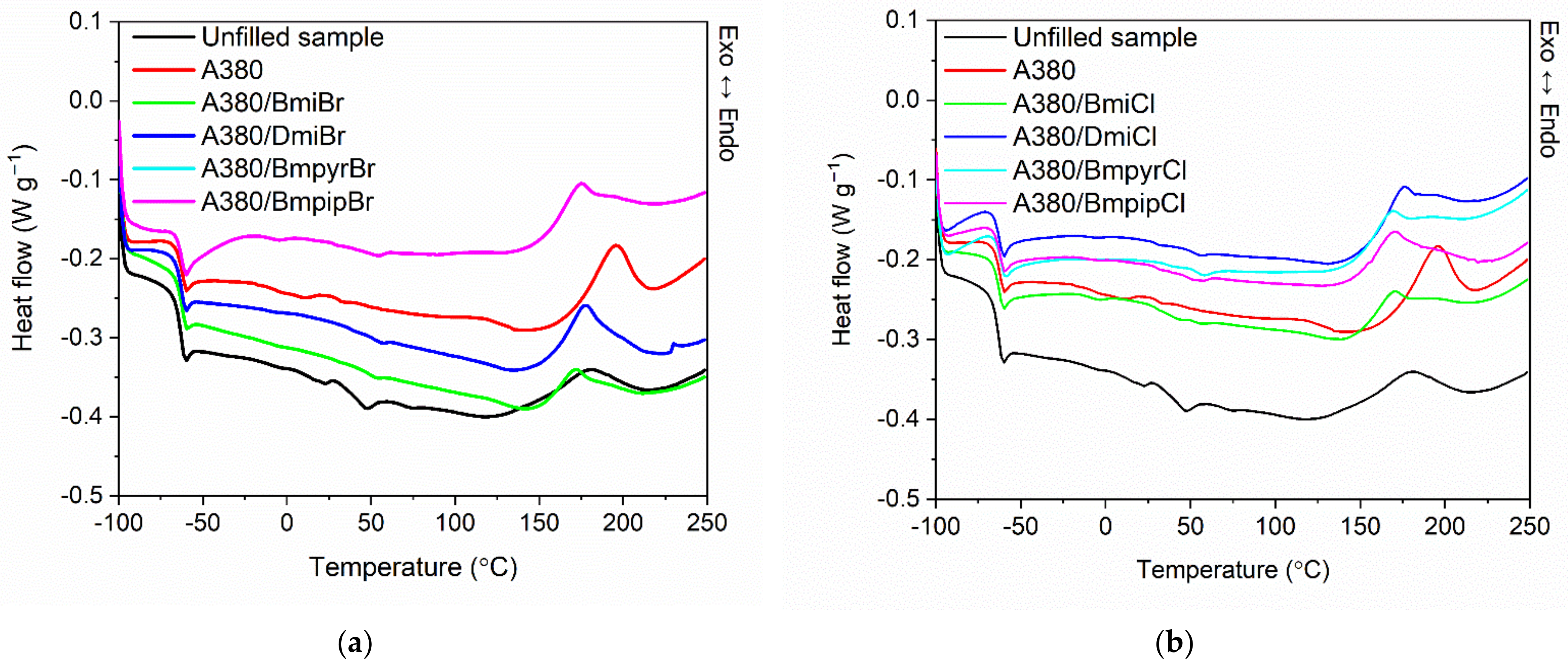
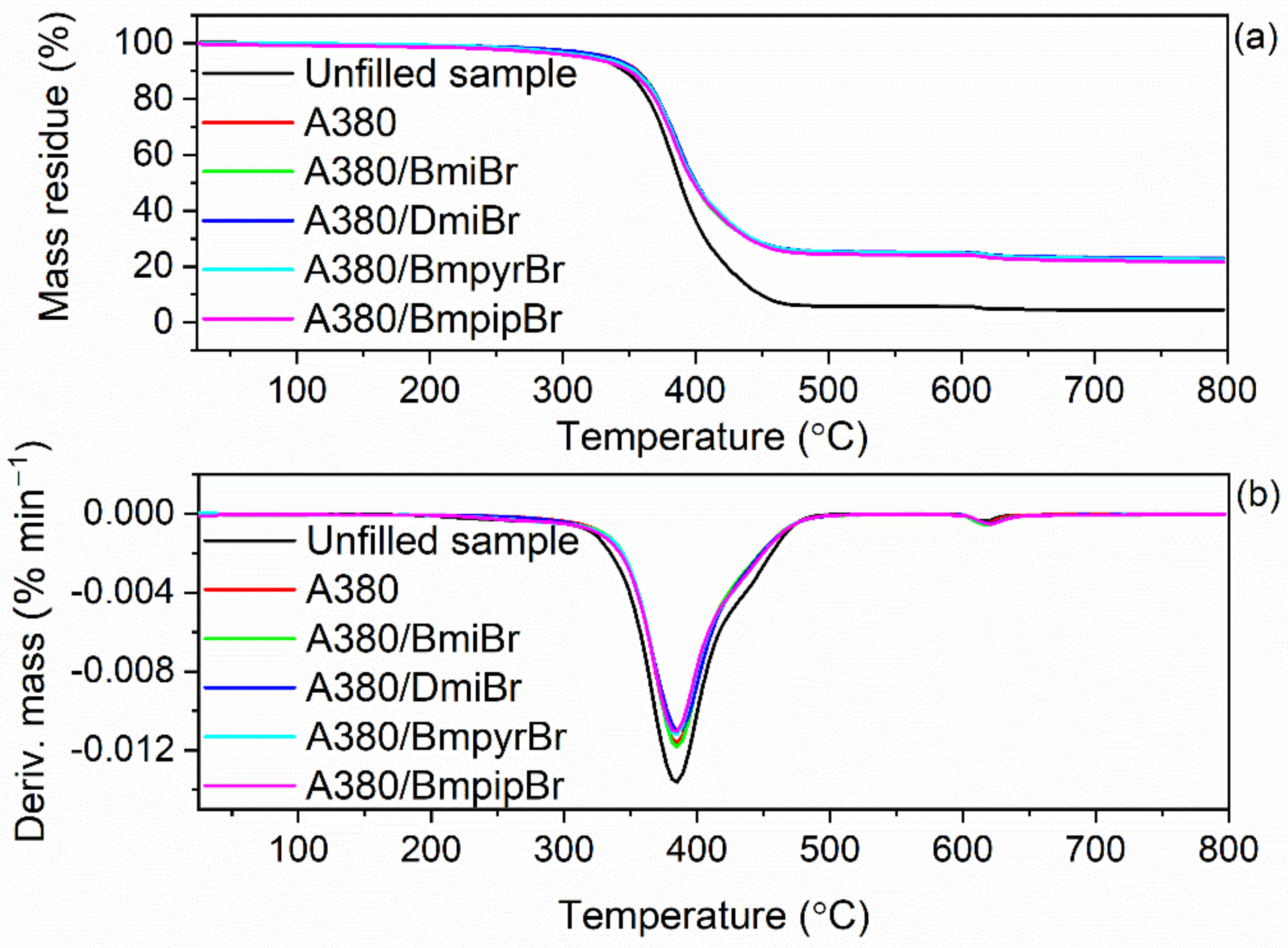
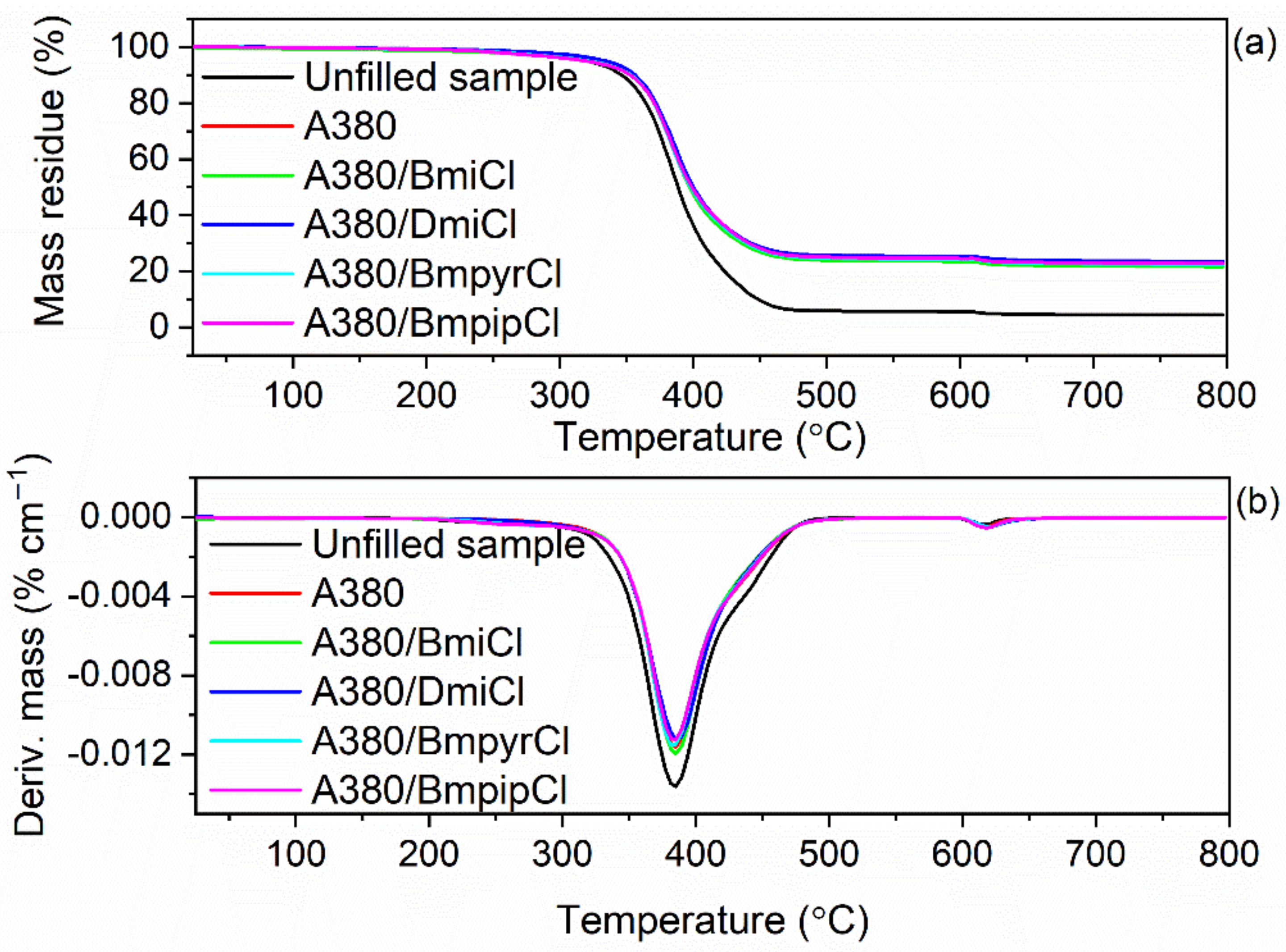
3.5. Effect of Ionic Liquids on the Thermal Stability of NR Composites Filled with Nanosized Silica
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, A.H.; Jakarni, F.M.; Muniandy, R.; Hassim, S.; Elahi, Z. Natural Rubber as a Renewable and Sustainable Bio-modifier For Pavement Applications: A Review. J. Clean. Prod. 2021, 289, 125727. [Google Scholar] [CrossRef]
- Greve, H. Rubber, 2. Natural. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2000; pp. 583–596. [Google Scholar]
- Umunakwe, R.; Oyetunji, A.; Adewuyi, B.O.; Uzochukwu EZE, W.; Nwigwe, U.S.; Umunakwe, I.J. Mechanical Properties and Microstructure of Hybrid Vulcanized Natural Rubber Filled with Carbon Black and Nano-CaCO3 from Achatina Achatina Shells. J. Met. Mater. Miner. 2019, 29, 80–89. [Google Scholar] [CrossRef]
- Hurley, P.E. History of Natural Rubber. J. Macromol. Sci. A 2006, 15, 1279–1287. [Google Scholar] [CrossRef]
- Mathew, N.W. Natural Rubber. In Rubber Technologist’s Handbook; White, J.R., De, S.K., Eds.; Rapra Technology Ltd.: Shawbury, UK, 2001. [Google Scholar]
- Mooibroek, H.; Cornish, K. Alternative sources of Natural Rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and Recycling of Waste Rubber. Prog. Polym. Sci. 2000, 25, 909–947. [Google Scholar] [CrossRef]
- Yarra, S.; Gordaninejad, F.; Behrooz, M.; Pekcan, G. Performance of Natural Rubber and Silicone-Based Magnetorheological Elastomers Under Large-Strain Combined Axial and Shear Loading. J. Intell. Mater. Syst. Struct. 2019, 30, 228–242. [Google Scholar] [CrossRef]
- Hayashi, Y. Production of Natural Rubber from Para Rubber Tree. Plant Biotechnol. 2009, 26, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Ruscinska, K.; Dabrowski, K.; Debek, C. Innovations in the Tire Industry. Elastomers 2020, 24, 15–27. [Google Scholar]
- Moreno, R.M.B.; Ferreira, M.; Goncalves, P.; Capparelli, M.L.H. Technological Properties of Latex and Natural Rubber of Hevea Brasiliensis Clones. Sci. Agric. 2005, 62, 112–126. [Google Scholar] [CrossRef] [Green Version]
- Yip, E.; Cacioli, P. The Manufacture of Gloves from Natural Rubber Latex. J. Allergy Clin. Immunol. 2002, 110, S3–S14. [Google Scholar] [CrossRef]
- Boonmahitthisud, A.; Chuayjuljit, S. NR/XSBR Nanocomposites with Carbon Black and Carbon Nanotube Prepared by Latex Compounding. J. Met. Mater. 2012, 22, 77–85. [Google Scholar]
- Boonkerd, K.; Chuayjuljit, S.; Abdulraman, D.; Jaranrangsup, W. Silica-Rich Filler for The Reinforcement in Natural Rubber. Rubber Chem. Technol. 2012, 85, 1–13. [Google Scholar] [CrossRef]
- Ngamsurat, S.; Boonkerd, K.; Leela-adisorn, U.; Potiyaraj, P. Curing Characteristics of Natural Rubber Filled with Gypsum. Energy. Energy Procedia. 2011, 9, 452–458. [Google Scholar] [CrossRef] [Green Version]
- George, S.C.; Rajan, R.; Aprem, A.S.; Thomas, S.; Kim, S.S. The Fabrication and Properties of Natural Rubber-Clay Nano-Composites. Polym. Test. 2016, 51, 165–173. [Google Scholar] [CrossRef]
- Mariano, R.M.; Picciani, P.H.S.; Nunes, R.C.R.; Visconte, L.L.Y. Preparation, Structure, and Properties of Montmorillonite/Cellulose II/Natural Rubber Nanocomposites. J. Appl. Polym. Sci. 2011, 120, 458–465. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Ishak, Z.A.M. Processing and Mechanical Properties of Organic Filler–Polypropylene Composites. J. Appl. Polym. Sci. 2005, 96, 1906–1913. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Doroftei, F. Polymeric Composites Based on Natural Rubber and Hemp Fibers. Iran. Polym. J. 2015, 24, 35–148. [Google Scholar] [CrossRef]
- Maslowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 971. [Google Scholar] [CrossRef] [Green Version]
- La Mantia, F.P.; Morreale, M. Green Composites: A Brief Review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Ichazo, M.N.; Hernandez, M.; Albano, C.; Gonzalez, J. Curing and Physical Properties of Natural Rubber/Wood Flour Composites. Macromol. Symp. 2006, 239, 192–200. [Google Scholar] [CrossRef]
- Srirachya, N.; Boonkerd, K.; Nakajima, L.; Kobayashi, T. Bio-Composite Hydrogels of Cellulose and Vulcanized Natural Rubber with Nanointerconnected Layers for Reinforced Water-retaining Materials. Polym. Bull. 2018, 75, 5493–5512. [Google Scholar] [CrossRef]
- Srirachya, N.; Boonkerd, K.; Kobayashi, T. Effective Elongation Properties of Cellulose–Natural Rubber Composite Hydrogels Having Interconnected Domain. J. Elastomers Plast. 2020, 52, 337–355. [Google Scholar] [CrossRef]
- Kumagai, A.; Tajima, N.; Iwamoto, S.; Morimoto, T.; Nagatani, A.; Okazaki, T.; Endo, T. Properties of Natural Rubber Reinforced with Cellulose Nanofibers Based on Fiber Diameter Distribution as Estimated by Differential Centrifugal Sedimentation. Int. J. Biol. Macromol. 2019, 121, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.G.; Dufresne, A. Crab Shell Chitin Whisker Reinforced Natural Rubber Nanocomposites. 1. Processing and Swelling Behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Rattanasom, N.; Prasertsri, S.; Ruangritnumchai, T. Comparison of The Mechanical Properties at Similar Hardness Level of Natural Rubber Filled with Various Reinforcing-Fillers. Polym. Test. 2009, 28, 8–12. [Google Scholar] [CrossRef]
- Hayichelaev, C.; Reuvekamp, L.; Dierkes, W.K.; Blume, A.; Noordermeer, J.W.M.; Sahakaro, K. Silica-Reinforced Natural Rubber Tire Tread Compounds Containing Bio-based Process Oils: Influence of Epoxide and Amino Functional Groups. Rubber Chem. Technol. 2020, 93, 195–207. [Google Scholar] [CrossRef]
- Xu, H.; Fan, T.; Ye, N.; Wu, W.; Huang, D.; Wang, D.; Wang, Z.; Zhang, L. Plasticization Effect of Bio-based Plasticizers from Soybean Oil For Tire Tread Rubber. Polymers 2020, 12, 623. [Google Scholar] [CrossRef] [Green Version]
- Boonmahitthisud, A.; Boonkerd, K. Sustainable Development of Natural Rubber and its Environmentally Friendly Composites. Green Sustain. Chem. 2021, 28, 100446–100452. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal-Organic Framework for CO2 Capture And Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Marinkovic, J.M.; Riisager, A.; Franke, R.; Wasserscheid, P.; Haumann, M. Fifteen Years of Supported Ionic Liquid Phaze-Catalyzed Hydroformylation: Material and Process Developments. Ind. Eng. Chem. Res. 2019, 58, 2409–2420. [Google Scholar] [CrossRef]
- Kreyenschulte, H.; Richter, S.; Goötze, T.; Fischer, D.; Steinhauser, D.; Kluppel, M.; Heinrich, G. Interaction of 1-Allyl-3- methyl-imidazolium Chloride and Carbon Black and its Influence on Carbon Black Filled Rubbers. Carbon 2012, 50, 3649–3658. [Google Scholar] [CrossRef]
- Sowinska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, Z.; Zhu, L.; Guo, B.; Jia, D. Thiol-Containing Ionic liquid for the modification of Styrene−Butadiene Rubber/Silica Composites. J. Appl. Polym. Sci. 2012, 123, 1252–1260. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Ionic liquids As Coagents for sulfur vulcanization of Butadiene−Styrene Elastomer Filled with Carbon Black. Polym. Bull. 2018, 75, 4499–4514. [Google Scholar] [CrossRef] [Green Version]
- Laskowska, A.; Marzec, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Effect of Imidazolium Ionic Liquid Type on the Properties of Nitrile Rubber Composites. Polym. Int. 2013, 62, 1575–1582. [Google Scholar] [CrossRef]
- Gan, S.; Wu, Z.L.; Xu, H.; Song, Y.; Zheng, Q. Viscoelastic behaviors of carbon black gel extracted from highly filled natural rubber compounds: Insights into the Payne Effect. Macromolecules 2016, 49, 1454–1463. [Google Scholar] [CrossRef]
- Hussain, M.; Yasin, S.; Akram, M.A.; Xu, H.; Song, Y.; Zheng, Q. Influence of Ionic Liquids on Structure and Rheological Behaviors of Silica-Filled Butadiene Rubber. Ind. Eng. Chem. Res. 2019, 58, 18205–18212. [Google Scholar] [CrossRef]
- Sowinska, A.; Maciejewska, M.; Grajewska, A. Bis(trifluoromethylsulfonyl)imide Ionic Liquids Applied for Fine-Tuning the Cure Characteristics and Performance of Natural Rubber Composites. Int. J. Mol. Sci. 2021, 22, 3678. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A.; Hadj-Kalie, M.K. Physicochemical Properties of Piperidinium, Ammonium, Pyrrolidinium and Morpholinium Cations Based Ionic Liquids Paired with Bis(trifluoromethylsulfonyl)imide Anion. Fluid Phase Equilib. 2016, 427, 18–26. [Google Scholar] [CrossRef]
- ISO 6502-3:2018; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Rheometer. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11357-1:2016; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 1817:2015; Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids. International Organization for Standardization: Geneva, Switzerland, 2017.
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-linked Polymer Networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Properties of Chemically Modified (Selected Silanes) Lignocellulosic Filler and Its Application in Natural Rubber Biocomposites. Materials 2020, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 188:2011; Rubber, Vulcanized or Thermoplastic—Accelerated Ageing and Heat Resistance Tests. International Organization for Standardization: Geneva, Switzerland, 2011.
- Maslowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common Nettle (Urtica dioica L.) as an Active Filler of Natural Rubber Biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef] [PubMed]
- Kosmalska, A.; Zaborski, M.; Slusarski, L. Adsorption of Curatives and Activity of Silica Toward Elastomers. Macromol. Symp. 2003, 194, 269–275. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of Natural Rubber with Silica/Carbon Black Hybrid Filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Zaborski, M.; Donnet, J.B. Activity of Fillers in Elastomer Networks of Different Structures. Macromol Symp. 2003, 194, 87–99. [Google Scholar] [CrossRef]
- Noordermeer, J.W.M. Vulcanization. In Encyclopedia of Polymeric Nanomaterials, 1st ed.; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowinska, A. Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites. Polymers 2021, 13, 1656. [Google Scholar] [CrossRef]
- Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Thermal Analysis and SEM Microscopy Applied to Studying the Efficiency of Ionic Liquid Immobilization on Solid Supports. Materials 2019, 12, 1579. [Google Scholar] [CrossRef] [Green Version]
- Fontana, J.P.; Camilo, F.C.; Bizeto, M.A.; Faez, R. Evaluation of the Role of an Ionic Liquid as Organophilization Agent into Montmorillonite for NBR Rubber Composite Production. Appl. Clay Sci. 2013, 83, 203–209. [Google Scholar] [CrossRef]
- Du, M.L.; Guo, B.C.; Jia, D.M. Carboxylated Butadiene-Styrene Rubber/Halloysite Nanotube: Nanocomposites: Interfacial Interaction and Performance. Polymer 2008, 49, 4871–4876. [Google Scholar] [CrossRef]
- Mrozik, W.; Jungnickel, C.; Skup, M.; Urbaszek, P.; Stepnowski, P. Determination of the Adsorption Mechanism of Imidazolium-Type Ionic Liquids onto Kaolinite, Implications for Their Fate and Transport in the Soil Environment. Environ. Chem. 2008, 5, 299. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, Z.; Guo, B.; Jia, D. Functional Thiol Ionic Liquids as a Novel Interfacial Modifiers in SBR/HNTs Composites. Polymer 2011, 52, 1337–1344. [Google Scholar] [CrossRef]
- Szepiński, E.; Smolarek, P.; Milewska, M.; Łuczak, J. Application of Surface Active Amino Acid Ionic Liquids as Phase-Transfer Catalyst. J. Mol. Liq. 2020, 303, 112607. [Google Scholar] [CrossRef]
- Steinrück, H.P.; Wasserscheid, P. Ionic Liquids in Catalysis. Catal. Lett. 2015, 145, 380–397. [Google Scholar] [CrossRef]
- Sowinska-Baranowska, A.; Maciejewska, M. Influence of the Silica Specific Surface Area and Ionic Liquids on the Curing Characteristics and Performance of Styrene–Butadiene Rubber Composites. Materials 2021, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bi, W.; Zhao, S. Influence of Crosslink Density on Mechanical Properties of Natural Rubber Vulcanizates. J. Macromol. Sci. Part B 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and Crosslinking in Elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Candau, N.; Oguz, O.; Eloy, C.E.; Stoclet, G.; Tahon, J.-F.; Maspoch, M.L. Strain Induced Crystallization in Vulcanized Natural Rubber Containing Ground Tire Rubber Particles with Reinforcement and Nucleation Abilities. Polym. Test. 2021, 101, 107313–107322. [Google Scholar] [CrossRef]
- Ulfah, I.M.; Fidyaningsih, R.; Rahayu, S.; Fitriani, D.A.; Saputra, D.A.; Winarto, D.A.; Wisojodharmo, L.A. Influence of Carbon Black and Silica Filler on the Rheological and Mechanical Properties of Natural Rubber Compound. Procedia Chem. 2015, 16, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Joy, J.; George, E.; Thomas, S.; Anas, S. Effect of Filler Loading on Polymer Chain Confinement and Thermomechanical Properties of Epoxy/Boron Nitride (h-BN) Nanocomposites. New J. Chem. 2020, 44, 4494–4503. [Google Scholar] [CrossRef]
- Yoon, B.; Kim, J.Y.; Hong, U.; Oh, M.K.; Kim, M.; Han, S.B.; Nam, J.-D.; Suhr, J. Dynamic Viscoelasticity of Silica-filled Styrene-Butadiene Rubber/Polybutadiene Rubber (SBR/BR) Elastomer Composites. Compos. Part B Eng. 2020, 187, 107865–107871. [Google Scholar] [CrossRef]
- Choi, S.S. Influence of Thermal Aging in Change of Crosslink Density and Deformation of Natural Rubber Vulcanizates. Bull. Korean Chem. Soc. 2000, 21, 628–634. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, J.C. Influence of the 1,2-Unit Content of SBR and Filler Systems on Thermal Aging Behaviors of SBR Composites. J. Ind. Eng. Chem. 2007, 13, 950–955. [Google Scholar]
- Debnath, S.C.; Basu, D.K. Studies on Cure Synergism: Use of Safe Thiocarbamyl Sulfenamide-Dibenzothiazyl Disulfide Accelerator System in the Vulcanization of Natural Rubber. Polym. Plast. Technol. Eng. 1995, 34, 539–550. [Google Scholar] [CrossRef]
- Coran, A.Y. Chemistry of the Vulcanization and Protection of Elastomers: A Review of the Achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, Electrochemical and Radiolytic Stabilities of Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, Q.; Cheng, H.; Frost, R.L. Thermal Stability of Styrene Butadiene Rubber (SBR) Composites Filled with Kaolinite/Silica Hybrid Filler. J. Therm. Anal. Calorim. 2014, 115, 1013–1020. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wei, W.L.; Hsu, K.Y.; Ho, W.H. Thermal Stability of Epoxy-Silica Hybrid Materials by Thermogravimetric Analysis. Thermochim. Acta 2004, 412, 139–147. [Google Scholar] [CrossRef]


| Name | Abbreviation | CAS Number | Purity (%) | Water Content (wt. %) |
|---|---|---|---|---|
| Imidazolium ionic liquids | ||||
| 1-butyl-3-methylimidazolium bromide | BmiBr | 85100-77-2 | ≥99.0% | <0.5% |
| 1-butyl-1-methylimidazolium chloride | BmiCl | 79917-90-1 | ≥99.0% | ≤0.2% |
| 1-decyl-3-methylimidazolium bromide | DmiBr | 188589-32-4 | >98% | <1% |
| 1-decyl-3-methylimidazolium chloride | DmiCl | 171058-18-7 | 96.0% | ≤2.0% |
| Pyrrolidinium ionic liquids | ||||
| 1-butyl-1-methylpyrrolidinium bromide | BmpyrBr | 93457-69-3 | ≥99.0% | ≤0.5% |
| 1-butyl-1-methylpyrrolidinium chloride | BmpyrCl | 479500-35-1 | ≥99.0% | ≤0.5% |
| Piperidinium ionic liquids | ||||
| 1-butyl-1-methylpiperidinium bromide | BmpipBr | 94280-72-5 | 99% | 0.5% |
| 1-butyl-1-methylpiperidinium chloride | BmpipCl | 845790-13-8 | 99% | <1% |
| Ingredient, phr | Unfilled Sample (R1) | NR/A380 (R2) | NR/A380/IL (R3–R10) |
|---|---|---|---|
| NR | 100 | 100 | 100 |
| Sulfur | 2 | 2 | 2 |
| ZnO | 5 | 5 | 5 |
| MBT | 2 | 2 | 2 |
| St.A. | 1 | 1 | 1 |
| Silica A380 | ₋ | 30 | 30 |
| IL * | ₋ | ₋ | 3 |
| Compounds | Smin (dNm) | Smax (dNm) | ∆S (dNm) | t02 (min) | t90 (min) |
|---|---|---|---|---|---|
| Unfilled sample | 0.3 | 5.0 | 4.7 | 1 | 2 |
| NR compound without ionic liquid | |||||
| A380 | 6.0 | 11.4 | 5.4 | 2 | 12 |
| NR compounds with imidazolium ionic liquids | |||||
| A380/BmiBr | 6.1 | 13.5 | 7.4 | 1 | 2 |
| A380/DmiBr | 5.8 | 15.0 | 9.2 | 1 | 3 |
| A380/BmiCl | 5.8 | 14.5 | 8.7 | 1 | 2 |
| A380/DmiCl | 5.4 | 16.8 | 11.8 | 1 | 3 |
| NR compounds with pyrrolidinium ionic liquids | |||||
| A380/BmpyrBr | 6.7 | 15.2 | 8.5 | 1 | 3 |
| A380/BmpyrCl | 5.6 | 12.4 | 6.8 | 1 | 1 |
| NR compounds with piperidinium ionic liquids | |||||
| A380/BmpipBr | 6.9 | 13.8 | 6.9 | 1 | 2 |
| A380/BmpipCl | 6.4 | 13.6 | 7.2 | 1 | 2 |
| Compounds | Tg (°C) | ∆Cp (J/g × K) | Temperature of Crosslinking (°C) | ∆Hcross (J/g) |
|---|---|---|---|---|
| Unf illed sample | −64.2 | 0.44 | 146–208 | 9.0 |
| NR compound without ionic liquid | ||||
| A380 | −63.6 | 0.31 | 171–211 | 10.1 |
| NR compounds with imidazolium ionic liquids | ||||
| A380/BmiBr | −63.6 | 0.37 | 154–190 | 7.0 |
| A380/DmiBr | −64.1 | 0.37 | 157–197 | 12.7 |
| A380/BmiCl | −63.9 | 0.32 | 151–190 | 7.9 |
| A380/DmiCl | −64.3 | 0.31 | 156–194 | 10.5 |
| NR compounds with pyrrolidinium ionic liquids | ||||
| A380/BmpyrBr | −63.5 | 0.30 | 154–195 | 11.0 |
| A380/BmpyrCl | −63.0 | 0.30 | 150–189 | 9.1 |
| NR compounds with piperidinium ionic liquids | ||||
| A380/BmpipBr | −63.2 | 0.30 | 153–194 | 11.4 |
| A380/BmpipCl | −63.4 | 0.29 | 150–191 | 9.8 |
| NR Vulcanizates | Qt (−) | νt × 10−5 (mol/cm3) |
|---|---|---|
| Unfilled sample | 4.47 | 0.98 |
| NR vulcanizate without ionic liquid | ||
| A380 | 5.41 | 0.72 |
| NR vulcanizates with imidazolium ionic liquids | ||
| A380/BmiBr | 3.58 | 1.47 |
| A380/DmiBr | 3.71 | 1.38 |
| A380/BmiCl | 3.53 | 1.51 |
| A380/DmiCl | 3.62 | 1.44 |
| NR vulcanizates with pyrrolidinium ionic liquids | ||
| A380/BmpyrBr | 3.87 | 1.37 |
| A380/BmpyrCl | 3.72 | 1.37 |
| NR vulcanizates with piperidinium ionic liquids | ||
| A380/BmpipBr | 3.72 | 1.37 |
| A380/BmpipCl | 3.70 | 1.40 |
| NR Vulcanizates | Se100 (MPa) | Se300 (MPa) | TS () | Eb (%) | H (Shore A) |
|---|---|---|---|---|---|
| Unfilled sample | 1.1 ± 0.1 | 1.6 ± 0.1 | 9.5 ± 0.4 | 829 ± 14 | 30 ± 1 |
| NR vulcanizate without ionic liquid | |||||
| A380 | 1.6 ± 0.1 | 2.2 ± 0.1 | 5.1 ± 0.3 | 634 ± 18 | 52 ± 1 |
| NR vulcanizates with imidazolium ionic liquids | |||||
| A380/BmiBr | 1.8 ± 0.1 | 3.4 ± 0.1 | 8.6 ± 0.2 | 605 ± 20 | 57 ± 1 |
| A380/DmiBr | 1.7 ± 0.1 | 3.4 ± 0.1 | 8.4 ± 0.3 | 584 ± 9 | 56 ± 1 |
| A380/BmiCl | 1.8 ± 0.1 | 3.8 ± 0.1 | 9.1 ± 0.8 | 569 ± 16 | 60 ± 1 |
| A380/DmiCl | 1.8 ± 0.1 | 3.7 ± 0.1 | 10.9 ± 0.6 | 608 ± 11 | 59 ± 1 |
| NR vulcanizates with pyrrolidinium ionic liquids | |||||
| A380/BmpyrBr | 1.7 ± 0.1 | 3.2 ± 0.1 | 8.7 ± 0.5 | 643 ± 6 | 55 ± 1 |
| A380/BmpyrCl | 1.8 ± 0.1 | 3.6 ± 0.1 | 8.3 ± 0.8 | 565 ± 11 | 56 ± 1 |
| NR vulcanizates with piperidinium ionic liquids | |||||
| A380/BmpipBr | 1.8 ± 0.1 | 3.5 ± 0.1 | 8.1 ± 0.9 | 578 ± 19 | 59 ± 1 |
| A380/BmpipCl | 1.8 ± 0.1 | 3.7 ± 0.1 | 11.3 ± 0.7 | 622 ± 14 | 56 ± 1 |
| NR Vulcanizates | Tg (°C) | Tan δTg (−) | Tan δ25 °C (−) | Tan δ50 °C (−) |
|---|---|---|---|---|
| Unfilled sample | −69 ± 1 | 2.10 ± 0.1 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| NR vulcanizate without ionic liquid | ||||
| A380 | −72 ± 1 | 1.10 ± 0.1 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| NR vulcanizates with imidazolium ionic liquids | ||||
| A380/BmiBr | −74 ± 1 | 0.81 ± 0.1 | 0.11 ± 0.01 | 0.11 ± 0.01 |
| A380/DmiBr | −72 ± 1 | 0.90 ± 0.1 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| A380/BmiCl | −73 ± 1 | 0.79 ± 0.1 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| A380/DmiCl | −70 ± 1 | 0.90 ± 0.1 | 0.10 ± 0.02 | 0.10 ± 0.02 |
| NR vulcanizates with pyrrolidinium ionic liquids | ||||
| A380/BmpyrBr | −73 ± 1 | 0.90 ± 0.1 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| A380/BmpyrCl | −74 ± 1 | 0.89 ± 0.1 | 0.11 ± 0.02 | 0.13 ± 0.02 |
| NR vulcanizates with piperidinium ionic liquids | ||||
| A380/BmpipBr | −74 ± 1 | 0.91 ± 0.1 | 0.10± 0.01 | 0.10 ± 0.01 |
| A380/BmpipCl | −71 ± 1 | 0.91 ± 0.1 | 0.09 ± 0.01 | 0.10 ± 0.01 |
| SBR Vulcanizates | Af (−) |
|---|---|
| Unfilled sample | 0.5 ± 0.1 |
| NR vulcanizate without ionic liquid | |
| A380 | 1.2 ± 0.1 |
| NR vulcanizates with imidazolium ionic liquids | |
| A380/BmiBr | 0.8 ± 0.1 |
| A380/DmiBr | 0.8 ± 0.1 |
| A380/BmiCl | 0.7 ± 0.1 |
| A380/DmiCl | 0.8 ± 0.1 |
| NR vulcanizates with pyrrolidinium ionic liquids | |
| A380/BmpyrBr | 0.7 ± 0.1 |
| A380/BmpyrCl | 0.7 ± 0.1 |
| NR vulcanizates with piperidinium ionic liquids | |
| A380/BmpipBr | 0.9 ± 0.1 |
| A380/BmpipCl | 0.8 ± 0.1 |
| SBR Vulcanizates | T5% (°C) | TDTG (°C) | ∆m25–600 °C (%) | ∆m600–800 °C (%) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| Unfilled sample | 317 | 401 | 94.0 | 1.8 | 4.2 |
| NR vulcanizate without ionic liquid | |||||
| A380 | 333 | 401 | 75.2 | 1.9 | 22.9 |
| NR vulcanizates with imidazolium ionic liquids | |||||
| A380/BmiBr | 329 | 402 | 75.3 | 2.4 | 22.3 |
| A380/DmiBr | 333 | 403 | 74.9 | 2.2 | 22.9 |
| A380/BmiCl | 327 | 402 | 75.9 | 2.0 | 22.1 |
| A380/DmiCl | 333 | 403 | 75.1 | 2.0 | 22.9 |
| NR vulcanizates with pyrrolidinium ionic liquids | |||||
| A380/BmpyrBr | 321 | 401 | 75.1 | 2.3 | 22.6 |
| A380/BmpyrCl | 321 | 400 | 75.7 | 2.1 | 22.2 |
| NR/A380 vulcanizates with piperidinium ionic liquids | |||||
| A380/BmpipBr | 315 | 401 | 75.3 | 2.3 | 22.4 |
| A380/BmpipCl | 317 | 400 | 75.3 | 2.0 | 22.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska, M.; Sowińska-Baranowska, A. Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica. Nanomaterials 2022, 12, 1209. https://doi.org/10.3390/nano12071209
Maciejewska M, Sowińska-Baranowska A. Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica. Nanomaterials. 2022; 12(7):1209. https://doi.org/10.3390/nano12071209
Chicago/Turabian StyleMaciejewska, Magdalena, and Anna Sowińska-Baranowska. 2022. "Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica" Nanomaterials 12, no. 7: 1209. https://doi.org/10.3390/nano12071209
APA StyleMaciejewska, M., & Sowińska-Baranowska, A. (2022). Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica. Nanomaterials, 12(7), 1209. https://doi.org/10.3390/nano12071209