Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes
Abstract
:1. Introduction
pH <≈ 8
pH >≈ 8
- (i).
- To destabilize an oxidant such as ozone, promoting its decomposition, an antioxidant is necessary, and L-cysteine will be used as carbon precursor. Moreover, it has been observed that antioxidant activity is related to –SH functional groups on CD [25];
- (ii).
- To increase the surface reactivity of the CD, doping with transition metal ions, that are rich in electrons, will provide a means for emerging chemical reactivities [26]. For example, copper(II) doping of CD can boost electron transfer and photooxidation [27] and has already been used in several applications [26,27,28,29];
- (iii).
- To increase the water solubility and to coat the CD with a soft surface, allowing increased affinity of ozone and/or dye molecules, polyethylene glycol (PEG) will be used as a carbon precursor (together with L-cysteine) and as a passivating agent. Indeed, PEG undergoes caramelization, leading to the production of PEGylated nanoparticles [30]. Moreover, PEG is a highly water-soluble polymer and has the capability to enhance the CD quantum yield [31,32,33].
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Copper(II)-Doped Carbon Dots (Cu-CDs and CuCys-CDs)
2.3. Ozone Advanced Oxidation Process (AOP)
2.4. Equipment
2.5. Data Analysis
3. Results
3.1. Ozone AOP of Dyes
3.2. Preliminary Analysis of the Catalytic Performance of the Cu-CD
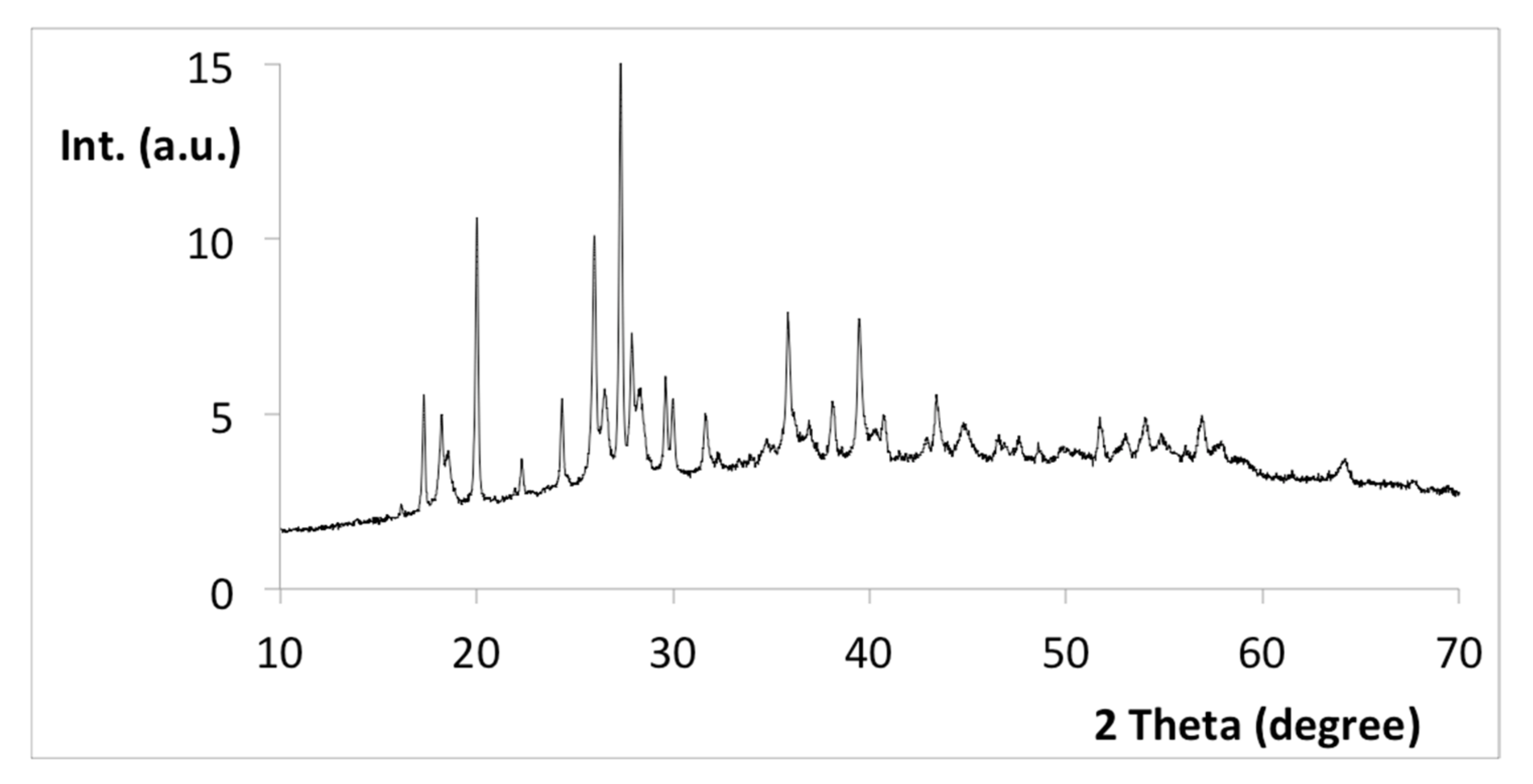
3.3. Characterization of the Cu-CD
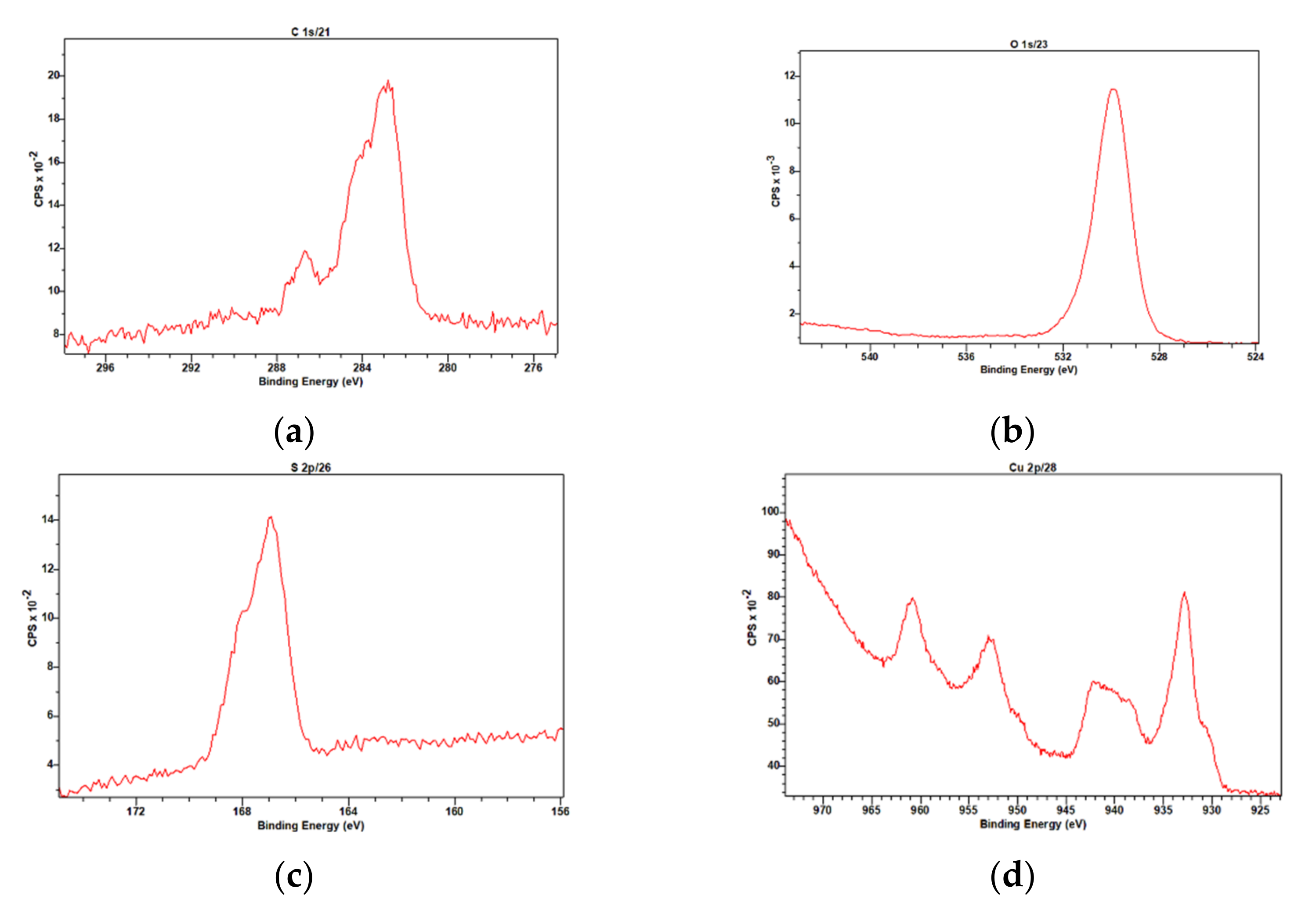
3.4. Characterization of the CuCys-CD
3.5. Ozone AOP in the Presence of Cu-CD
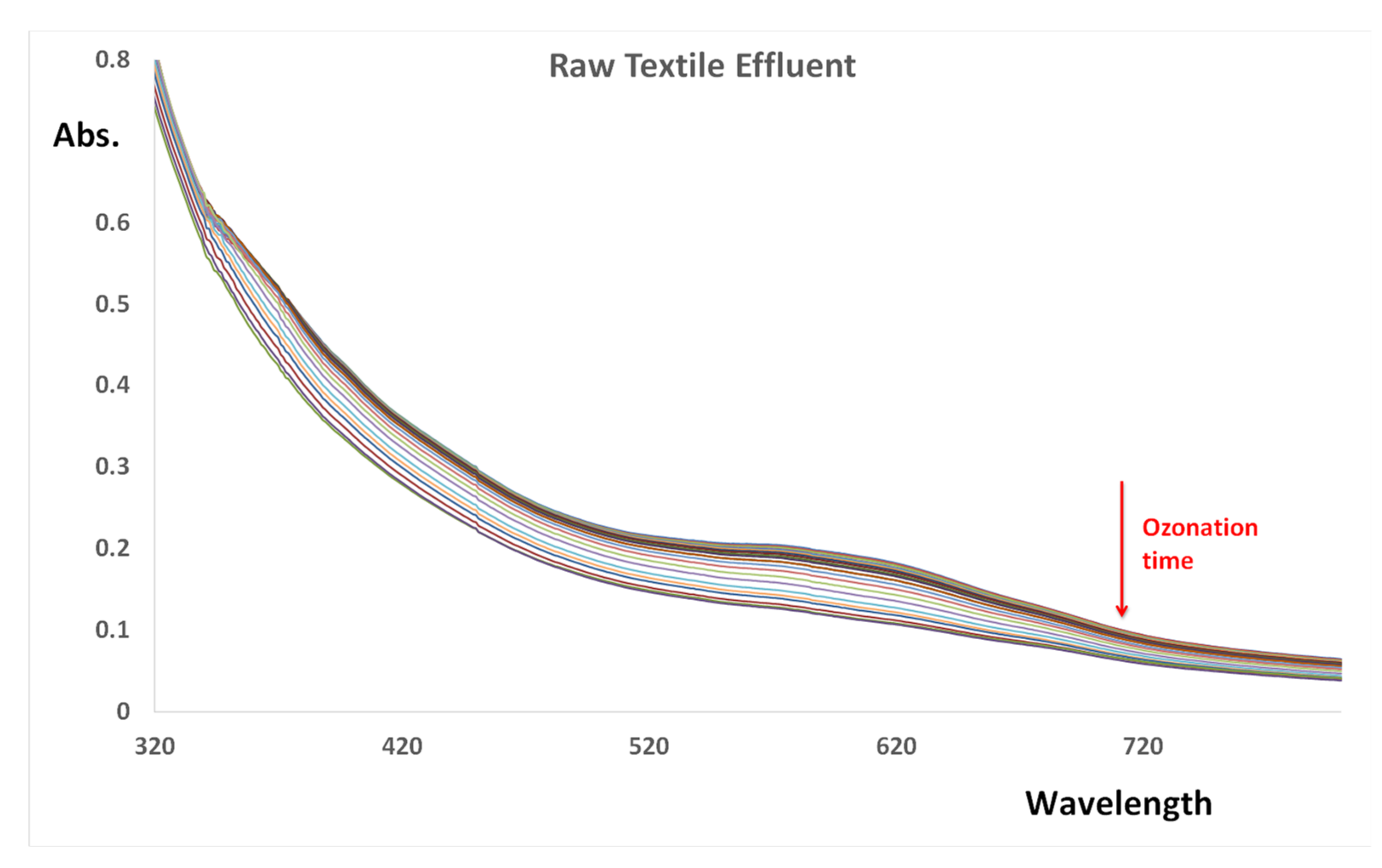
3.6. Real Textile Effluent Analysis
3.7. Mechanism of Ozone plus Cu-CD
- (i).
- Taking into consideration the high specific surface area of the nanocomposite and that the carbon-based core is passivated by a hydrated soft caramelized PEG coating, the dyes can become adsorbed on the surface.
- (ii).
- Secondly, on the same surface, ozone is catalytically transformed into hydroxyl radicals due to the existence of copper and sulfur atoms.
- (iii).
- Thirdly, the adsorbed dyes react with the hydroxyl radicals being degraded.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcamo, J. Water quality and its interlinkages with the Sustainable Development Goals. Curr. Opin. Environ. Sustain. 2019, 36, 126–140. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Ikehata, K.; Li, Y. Advanced Oxidation Processes for Wastewater Treatment. Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: London, UK, 2018; Chapter 5. [Google Scholar]
- Von Gunten, U. Ozonation of drinking water: Part, I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Bilińska, M.; Gmurek, M. Industrial Textile Wastewater Ozone Treatment: Catalyst Selection. Catalysts 2020, 10, 611. [Google Scholar] [CrossRef]
- Turhan, K.; Durukan, I.; Ozturkcan, S.; Turgut, Z. Decolorization of textile basic dye in aqueous solution by ozone. Dye. Pigments 2012, 92, 897–901. [Google Scholar] [CrossRef]
- Karrew, N.; Ryhiner, G.; Heinzle, E. Applicability test for combined biological-chemical treatment of wastewaters containing biorefractory compounds. Water Res. 1997, 31, 1013–1020. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Liang, C.; Luo, X.; Hu, Y. Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen. Water 2022, 14, 19. [Google Scholar] [CrossRef]
- Jin, X.; Wu, C.; Fu, L.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. J. Environ. Sci. 2023, 124, 330–349. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Da Silva, J.C.G.E.; Gonçalves, H. Analytical and bioanalytical applications of carbon dots. Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Simões, E.; Leitão, J.; da Silva, J.C.G.E. Carbon dots from tryptophan doped glucose for peroxynitrite sensing. Anal. Chim. Acta. 2014, 852, 174–180. [Google Scholar] [CrossRef]
- Algarra, M.; Martin, M.P.; Rueda, M.C.; Jiménez-Jiménez, J.; da Silva, J.C.G.E.; Bandosz, T.; Castellón, E.R.; Casado, J.; López-Navarrete, J.T. Carbon Dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in-vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mat. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Campos, B.B.; Contreras-Cáceres, R.; Bandosz, T.J.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; da Silva, J.C.E.; Algarra, M. Carbon Dots as Fluorescent Sensor for Detection of Explosive Nitrocompounds. Carbon 2016, 106, 171–178. [Google Scholar] [CrossRef]
- Bin Chen, B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Coll. Interf. Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Sendão, R.M.; Crista, D.M.; Afonso, A.C.P.; de Yuso, M.D.V.M.; Algarra, M.; da Silva, J.C.E.; da Silva, L.P. Insight into the Hybrid Luminescence Showed by Carbon Dots and Molecular Fluorophores in Solution. Phy. Chem. Chem. Phys. 2019, 21, 20919–20926. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse carbon Quantum Dots from fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lin, Y.; Zhang, L.; Yan, Z.; Wang, Y.; Liu, Y. Synthesis of Sulfur-Selenium Doped Carbon Quantum Dots for Biological Imaging and Scavenging Reactive Oxygen Species. Sci. Rep. 2019, 9, 19651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, M.; Wang, D.; Li, Z.; Primo, F.L.; Tedesco, A.C.; Bi, H. Copper-Doped Carbon Dots for Optical Bioimaging and Photodynamic Therapy. Inorg. Chem. 2019, 58, 13394–13402. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu–N Dopants Boost Electron Transfer and Photooxidation Reactions of Carbon Dots. Angew. Chem. Int. Ed. 2015, 54, 6540–6544. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, Y.; Chen, S.; Zuo, W.; Shi, B. Cu-Doped Carbon Dots as Catalysts for the Chemiluminescence Detection of Glucose. ACS Omega 2019, 4, 9911–9917. [Google Scholar] [CrossRef]
- Guo, J.; Lu, W.; Zhang, H.; Meng, Y.; Du, F.; Shuang, S.; Dong, C. Copper doped carbon dots as the multi- functional fluorescent sensing platform for tetracyclines and pH. Sens. Actuators B Chem. 2021, 330, 129360. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mat. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Chen, S.; Jia, Q.; Zheng, X.; Wen, Y.; Liu, W.; Zhang, H.; Ge, J.; Wang, P. PEGylated carbon dot/MnO2 nanohybrid: A new pH/ H2O2-driven, turn-on cancer nanotheranostics. Sci. China Mater. 2018, 61, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Arsalani, N.; Nezhad-Mokhtari, P.; Jabbari, E. Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basavaraj, N.; Sekar, A.; Yadav, R. Review on green carbon dot-based materials for the photocatalytic degradation of dyes: Fundamentals and future perspective. Mater. Adv. 2021, 2, 7559. [Google Scholar] [CrossRef]
- Akbar, K.; Moretti, E.; Vomiero, A. Carbon Dots for Photocatalytic Degradation of Aqueous Pollutants: Recent Advancements. Adv. Opt. Mater. 2021, 9, 2100532. [Google Scholar] [CrossRef]
- Patel, S.K.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.K.; Kalia, V.C.; Kang, Y.C.; Lee, J.K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef]
- Tjahjanto, R.T.; Galuh, D.; Wardani, S. Ozone Determination: A Comparison of Quantitative Analysis Methods. J. Pure Appl. Chem. Res. 2012, 1, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Eastman, J.W. Quantitative Spectrofluorimetry—The Fluorescence Quantum Yield of Quinine Sulfate. Photochem. Photobiol. 1967, 6, 55–72. [Google Scholar] [CrossRef]
- El Hassani, K.; Kalnina, D.; Turks, M.; Beakou, B.H.; Anouar, A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Sep. Pur. Technol. 2019, 210, 764–774. [Google Scholar] [CrossRef]
- Razumovskii, S.D.; Konstantinova, M.L.; Grinevich, T.V.; Korovina, G.V.; Zaitsev, V.Y. Mechanism and Kinetics of the Reaction of Ozone with Sodium Chloride in Aqueous Solutions. Kin. Cat. 2010, 51, 492–496. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef]
- Massad-Ivanir, N.; Bhunia, S.K.; Raz, N.; Segal, E.; Jelinek, R. Synthesis and characterization of a nanostructured porous silicon/carbon dot-hybrid for orthogonal molecular detection. NPG Asia Mater. 2018, 10, e463. [Google Scholar] [CrossRef] [Green Version]
- Vrandecic, S.; Erceg, M.; Klaric, M. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Thermo Scientific Avantage Data System for XPS. Available online: https://www.jp.xpssimplified.com/periodictable.php (accessed on 11 March 2022).
- Qiu, T.; Yang, J.G.; Bai, X.J.; Wang, Y.L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737. [Google Scholar] [CrossRef] [Green Version]
- Ambalagi, S.M.; Devendrappa, M.; Nagaraja, S.; Sannakki, B. Dielectric Properties of PANI/CuO Nanocomposites. Mater. Sci. Eng. 2018, 310, 012081. [Google Scholar] [CrossRef]
- Szeto, W.; Li, J.; Huang, H.; Leung, D.Y.C. VUV/TiO2 photocatalytic oxidation process of methyl orange and simultaneous utilization of the lamp-generated ozone. Chem. Eng. Sci. 2018, 177, 380–390. [Google Scholar] [CrossRef]
- Medina, J.C.; Bizarro, M.; Silva-Bermudez, P.; Giorcelli, M.; Tagliaferro, A.; Rodil, S.E. Photocatalytic discoloration of methyl orange dye by δ-Bi2O3 thin films. Thin Solid Film. 2016, 612, 72–81. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, H.; Ma, J.; Kong, Y.; Qin, Y.; Komarneni, S. Decolorization of methyl orange by MnO2/organic acid system: The role of T Mn(III). Mat. Res. Bull. 2020, 122, 110670. [Google Scholar] [CrossRef]
- Quadrado, R.F.N.; Fajardo, A.R. Fast decolorization of azo methyl orange via heterogeneous Fenton and Fenton-like reactions using alginate-Fe2+/Fe3+ films as catalysts. Carbohydr. Polym. 2017, 177, 443–450. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.L. Discoloration and Mineralization of Orange II Using Different Heterogeneous Catalysts Containing Fe: A Comparative Study. Environ. Sci. Technol. 2004, 38, 5773–5778. [Google Scholar] [CrossRef]
- Feng, J.; Hu, X.; Yue, P.L. Novel Bentonite Clay-Based Fe-Nanocomposite as a Heterogeneous Catalyst for Photo-Fenton Discoloration and Mineralization of Orange II. Environ. Sci. Technol. 2004, 38, 269–275. [Google Scholar] [CrossRef]
- Feng, J.; Wong, R.S.K.; Hu, X.; Yue, P.L. Discoloration and mineralization of Orange II by using Fe3+-doped TiO2 and bentonite clay-based Fe nanocatalysts. Cat. Today 2004, 98, 441–446. [Google Scholar] [CrossRef]
- Domenzain-Gonzalez, J.; Castro-Arellano, J.J.; Galicia-Luna, L.A.; Lartundo-Rojas, L. Photo-Fenton Degradation of RB5 Dye in Aqueous Solution Using Fe Supported on Mexican Natural Zeolite. Int. J. Photoenergy 2019, 2019, 4981631. [Google Scholar] [CrossRef] [Green Version]
- Chong, M.N.; Tneu, Z.Y.; Poh, P.E.; Jin, B.; Aryal, R. Synthesis, characterisation and application of TiO2–zeolite nanocomposites for the advanced treatment of industrial dye wastewater. J. Taiwan Inst. Chem. Eng. 2015, 50, 288–296. [Google Scholar] [CrossRef]
- Alam, R.; Ardiati, F.C.; Solihat, N.N.; Alam, B.; Lee, S.H.; Yanto, D.H.Y.; Watanabe, T.; Kim, S. Biodegradation and metabolic pathway of anthraquinone dyes by Trametes hirsuta D7 immobilized in light expanded clay aggregate and cytotoxicity assessment. J. Hazard. Mater. 2021, 405, 124176. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Fulazzaky, M.A. Decolorization kinetics and mass transfer mechanisms of Remazol Brilliant Blue R dye mediated by different fungi. Biotech. Rep. 2021, 29, e00573. [Google Scholar] [CrossRef]
- Miranda, M.O.; Viana, B.C.; Honório, L.M.; Trigueiro, P.; Fonseca, M.G.; Franco, F.; Osajima, J.A.; Silva-Filho, E.C. Oxide-Clay Mineral as Photoactive Material for Dye Discoloration. Minerals 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Karcher, S.; Kornmüller, A.; Jekel, M. Screening of commercial sorbents for the removal of reactive dyes. Dyes Pigm. 2001, 51, 111–125. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Wat. Res. 2016, 98, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Attila Nemes, A.; Fábián, I.; van Eldik, R. Kinetics and Mechanism of the Carbonate Ion Inhibited Aqueous Ozone Decomposition. J. Phys. Chem. A 2000, 104, 7995–8000. [Google Scholar] [CrossRef]
- Aadnan, I.; Zegaoui, O.; El Mragui, A.; da Silva, J.C.G.G. Physicochemical and photocatalytic properties under visible light of ZnO-Bentonite/Chitosan hybrid-biocomposite for water remediation. Nanomaterials 2022, 12, 102. [Google Scholar] [CrossRef]
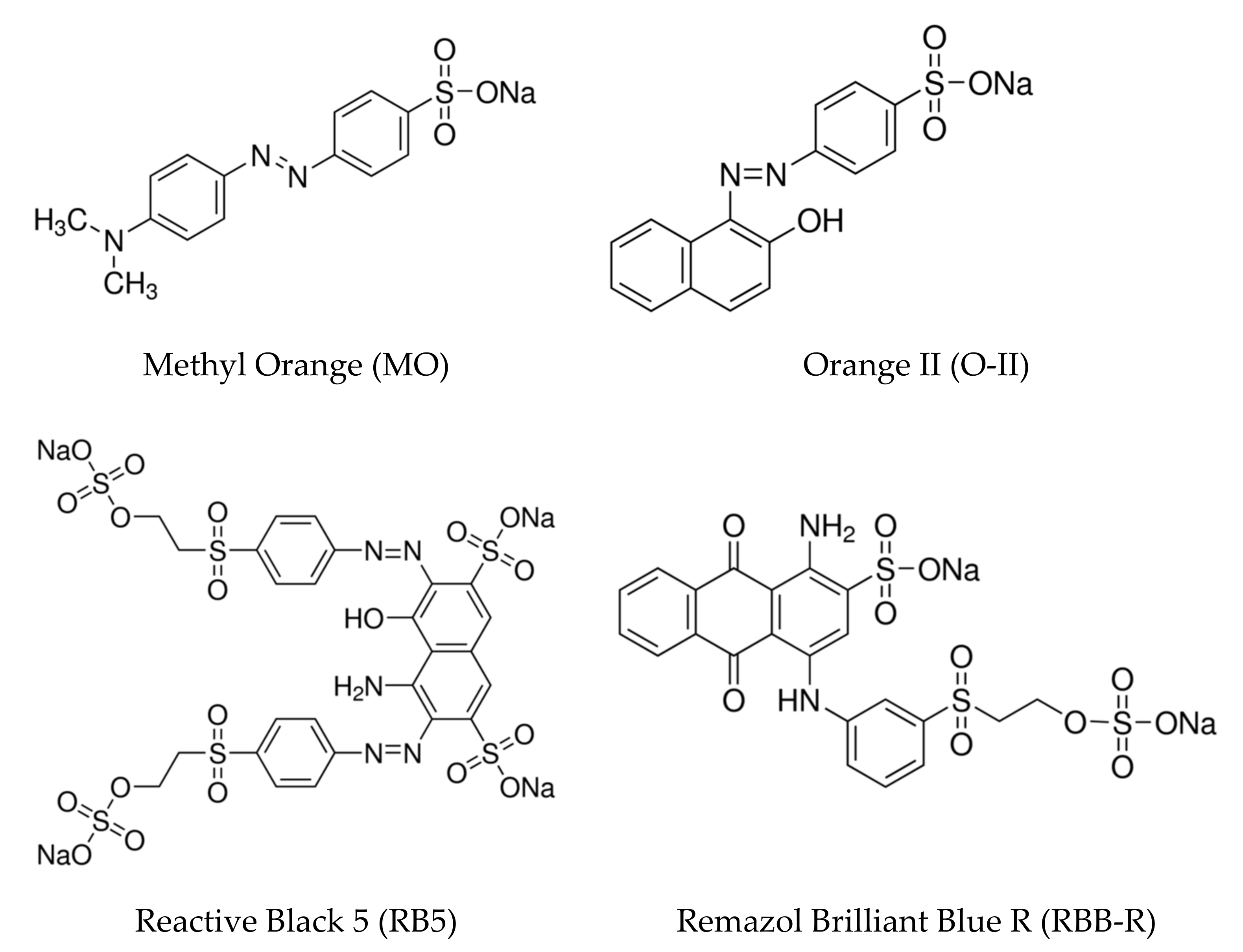
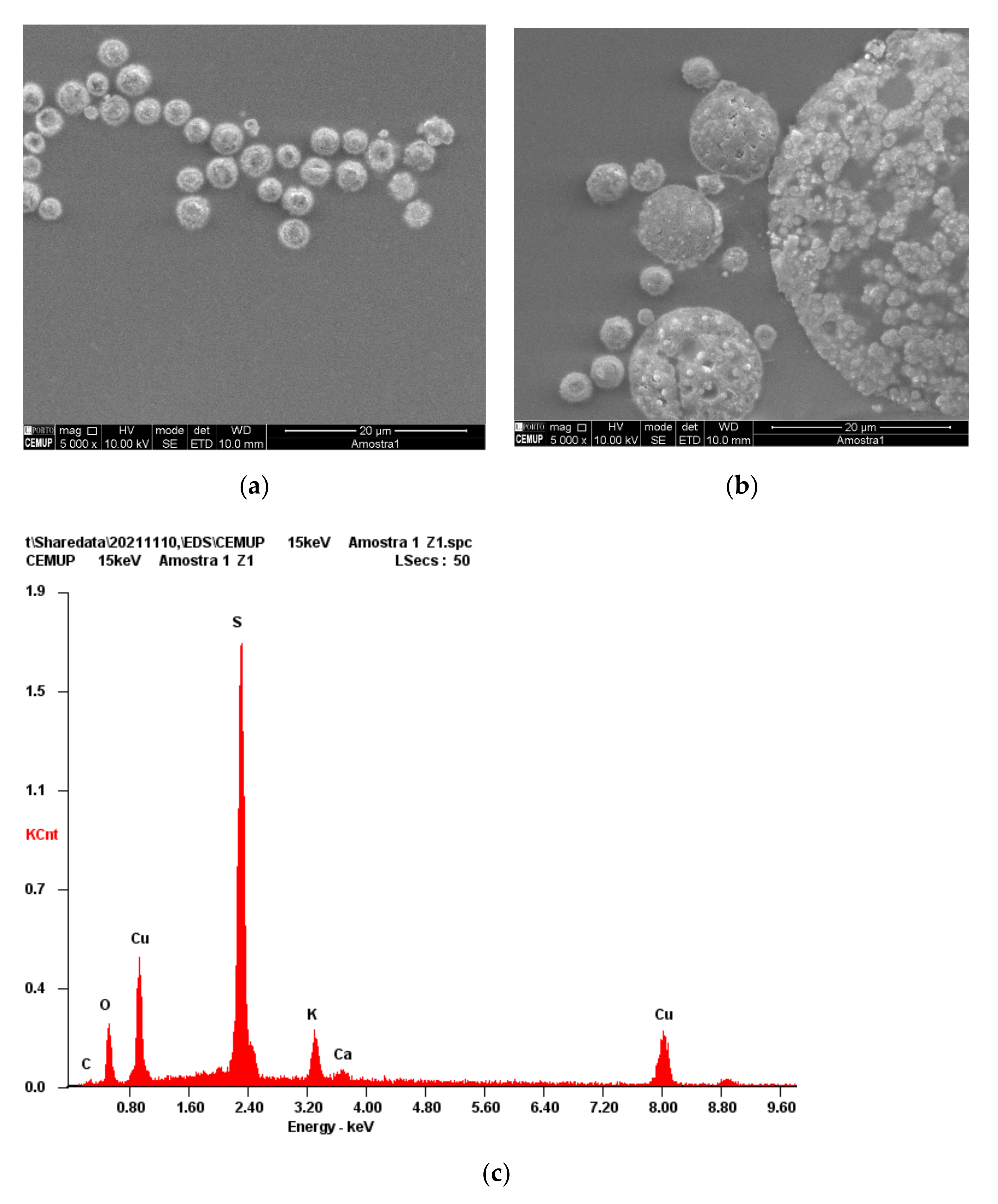
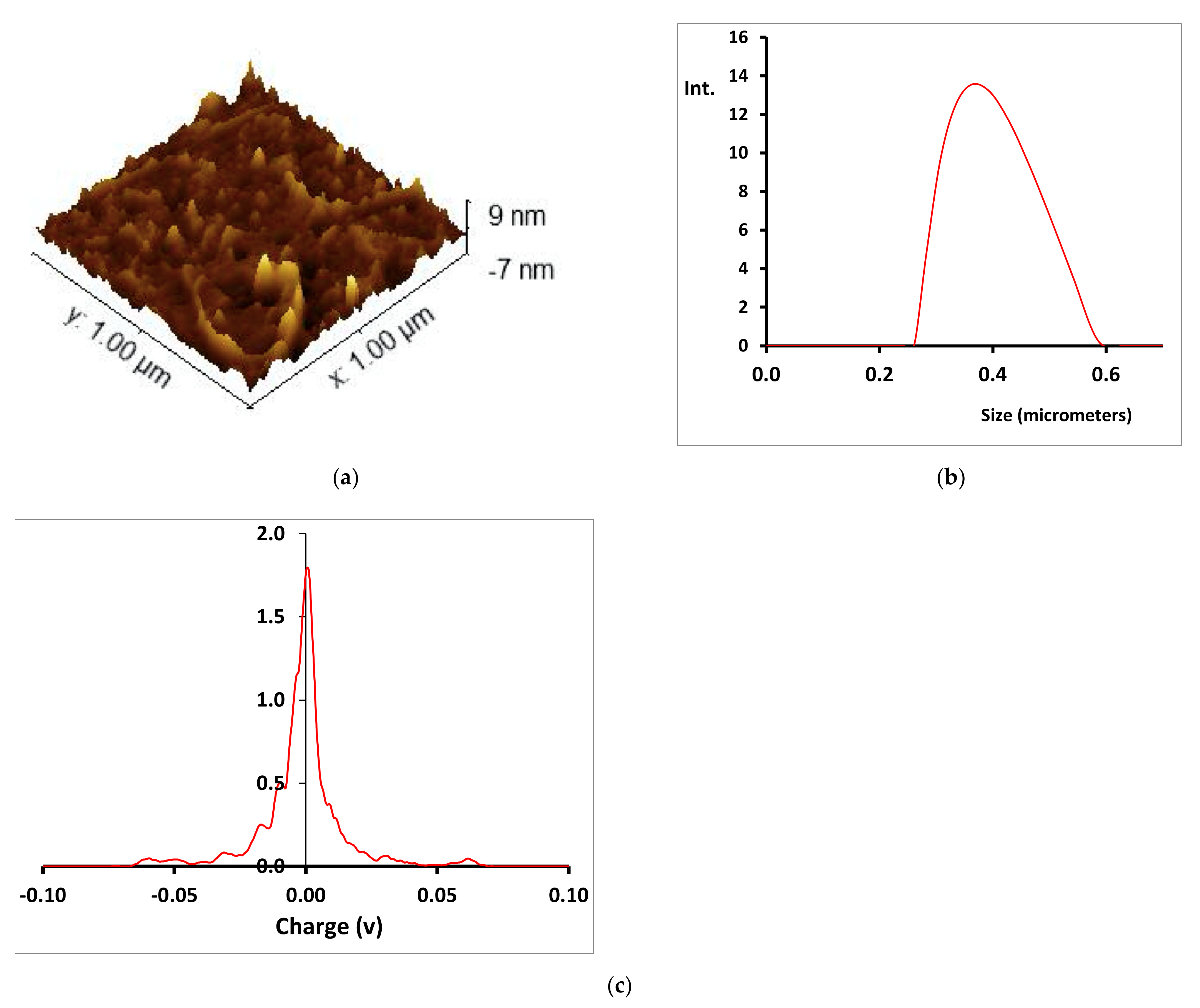

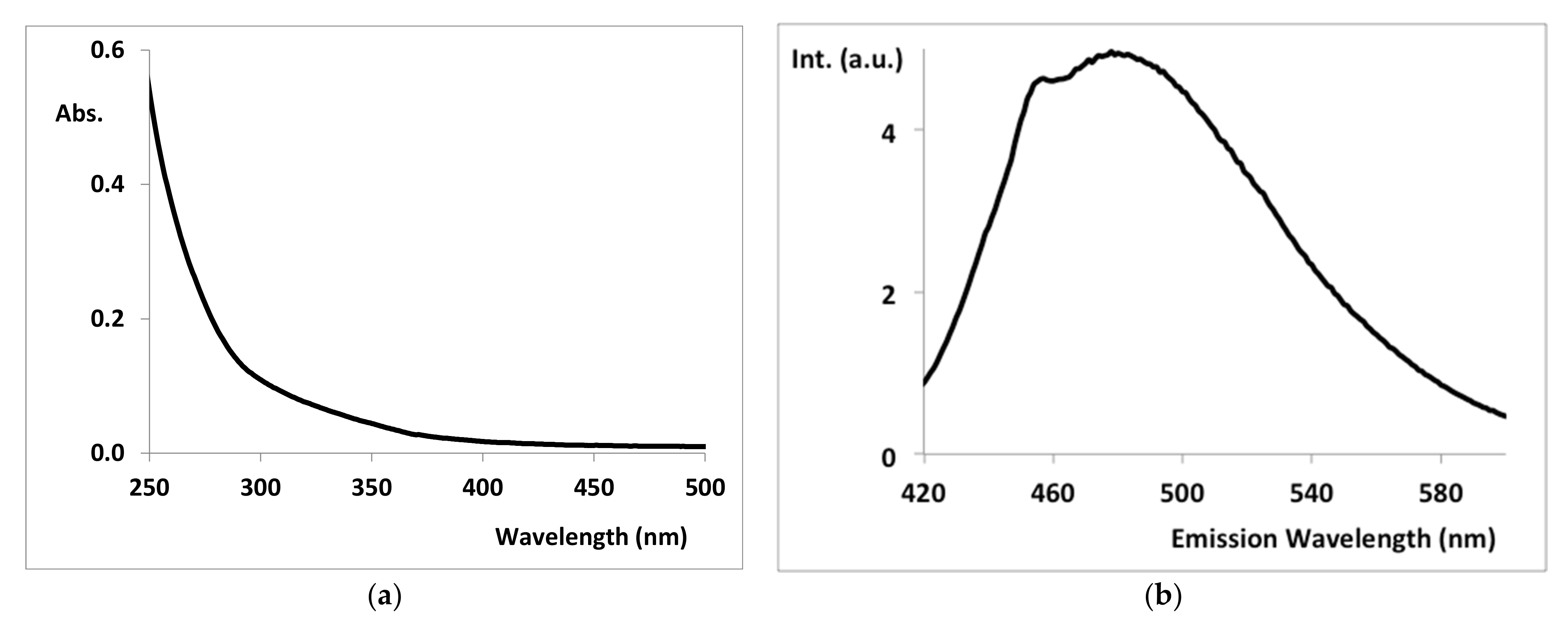

| Dye | kap (min−1) | %DR |
|---|---|---|
| MO | 0.210 ± 0.013 | 99.4 ± 0.3 (30 min) |
| MO + NACL | 0.631 ± 0.046 | 99.4 ± 0.1 (10 min) |
| O-II | 0.133 ± 0.021 | 97.9 ± 0.7 (30 min) |
| O-II + NACL | 0.642 ± 0.034 | 99.0 ± 0.4 (10 min) |
| RB-5 | 0.177 ± 0.031 | 98.4 ± 0.5 (30 min) |
| RB-5 + NACL | 1.013 ± 0.010 | 98.1 ± 0.1 (4 min) |
| RBB-R | 0.086 ± 0.009 | 91.4 ± 0.9 (30 min) |
| RBB-R + NACL | 0.123 ± 0.003 | 96.7 ± 0.5 (30 min) |
| Dye | kapc (min−1) | %Inc | %DR |
|---|---|---|---|
| MO + CU-CD | 1.184 ± 0.003 | 429 | 99.8 ± 0.2 (6 min) |
| MO + NACL + CU-CD | 0.705 ± 0.006 | 12 | 99.1 ± 0.1 (10 min) |
| O-II + CU-CD | 1.002 ± 0.036 | 653 | 99.3 ± 0.2 (6 min) |
| O-II + NACL + CU-CD | 0.971 ± 0.009 | 51 | 99.7 ± 0.2 (10 min) |
| RB-5 + CU-CD | 0.709 ± 0.029 | 301 | 80.1 ± 1.6 (6 min) |
| RB-5 + NACL + CU-CD | 1.057 ± 0.047 | 4 | 86.0 ± 3.1 (6 min) |
| RBB-R + CU-CD | 0.230 ± 0.004 | 167 | 99.1 ± 0.1 (30 min) |
| RBB-R + NACL + CU-CD | 0.221 ± 0.004 | 80 | 98.7 ± 0.2 (30 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, R.M.F.; Cardoso, I.M.F.; da Silva, L.P.; Esteves da Silva, J.C.G. Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes. Nanomaterials 2022, 12, 1211. https://doi.org/10.3390/nano12071211
Cardoso RMF, Cardoso IMF, da Silva LP, Esteves da Silva JCG. Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes. Nanomaterials. 2022; 12(7):1211. https://doi.org/10.3390/nano12071211
Chicago/Turabian StyleCardoso, Rita M. F., Inês M. F. Cardoso, Luís Pinto da Silva, and Joaquim C. G. Esteves da Silva. 2022. "Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes" Nanomaterials 12, no. 7: 1211. https://doi.org/10.3390/nano12071211
APA StyleCardoso, R. M. F., Cardoso, I. M. F., da Silva, L. P., & Esteves da Silva, J. C. G. (2022). Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes. Nanomaterials, 12(7), 1211. https://doi.org/10.3390/nano12071211








