Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Conditions
2.3. Determination of the Growth Indicators
2.4. Na+, K+, and Ca2+ Analysis
2.5. Statistical Analysis
3. Results
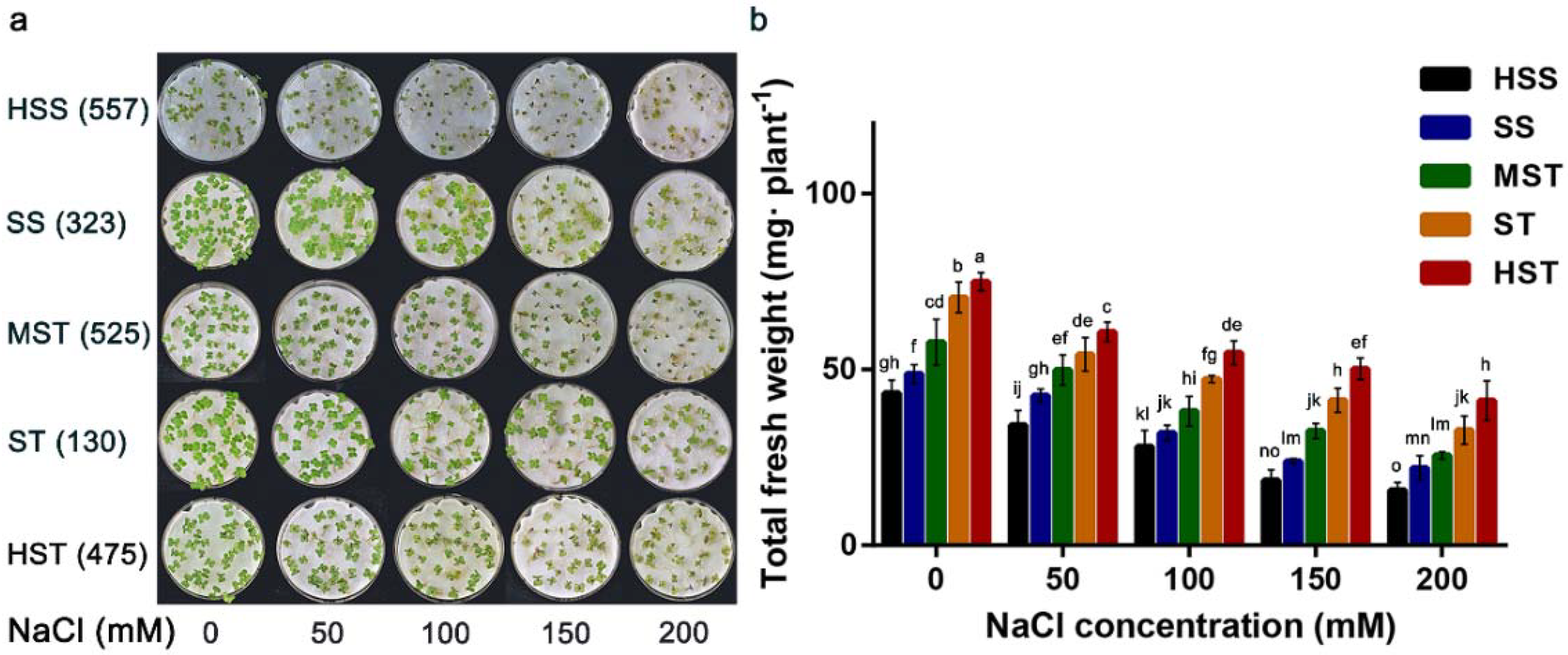
3.1. Growth under Varying Degrees of NaCl Stress
3.2. Correlation between the Relative Values of the Growth Indicators and NaCl Concentrations
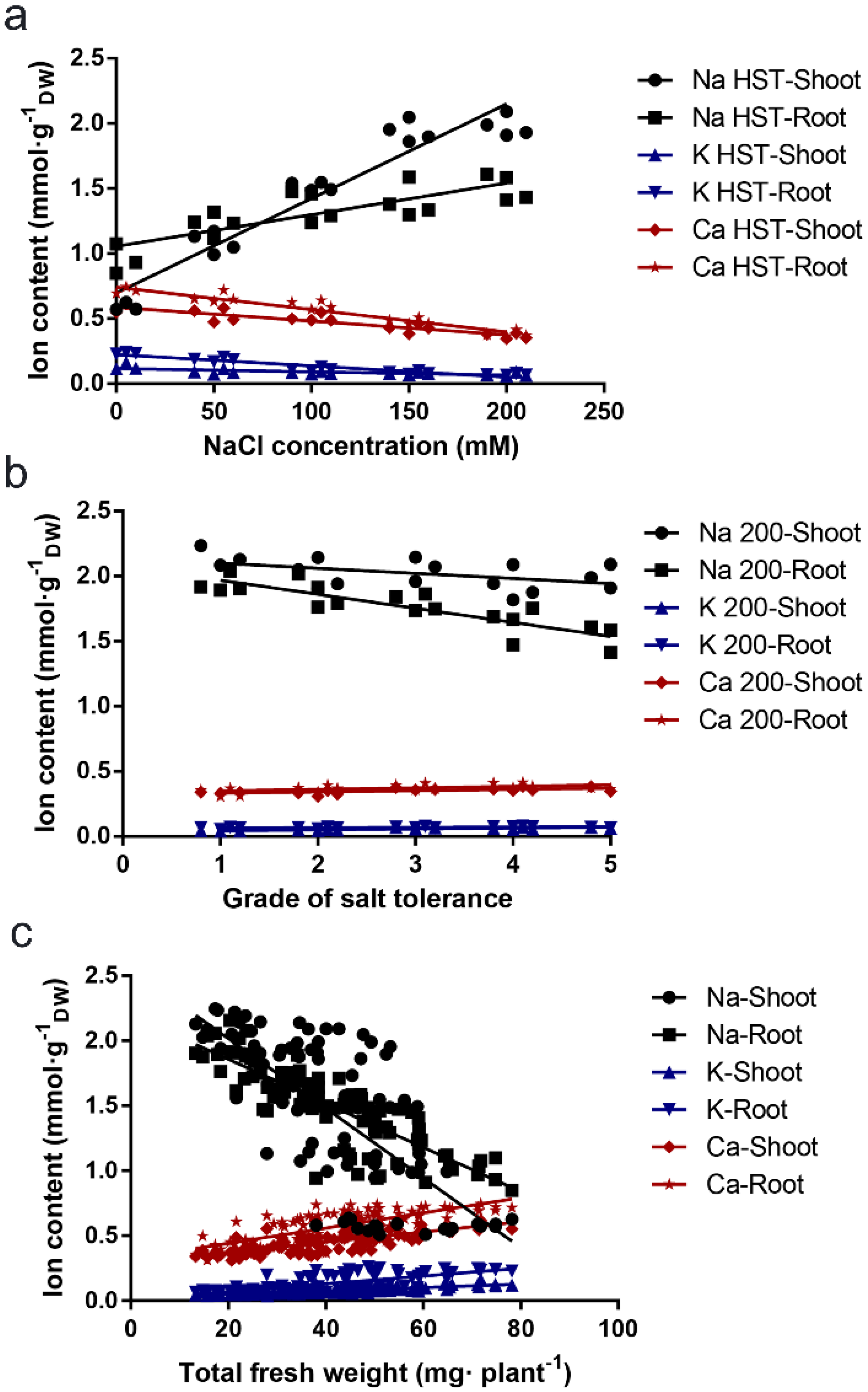
3.3. Correlation between the Na+, Ca2+, and K+ Content Parameters and the B. napus Salt Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, X.D.; Zhou, J.X.; Liu, G.H.; Li, D.X. World resources of saline soil and main amelioration measures. Res. Soil Water Conserv. 2005, 12, 28–30. [Google Scholar]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Kovda, V.A. Loss of productive land due to salinization. AMBIO A J. Hum. Environ. 1983, 12, 91–93. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ayyub, C.M.; Ali, M.; Shaheen, M.R.; Qadri, R.W.K.; Khan, I.; Jahangir, M.M.; Abbasi, K.Y.; Kamal, S.; Zain, M. Enhancing the salt tolerance potential of watermelon (Citrullus lanatus) by exogenous application of salicylic acid. Am. J. Plant Sci. 2015, 6, 3267–3271. [Google Scholar] [CrossRef][Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Wang, B.S.; Zhao, K.F. Comparison of extractive methods of Na, K in wheat leaves. Plant Physiol. Commun. 1995, 31, 50–52. [Google Scholar]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in china: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Zhong, T.; Chen, Z. Review on sustainable utilization of salt-affected land. Acta Geogr. Sin. 2011, 66, 673–684. [Google Scholar]
- Tarchoune, I.; Degl’Innocenti, E.; Kaddour, R.; Guidi, L.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. Acta Physiol. Plant. 2012, 34, 607–615. [Google Scholar] [CrossRef]
- Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Fouilleux, E.; Bricas, N.; Alpha, A. ‘Feeding 9 billion people’: Global food security debates and the productionist trap. J. Eur. Public Policy 2017, 24, 1658–1677. [Google Scholar] [CrossRef]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive physiology of halophytes: Current standing. Front. Plant Sci. 2019, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Yuan, F.; Guo, J.; Zhang, Y.; Sui, N.; Wang, B. Atsiz1 improves salt tolerance by maintaining ionic homeostasis and osmotic balance in Arabidopsis. Plant Sci. 2019, 285, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Bañuelos, G.S. Selection of salt and boron tolerant selenium hyperaccumulator stanleya pinnata genotypes and characterization of se phytoremediation from agricultural drainage sediments. Environ. Sci. Technol. 2011, 45, 9703–9710. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Ma, L.; Yang, Z.; Dong, Q.; Li, Q.; Ni, X.; Kudla, J.; Song, C.; Guo, Y. The Ca2+ sensor SCaBP3/CBL7 modulates plasma membrane H+-ATPase activity and promotes alkali tolerance in Arabidopsis. Plant Cell 2019, 31, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.H.; Foster, K.J.; et al. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Shannon, M.C. Salinity effects on seedling growth and yield components of rice. Crop Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Liu, C.; Tang, D.Y.; Yan, L.; Wang, D.; Yang, Y.Z.; Gui, J.S.; Zhao, X.Y.; Li, L.G.; Tang, X.D.; et al. The receptor-like cytoplasmic kinase STRK1 phosphorylates and activates CatC, thereby regulating H2O2 homeostasis and improving salt tolerance in rice. Plant Cell 2018, 30, 1100–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001, 4, 401–406. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 1997, 94, 14960–14964. [Google Scholar] [CrossRef]
- Jamil, M.; Lee, C.C.; Rehman, S.U.; Lee, D.B.; Ashraf, M.; Rha, E.S. Salinity (NaCl) tolerance of Brassica species at germination and early seedling growth. Electron. J. Environ. Agric. Food Chem. 2005, 4, 970–976. [Google Scholar]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.A.; Garcia, M.; Tilbrook, J.; Brien, C.; Dowling, K.; Berger, B.; Schilling, R.K.; Short, L.; Trittermann, C.; Gilliham, M.; et al. Identification of salt tolerance qtl in a wheat ril mapping population using destructive and non-destructive phenotyping. Funct. Plant Biol. 2021, 48, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-S.; Liu, H.-Y.; Long, X.-H.; Liu, Z.-P.; Niu, D.-D.; Gao, Y.-Y. Effects of salt stress on ionic absorption and distribution of rapeseed seedlings. Chin. J. Oil Crop. Sci. 2010, 32, 65–70. [Google Scholar]
- Shang, X.F.; Dong, S.T.; Zheng, S.Y.; Wang, L.Y. Relationship between changes of Na+, K+, and Ca2+ contents during seed germination and salt tolerance in maize. Acta Agron. Sin. 2008, 34, 333–336. [Google Scholar] [CrossRef]
- Akter, M.; Oue, H. Effect of saline irrigation on accumulation of Na+, K+, Ca2+, and Mg2+ ions in rice plants. Agriculture 2018, 8, 164. [Google Scholar] [CrossRef]
- Zhang, F.; Sapkota, S.; Neupane, A.; Yu, J.; Wang, Y.; Zhu, K.; Lu, F.; Huang, R.; Zou, J. Effect of salt stress on growth and physiological parameters of sorghum genotypes at an early growth stage. Indian J. Exp. Biol. 2020, 58, 404–411. [Google Scholar]
- Harwood, J.L.; Woodfield, H.K.; Chen, G.; Weselake, R.J. Chapter 6—Modification of oil crops to produce fatty acids for industrial applications. In Fatty Acids; Ahmad, M.U., Ed.; AOCS Press, Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 187–236. [Google Scholar]
- Zubair, M.; Maqbool, F.; Mehmood, I.; Muzammil, S.; Waseem, M.; Imran, M.; Nadeem, H.U.; Azeem, F.; Siddique, M.H. Chapter 4–Rapeseed oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–55. [Google Scholar]
- Sun, R. Economic/academic importance of Brassica rapa. In The Brassica Rapa Genome; Wang, X., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–15. [Google Scholar]
- Neik, T.X.; Barbetti, M.J.; Batley, J. Current status and challenges in identifying disease resistance genes in Brassica napus. Front. Plant Sci. 2017, 8, 1–37. [Google Scholar] [CrossRef]
- Ashraf, M.; Mcneilly, T. Salinity tolerance in brassica oilseeds. Crit. Rev. Plant Sci. 2004, 23, 157–174. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual exposure to salinity improves tolerance to salt stress in rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Rezaei, H.; Khosh Kholgh Sima, N.A.; Malakouti, M.J.; Pessarakli, M. Salt tolerance of canola in relation to accumulation and xylem transportation of cations. J. Plant Nutr. 2006, 29, 1903–1917. [Google Scholar] [CrossRef]
- Yahya, A. Salinity effects on growth and on uptake and distribution of sodium and some essential mineral nutrients in sesame. J. Plant Nutr. 1998, 21, 1439–1451. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.; Wang, C.; Li, K.; Zhang, X.; Yang, Z.; Li, M.; Wang, B. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Flower, T.J.; Yeo, A.R. Ion relations of salt tolerance. In Solute Transport in Plant Cells and Tissues; Baker, D.A., Hall, J.L., Eds.; John Wiley & Sons: New York, NY, USA, 1988; pp. 392–416. [Google Scholar]
- Pitman, M.G. Transport across the root and shoot/root interactions. In Salinity Tolerance in Plants; Staples, R.C., Toenniessen, G.H., Eds.; John Wiley & Sons: New York, NY, USA, 1984; pp. 93–124. [Google Scholar]
- Swift, M.L. Graphpad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Han, G.; Wei, X.; Dong, X.; Wang, C.; Sui, N.; Guo, J.; Yuan, F.; Gong, Z.; Li, X.; Zhang, Y. Arabidopsis zinc finger protein1 acts downstream of GL2 to repress root hair initiation and elongation by directly suppressing bHLH genes. Plant Cell 2020, 32, 206–225. [Google Scholar] [CrossRef]
- Nissen, P. Multiphasic uptake mechanisms in plants. Int. Rev. Cytol. Surv. Cell Biol. 1991, 126, 89–134. [Google Scholar]
- Mäser, P.; Gierth, M.; Schroeder, J.I. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 2002, 247, 43–54. [Google Scholar] [CrossRef]
- Kudo, N.; Sugino, T.; Oka, M.; Fujiyama, H. Sodium tolerance of plants in relation to ionic balance and the absorption ability of microelements. Soil Sci. Plant Nutr. 2010, 56, 225–233. [Google Scholar] [CrossRef]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.-W.; Li, X.-W.; Li, H.-Y. Adaptation of plants to salt stress: Characterization of Na+ and K+ transporters and role of cbl gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Errabii, T.; Gandonou, C.B.; Bouhdid, S.; Abrini, J.; Skali-Senhaji, N. Callus growth and ion composition in response to long-term NaCl-induced stress in two sugarcane (Saccharum sp.) cultivars. Int. J. Biotechnol. Mol. Biol. Res. 2017, 8, 1–9. [Google Scholar]
- Zhang, J.-L.; Flowers, T.J.; Wang, S.-M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Wang, B.S. Plant Biology under Stress, 1st ed.; Higher Education Press: Beijing, China, 2010; p. 57. [Google Scholar]
- Peng, Y.-H.; Zhu, Y.-F.; Mao, Y.-Q.; Wang, S.-M.; Su, W.-A.; Tang, Z.-C. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J. Exp. Bot. 2004, 55, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, S.M.; Kim, B.K.; Yoon, I.S.; Kwon, T.R. Identification of rice accessions associated with K+/Na+ ratio and salt tolerance based on physiological and molecular responses. Rice Sci. 2017, 24, 360–364. [Google Scholar] [CrossRef]
- Tahal, R.; Mills, D.; Heimer, Y.; Tal, M. The relation between low K+/Na+ ratio and salt-tolerance in the wild tomato species Lycopersicon pennellii. J. Plant Physiol. 2000, 157, 59–64. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.A.; Desoky, E.-S.M.; Ali, M.M.A.; Yasin, M.A.T.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Rameeh, V.; Cherati, A. Salinity stress indices of seed yield and nutrient compositions in rapeseed (Brassica napus L.). Int. J. Biol. 2012, 4, 154. [Google Scholar] [CrossRef]
- Long, W.H.; Pu, H.M.; Chen, S.; Zhang, J.F.; Qi, C.K.; Zhang, X.K. Evaluation for salt tolerance of three cultivated species of rapeseeds at germination stage. J. Plant Genet. Resour. 2014, 15, 32–37. [Google Scholar]
- Liu, Y.; Wang, X.; Liu, G.; Sun, J.; Yao, Y.; Yang, Y. Ions absorption and accumulation in four typical salt-tolerant plants in coastal saline soil region. Soils 2017, 49, 782–788. [Google Scholar]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric oxide regulates plant growth, physiology, antioxidant defense, and ion homeostasis to confer salt tolerance in the mangrove species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef]
- Rameeh, V. Ions uptake, yield and yield attributes of rapeseed exposed to salinity stress. J. Soil Sci. Plant Nutr. 2012, 12, 851–861. [Google Scholar] [CrossRef]
- Robbins, N.E.; Trontin, C.; Duan, L.; Dinneny, J.R. Beyond the barrier: Communication in the root through the endodermis. Plant Physiol. 2014, 166, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Muthert, L.W.F.; Izzo, L.G.; Zanten, M.V.; Aronne, G. Root tropisms: Investigations on earth and in space to unravel plant growth direction. Front. Plant Sci. 2020, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Elsheikh, E.A.E.; Wood, M. Effect of salinity on growth, nodulation and nitrogen yield of chickpea (Cicer arietinum L.). J. Exp. Bot. 1990, 41, 1263–1269. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, W.; Ren, J.; Zhang, C. Selectivity of various types of salt-resistant plants for K+ over Na+. J. Arid. Environ. 2002, 52, 457–472. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H⁺-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. Ros homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2013, 65, 1241–1257. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T.S. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; Boer, G.J.D.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef]
- Ottow, E.A.; Brinker, M.; Teichmann, T.; Fritz, E.; Kaiser, W.; Brosché, M.; Kangasjärvi, J.; Jiang, X.; Polle, A. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol. 2005, 139, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Dahuja, A.; Kumar, R.R.; Sakhare, A.; Watts, A.; Singh, B.; Goswami, S.; Sachdev, A.; Praveen, S. Role of atp-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol. Plant. 2021, 171, 785–801. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wei, X.; Fan, H.; Wang, B. Adaptation mechanism of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhao, S.; Gao, H.; Sun, S. The salt resistance of wild soybean (Glycine soja sieb. Et zucc. Zyd 03262) under NaCl stress is mainly determined by Na+ distribution in the plant. Acta Physiol. Plant. 2014, 36, 61–70. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ranathunge, K.; Franke, R.; Prakash, H.S.; Schreiber, L.; Mathew, M.K. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 2009, 230, 119–134. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ranathunge, K.; Nayak, S.; Schreiber, L.; Mathew, M.K. Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 4215–4228. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Jyothi-Prakash, P.A.; Qin, L.; He, J.; Lin, Q.; Loh, C.-S.; Kumar, P.P. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ. 2014, 37, 1656–1671. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Matoh, T. Characterization of the na+ delivery from roots to shoots in rice under saline stress: Excessive salt enhances apoplastic transport in rice plants. Soil Sci. Plant Nutr. 2002, 48, 371–378. [Google Scholar] [CrossRef]
- Cui, B.; Liu, R.; Flowers, T.J.; Song, J. Casparian bands and suberin lamellae: Key targets for breeding salt tolerant crops? Environ. Exp. Bot. 2021, 191, 104600. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Mishra, A.K.; Kumar, K. Molecular insights into the role of plant transporters in salt stress response. Physiol. Plant. 2021, 173, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Cai, J.; Zhan, E.; Yang, Y.; Zhao, J.; Guo, Y.; Zhou, H. Stability and localization of 14-3-3 proteins are involved in salt tolerance in Arabidopsis. Plant Mol. Biol. 2016, 92, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Brini, F.; Masmoudi, K. Ion transporters and abiotic stress tolerance in plants. ISRN Mol. Biol. 2012, 2012, 927436. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Chattaopadhyay, K.; Nayak, L.; Ray, S.; Yeasmin, L.; Jena, P.; Gupta, S.; Mohanty, S.K.; Swain, P.; Sarkar, R.K. Ionic selectivity and coordinated transport of Na+ and K+ in flag leaves render differential salt tolerance in rice at the reproductive stage. Planta 2019, 250, 1637–1653. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Naz, S.; Sehar, S.; Cao, F.; Wu, F. Resemblance and difference of seedling metabolic and transporter gene expression in high tolerance wheat and barley cultivars in response to salinity stress. Plants 2020, 9, 519. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Wang, R.L.; Liu, Y.L. Effects of Ca2+ on absorption and distribution of ions in salt-treated cotton seedlings. Acta Phytophysiol. Sin. 2001, 27, 325–330. [Google Scholar]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Chen, T.; Wang, W.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. K+ and Na+ transport contribute to K+/Na+ homeostasis in Pyropia haitanensis under hypersaline stress. Algal Res. 2019, 40, 101526. [Google Scholar] [CrossRef]
- Sen, A. Integrative expressional regulation of TaHKT2;1, TaNa+/H+ vacuolar antiporter, and TaSOS1 genes improve salt tolerance in gamma-ray induced bread wheat mutants. Cereal Res. Commun. 2021, 49, 599–606. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium transport in plant cells. Biochim. Biophys. Acta 2000, 1465, 140–151. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2014, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]

| Na | K | Ca | ||||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | |
| HSS | 0.94 ** | 0.85 ** | 0.71 ** | 0.89 ** | 0.90 ** | 0.95 ** |
| SS | 0.91 ** | 0.80 ** | 0.75 ** | 0.88 ** | 0.88 ** | 0.93 ** |
| MST | 0.95 ** | 0.85 ** | 0.72 ** | 0.89 ** | 0.90 ** | 0.93 ** |
| ST | 0.92 ** | 0.79 ** | 0.76 ** | 0.84** | 0.89 ** | 0.90 ** |
| HST | 0.94 ** | 0.71 ** | 0.56 ** | 0.91 ** | 0.85 ** | 0.93 ** |
| K/Na | Ca/Na | SK, Na | SCa, Na | |||
| Shoot | Root | Shoot | Root | |||
| HSS | 0.70 ** | 0.82 ** | 0.78 ** | 0.90 ** | 0.10 | 0.57 ** |
| SS | 0.68 ** | 0.82 ** | 0.76 ** | 0.90 ** | 0.04 | 0.57 ** |
| MST | 0.74 ** | 0.84 ** | 0.81 ** | 0.90 ** | 2.51 × 10−4 | 0.65 ** |
| ST | 0.72 ** | 0.81 ** | 0.81 ** | 0.94 ** | 0.20 | 0.65 ** |
| HST | 0.67 ** | 0.86 ** | 0.80 ** | 0.92 ** | 0.02 | 0.60 ** |
| SNa | SK | SCa | ||||
| HSS | 0.85 ** | 0.49 ** | 0.30 * | |||
| SS | 0.86 ** | 0.70 ** | 0.30 * | |||
| MST | 0.88 ** | 0.71 ** | 0.64 ** | |||
| ST | 0.90 ** | 0.60 ** | 0.31 * | |||
| HST | 0.80 ** | 0.69 ** | 0.41 ** | |||
| Na | K | Ca | ||||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | |
| 0 | 6.04 × 10−6 | 0.06 | 0.34 ** | 0.02 | 0.01 | 0.08 |
| 50 | 0.09 | 0.67 ** | 0.32 ** | 0.02 | 0.08 | 0.15 |
| 100 | 0.25 * | 0.61 ** | 0.56 ** | 0.10 | 0.35 ** | 0.20 * |
| 150 | 0.19 | 0.74 ** | 0.60 ** | 0.18 | 0.35 ** | 0.27 * |
| 200 | 0.25 * | 0.75 ** | 0.65 ** | 0.26 * | 0.44 ** | 0.33 ** |
| K/Na | Ca/Na | SK, Na | SCa, Na | |||
| Shoot | Root | Shoot | Root | |||
| 0 | 0.30 * | 0.10 | 1.54 × 10−3 | 0.10 | 0.11 | 0.07 |
| 50 | 0.31 * | 0.53 ** | 0.15 | 0.69 ** | 0.06 | 0.13 |
| 100 | 0.59 ** | 0.36 ** | 0.44 ** | 0.77 ** | 0.13 | 0.02 |
| 150 | 0.65 ** | 0.69 ** | 0.41 ** | 0.78 ** | 0.02 | 0.54 ** |
| 200 | 0.74 ** | 0.73 ** | 0.49 ** | 0.72 ** | 0.19 | 0.34 * |
| SNa | SK | SCa | ||||
| 0 | 0.07 | 0.26 * | 4.28 × 10−3 | |||
| 50 | 0.24 * | 0.40 ** | 0.01 | |||
| 100 | 0.32 ** | 0.35 ** | 0.11 | |||
| 150 | 0.50 ** | 0.39 ** | 0.01 | |||
| 200 | 0.45 ** | 0.44 ** | 2.93 × 10−3 | |||
| Na | K | Ca | ||||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | |
| TFW | 0.53 ** | 0.71 ** | 0.64 ** | 0.49 ** | 0.55 ** | 0.50 ** |
| TDW | 0.38 ** | 0.48 ** | 0.44 ** | 0.31 ** | 0.34 ** | 0.38 ** |
| SFW | 0.38 ** | 0.58 ** | 0.55 ** | 0.34 ** | 0.40 ** | 0.36 ** |
| RFW | 0.69 ** | 0.73 ** | 0.63 ** | 0.68 ** | 0.68 ** | 0.64 ** |
| SDW | 0.19 ** | 0.27 ** | 0.25 ** | 0.14 ** | 0.15 ** | 0.20 ** |
| RDW | 0.65 ** | 0.72 ** | 0.62 ** | 0.63 ** | 0.67 ** | 0.62 ** |
| SL | 0.57 ** | 0.46 ** | 0.55 ** | 0.54 ** | 0.58 ** | 0.49 ** |
| RL | 0.76 ** | 0.70 ** | 0.63 ** | 0.75 ** | 0.73 ** | 0.75 ** |
| S-DW/FW | 0.41 ** | 0.66 ** | 0.55 ** | 0.39 ** | 0.51 ** | 0.45 ** |
| R-DW/FW | 0.44 ** | 0.36 ** | 0.22 ** | 0.42 ** | 0.36 ** | 0.41 ** |
| K/Na | Ca/Na | SK, Na | SCa, Na | |||
| Shoot | Root | Shoot | Root | |||
| TFW | 0.50 ** | 0.54 ** | 0.48 ** | 0.63 ** | 0.11 ** | 0.24 ** |
| TDW | 0.31 ** | 0.34 ** | 0.30 ** | 0.42 ** | 0.11 ** | 0.14 ** |
| SFW | 0.36 ** | 0.38 ** | 0.33 ** | 0.48 ** | 0.11 ** | 0.14 ** |
| RFW | 0.62 ** | 0.71 ** | 0.64 ** | 0.77 ** | 0.07 ** | 0.41 ** |
| SDW | 0.15 ** | 0.15 ** | 0.13 ** | 0.21 ** | 0.09 ** | 0.05 * |
| RDW | 0.58 ** | 0.68 ** | 0.60 ** | 0.76 ** | 0.07 ** | 0.34 ** |
| SL | 0.57 ** | 0.54 ** | 0.58 ** | 0.55 ** | 0.16 ** | 0.49 ** |
| RL | 0.64 ** | 0.72 ** | 0.68 ** | 0.78 ** | 0.07 ** | 0.47 ** |
| S-DW/FW | 0.36 ** | 0.42 ** | 0.36 ** | 0.53 ** | 0.06 * | 0.14 ** |
| R-DW/FW | 0.28 ** | 0.36 ** | 0.34 ** | 0.38 ** | 1.81 × 10−3 | 0.27 ** |
| SNa | SK | SCa | ||||
| TFW | 0.24 ** | 0.08 ** | 0.11 ** | |||
| TDW | 0.17 ** | 0.04 | 0.14 ** | |||
| SFW | 0.13 ** | 0.02 | 0.08 ** | |||
| RFW | 0.44 ** | 0.25 ** | 0.14 ** | |||
| SDW | 0.07 ** | 2.19 × 10−3 | 0.10 ** | |||
| RDW | 0.38 ** | 0.20 ** | 0.13 ** | |||
| SL | 0.46 ** | 0.18 ** | 0.09 ** | |||
| RL | 0.54 ** | 0.32 ** | 0.23 ** | |||
| S-DW/FW | 0.13 ** | 0.04 * | 0.10 ** | |||
| R-DW/FW | 0.33 ** | 0.31 ** | 0.14 ** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-F.; Han, G.-L.; Qiao, Z.-Q.; Li, Y.-X.; Yang, Z.-R.; Wang, B.-S. Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings. Plants 2022, 11, 906. https://doi.org/10.3390/plants11070906
Wang C-F, Han G-L, Qiao Z-Q, Li Y-X, Yang Z-R, Wang B-S. Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings. Plants. 2022; 11(7):906. https://doi.org/10.3390/plants11070906
Chicago/Turabian StyleWang, Cheng-Feng, Guo-Liang Han, Zi-Qi Qiao, Yu-Xia Li, Zong-Ran Yang, and Bao-Shan Wang. 2022. "Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings" Plants 11, no. 7: 906. https://doi.org/10.3390/plants11070906
APA StyleWang, C.-F., Han, G.-L., Qiao, Z.-Q., Li, Y.-X., Yang, Z.-R., & Wang, B.-S. (2022). Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings. Plants, 11(7), 906. https://doi.org/10.3390/plants11070906