Monitoring Colletotrichum Colonization and Reproduction in Different Rubber Tree Clones
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials from Rubber Trees and C. tamarilloi Isolate
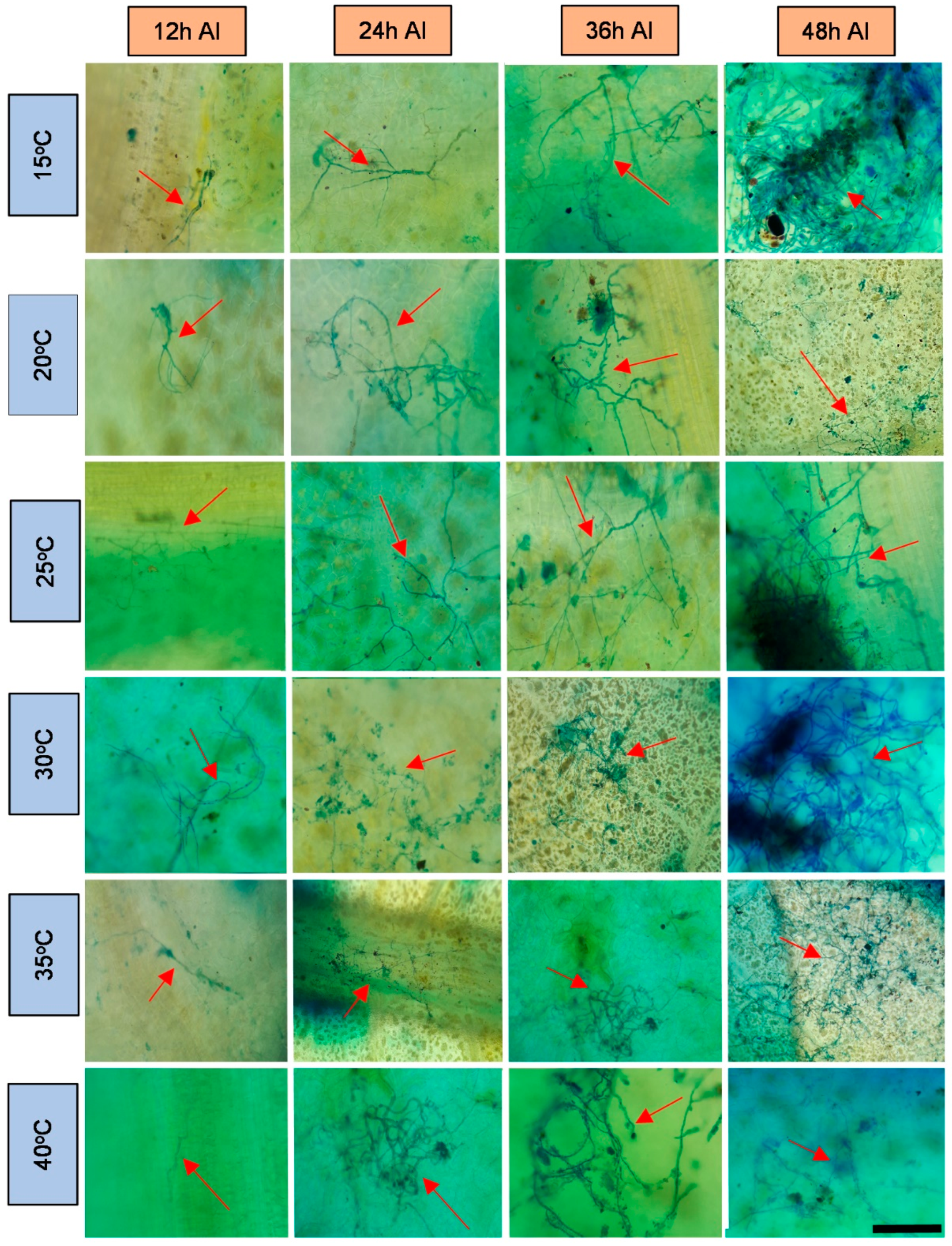
2.2. Preparation and Evaluation of Samples under a Light Optical Microscope
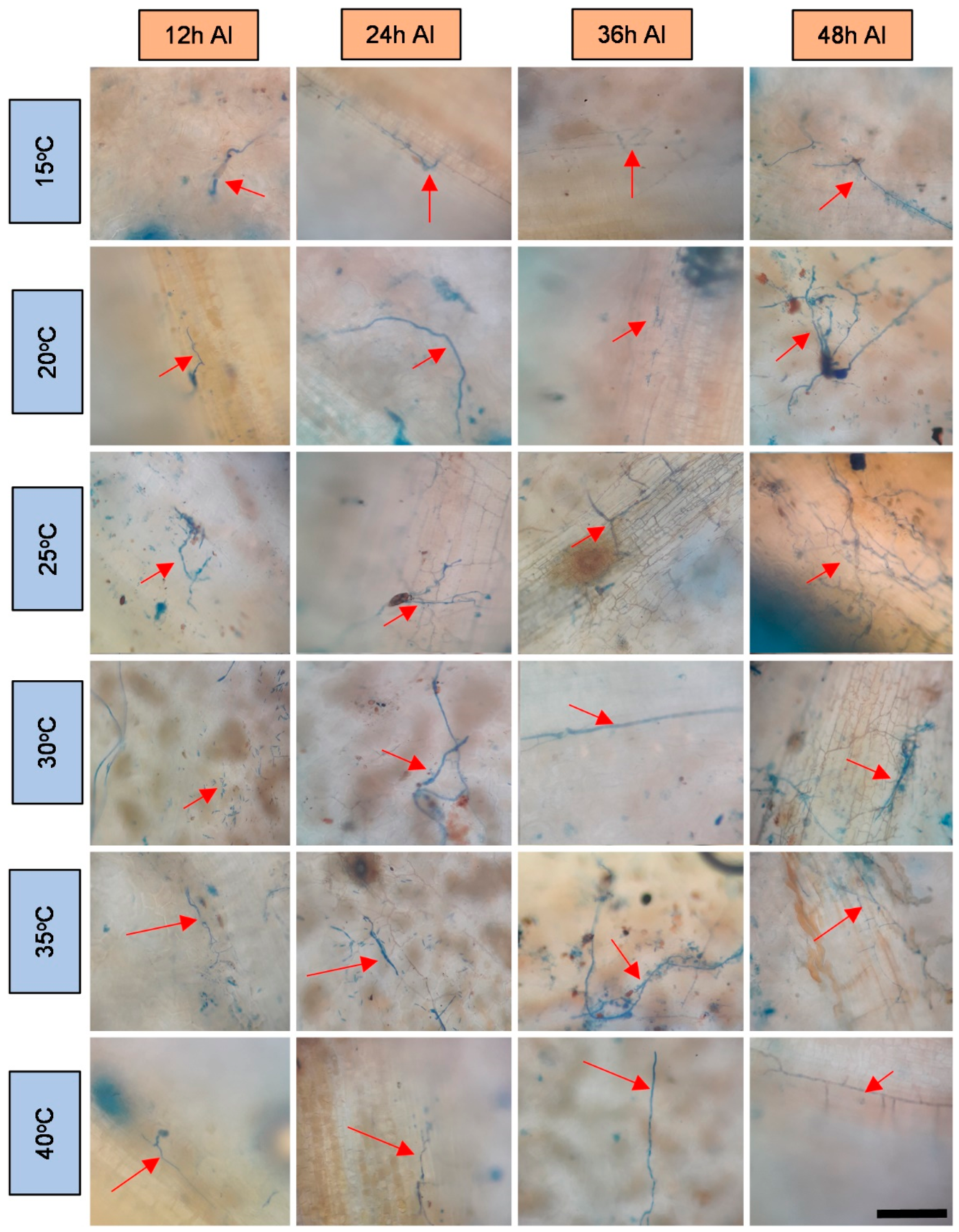
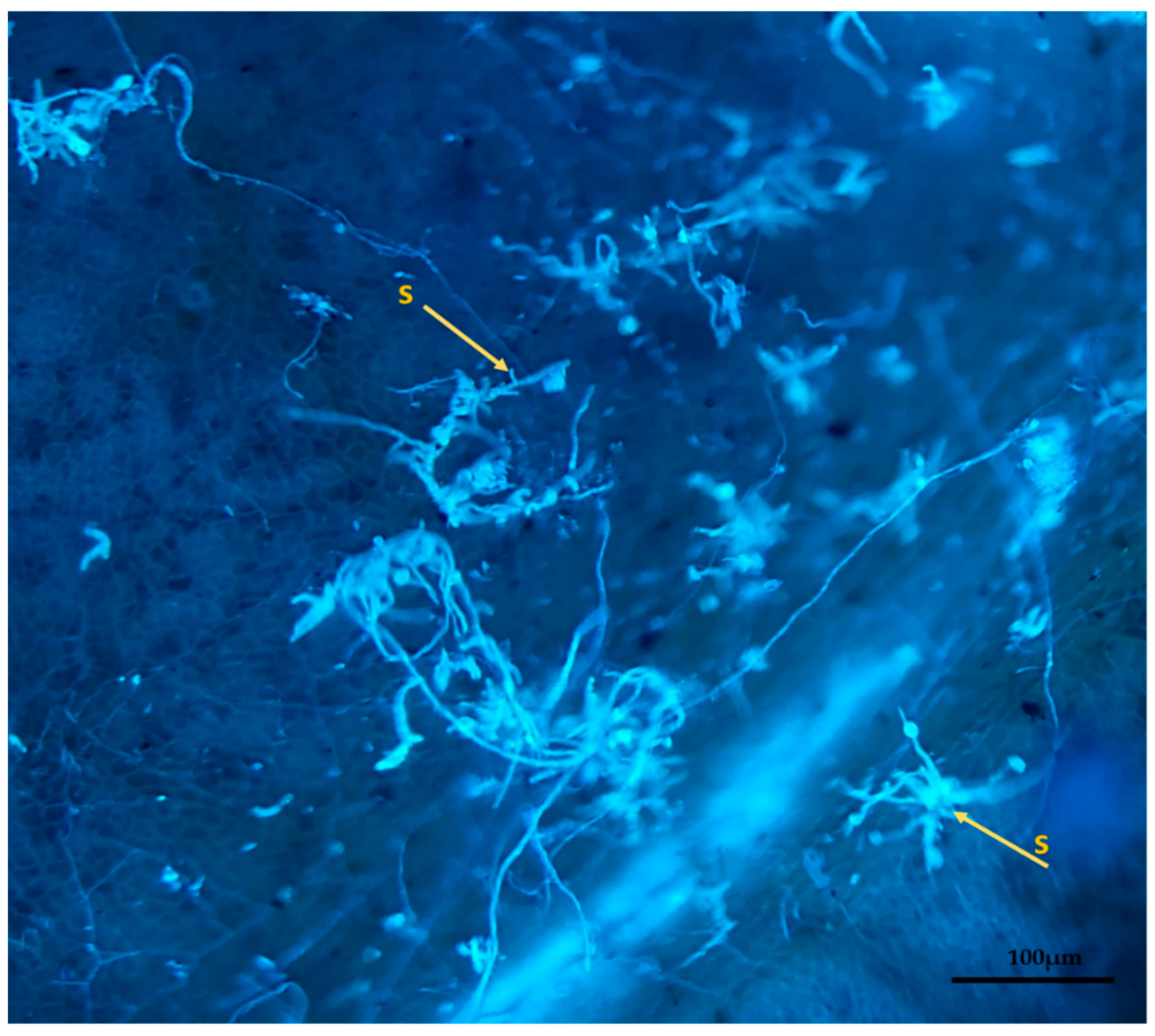
2.3. Preparation and Evaluation of Samples under Ultraviolet Light Microscopy
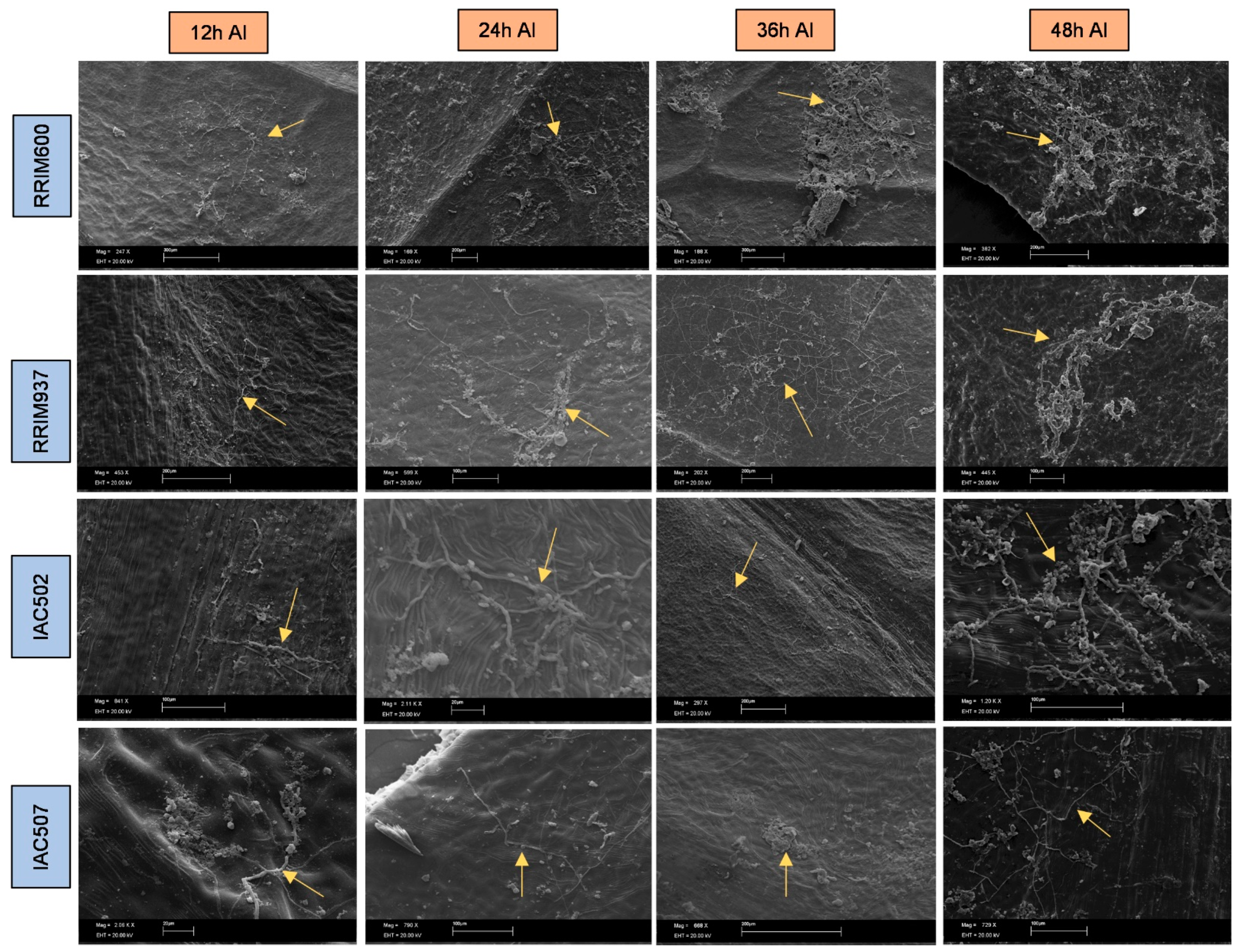
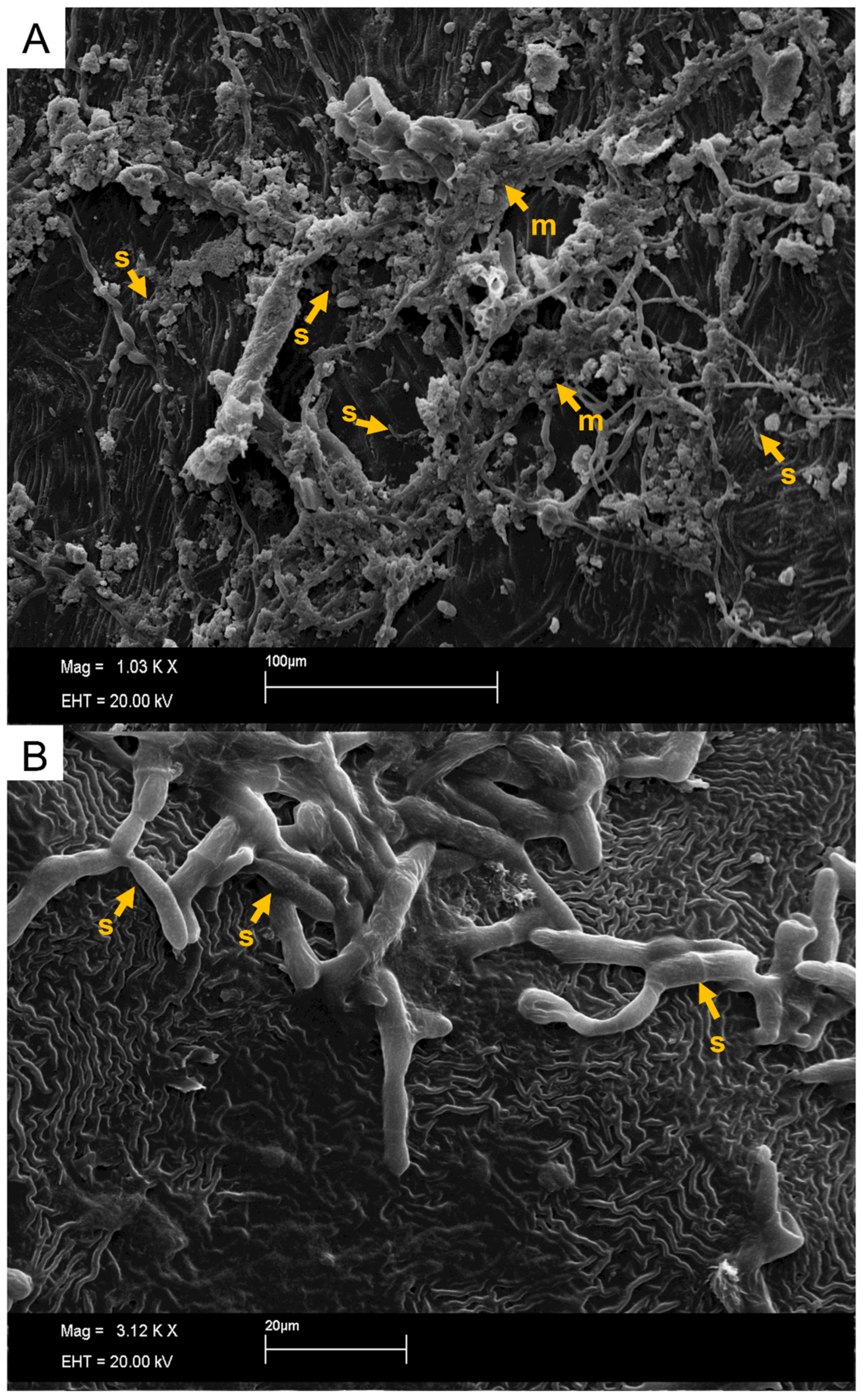
2.4. Preparation and Evaluation of Samples under a Scanning Electron Microscope (SEM)
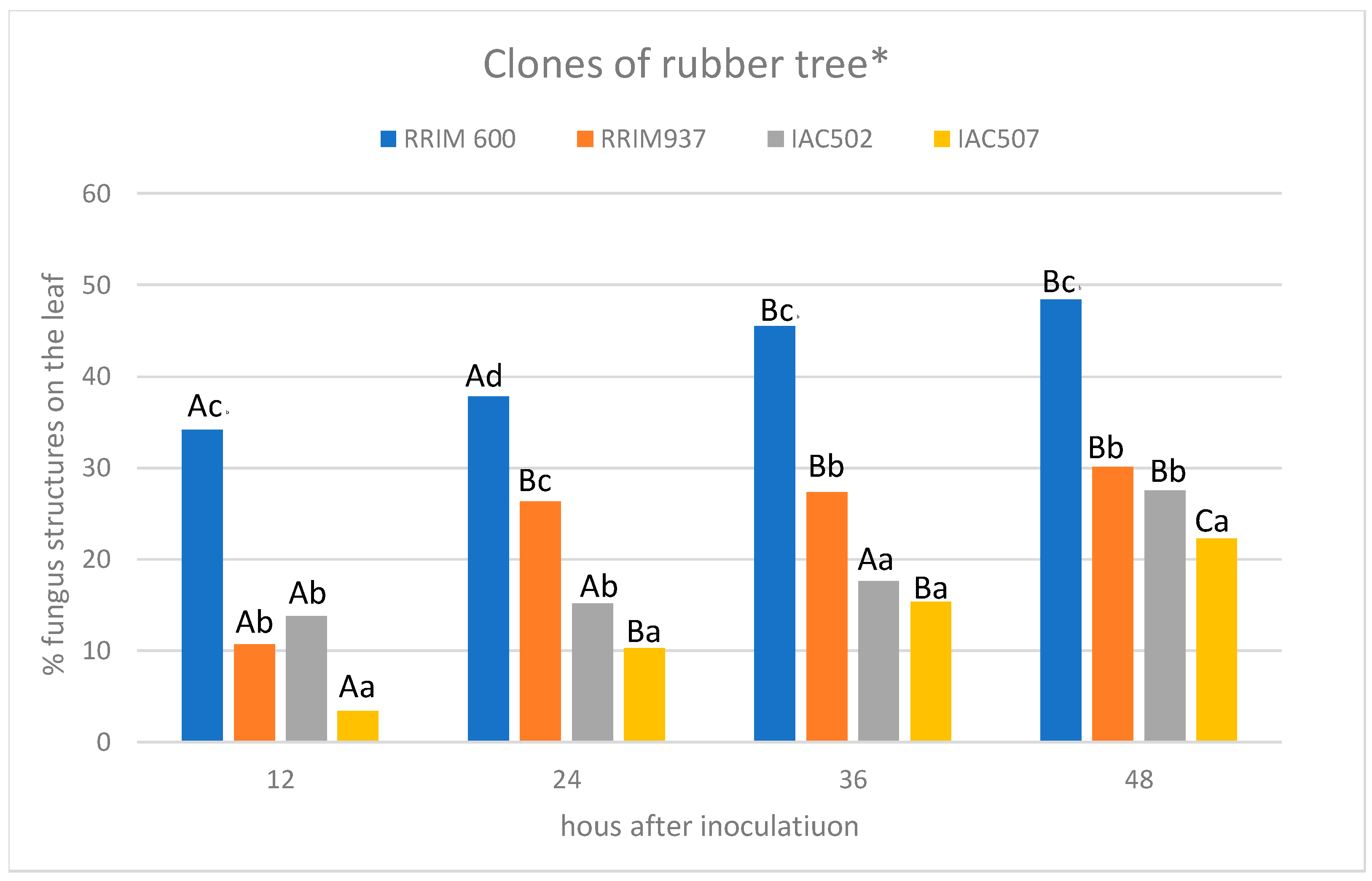
2.5. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasparotto, L.; Rezende Pereira, J.C.R. (Eds.) Doenças da Seringueira No Brasil; Embrapa: Manaus, Brazil, 2012. [Google Scholar]
- Furtado, E.L.; Gasparotto, L.; Pereira, J.C.R. Doenças da Seringueira. In Manual de Fitopatologia: Doenças das Culturas, 5th ed.; Amorim, L., Rezende, J.A.M., Filho, A.B., Camargo, L.E.A., Eds.; Agronomica Ceres: Piracicaba, Brazil, 2016; pp. 647–656. [Google Scholar]
- Antonio, G.L.; Scaloppi, E.J., Jr.; Fischer, I.H.; Furtado, E.L.; Firmino, A.C. Clonal resistance of rubber tree to Colletotrichum spp. For. Pathol. 2021, 51, e12685. [Google Scholar] [CrossRef]
- Magalhães, I.P.; Marques, J.P.R.; Gomes, M.E.; Scaloppi, E.J., Jr.; Fischer, I.H.; Furtado, E.L.; Firmino, A.C. Structural and biochemical aspects related to resistance and susceptibility of rubber tree clones to anthracnose. Plants 2021, 10, 985. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.R.; Herculano, L.M.; Silva, J.M.D.; Oliveira, S.M.A.D. Fosfitos no manejo da antracnose do jiló. Pesqui. Agropecu. Bras. 2014, 49, 930–938. [Google Scholar] [CrossRef]
- Silveira, A.P.; Furtado, E.L.; Lopes, M.E.B.M. Antracnose: Nova doença do painel de sangria da seringueira. Summa Phytopathol. 2009, 18, 195–200. [Google Scholar]
- Guyot, J.; Omanda, E.N.; Ndoutoume, A.; Otsaghe, A.A.M.; Enjalric, F.; Assoumou, H.G.N. Effect of controlling Colletotrichum leaf fall of rubber tree on epidemic development and rubber production. Crop Prot. 2001, 20, 581–590. [Google Scholar] [CrossRef]
- Qianchun, X. Resistant behavior of popular rubber clones in China to Colletotrichum. Phytopathologica 1992, 18, 107–113. [Google Scholar]
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D. QTL mapping and quantitative disease resistance in plants. Annu. Rev. Phytopathol. 1994, 34, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Lourenço, S.A.; Amorim, L. Infecção de goiabas por Colletotrichum gloeosporioides e Colletotrichum acutatum sob diferentes temperaturas e períodos de molhamento. Trop. Plant Pathol. 2008, 33, 265–272. [Google Scholar]
- Guyot, J.; Omanda, E.N.; Pinard, F. Some epidemiological investigations on Colletotrichum leaf disease on rubber tree. Crop Prot. 2005, 24, 65–77. [Google Scholar] [CrossRef]
- King, W.T.; Madden, L.V.; Ellis, M.A.; Wilson, L.L. Effects of temperature on sporulation and latent period of Colletotrichum spp. infecting strawberry fruit. Plant Dis. 1997, 81, 77–84. [Google Scholar] [CrossRef][Green Version]
- Wastie, R.L. Secondary leaf fall of Hevea brasiliensis: Factors affecting the production, germination and viability of spores of Colletotrichum gloeosporioides. Ann. Appl. Biol. 1972, 72, 273–282. [Google Scholar] [CrossRef]
- Magalhães, D.M.A.; Gomes, M.E.; Scaloppi, E.J., Jr.; Fischer, I.H.; Furtado, E.L.; Moreira, B.R.A.; Prado, E.P.; Firmino, A.C. Pré penetração e período de latência de Colletotrichum tamarilloi em clones de seringueira resistentes e suscetível sob diferentes condições ambientais. Sci. For. 2021, 49, e3630. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Ed.; Academic press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Crous, P.W.; Damm, U.; Goodwin, P.H.; Chen, H.; Johnston, P.R.; Jones, E.B.G.; et al. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Celio, G.J.; Hausbeck, M.K. Conidial germination, infection structure formation, and early colony development of powdery mildew on poinsettia. Phytopathology 1998, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Firmino, A.C.; Viana, C.M.; Soliman, E.P.; de Souza, I.C.G.; Silva, M.R.; Furtado, E.L. Análise histológica de plantas de eucalipto resistentes e suscetíveis inoculadas com de Ceratocystis fimbriata. Sci. For. 2018, 46, 209–216. [Google Scholar] [CrossRef]
- Labno, C. Basic Intensity Quantification with ImageJ. Integrated Light Microscopy Core; University of Chicago: Chicago, IL, USA, 2019. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Pereira, I.S.; Abreu, M.S.; Alves, E.; Ferreira, J.B. Estudos Histopatológicos da Interação Colletotrichum gloeosporioides: Cafeeiro. Bragantia 2009, 68, 53–61. [Google Scholar] [CrossRef]
- Wharton, P.S.; Diéguez-Uribeondo, J. The Biology of Colletotrichum acutatum. 2004. Available online: file:///C:/Users/User/Downloads/61-Article%20Text-60-1-10-20071114.pdf (accessed on 15 February 2022).
- Amorim, L.; Rezende, J.A.M.; Filho, A.B. (Eds.) Manual de Fitopatologia: Princípios e Conceitos; Agronômica Ceres Ltd.: Piracicaba, Brazil, 2011. [Google Scholar]
- Xie, L.; Zhang, J.Z.; Cai, L.; Hyde, K.D. Biology of Colletotrichum horii, the causal agent of persimmon anthracnose. Mycology 2010, 1, 242–253. [Google Scholar] [CrossRef]
- Pacholati, S.F.; Leite, B.; Stangarlin, J.R.; Cia, P. (Eds.) Interação Planta-Patógeno: Fisiologia, Bioquímica e Biologia Molecular; Fealq: Piracicaba, Brazil, 2008. [Google Scholar]
- Lorenzetti, E.; Stangarlin, J.R.; Kuhn, O.J.; Portz, R.L. Induction of resistance to Macrophomina phaseolina in soyben treated with rosemary extract. Summa Phytopathol. 2018, 44, 45–50. [Google Scholar] [CrossRef]
- Wharton, P.S.; Julian, A.M.; O’Connell, R.J. Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology 2001, 91, 149–158. [Google Scholar] [CrossRef]
- Wharton, P.S.; Julian, A.M. A cytological study of compatible and incompatible interactions between Sorghum bicolor and Colletotrichum sublineolum. New Phytol. 1996, 134, 25–34. [Google Scholar] [CrossRef]
- Miranda, M.J.; Pinto, H.S.; Zullo Júnior, J.; Fagundes, R.M.; Fonsechi, D.B.; Calve, L.; Pellegrino, G.Q. Clima dos Municípios Paulistas: A Classificação Climática de Koeppen para o Estado de São Paulo. 2011. Available online: http://www.cpa.unicamp.br/outras-informacoes/clima-dPS-municipiPS-paulistas.html (accessed on 15 February 2022).
- Moraes, S.R. Infecção e Colonização de Colletotrichum gloeosporioides em Goiaba e Infecção de Colletotrichum acutatum em Folhas de Citros. 2009. Available online: https://www.teses.usp.br/teses/disponiveis/11/11135/tde-15042009-085030/publico/Sylvia_Moraes.pdf (accessed on 15 February 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firmino, A.C.; Magalhães, I.P.; Gomes, M.E.; Fischer, I.H.; Junior, E.J.S.; Furtado, E.L. Monitoring Colletotrichum Colonization and Reproduction in Different Rubber Tree Clones. Plants 2022, 11, 905. https://doi.org/10.3390/plants11070905
Firmino AC, Magalhães IP, Gomes ME, Fischer IH, Junior EJS, Furtado EL. Monitoring Colletotrichum Colonization and Reproduction in Different Rubber Tree Clones. Plants. 2022; 11(7):905. https://doi.org/10.3390/plants11070905
Chicago/Turabian StyleFirmino, Ana Carolina, Izabela Ponso Magalhães, Marcela Eloi Gomes, Ivan Herman Fischer, Erivaldo José Scaloppi Junior, and Edson Luiz Furtado. 2022. "Monitoring Colletotrichum Colonization and Reproduction in Different Rubber Tree Clones" Plants 11, no. 7: 905. https://doi.org/10.3390/plants11070905
APA StyleFirmino, A. C., Magalhães, I. P., Gomes, M. E., Fischer, I. H., Junior, E. J. S., & Furtado, E. L. (2022). Monitoring Colletotrichum Colonization and Reproduction in Different Rubber Tree Clones. Plants, 11(7), 905. https://doi.org/10.3390/plants11070905





