Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants
Abstract
1. Introduction
2. Distribution of Si in Plants
3. Uptake, Translocation and Accumulation of Si in Plants
4. Si Transporters
4.1. Influx and Efflux of Si Transporters in Rice
4.2. Brief Introduction of Si Transporters in Some Other Plants
4.2.1. Barley and Maize
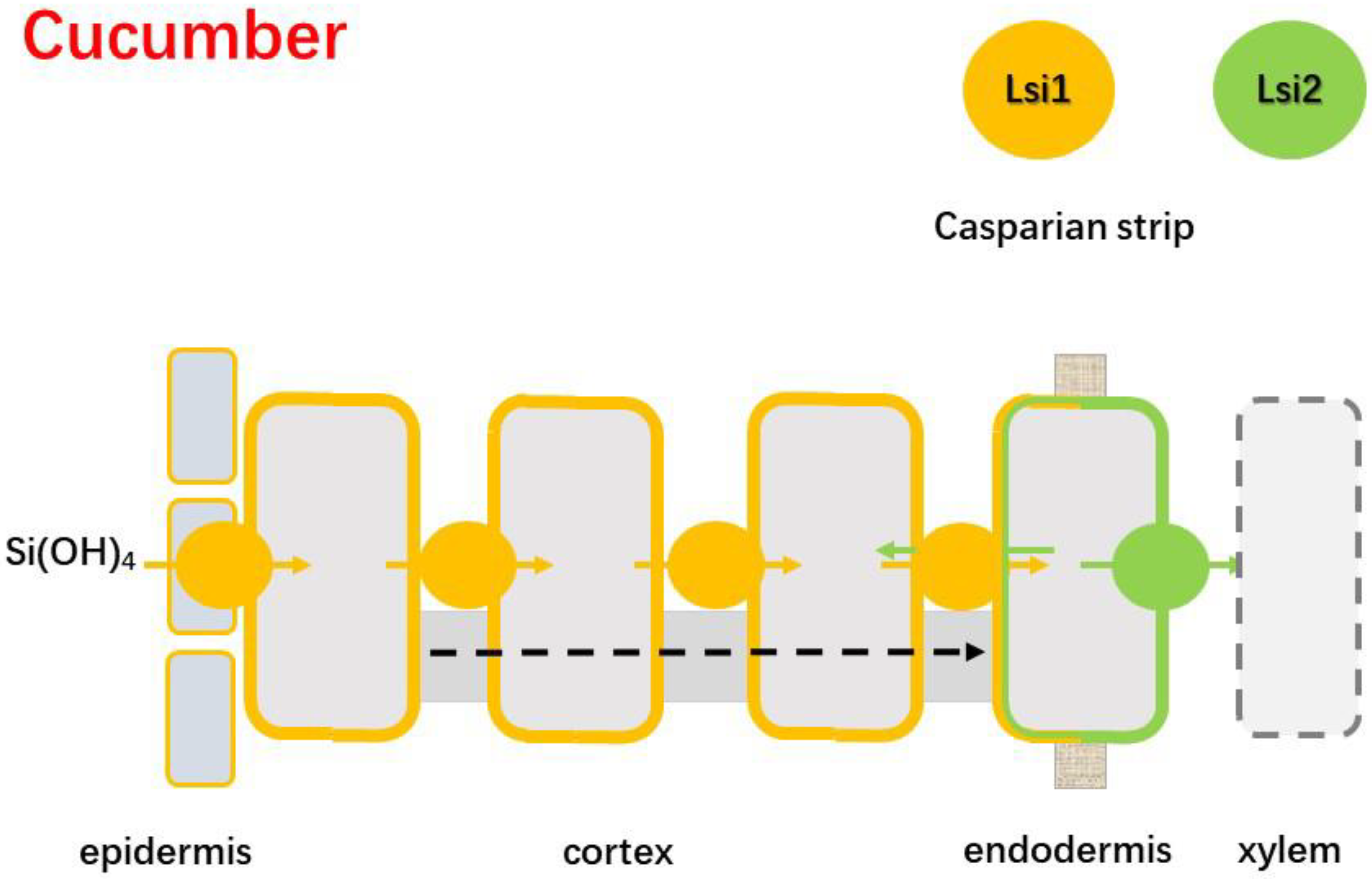
4.2.2. Cucumber
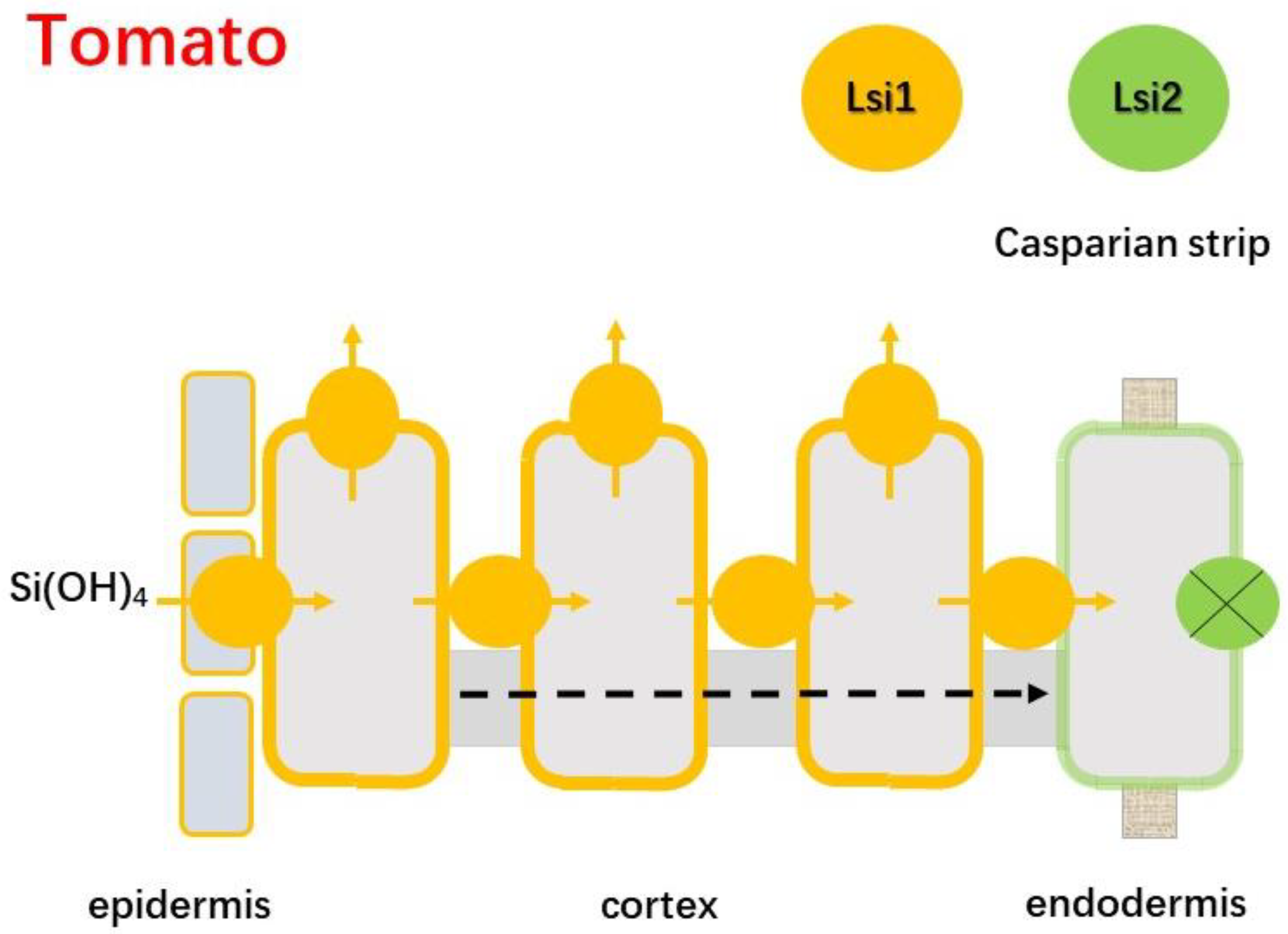
4.2.3. Tomato
4.2.4. Horsetail
4.2.5. Potato
4.2.6. Soybean
5. Silicon Accumulation Improves Plant Disease Resistance
5.1. Formation of Physical Barriers as Influenced by Si
5.2. Formation of Biochemical Barriers as Influenced by Si
5.3. Application of Si in Plant Diseases
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Wei, Q. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef] [PubMed]
- Vibhor Kumar, V.; Krishan, K.; Brajesh, K. Plant disease detection using computational intelligence and image processing. J. Plant Dis. Prot. 2021, 128, 19–53. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Zhou, T.; Wei, B.; Zhou, Y. Resistance evaluation of major commercial rice varieties in Jiangsu province to rice stripe disease and small brown planthopper. J. Nanjing Agric. Univ. 2010, 81, 1121–1130. [Google Scholar] [CrossRef]
- Scalenghe, R.; Davis, C.E.; Marino, P.; Peirano, D.J. Proposal of a citrus translational genomic approach for early and infield detection of flavescence doree in vitis. Plant Biosyst. 2016, 150, 43–53. [Google Scholar] [CrossRef]
- Dan, Y.; Liu, M.; Cui, X.; Yin, F. The main diseases of potato and their control measures in Chongqing. South China Agric. 2021, 15, 35–36. [Google Scholar] [CrossRef]
- Li, B.; Tan, Z.; Wei, Y. Occurrence regularity and green prevention and control technology of main tomato diseases in southern Shandong. Mod. Agric. Sci. Technol. 2021, 17, 110–111. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Hu, C.; Zeng, Z.; Wu, H.; Cui, B.; Fan, X.; Liu, Y.; Ma, H.; Ma, T. Advances in the regulation effects of silicon fertilizer under adversity stress. Soil Fertil. Sci. China 2021, 4, 337–346. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Verma, K.K.; Liu, X.-H.; Wu, K.-C.; Singh, R.K.; Song, Q.-Q.; Malviya, M.K.; Song, X.-P.; Singh, P.; Verma, C.L.; Li, Y.-R. The impact of silicon on photosynthetic and biochemical responses of sugarcane under different soil moisture levels. Silicon 2019, 12, 1355–1367. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Cai, K.; Liu, L.; Shi, J.; Geng, W. Silicon improves the tolerance of wheat seedlings to ultraviolet-B stress. Biol. Trace Elem. Res. 2011, 143, 507–517. [Google Scholar] [CrossRef]
- Gou, T.; Chen, X.; Han, R.; Liu, J.; Gong, H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol. Plant. 2020, 42, 12–23. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Corsi, D.; Stephan, N.A.; Sitarama, P.A.; Eduardo, A. Silicon rates and beneficial microorganism on blast suppression and productivity of upland rice. J. Plant Sci. Phytopathol. 2021, 5, 20–27. [Google Scholar] [CrossRef]
- Araujo, L.; Paschoalino, R.S.; Rodrigues, F. Microscopic aspects of silicon-mediated rice resistance to leaf scald. Phytopathology 2016, 106, 132–141. [Google Scholar] [CrossRef]
- Lepolu Torlon, J.; Heckman, J.; Simon, J.; Wyenandt, C. Silicon soil amendments for suppressing powdery mildew on pumpkin. Sustainability 2016, 8, 293. [Google Scholar] [CrossRef]
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Aconitate and methyl aconitate are modulated by silicon in powdery mildew-infected wheat plants. J. Plant Physiol. 2009, 166, 1413–1422. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Wang, L.; Lin, W.; Fan, X.; Cai, K. Proteomic characterization of silicon-mediated resistance against Ralstonia solanacearumin tomato. Plant Soil 2015, 387, 425–440. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 is a silicon influx transporter in barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef]
- Mitani, N.; Yamaji, N.; Ma, J.F. Identification of maize silicon influx transporters. Plant Cell Physiol. 2008, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M.; Naoki, Y.; Yukiko, A.; Kozo, I.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef]
- Vivancos, J.; Deshmukh, R.; Gregoire, C.; Remus-Borel, W.; Belzile, F.; Belanger, R.R. Identification and characterization of silicon efflux transporters in horsetail (Equisetum arvense). J. Plant Physiol. 2016, 200, 82–89. [Google Scholar] [CrossRef]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Remus-Borel, W.; Belzile, F.; Ma, J.F.; Belanger, R.R. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Vivancos, J.; Guerin, V.; Sonah, H.; Labbe, C.; Belzile, F.; Belanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, C.; Fan, P.; Bao, B.; Li, T.; Zhu, Z. Identification of two cucumber putative silicon transporter genes in Cucumis sativus. J. Plant Growth Regul. 2015, 34, 332–338. [Google Scholar] [CrossRef]
- Sun, H.; Duan, Y.; Mitani-Ueno, N.; Che, J.; Jia, J.; Liu, J.; Guo, J.; Ma, J.F.; Gong, H. Tomato roots have a functional silicon influx transporter but not a functional silicon efflux transporter. Plant Cell Environ. 2020, 43, 732–744. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef]
- Rios, J.J.; Martínez-Ballesta, M.C.; Ruiz, J.M.; Begoña, B.; Micaela, C. Silicon-mediated improvement in plant salinity tolerance: The role of aquaporins. Front. Plant Sci. 2017, 8, 948. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K.; Wang, S.; Zhang, C. Advances in silicon nutrition of plants. Acta Bot. Boreali-Occident. Sin. 2004, 24, 2385–2392. [Google Scholar]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Shivaraj, S.M.; Mandlik, R.; Bhat, J.A.; Raturi, G.; Elbaum, R.; Alexander, L.; Tripathi, D.K.; Deshmukh, R.; Sonah, H. Outstanding questions on the beneficial role of silicon in crop plants. Plant Cell Physiol. 2021, 63, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Casey, W.H.; Kinrade, S.D.; Knight, C.T.G.; Rains, D.W.; Epstein, E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 2004, 27, 51–54. [Google Scholar] [CrossRef]
- Ma, J.F. Silicon-accumulating plants in the plant kingdom. Soil Fertil. Plant Silicon Res. Jpn. 2002, 63–71. [Google Scholar] [CrossRef]
- Laane, H.-M. The effects of foliar sprays with different silicon compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef]
- Jayawardana, H.A.R.K.; Weerahewa, H.L.D.; Saparamadu, M.D.J.S. Effect of root or foliar application of soluble silicon on plant growth, fruit quality and anthracnose development of capsicum. Trop. Agric. 2015, 26, 74–81. [Google Scholar] [CrossRef]
- Prakash, N.B.; Chandrashekar, N.; Mahendra, C.; Patil, S.U.; Thippeshappa, G.N.; Laane, H.M. Effect of foliar spray of soluble silicic acid on growth and yield parameters of wetland rice in hilly and coastal zone soils of karnataka, south india. J. Plant Nutr. 2011, 34, 1883–1893. [Google Scholar] [CrossRef]
- Buck, G.B.; Korndörfer, G.H.; Nolla, A.; Coelho, L. Potassium silicate as foliar spray and rice blast control. J. Plant Nutr. 2008, 31, 231–237. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; Duarte, H.S.S.; Rezende, D.C.; Filho, J.A.W.; Zambolim, L. Foliar spray of potassium silicate on the control of angular leaf spot on beans. J. Plant Nutr. 2010, 33, 2082–2093. [Google Scholar] [CrossRef]
- Exley, C.; Guerriero, G.; Lopez, X. Silicic acid: The omniscient molecule. Sci. Total Environ. 2019, 665, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, K.R.; Bansal, A.; Rout, G.R. Silicon amendment induces synergistic plant defense mechanism against pink stem borer (Sesamia inferens Walker.) in finger millet (Eleusine coracana Gaertn.). Sci. Rep. 2020, 10, 4229–4244. [Google Scholar] [CrossRef]
- Labun, P.; Grulova, D.; Salamon, I.; Sersen, F. Calculating the silicon in horsetail (Equisetum arvense L.) during the vegetation season. Food Nutr. Sci. 2013, 4, 510–514. [Google Scholar] [CrossRef][Green Version]
- Grégoire, C.; Rémus-Borel, W.; Vivancos, J.; Labbé, C.; Bélanger, R.R. Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J. Cell Mol. Biol. 2012, 72, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef]
- Sheng, H.; Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Konishi, N.; Ma, J.F. A pericycle-localized silicon transporter for efficient xylem loading in rice. New Phytol. 2022, 234, 197–208. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; WU, L. The distribution of silicon, nitrogen, phosphorus and potassium in the organs of different silicon-absorbing plants. Chin. J. Soil Sci. 2014, 45. [Google Scholar] [CrossRef]
- Mitani, N.; Chiba, Y.; Ma, Y.J.F. Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 2009, 21, 2133–2142. [Google Scholar] [CrossRef]
- Yamaji, N.; Chiba, Y.; Mitani-Ueno, N.; Ma, J.F. Functional characterization of a silicon transporter gene implicated in silicon distribution in barley. Plant Physiol. 2012, 160, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Duan, Y.; Qi, X.; Zhang, L.; Huo, H. Isolation and functional characterization of CsLsi2, a cucumber silicon efflux transporter gene. Ann. Bot. 2018, 122, 641–648. [Google Scholar] [CrossRef]
- Soratto, R.P.; Fernandes, A.M.; Pilon, C.; Souza, M.R. Phosphorus and silicon effects on growth, yield, and phosphorus forms in potato plants. J. Plant Nutr. 2019, 42, 218–233. [Google Scholar] [CrossRef]
- Vulavala, V.K.R.; Elbaum, R.; Yermiyahu, U.; Fogelman, E.; Kumar, A.; Ginzberg, I. Silicon fertilization of potato: Expression of putative transporters and tuber skin quality. Planta 2016, 243, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Felisberto, G.; de Mello Prado, R.; de Oliveira, R.L.L.; de Carvalho, F.; Patrícia, A. Are nanosilica, potassium silicate and new soluble sources of silicon effective for silicon foliar application to soybean and rice plants? Silicon 2021, 13, 3217–3228. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007, 143, 1306–1313. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Tan, L.; Yin, C.; Li, T.; Liang, Y. Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiol. Biochem. 2021, 158, 219–227. [Google Scholar] [CrossRef]
- Sakurai, G.; Satake, A.; Yamaji, N.; Mitani-Ueno, N.; Yokozawa, M.; Feugier, F.G.; Ma, J.F. In silico simulation modeling reveals the importance of the casparian strip for efficient silicon uptake in rice roots. Plant Cell Physiol. 2015, 56, 631–639. [Google Scholar] [CrossRef]
- Wang, Z.; Yamaji, N.; Huang, S.; Zhang, X.; Xia, J. OsCASP1 is required for casparian strip formation at endodermal cells of rice roots for selective uptake of mineral elements. Plant Cell 2019, 31, 2636–2648. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Ma, J.F. High silicon accumulation in the shoot is required for down-regulating the expression of Si transporter genes in rice. Plant Cell Physiol. 2016, 57, 2510–2518. [Google Scholar] [CrossRef]
- Nakata, Y.; Ueno, M.; Kihara, J.; Ichii, M.; Taketa, S.; Arase, S. Rice blast disease and susceptibility to pests in a silicon uptake-deficient mutant lsi1 of rice. Crop Prot. 2008, 27, 865–868. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Mielli, M.V.B.; Ma, J.F. Rice grain resistance to brown spot and yield are increased by silicon. Trop. Plant Pathol. 2014, 39, 56–63. [Google Scholar] [CrossRef]
- Somapala, K.; Weerahewa, D.; Thrikawala, S. Silicon rich ricehull amended soil enhances anthracnose resistance in tomato. Procedia Food Sci. 2016, 6, 190–193. [Google Scholar] [CrossRef]
- Huang, C.-H.; Roberts, P.D.; Datnoff, L.E. Silicon suppresses fusarium crown and root rot of tomato. J. Phytopathol. 2011, 159, 546–554. [Google Scholar] [CrossRef]
- Gulzar, N.; Ali, S.; Shah, M.; Kamili, A.N. Silicon supplementation improves early blight resistance in Lycopersicon esculentum Mill. by modulating the expression of defense-related genes and antioxidant enzymes. 3 Biotech 2021, 11, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Najihah, N.I.; Hanafi, M.M.; Idris, A.S.; Hakim, M.A. Silicon treatment in oil palms confers resistance to basal stem rot disease caused by Ganoderma boninense. Crop Prot. 2015, 67, 151–159. [Google Scholar] [CrossRef]
- Carré-Missio, V.; Rodrigues, F.A.; Schurt, D.A.; Resende, R.S.; Souza, N.F.A.; Rezende, D.C.; Moreira, W.R.; Zambolim, L. Effect of foliar-applied potassium silicate on coffee leaf infection by Hemileia vastatrix. Ann. Appl. Biol. 2014, 164, 396–403. [Google Scholar] [CrossRef]
- French-Monar, R.D.; Rodrigues, F.A.; Korndörfer, G.H.; Datnoff, L.E. Silicon suppresses phytophthora blight development on bell pepper. J. Phytopathol. 2010, 158, 554–560. [Google Scholar] [CrossRef]
- Bathoova, M.; Bokor, B.; Soukup, M.; Lux, A.; Martinka, M. Silicon-mediated cell wall modifications of sorghum root exodermis and suppression of invasion by fungus Alternaria alternata. Plant Pathol. 2018, 67, 1891–1900. [Google Scholar] [CrossRef]
- Siregar, A.F.; Husnain, H.; Sato, K.; Wakatsuki, T.; Masunaga, T. Empirical study on effect of silicon application on rice blast disease and plant morphology in indonesia. J. Agric. Sci. 2016, 8, 137–148. [Google Scholar] [CrossRef][Green Version]
- Sathe, A.P.; Kumar, A.; Mandlik, R.; Raturi, G.; Yadav, H.; Kumar, N.; Shivaraj, S.M.; Jaswal, R.; Kapoor, R.; Gupta, S.K.; et al. Role of silicon in elevating resistance against sheath blight and blast diseases in rice (Oryza sativa L.). Plant Physiol. Biochem. 2021, 166, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Filha, M.S.X.; Rodrigues, F.A.; Domiciano, G.P.; Oliveira, H.V.; Silveira, P.R.; Moreira, W.R. Wheat resistance to leaf blast mediated by silicon. Australas. Plant Pathol. 2010, 40, 28–38. [Google Scholar] [CrossRef]
- Pazdiora, P.C.; Dorneles, K.; Morello, T.N.; Nicholson, P.; Dallagnol, L.J. Silicon soil amendment as a complement to manage tan spot and fusarium head blight in wheat. Agron. Sustain. Dev. 2021, 41, 1–13. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Labbe, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G.; Belanger, R.R. Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol. 2018, 18, 97. [Google Scholar] [CrossRef]
- Telles Nascimento, K.J.; Debona, D.; Silveira, P.R.; Silva, L.C.; DaMatta, F.M.; Rodrigues, F.Á. Silicon-induced changes in the antioxidant system reduce soybean resistance to frogeye leaf spot. J. Phytopathol. 2016, 164, 768–778. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Polanco, L.R.; Duarte, H.S.S.; Resende, R.S.; do Vale, F.X.R. Photosynthetic gas exchange in common bean submitted to foliar sprays of potassium silicate, sodium molybdate and fungicide and infected with Colletotrichum lindemuthianum. J. Phytopathol. 2015, 163, 554–559. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef]
- Ratnayake, R.M.R.N.K.; Daundasekera, W.A.M.; Ariyarathne, H.M.; Ganehenege, M.Y.U. Some biochemical defense responses enhanced by soluble silicon in bitter gourd-powdery mildew pathosystem. Australas. Plant Pathol. 2016, 45, 425–433. [Google Scholar] [CrossRef]
- Hawerroth, C.; Araujo, L.; Bermúdez-Cardona, M.B.; Silveira, P.R.; Wordell Filho, J.A.; Rodrigues, F.A. Silicon-mediated maize resistance to macrospora leaf spot. Trop. Plant Pathol. 2018, 44, 192–196. [Google Scholar] [CrossRef]
- Whan, J.A.; Dann, E.K.; Aitken, E.A. Effects of silicon treatment and inoculation with Fusarium oxysporum f. sp. vasinfectum on cellular defences in root tissues of two cotton cultivars. Ann. Bot. 2016, 118, 219–226. [Google Scholar] [CrossRef]
- Shetty, R.; Jensen, B.; Shelton, D.; Jrgensen, K.; Jrgensen, H. Site-specific, silicon-induced structural and molecular defence responses against powdery mildew infection in roses. Pest Manag. Sci. 2021, 77, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Geng, T.; Liu, H.; Yang, W.; Zhong, W.; Zhang, Z.; Zhu, C.; Chu, Z. Foliar application of silicon enhances resistance against Phytophthora infestans through the ET/JA- and NPR1-dependent signaling pathways in potato. Front. Plant Sci. 2021, 12, 609870. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, H.; Bozsó, Z.; Ott, P.G.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Fan, X.; Lin, W.; Liu, R.; Jiang, N.; Cai, K. Physiological response and phenolic metabolism in tomato (Solanum lycopersicum) mediated by silicon under Ralstonia solanacearum infection. J. Integr. Agric. 2018, 17, 2160–2171. [Google Scholar] [CrossRef]
- Ferreira, H.A.; do Nascimento, C.W.A.; Datnoff, L.E.; de Sousa Nunes, G.H.; Preston, W.; de Souza, E.B.; Mariano, R.D.L.R. Effects of silicon on resistance to bacterial fruit blotch and growth of melon. Crop Prot. 2015, 78, 277–283. [Google Scholar] [CrossRef]
- Kablan, L.; Lagauche, A.; Delvaux, B.; Legr Ve, A. Silicon reduces black sigatoka development in banana. Plant Dis. 2012, 96, 273–278. [Google Scholar] [CrossRef]
- Fauteux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Hayasaka, T.; Fujii, H.; Ishiguro, K. The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopathology 2008, 98, 1038–1044. [Google Scholar] [CrossRef][Green Version]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; DaMatta, F.M.; Mielli, M.V.B.; Pereira, S.C. Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice-Bipolaris oryzae interaction. Phytopathology 2011, 101, 92–104. [Google Scholar] [CrossRef]
- Schurt, D.A.; Cruz, M.F.A.; Nascimento, K.J.T.; Filippi, M.C.C.; Rodrigues, F.Á. Silicon potentiates the activities of defense enzymes in the leaf sheaths of rice plants infected by Rhizoctonia solani. Trop. Plant Pathol. 2014, 39, 457–463. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Shetty, R.; Frette, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jorgensen, H.; Newman, M.A.; Christensen, L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Xue, G.; Cui, P.; Fan, F.; Liu, H.; Yin, C.; Sun, W.; Liang, Y. The role of silicon in enhancing resistance to bacterial blight of hydroponic-and soil-cultured rice. Sci. Rep. 2016, 6, 24640–24653. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Vilela, A.; Ferreira-Cardoso, J.; Marques, T.; Anjos, R.; Gomes-Laranjo, J.; Pinto, T. Productivity, chemical composition and sensory quality of "Martaínha" chestnut variety treated with Silicon. CyTA-J. Food 2019, 17, 316–323. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; Jurick, W.M.; Datnoff, L.E.; Jones, J.B.; Rollins, J.A. Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol. Mol. Plant Pathol. 2005, 66, 144–159. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, M.; Teixeira da Silva, J.A.; Zhang, Y.; Yuan, Y.; Jia, Y.; Xiao, Y.; Li, Y.; Fang, L.; Zeng, S. Identification and functional characterization of three new terpene synthase genes involved in chemical defense and abiotic stresses in Santalum album. BMC Plant Biol. 2019, 19, 115–133. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Mcnally, D.J.; Datnoff, L.E.; Jones, J.B.; Bélanger, R. Silicon enhances the accumulation of diterpenoid phytoalexins in rice: A potential mechanism for blast resistance. Phytopathology 2004, 94, 177–183. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, H.; Bozsó, Z.; Ott, P.G.; Wydra, K. Silicon and Ralstonia solanacearum modulate expression stability of housekeeping genes in tomato. Physiol. Mol. Plant Pathol. 2011, 75, 176–179. [Google Scholar] [CrossRef]
- Ayana, G.; Fininsa, C.; Ahmed, S.; Wydra, K. Effects of soil amendment on bacterial wilt caused by Ralstonia solanacerum and tomato yields in ethiopia. J. Plant Prot. Res. 2011, 51, 72–76. [Google Scholar] [CrossRef]
- Conceição, C.S.; Felix, K.; Mariano, R.; Medeiros, E.V.; Souza, E.B. Combined effect of yeast and silicon on the control of bacterial fruit blotch in melon. Sci. Hortic. 2014, 174, 164–170. [Google Scholar] [CrossRef]
- Polanco, L.R.; Rodrigues, F.A.; Nascimento, K.J.T.; Cruz, M.F.A.; Curvelo, C.R.S.; DaMatta, F.M.; Vale, F.X.R. Photosynthetic gas exchange and antioxidative system in common bean plants infected by Colletotrichum lindemuthianum and supplied with silicon. Trop. Plant Pathol. 2014, 39, 35–42. [Google Scholar] [CrossRef]
- Yuxia, F.; Yuxin, H.; Pengpeng, F.; Xiangjun, Z.; Jinxiong, W.; Jiana, L.; Wei, Q.; Jiaqin, M. Silicon alleviates the disease severity of sclerotinia stem rot in rapeseed. Front. Plant Sci. 2021, 12, 721346. [Google Scholar] [CrossRef]
- Nachaat, S. Soluble silicon controls fusarium head blight in bread and durum wheat plants. Gesunde Pflanz. 2021, 73, 479–493. [Google Scholar] [CrossRef]
- Hong, D.; Talha, J.; Yao, Y.; Zou, Z.; Fu, H.; Gao, S.; Xie, Y.; Wang, J. Silicon enhancement for endorsement of Xanthomonas albilineans infection in sugarcane. Ecotoxicol. Environ. Saf. 2021, 220, 112380. [Google Scholar] [CrossRef]
- Ning, D.; Song, A.; Fan, F.; Li, Z.; Liang, Y. Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 2014, 9, e102681. [Google Scholar] [CrossRef]


| Si Accumulation | Species | Si Content (%d.w.) | Si Transporter | Reference |
|---|---|---|---|---|
| High | Horsetail | 10% [44] | EaLsi2-1; EaLsi2-2 | [23,45] |
| Rice | 10% [35] | OsLsi1; OsLsi2; OsLsi3; OsLsi6 | [19,46,47,48] | |
| Maize | 4–7.5% [49] | ZmLsi1; ZmLsi2; ZmLsi6 | [50] | |
| Barely | 2–4% [49] | HvLsi1; HvLsi2; HvLsi6 | [20,51] | |
| Intermediate | Cucumber | 2–4% [49] | CmLsi1; CmLsi2 | [26,52] |
| Low | Tomato | <0.2% [49] | SlLsi1; SlLsi2 | [27] |
| Potato | <0.5% [53] | StLsi1; StLsi2 | [54] | |
| Soybean | <0.5% [55] | GmNIP2-1; GmNIP2-2 | [25] |
| Types of Disease | Plant | Disease Response of Si Deposition | Pathway | References |
|---|---|---|---|---|
| Fungal pathogen | Tomato | Improve resistance to anthracnose | Physical and Biochemical Barriers | [63] |
| Improve resistance to root rot | Physical Barriers | [64] | ||
| Improve resistance to early blight | Biochemical Barriers | [65] | ||
| Pumpkin | Improve resistance to powdery mildew | Physical Barriers | [16] | |
| Oil palms | Improve resistance to powdery mildew | Physical Barriers | [66] | |
| Coffee | Improve resistance to leaf rust | Physical Barriers | [67] | |
| Pepper | Improve resistance to Phytophthora blight | Physical Barriers | [68] | |
| Sorghum | Improve resistance to leaf spot | Physical Barriers | [69] | |
| Rice | Improve resistance to brown spot | Physical Barriers | [62] | |
| Improve resistance to leaf Scald | Physical Barriers | [15] | ||
| Improve resistance to rice blast | Physical Barriers | [14,70] | ||
| Improve resistance to sheath blight | Physical and Biochemical Barriers | [71] | ||
| Wheat | Improve resistance to leaf blast | Physical Barriers | [72] | |
| Improve resistance to tan spot and fusarium head blight | Physical Barriers | [73] | ||
| Beans | Improve resistance to Phytophthora blight | Biochemical Barriers | [74] | |
| Improve resistance to frogeye leaf spot | Biochemical Barriers | [75] | ||
| Improve resistance to anthracnose | Biochemical Barriers | [76] | ||
| PerennialRyegrass | Improve resistance to leaf spot | Biochemical Barriers | [77] | |
| Bittergourd | Improve resistance to powdery mildew | Biochemical Barriers | [78] | |
| Maize | Improve resistance to leaf spot | Physical and Biochemical Barriers | [79] | |
| Cotton | Improve resistance to Fusarium oxysporum | Physical and Biochemical Barriers | [80] | |
| Rose | Improve resistance to powdery mildew | Physical and Biochemical Barriers | [81] | |
| Potato | Improve resistance to late blight | Biochemical Barriers | [82] | |
| Bacterial pathogen | Tomato | Improve resistance to bacterial wilt | Biochemical Barriers | [18,83,84] |
| Melon | Improve resistance to bacterial fruit blotch | Physical Barriers | [85] | |
| Banana | Improve resistance to black sigatoka | Physical Barriers | [86] | |
| Beans | Improve resistance to leaf spot | Physical Barriers | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Sun, Y.; Wang, H.; Wang, H. Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants. Appl. Sci. 2022, 12, 3282. https://doi.org/10.3390/app12073282
Li R, Sun Y, Wang H, Wang H. Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants. Applied Sciences. 2022; 12(7):3282. https://doi.org/10.3390/app12073282
Chicago/Turabian StyleLi, Ruonan, Yihan Sun, Hongzhen Wang, and Huasen Wang. 2022. "Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants" Applied Sciences 12, no. 7: 3282. https://doi.org/10.3390/app12073282
APA StyleLi, R., Sun, Y., Wang, H., & Wang, H. (2022). Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants. Applied Sciences, 12(7), 3282. https://doi.org/10.3390/app12073282






