Effect of Soybean Protein Isolate-7s on Delphinidin-3-O-Glucoside from Purple Corn Stability and Their Interactional Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of SPI-7s
2.3. Extraction of D3G
2.4. Optimum Design of SPI-7s-D3G Stable System
2.5. Stability of SPI-7s-D3G
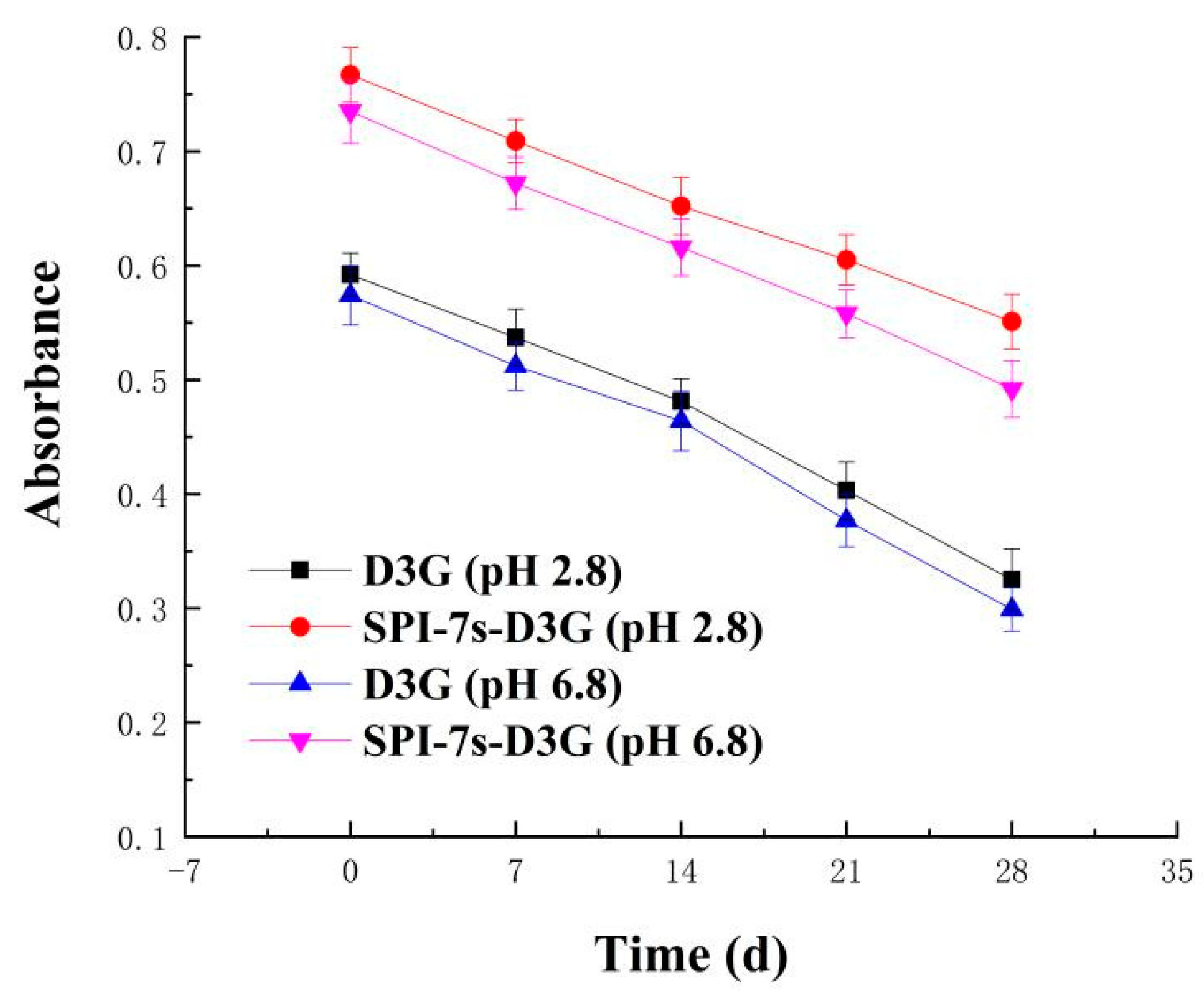
2.5.1. Light Stability
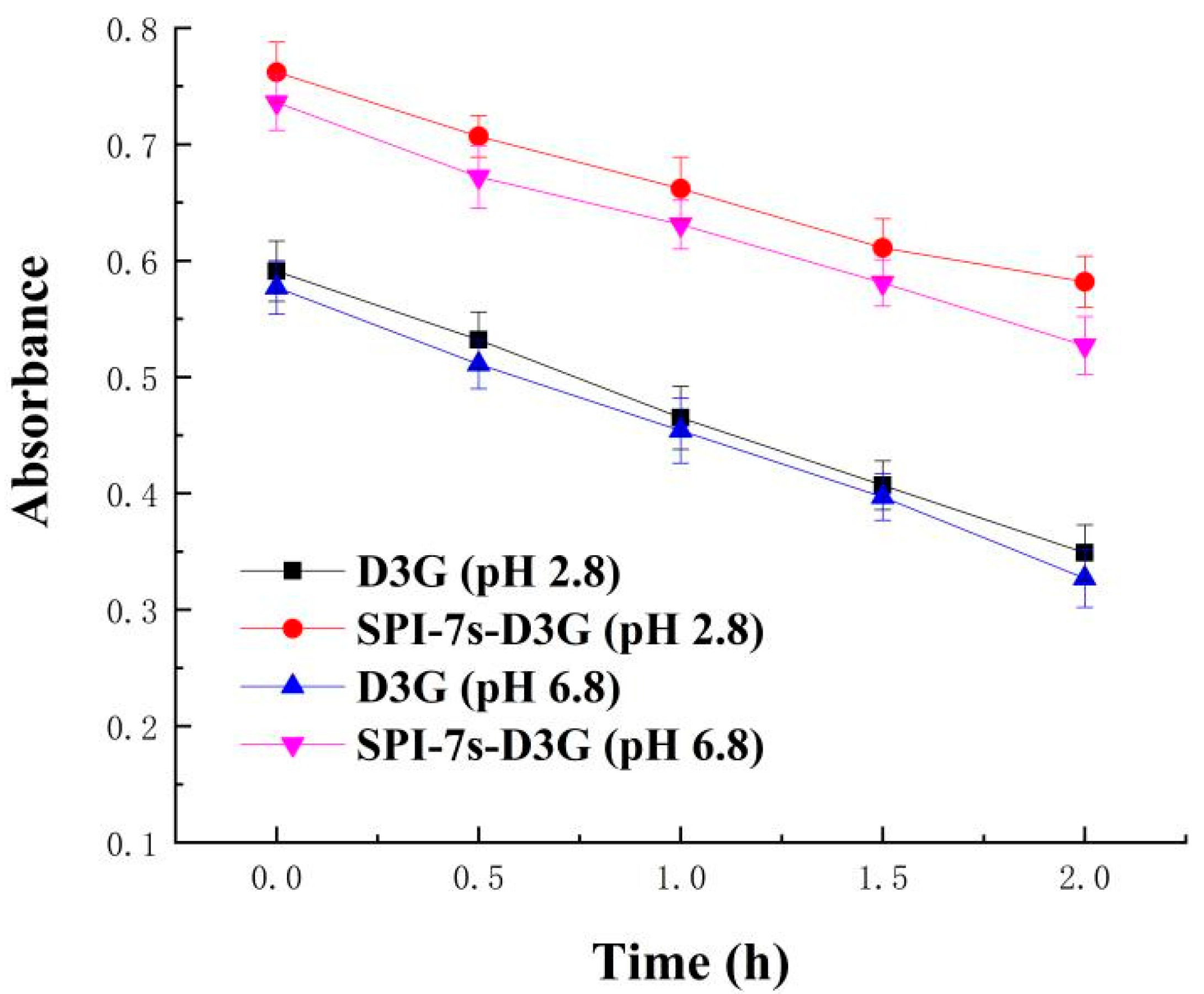
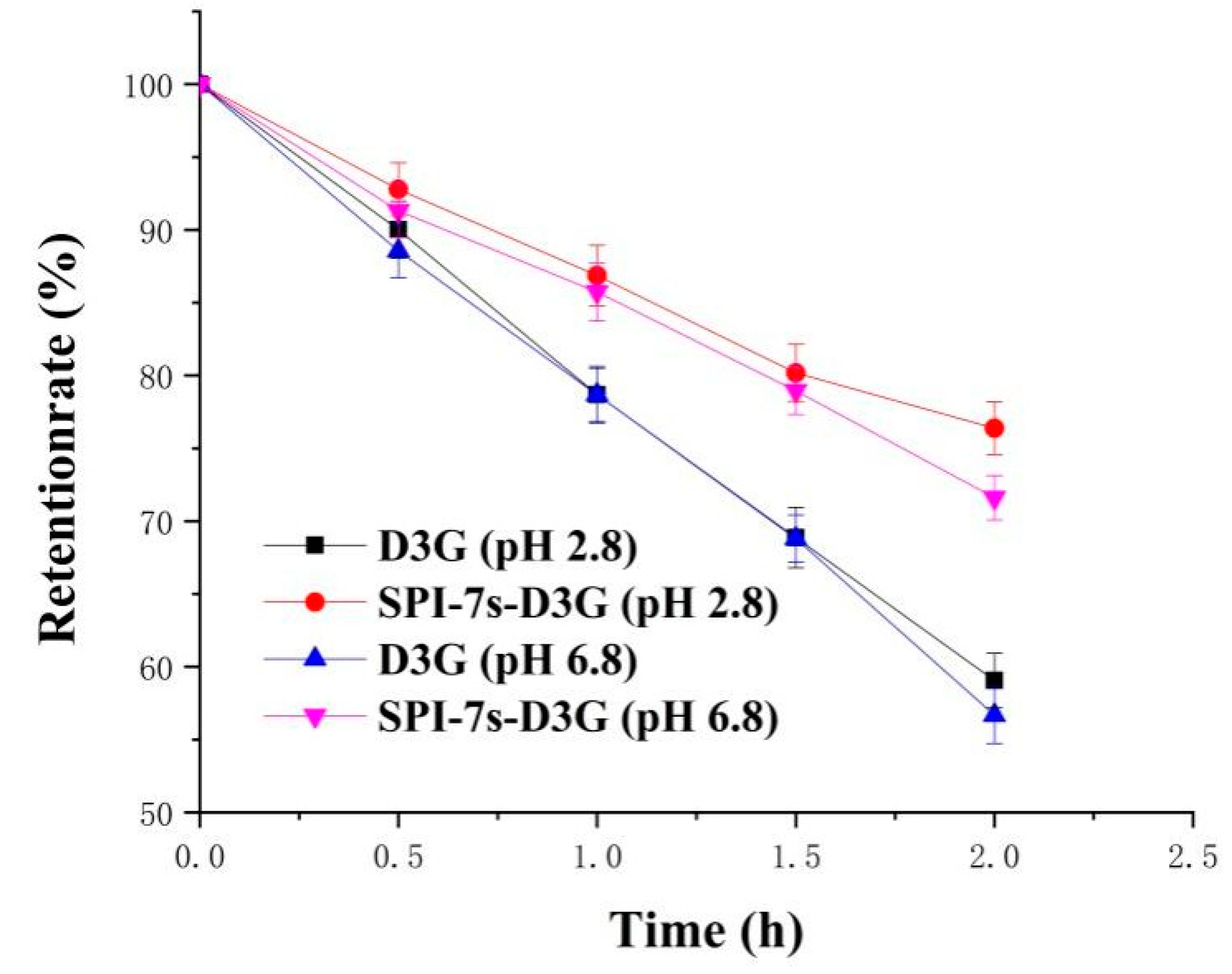
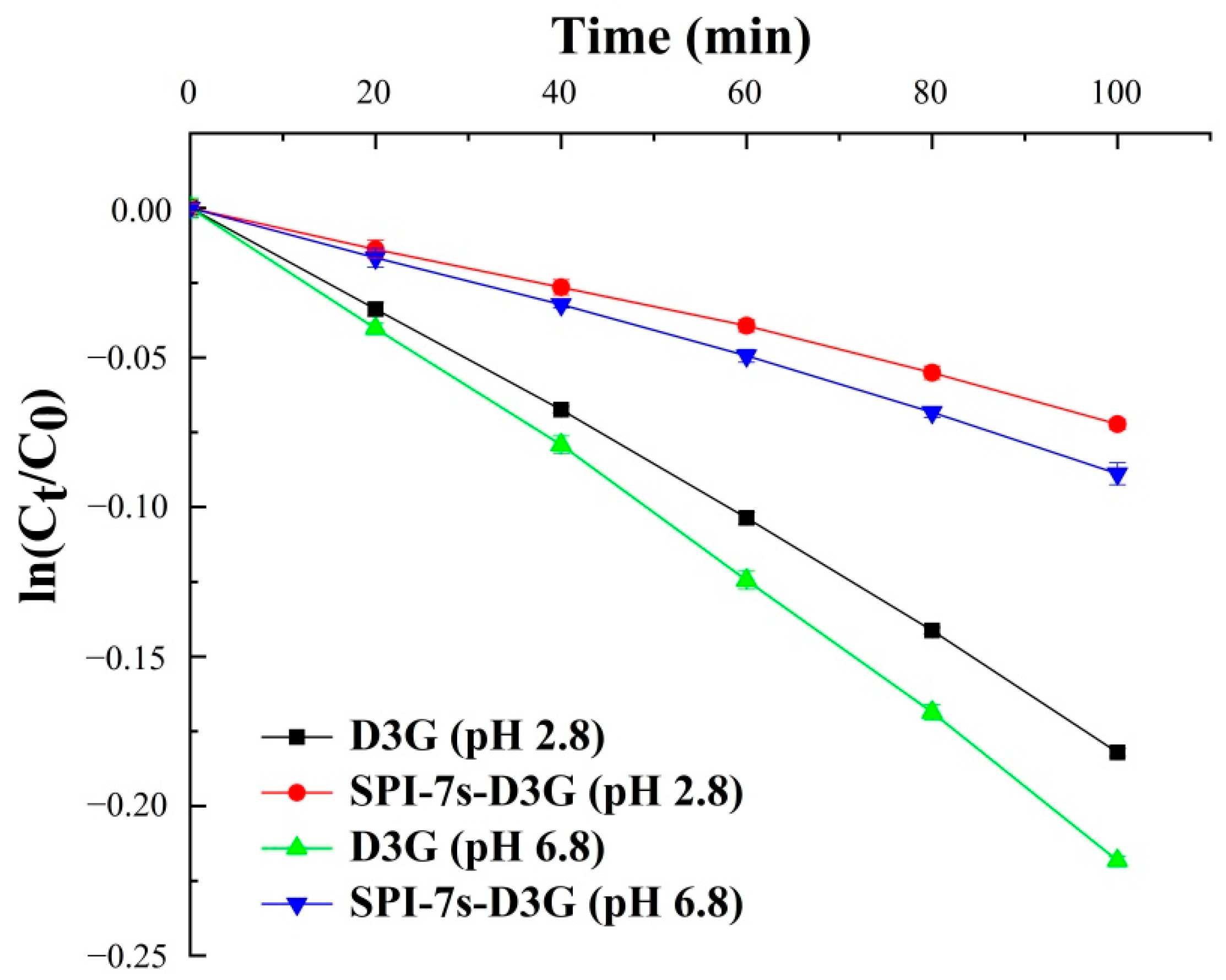
2.5.2. Thermal Stability
2.6. Characterization of SPI-7s-D3G
2.6.1. UV-Vis
2.6.2. CD Spectroscopy
2.6.3. Fluorescence Spectroscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimum Design of SPI-7s-D3G Stable System
3.2. Stability of SPI-7s-D3G
3.2.1. Light Stability
3.2.2. Thermal Stability
3.3. Characterization of SPI-7s-D3G
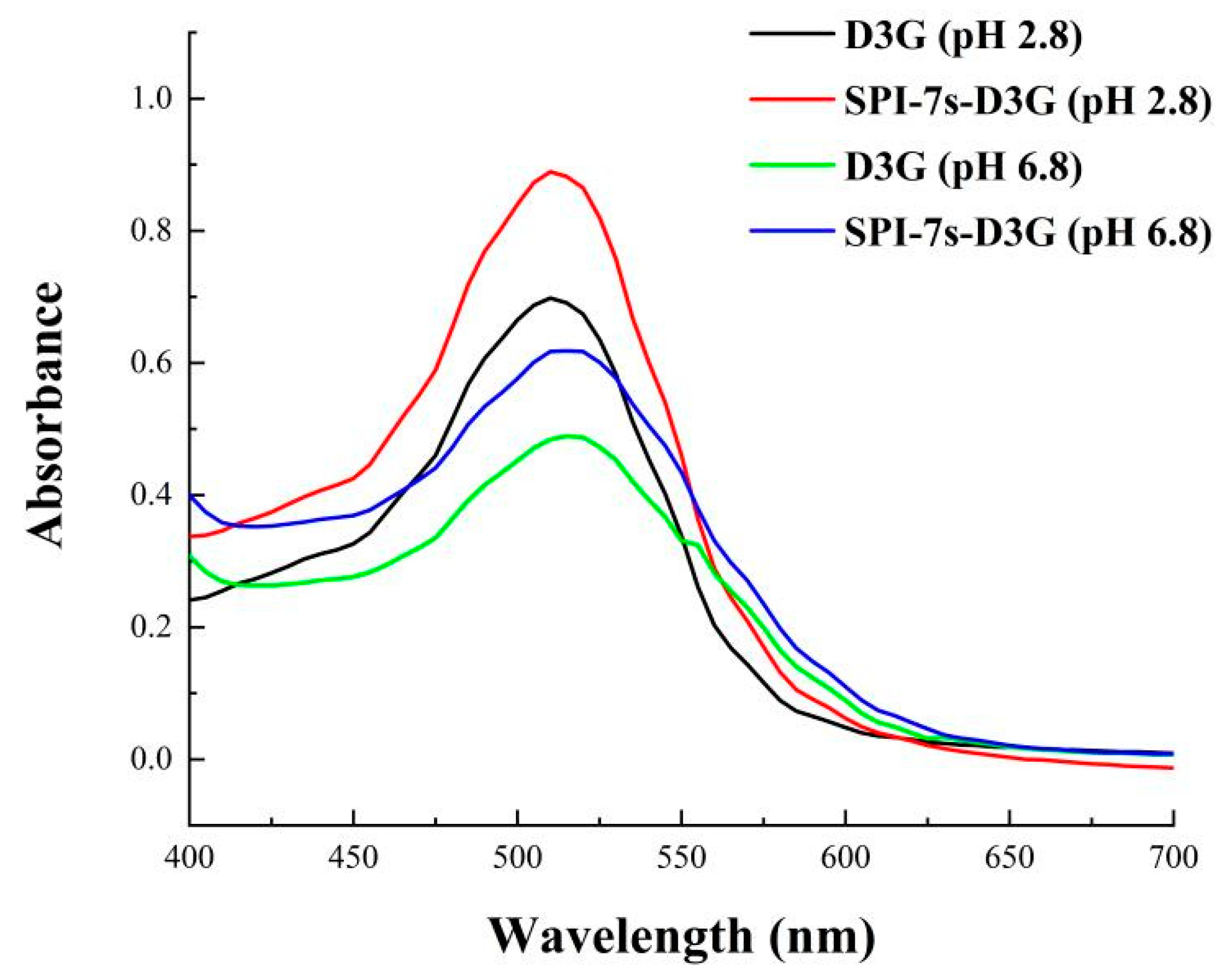
3.3.1. UV-Vis Spectra
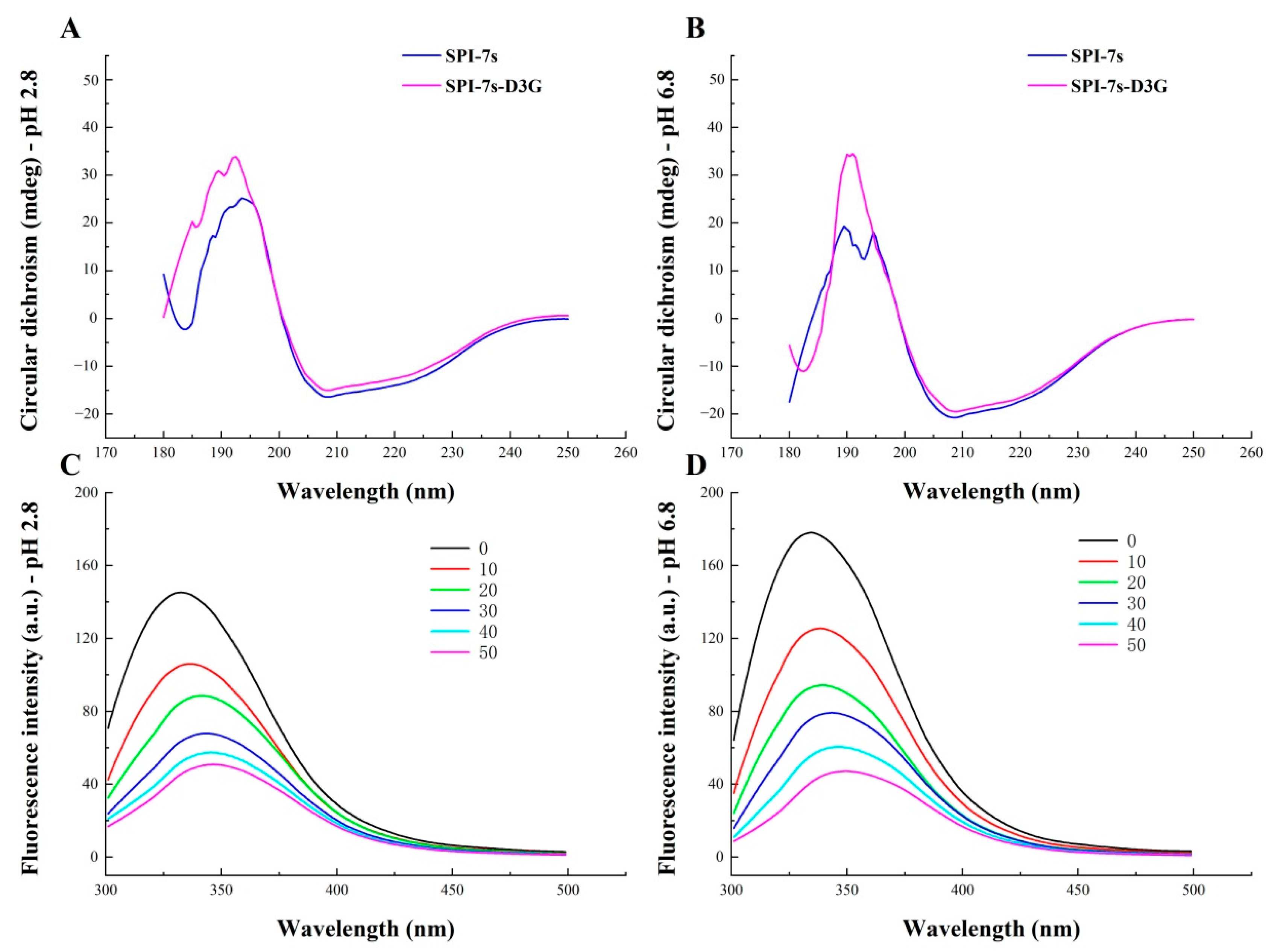
3.3.2. CD Spectra
3.3.3. Fluorescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.; Kocić, G.; Katić, V.; Jović, Z.; Zarubica, A.; Janković Veličković, L.; Nikolić, V.; Jović, A.; Kundalić, B.; Rakić, V.; et al. Protective effects of anthocyanins from bilberry extract in rats exposed to nephrotoxic effects of carbon tetrachloride. Chemico-Biol. Interact. 2019, 304, 61–72. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Liu, H.; Zhao, B.; Zheng, M.; Liu, J. Anthocyanins from purple corn ameliorated obesity in high fat diet-induced obese mice through activating hepatic ampk. J. Funct. Foods 2021, 84, 104582. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Wang, Z.; West, M.; Singh, V.; West, L.; de Mejia, E.G. Processing method and corn cultivar affected anthocyanin concentration from dried distillers grains with solubles. J. Agric. Food Chem. 2015, 63, 3205–3218. [Google Scholar] [CrossRef] [PubMed]
- Priprem, A.; Limsitthichaikoon, S.; Sukkhamduang, W.; Limphirat, W.; Thapphasaraphong, S.; Nualkaew, N. Anthocyanin complex improves stability with in vitro localized uva protective effect. Curr. Bioact. Compd. 2017, 13, 333–339. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, L.; Li, J.; Oliveira, H.; Yang, N.; Jin, W.; Zhu, Z.; Li, S.; He, J. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. LWT 2020, 123, 109097. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from thai rice bran extracts. Food Chem. 2020, 329, 127157. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Giusti, M.M. The effect of whey protein concentration and preheating temperature on the color and stability of purple corn, grape and black carrot anthocyanins in the presence of ascorbic acid. Food Res. Int. 2021, 144, 110350. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, N.; Kimura, Y.; Ohno, T. Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chem. 2014, 143, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mukherjee, D.; Chang, S.K.C. Physicochemical properties and storage stability of soybean protein nanoemulsions prepared by ultra-high pressure homogenization. Food Chem. 2018, 240, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-reacted whey protein isolates enhance thermal stability of anthocyanins over a wide ph range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Preheated milk proteins improve the stability of grape skin anthocyanins extracts. Food Chem. 2016, 210, 221–227. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Hsieh, J.-F. The conversion and deglycosylation of isoflavones and anthocyanins in black soymilk process. Food Chem. 2018, 261, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Gao, X.; Chen, Y.; Kumar Santhanam, R.; Wang, C.; Xu, L.; Chen, H. Interaction characterization of preheated soy protein isolate with cyanidin-3-o-glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts. Food Chem. 2019, 271, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Cui, Z.; He, X.; Wang, X.; Zeng, X.; Ma, H. Optimization of extraction and isolation for 11s and 7s globulins of soybean seed storage protein. Food Chem. 2007, 102, 1310–1316. [Google Scholar] [CrossRef]
- He, Y.; Wen, L.; Yu, H.; Zheng, F.; Wang, Z.; Xu, X.; Zhang, H.; Cao, Y.; Wang, B.; Chu, B.; et al. Effects of high hydrostatic pressure-assisted organic acids on the copigmentation of vitis amurensis rupr anthocyanins. Food Chem. 2018, 268, 15–26. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, L.; Yu, H.; Cao, Y.; Nan, H.; Gou, M.; Xie, C.; Xue, H. Isolation and structural identification of the main anthocyanin monomer in vitis amurensis rupr. Nat. Prod. Res. 2018, 32, 867–870. [Google Scholar] [CrossRef]
- He, W.; Yin, Z.; Liu, S.; Chen, Y.; Qie, X.; Chen, J.; Zeng, M.; Qin, F.; He, Z. Effect of preheated milk proteins and bioactive compounds on the stability of cyanidin-3-o-glucoside. Food Chem. 2021, 345, 128829. [Google Scholar] [CrossRef] [PubMed]
- Attaribo, T.; Jiang, X.; Huang, G.; Zhang, B.; Xin, X.; Zhang, Y.; Zhang, N.; Gui, Z. Studies on the interactional characterization of preheated silkworm pupae protein (spp) with anthocyanins (c3g) and their effect on anthocyanin stability. Food Chem. 2020, 326, 126904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.-H.; Prasanna, G.; Jing, P. Spectrofluorimetric and molecular docking studies on the interaction of cyanidin-3-o-glucoside with whey protein, β-lactoglobulin. Int. J. Biol. Macromol. 2017, 105, 965–972. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, X.; Yu, P.; Chen, Z. Complexation of rice glutelin fibrils with cyanidin-3-o-glucoside at acidic condition: Thermal stability, binding mechanism and structural characterization. Food Chem. 2021, 363, 130367. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. Interaction and binding mechanism of cyanidin-3-o-glucoside to ovalbumin in varying ph conditions: A spectroscopic and molecular docking study. Food Chem. 2020, 320, 126616. [Google Scholar] [CrossRef]
- Quan, W.; He, W.; Qie, X.; Chen, Y.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of β-cyclodextrin, whey protein, and soy protein on the thermal and storage stability of anthocyanins obtained from purple-fleshed sweet potatoes. Food Chem. 2020, 320, 126655. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, F.; Li, Y.; Chen, H.; Liao, X.; Zhang, Y. Hydrophobic interaction driving the binding of soybean protein isolate and chlorophyll: Improvements to the thermal stability of chlorophyll. Food Hydrocoll. 2021, 113, 106465. [Google Scholar] [CrossRef]
- Lang, Y.; Li, E.; Meng, X.; Tian, J.; Ran, X.; Zhang, Y.; Zang, Z.; Wang, W.; Li, B. Protective effects of bovine serum albumin on blueberry anthocyanins under illumination conditions and their mechanism analysis. Food Res. Int. 2019, 122, 487–495. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stănciuc, N.; Grigore-Gurgu, L.; Aprodu, I. Investigation on the interaction of heated soy proteins with anthocyanins from cornelian cherry fruits. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118114. [Google Scholar] [CrossRef]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of malbec and bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef]
- Ren, C.; Xiong, W.; Li, J.; Li, B. Comparison of binding interactions of cyanidin-3-o-glucoside to β-conglycinin and glycinin using multi-spectroscopic and thermodynamic methods. Food Hydrocoll. 2019, 92, 155–162. [Google Scholar] [CrossRef]
- Gao, D.; Tian, Y.; Bi, S.; Chen, Y.; Yu, A.; Zhang, H. Studies on the interaction of colloidal gold and serum albumins by spectral methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hao, L.; Tan, Y.; Yang, Y.; Liu, L.; Li, C.; Du, P. Noncovalent interaction of cyanidin-3-o-glucoside with whey protein isolate and β-lactoglobulin: Focus on fluorescence quenching and antioxidant properties. LWT 2021, 137, 110386. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res. Int. 2015, 76, 761–768. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]

| No. | A: Complex Proportion | B: Reaction Temperature (°C) | C: Reaction Time (min) | Abs ‡ | ||
|---|---|---|---|---|---|---|
| 1 | 1 (30:1) | 0 (60) | 0 (60) | 0.774 | ||
| 2 | 1 | −1 (50) | −1 (30) | 0.689 | ||
| 3 | −1 (40:1) | 1 (70) | 0 | 0.721 | ||
| 4 | 0 (20:1) | 1 | 0 | 0.694 | ||
| 5 | −1 | 0 | −1 | 0.703 | ||
| 6 | 1 | 0 | 0 | 0.787 | ||
| 7 | 1 | 0 | 0 | 0.768 | ||
| 8 | 1 | 0 | 0 | 0.771 | ||
| 9 | 1 | 0 | 0 | 0.774 | ||
| 10 | 1 | 1 | 1 (90) | 0.759 | ||
| 11 | 0 | 0 | −1 | 0.655 | ||
| 12 | 0 | 0 | 1 | 0.677 | ||
| 13 | 1 | 1 | −1 | 0.721 | ||
| 14 | 1 | −1 | 1 | 0.675 | ||
| 15 | −1 | −1 | 0 | 0.697 | ||
| 16 | 0 | −1 | 0 | 0.625 | ||
| 17 | −1 | 0 | 1 | 0.719 | ||
| Source | Sum of square | df | Mean square | F value | p value | Significant |
| Model | 0.021 | 9 | 0.002354 | 24.24 | 0.0002 | ** |
| A | 0.003698 | 1 | 0.003698 | 38.07 | 0.0005 | ** |
| B | 0.005202 | 1 | 0.005202 | 53.55 | 0.0002 | ** |
| C | 0.000008 | 1 | 0.000008 | 0.082 | 0.7824 | |
| AB | 0.000625 | 1 | 0.000625 | 6.43 | 0.0389 | * |
| AC | 0.000049 | 1 | 0.000049 | 0.50 | 0.5005 | |
| BC | 0.000036 | 1 | 0.000036 | 0.37 | 0.5619 | |
| A2 | 0.004725 | 1 | 0.004725 | 48.64 | 0.0002 | ** |
| B2 | 0.003917 | 1 | 0.003917 | 40.32 | 0.0004 | ** |
| C2 | 0.001769 | 1 | 0.001769 | 18.22 | 0.0037 | ** |
| Residual | 0.00068 | 7 | 0.00009714 | |||
| Lack of Fit | 0.00005 | 3 | 0.00001667 | 0.11 | 0.9524 | Not significant |
| Pure Error | 0.00063 | 4 | 0.001575 | |||
| Cor Total | 0.022 | 16 | ||||
| R2adj | 0.9289 | |||||
| R2Pred | 0.9184 | |||||
| C.V./% | 1.42 | |||||
| Sample | k (min−1) | R2 | t1/2 (min) | |
|---|---|---|---|---|
| D3G (pH 2.8) | 0.00185 ± 3.53 × 10−5 | 0.998 | 374.67 ± 7.15 | |
| SPI-7s-D3G † (pH 2.8) | 0.00057 ± 1.46 × 10−5 | 0.997 | 1216.04 ± 31.17 | |
| D3G (pH 6.8) | 0.00221 ± 3.64 × 10−5 | 0.998 | 313.64 ± 5.17 | |
| SPI-7s-D3G (pH 6.8) | 0.00066 ± 1.69 × 10−5 | 0.997 | 1050.22 ± 26.91 | |
| Sample | T (K) | ΔH (kJ·mol−1) | ΔG (kJ·mol−1) | ΔS (J·mol−1·K−1) |
| SPI-7s-D3G (pH 2.8) | 298 | −23.22 | −31.54 | 27.93 |
| 308 | −31.82 | |||
| 318 | −32.10 | |||
| SPI-7s-D3G (pH 6.8) | 298 | −21.98 | −31.10 | 30.59 |
| 308 | −30.40 | |||
| 318 | −31.71 | |||
| Sample | α-Helix (%) | β-Sheets (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| SPI-7s-D3G † (pH 2.8) | 38.13 | 20.78 | 21.02 | 20.07 |
| SPI-7s (pH 2.8) | 22.73 | 32.48 | 19.87 | 24.92 |
| SPI-7s-D3G (pH 6.8) | 38.03 | 23.64 | 20.96 | 17.37 |
| SPI-7s (pH 6.8) | 24.36 | 32.98 | 19.65 | 23.01 |
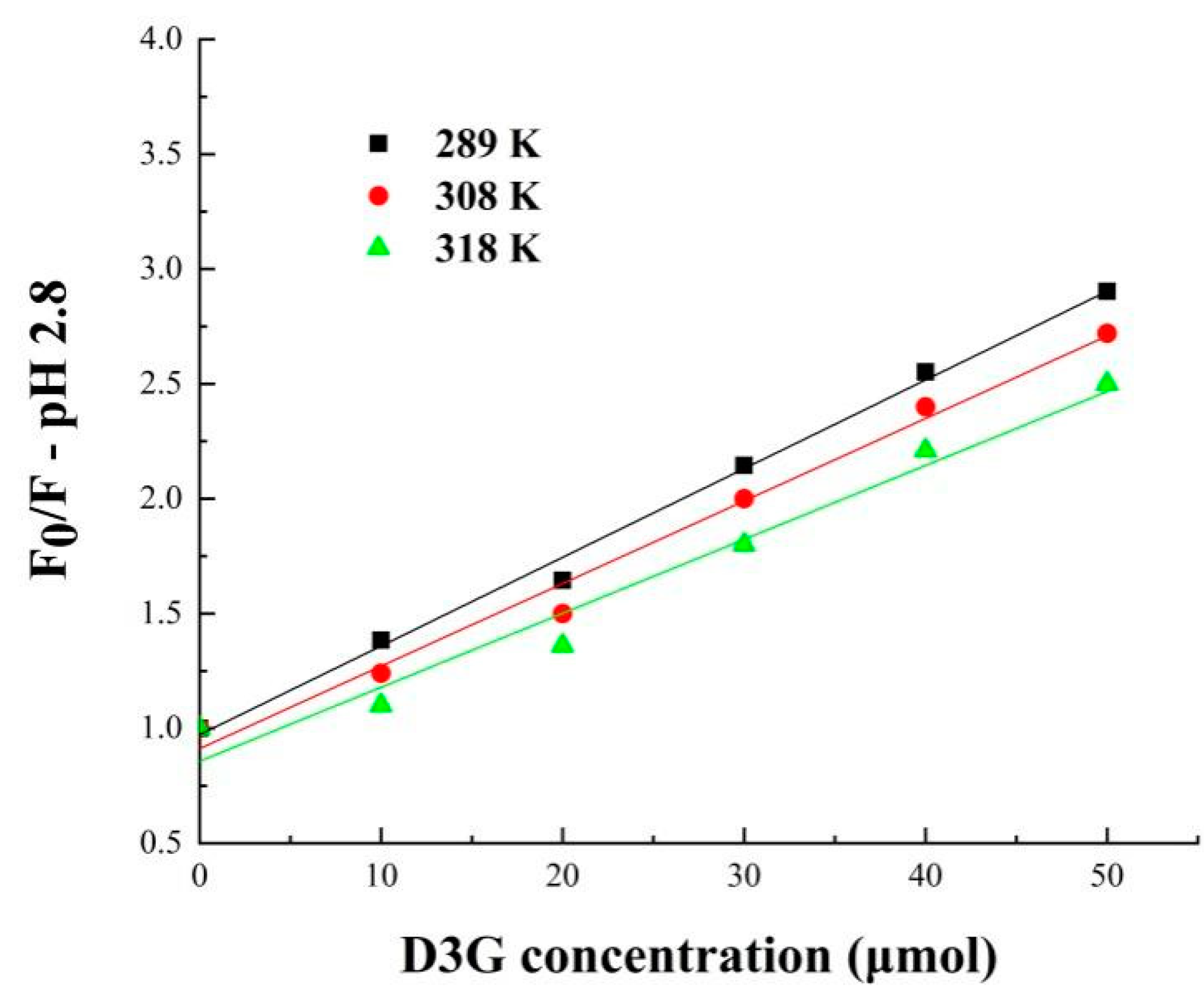
| Sample | T (K) | Ksv (×103 L·mol−1) | Kq (×1011 L·mol−1·s−1) | n |
|---|---|---|---|---|
| SPI-7s-D3G † (pH 2.8) | 298 | 38.62 | 38.62 | 1.02 |
| 308 | 35.94 | 35.94 | 0.95 | |
| 318 | 32.20 | 32.20 | 0.95 | |
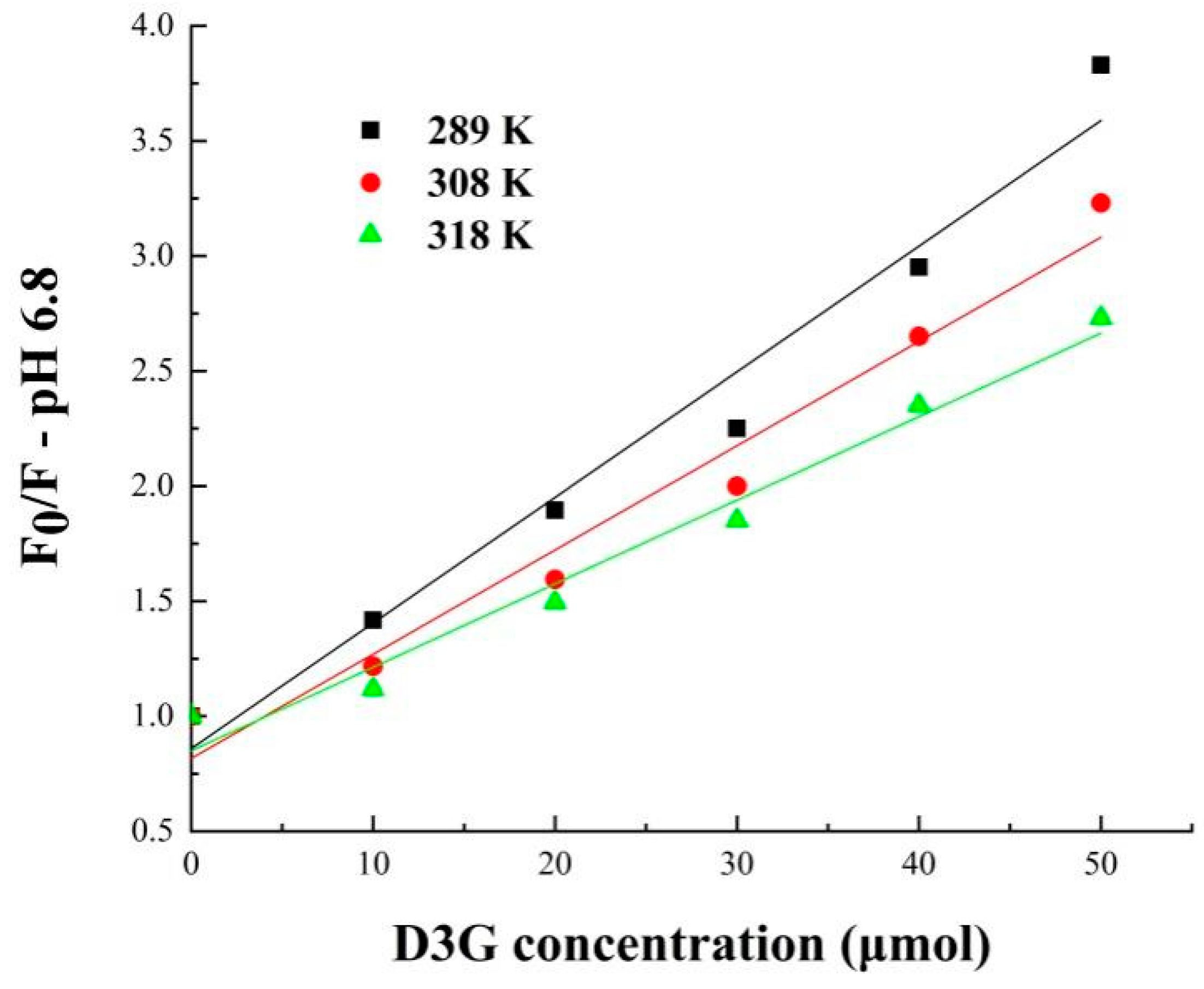
| SPI-7s-D3G (pH 6.8) | 298 | 54.58 | 54.58 | 1.15 |
| 308 | 45.30 | 45.30 | 1.16 | |
| 318 | 36.30 | 36.30 | 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Liu, Y.; Li, J.; Sun, X.; Gu, J.; He, Y.; Ci, H.; Wen, L.; Yu, H.; Xu, X. Effect of Soybean Protein Isolate-7s on Delphinidin-3-O-Glucoside from Purple Corn Stability and Their Interactional Characterization. Foods 2022, 11, 895. https://doi.org/10.3390/foods11070895
Chen D, Liu Y, Li J, Sun X, Gu J, He Y, Ci H, Wen L, Yu H, Xu X. Effect of Soybean Protein Isolate-7s on Delphinidin-3-O-Glucoside from Purple Corn Stability and Their Interactional Characterization. Foods. 2022; 11(7):895. https://doi.org/10.3390/foods11070895
Chicago/Turabian StyleChen, Dongxia, Yuheng Liu, Jia Li, Xiaozhen Sun, Jiadong Gu, Yang He, Hui Ci, Liankui Wen, Hansong Yu, and Xiuying Xu. 2022. "Effect of Soybean Protein Isolate-7s on Delphinidin-3-O-Glucoside from Purple Corn Stability and Their Interactional Characterization" Foods 11, no. 7: 895. https://doi.org/10.3390/foods11070895
APA StyleChen, D., Liu, Y., Li, J., Sun, X., Gu, J., He, Y., Ci, H., Wen, L., Yu, H., & Xu, X. (2022). Effect of Soybean Protein Isolate-7s on Delphinidin-3-O-Glucoside from Purple Corn Stability and Their Interactional Characterization. Foods, 11(7), 895. https://doi.org/10.3390/foods11070895







