Assessment of Phenotypic Diversity in the USDA Collection of Quinoa Links Genotypic Adaptation to Germplasm Origin
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Genetic Materials and Field Experiments
2.2. Measurements
2.3. Data Analysis
3. Results
3.1. Effects of Growing Conditions on Phenotypic Variation
3.2. Variance Components and Heritability
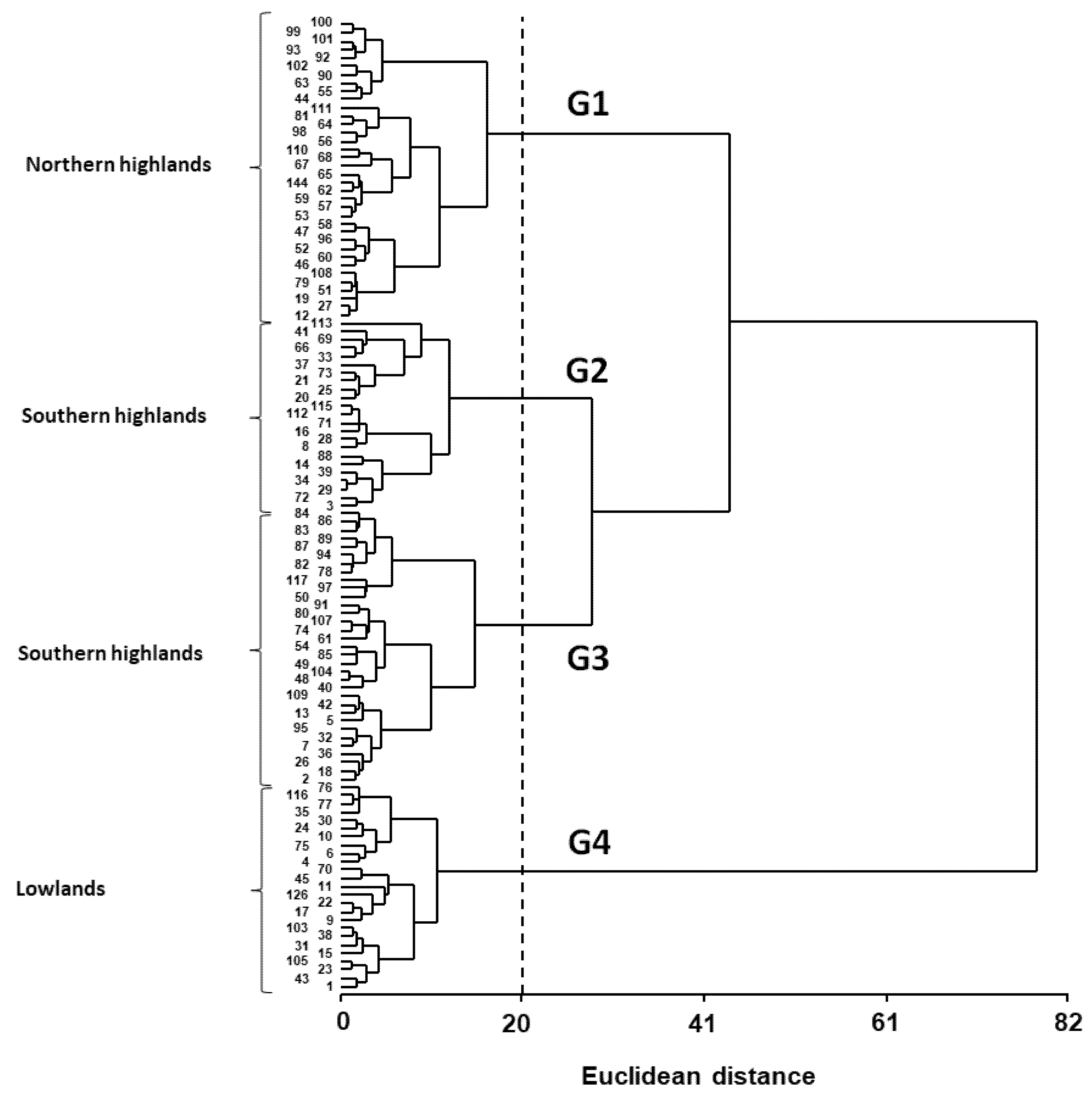
3.3. Phenotypic Variation Patterns in the USDA Germplasm Collection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, K.S.; Matanguihan, J. Quinoa: Improvement and Sustainable Production; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; Oficina Regional de la FAO para América Latina y el Caribe; FAO: Rome, Italy, 2015. [Google Scholar]
- Haros, C.M.; Schoenlechner, R. Pseudocereals: Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Economist, T. Big Agribusiness Wants to Make Quinoa More Mainstream. Traders Hope That Reliable Domestic Supply Will Entice Foodmakers to Use More of the Crop as an Ingredient in Processed Snacks. 2019. Available online: https://www.economist.com/business/2019/05/25/big-agribusiness-wants-to-make-quinoa-more-mainstream (accessed on 10 January 2019).
- Alandia, G.; Rodriguez, J.; Jacobsen, S.-E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Tapia, M.E. History of the Quinuas in South America. In The Quinoa Genome; Springer: Gewerbestrasse, Switzerland; Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenfell-Shaw, L.; Tester, M. Abiotic Stress Tolerance in Quinoa. In The Quinoa Genome; Springer: Gewerbestrasse, Switzerland; Cham, Switzerland, 2021; pp. 139–167. [Google Scholar]
- Yang, X.; Qin, P.; Guo, H.; Ren, G. Quinoa industry development in China. Cienc. Investig. Agrar. Rev. Latinoam. Cienc. Agric. 2019, 46, 208–219. [Google Scholar]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, R. Leveraging Agricultural Biodiversity for Crop Improvement and Food Security. In Routledge Handbook of Agricultural Biodiversity; Routledge: London, UK, 2017; pp. 285–297. [Google Scholar]
- Kilian, B.; Dempewolf, H.; Guarino, L.; Werner, P.; Coyne, C.; Warburton, M.L. Crop Science special issue: Adapting agriculture to climate change: A walk on the wild side. Crop Sci. 2021, 61, 32–36. [Google Scholar] [CrossRef]
- Bertero, H.; De la Vega, A.; Correa, G.; Jacobsen, S.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crops Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Curti, R.N.; De la Vega, A.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Multi-environmental evaluation for grain yield and its physiological determinants of quinoa genotypes across Northwest Argentina. Field Crops Res. 2014, 166, 46–57. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and interrelationship among various morphological and quality traits in quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2007, 101, 104–116. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M. Worldwide evaluations of quinoa: Preliminary results from post international year of quinoa FAO projects in nine countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef] [Green Version]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for marginal environments: Toward future food and nutritional security in MENA and Central Asia regions. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.P.; Ono, E.; Choukr-Allah, R.; Abdelaziz, H. Cultivation of quinoa (Chenopodium quinoa) in desert ecoregion. In Emerging Research in Alternative Crops; Springer: Gewerbestrasse, Switzerland; Cham, Switzerland, 2020; pp. 145–161. [Google Scholar]
- Maliro, M.F.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of central Malawi. Front. Plant Sci. 2017, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Maliro, M.; Njala, A.L. Agronomic performance and strategies of promoting Quinoa (Chenopodium quinoa Willd) in Malawi. Cienc. Investig. Agrar. Rev. Latinoam. Cienc. Agric. 2019, 46, 82–99. [Google Scholar] [CrossRef]
- Wilson, H.D. Quinua and relatives (Chenopodium sect. Chenopodium subsect. Celluloid). Econ. Bot. 1990, 44, 92–110. [Google Scholar] [CrossRef]
- Christensen, S.; Pratt, D.B.; Pratt, C.; Nelson, P.; Stevens, M.; Jellen, E.N.; Coleman, C.E.; Fairbanks, D.J.; Bonifacio, A.; Maughan, P.J. Assessment of genetic diversity in the USDA and CIP-FAO international nursery collections of quinoa (Chenopodium quinoa Willd.) using microsatellite markers. Plant Genet. Resour. 2007, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, N.; Toyoshima, M.; Fujita, M.; Fukuda, S.; Kobayashi, Y.; Ueno, M.; Tanaka, K.; Tanaka, T.; Nishihara, E.; Mizukoshi, H. The genotype-dependent phenotypic landscape of quinoa in salt tolerance and key growth traits. DNA Res. 2020, 27, dsaa022. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The global expansion of quinoa: Trends and limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.M. Response to selection for reduced grain saponin content in quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2000, 68, 157–163. [Google Scholar] [CrossRef]
- Jarvis, D.; Shwen, H.Y.; Lightfoot, D.J.; Schmöckel, S.M.; Bo, L.; Borm, T.J.; Hajime, O.; Katsuhiko, M.; Michell, C.T.; Noha, S.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.H.; Solgi, S.; Sepaskhah, A.R. Quinoa: A super or pseudo-super crop? Evidences from evapotranspiration, root growth, crop coefficients, and water productivity in a hot and semi-arid area under three planting densities. Agric. Water Manag. 2019, 225, 105784. [Google Scholar] [CrossRef]
- Ceccato, D.V.; Bertero, H.D.; Batlla, D. Environmental control of dormancy in quinoa (Chenopodium quinoa) seeds: Two potential genetic resources for pre-harvest sprouting tolerance. Seed Sci. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef]
- Ceccato, D.; Bertero, D.; Batlla, D.; Galati, B. Structural aspects of dormancy in quinoa (Chenopodium quinoa): Importance and possible action mechanisms of the seed coat. Seed Sci. Res. 2015, 25, 267–275. [Google Scholar] [CrossRef]
- Castellión, M.; Matiacevich, S.; Buera, P.; Maldonado, S. Protein deterioration and longevity of quinoa seeds during long-term storage. Food Chem. 2010, 121, 952–958. [Google Scholar] [CrossRef]
- Akram, M.Z.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Nazeer, S.; Iqbal, S.; Saddiq, M.S.; Zahra, N. Adaptability and yield potential of new quinoa lines under agro-ecological conditions of Faisalabad-Pakistan. Asian J. Agric. Biol. 2021, 2, 202005301. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Ward, J., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Lê, S.; Josse, J.; Mazet, F. Package ‘FactoMineR’. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Afiah, S.A.; Hassan, W.A.; Al Kady, A. Assessment of six quinoa (Chenopodium quinoa Willd.) genotypes for seed yield and its attributes under Toshka conditions. Zagazig J. Agric. Res. 2018, 45, 2281–2294. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A. Effect of Water Stress on Grain Yield and Physiological Characters of Quinoa Genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Isobe, K.; Sugiyama, H.; Okuda, D.; Murase, Y.; Harada, H.; Miyamoto, M.; Koide, S.; Higo, M.; Torigoe, Y. Effects of sowing time on the seed yield of quinoa (Chenopodium quinoa Willd) in South Kanto, Japan. Agric. Sci. 2016, 7, 146. [Google Scholar]
- Iqbal, S.; Basra, S.M.; Afzal, I.; Wahid, A.; Saddiq, M.S.; Hafeez, M.B.; Jacobsen, S.E. Yield potential and salt tolerance of quinoa on salt-degraded soils of Pakistan. J. Agron. Crop Sci. 2019, 205, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment. Plants 2020, 9, 1763. [Google Scholar] [CrossRef]
- De Santis, G.; Ronga, D.; Caradonia, F.; Ambrosio, T.D.; Troisi, J.; Rascio, A.; Fragasso, M.; Pecchioni, N.; Rinaldi, M. Evaluation of two groups of quinoa (Chenopodium quinoa Willd.) accessions with different seed colours for adaptation to the Mediterranean environment. Crop Pasture Sci. 2018, 69, 1264–1275. [Google Scholar] [CrossRef]
- Colque-Little, C.; Abondano, M.C.; Lund, O.S.; Amby, D.B.; Piepho, H.-P.; Andreasen, C.; Schmöckel, S.; Schmid, K. Genetic variation for tolerance to the downy mildew pathogen Peronospora variabilis in genetic resources of quinoa (Chenopodium quinoa). BMC Plant Biol. 2021, 21, 41. [Google Scholar] [CrossRef]
- De Santis, G.; D’Ambrosio, T.; Rinaldi, M.; Rascio, A. Heritabilities of morphological and quality traits and interrelationships with yield in quinoa (Chenopodium quinoa Willd.) genotypes in the Mediterranean environment. J. Cereal Sci. 2016, 70, 177–185. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Christiansen, J.; Jacobsen, S.-E.; Jørgensen, S. Photoperiodic effect on flowering and seed development in quinoa (Chenopodium quinoa Willd.). Acta Agric. Scand. Sect. B-Soil Plant Sci. 2010, 60, 539–544. [Google Scholar]
- Thiam, E.; Allaoui, A.; Benlhabib, O. Quinoa Productivity and Stability Evaluation through Varietal and Environmental Interaction. Plants 2021, 10, 714. [Google Scholar] [CrossRef]
- Curti, R.N.; De la Vega, A.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Adaptive responses of quinoa to diverse agro-ecological environments along an altitudinal gradient in North West Argentina. Field Crops Res. 2016, 189, 10–18. [Google Scholar] [CrossRef]
- Bertero, H.; King, R.; Hall, A. Modelling photoperiod and temperature responses of flowering in quinoa (Chenopodium quinoa Willd.). Field Crops Res. 1999, 63, 19–34. [Google Scholar] [CrossRef]
- EL-Harty, E.H.; Ghazy, A.; Alateeq, T.K.; Al-Faifi, S.A.; Khan, M.A.; Afzal, M.; Alghamdi, S.S.; Migdadi, H.M. Morphological and Molecular Characterization of Quinoa Genotypes. Agriculture 2021, 11, 286. [Google Scholar] [CrossRef]
- Gómez, M.B.; Curti, R.N.; Bertero, H.D. Seed weight determination in quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2022, 208, 243–254. [Google Scholar] [CrossRef]
- Gómez, M.B.; Castro, P.A.; Mignone, C.; Bertero, H.D. Can yield potential be increased by manipulation of reproductive partitioning in quinoa (Chenopodium quinoa)? Evidence from gibberellic acid synthesis inhibition using Paclobutrazol. Funct. Plant Biol. 2011, 38, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, J.L.; Borrás, L.; De Bruin, J.; Pedersen, P. Physiological strategies for seed number determination in soybean: Biomass accumulation, partitioning and seed set efficiency. Field Crops Res. 2012, 135, 58–66. [Google Scholar] [CrossRef]
- de la Vega, A.J.; Hall, A.J.; Kroonenberg, P.M. Investigating the physiological bases of predictable and unpredictable genotype by environment interactions using three-mode pattern analysis. Field Crops Res. 2002, 78, 165–183. [Google Scholar] [CrossRef]
- De La Vega, A.J.; Chapman, S.C.; Hall, A.J. Genotype by environment interaction and indirect selection for yield in sunflower: I. Two-mode pattern analysis of oil and biomass yield across environments in Argentina. Field Crops Res. 2001, 72, 17–38. [Google Scholar] [CrossRef]
- Chapman, S.C.; Crossa, J.; Edmeades, G.O. Genotype by environment effects and selection for drought tolerance in tropical maize. I. Two mode pattern analysis of yield. Euphytica 1997, 95, 1–9. [Google Scholar] [CrossRef]
- Risi, J.; Galwey, N. The pattern of genetic diversity in the Andean grain crop quinoa (Chenopodium quinoa Willd). I. Associations between characteristics. Euphytica 1989, 41, 147–162. [Google Scholar] [CrossRef]
- Nico, M.; Miralles, D.J.; Kantolic, A.G. Natural post-flowering photoperiod and photoperiod sensitivity: Roles in yield-determining processes in soybean. Field Crops Res. 2019, 231, 141–152. [Google Scholar] [CrossRef]
- Naneli, I.; Tanrikulu, A.; Dokuyucu, T. Response of the quinoa genotypes to different locations by grain yield and yield components. Int. J. Agric. Innov. Res. 2017, 6, 446–451. [Google Scholar]
- Khaitov, B.; Karimov, A.A.; Toderich, K.; Sultanova, Z.; Mamadrahimov, A.; Allanov, K.; Islamov, S. Adaptation, grain yield and nutritional characteristics of quinoa (Chenopodium quinoa) genotypes in marginal environments of the Aral Sea basin. J. Plant Nutr. 2020, 44, 1365–1379. [Google Scholar] [CrossRef]

| Season | |||
|---|---|---|---|
| 2016 | 2017 | ||
| Max. temp. (°C) | 17.6–37.7 | 21.5–36.8 | (27.3–35.4) a |
| Min. temp. (°C) | 8.2–20.9 | 5.5–20.8 | (2.2–13.0) a |
| Mean temp. (°C) | 12.9–29.3 | 13.5–28.8 | (12.5–27.7) a |
| Rainfall (mm) | 60 | 36 | (24.4) a |
| Ht (cm) b | 94.3 ± 1.6 | 97.6 ± 1.8 ** | |
| Pan Ht (cm) | 29.3 ± 0.5 | 29.4 ± 0.4 | |
| B (g m−2) | 67.9 ± 3.2 | 71.0 ± 3.1 * | |
| HI | 35.4 ± 0.6 | 35.7 ± 0.6 | |
| Pan Y (g m−2) | 17.0 ± 0.9 | 18.3 ± 0.9 ** | |
| Y (g m−2) | 24.6 ± 1.3 | 25.6 ± 1.3 | |
| SN (# m−2) | 7.7 × 106 ± 4 × 105 | 7.9 × 106 ± 3.9 × 105 | |
| SW (mg) | 3.1 × 10−3 ± 3.6 × 10−5 | 3.2 × 10−3 ± 4 × 10−5 ** | |
| E–-Ant (days) | 64.7 ± 0.3 | 67.9 ± 0.3 ** | |
| Ant–PM (days) | 51.3 ± 0.5 | 50.6 ± 0.5 | |
| Cycle (days) | 129.6 ± 0.5 | 134.9 ± 0.4 ** | |
| Trait | σ2g | σ2ge | σ2g/σ2ge | |
|---|---|---|---|---|
| Ht | 942.0 ** | 46.9 ** | 20.1 | 0.90 |
| Pan Ht | 60.1 ** | 0.15 ns | 0.87 | |
| B | 3364.0 ** | 7.8 ns | 0.97 | |
| HI | 89.5 ** | 11.0 ** | 8.1 | 0.75 |
| Pan Y | 17.7 * | 15.9 ns | 0.96 | |
| Y | 57.3 ** | 32.2 ns | 0.97 | |
| SN | 5.2 × 1013 ** | 2.0 ×1012 ** | 25.3 | 0.93 |
| SW | 4.3 × 10−7 ** | 8.4 × 10−8 ** | 5.1 | 0.82 |
| E–Ant | 35.1 ** | 4.0 ** | 8.8 | 0.87 |
| Ant–PM | 78.8 ** | 7.2 ** | 10.9 | 0.90 |
| Cycle | 63.8 ** | 5.2 ** | 12.3 | 0.92 |
| Group | Ht a (cm) | Pan Ht (cm) | B (g m−2) | HI | Pan Y (g m−2) | Y (g m−2) | SN (# m−2) | SW (mg) | E–Ant (days) | Ant–PM (days) | Cycle (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 79.7 a | 29.8 b | 26.2 a | 0.31 a | 5.0 a | 6.6 a | 2.8 × 106 a | 2.6 a | 63 a | 60 b | 138 a |
| G2 | 67.9 a | 25.0 a | 26.0 a | 0.37 bc | 7.7 a | 9.9 a | 3.2 ×106 a | 3.3 b | 63 a | 45 a | 123 c |
| G3 | 110.5 b | 29.3 b | 73.4 b | 0.35 b | 17.0 b | 23.8 b | 6.6 × 106 b | 3.6 b | 69 b | 46 a | 131 b |
| G4 | 121.4 b | 32.3 b | 150.5 c | 0.40 c | 41.2 c | 60.4 c | 1.9 × 107 c | 3.3 b | 69 b | 49 a | 133 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, M.B.; Iqbal, S.; Li, Y.; Saddiq, M.S.; Basra, S.M.A.; Zhang, H.; Zahra, N.; Akram, M.Z.; Bertero, D.; Curti, R.N. Assessment of Phenotypic Diversity in the USDA Collection of Quinoa Links Genotypic Adaptation to Germplasm Origin. Plants 2022, 11, 738. https://doi.org/10.3390/plants11060738
Hafeez MB, Iqbal S, Li Y, Saddiq MS, Basra SMA, Zhang H, Zahra N, Akram MZ, Bertero D, Curti RN. Assessment of Phenotypic Diversity in the USDA Collection of Quinoa Links Genotypic Adaptation to Germplasm Origin. Plants. 2022; 11(6):738. https://doi.org/10.3390/plants11060738
Chicago/Turabian StyleHafeez, Muhammad Bilal, Shahid Iqbal, Yuanyuan Li, Muhammad Sohail Saddiq, Shahzad M. A. Basra, Hui Zhang, Noreen Zahra, Muhammad Z. Akram, Daniel Bertero, and Ramiro N. Curti. 2022. "Assessment of Phenotypic Diversity in the USDA Collection of Quinoa Links Genotypic Adaptation to Germplasm Origin" Plants 11, no. 6: 738. https://doi.org/10.3390/plants11060738
APA StyleHafeez, M. B., Iqbal, S., Li, Y., Saddiq, M. S., Basra, S. M. A., Zhang, H., Zahra, N., Akram, M. Z., Bertero, D., & Curti, R. N. (2022). Assessment of Phenotypic Diversity in the USDA Collection of Quinoa Links Genotypic Adaptation to Germplasm Origin. Plants, 11(6), 738. https://doi.org/10.3390/plants11060738







