Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean
Abstract
1. Introduction
2. Results
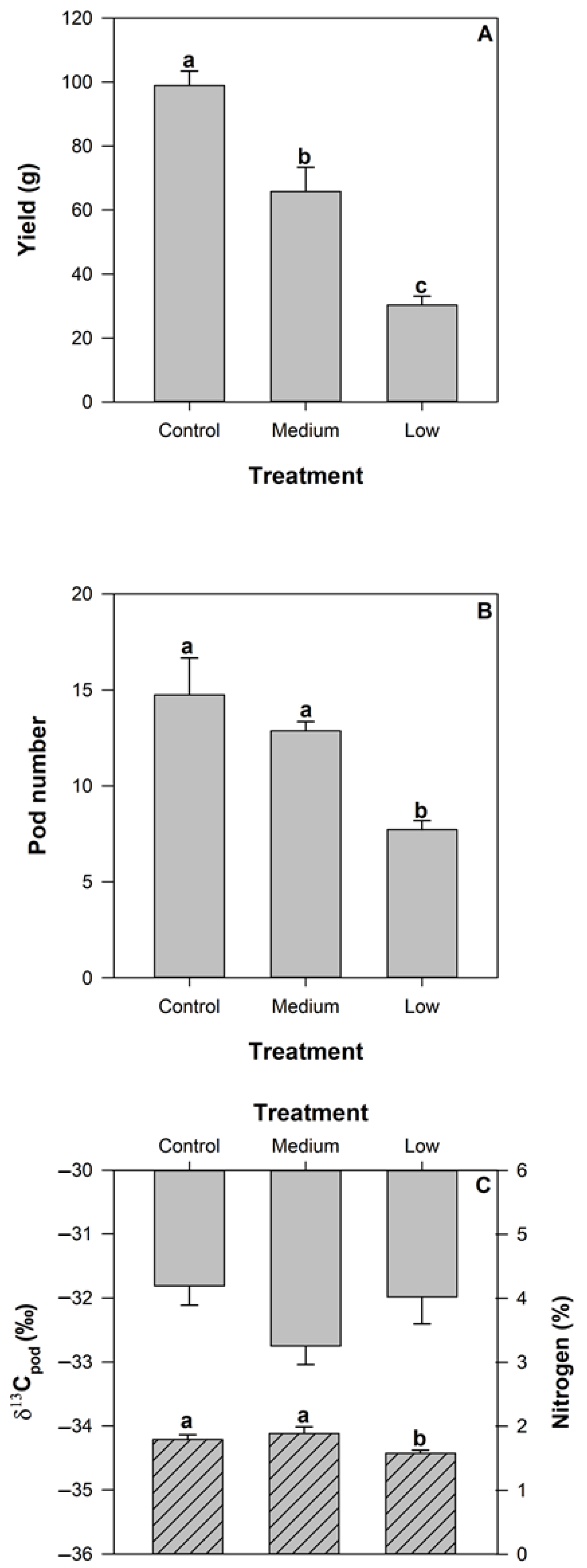
2.1. Effect of Nutrient Deficiency on Carbon Assimilation and Yield
2.2. Impact of Nutrient Deficiency on Carbon Isotope Abundance and Carbon and Nitrogen Content of Plant Tissues
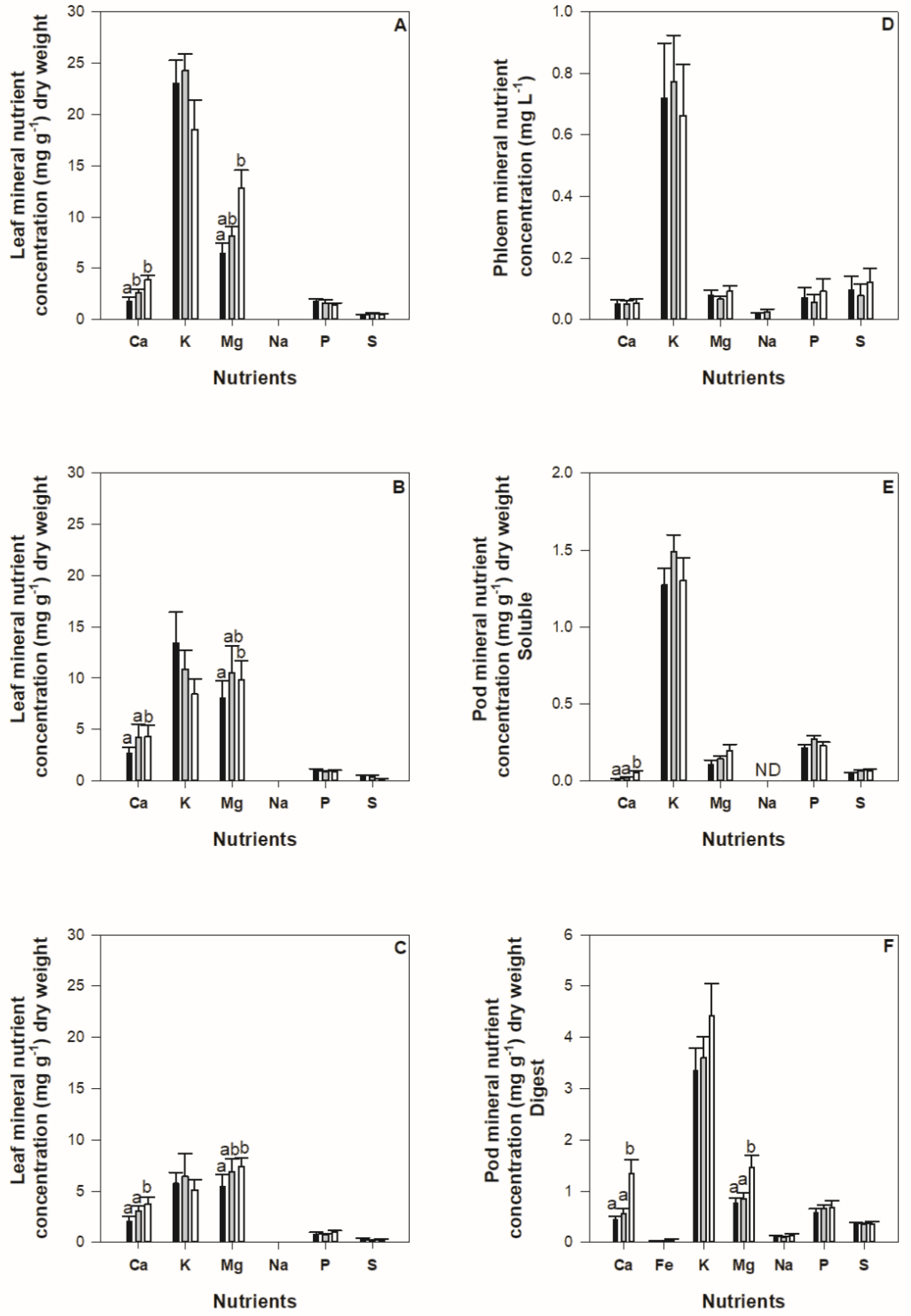
2.3. Impact of Nutrient Deficiency on Nutrient Concentrations and Content in Plant Tissues
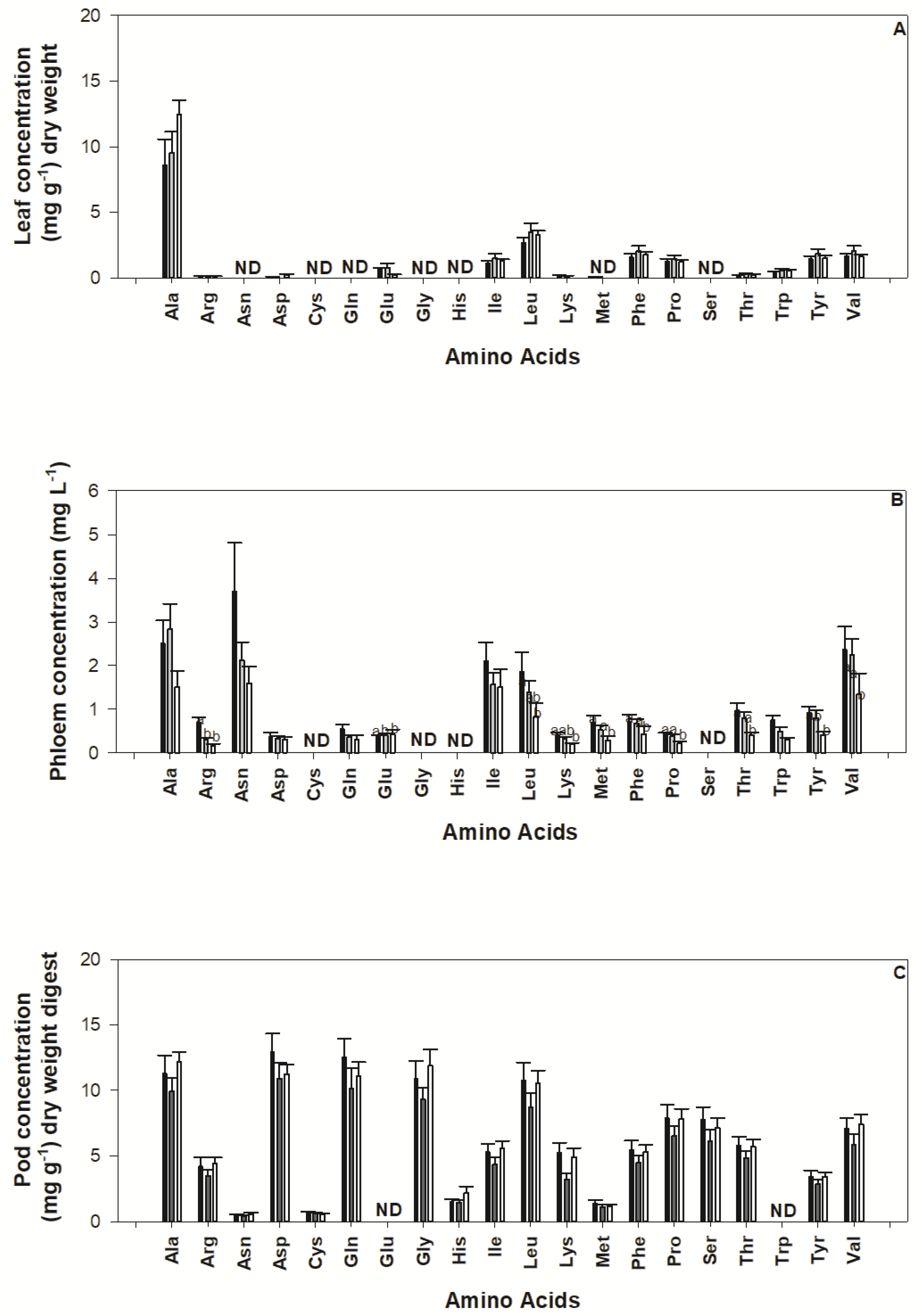
2.4. Impact of Nutrient Deficiency on Amino Acid Concentration
3. Discussion
3.1. Nutrient Supply Impacted Plant Growth and Yield Production
3.2. Nutrient Supply Did Not Alter Yield Nutrient Concentration
3.3. Soluble Extracts Did Not Reflect Digested Samples and Were Not Consistent throughout Development or Tissue Type
4. Materials and Methods
4.1. Experimental Design
4.2. Leaf Gas Exchange Measurements
4.3. Tissue Collection
4.4. Extractions of Leaf and Seed Material
4.5. Analysis of Carbon Isotope Abundance
4.6. Analysis of Plant Material for Amino Acids and Nutrients
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Ndakidemi, P.A.; Dakora, F.D.; Nkonya, E.M.; Ringo, D.; Mansoor, H. Yield and economic benefits of common bean (Phaseolus vulgaris) and soybean (Glycine max) inoculation in northern Tanzania. Aust. J. Exp. Agric. 2006, 46, 571–577. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Vadez, V. The future of grain legumes in cropping systems. Crop Pasture Sci. 2012, 63, 501–512. [Google Scholar] [CrossRef]
- Snapp, S.; Aggarwal, V.; Chirwa, R. Note on phosphorus and cultivar enhancement of biological nitrogen fixation and productivity of maize/bean intercrops in Malawi. Field Crop. Res. 1998, 58, 205–212. [Google Scholar] [CrossRef]
- Thung, M.; Rao, I.M. Integrated management of abiotic stresses. In Common Bean Improvement in the Twenty-First Century; Singh, S.P., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 331–370. [Google Scholar]
- Beebe, S.E.; Rao, I.M.; Devi, M.J.; Polania, J. Common beans, biodiversity, and multiple stresses: Challenges of drought resistance in tropical soils. Crop Pasture Sci. 2014, 65, 667–675. [Google Scholar] [CrossRef]
- Singh, S.P.; Terán, H.; Munoz-Perea, C.; Osorno, J.M.; Takegami, J.C.; Thung, M.D.T. Low Soil Fertility Tolerance in Landraces and Improved Common Bean Genotypes. Crop Sci. 2003, 43, 110–119. [Google Scholar] [CrossRef]
- Jansa, J.B.A.; Frossard, E.; Rao, I.M. Options for improving plant nutrition to increase common bean productivity in Africa. In Fighting Poverty in Sub-Saharan Africa: The Multiple Roles of Legumes in Integrated Soil Fertility Management; Bantonio, A.W.B., Okeyo, J.M., Maina, F., Kihara, F., Mokwunye, U., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 201–240. [Google Scholar]
- Beebe, S. Common Bean Breeding in the Tropics. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; Volume 36. [Google Scholar]
- Rychter, A.; Rao, I. Role of Phosphorus in Photosynthetic Carbon Metabolism. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 123–148. [Google Scholar] [CrossRef]
- Rao, I.M.; Miles, J.W.; Beebe, S.E.; Horst, W.J. Root adaptations to soils with low fertility and aluminium toxicity. Ann. Bot. 2016, 118, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.M.; Ricaurte, J.; Cajiao, C.; Galeano, C.H.; Rao, I.; Beebe, S.; Raatz, B. Phenotypic evaluation and QTL analysis of yield and symbiotic nitrogen fixation in a common bean population grown with two levels of phosphorus supply. Mol. Breed. 2017, 37, 76. [Google Scholar] [CrossRef]
- Araújo, S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic Stress Responses in Legumes: Strategies Used to Cope with Environmental Challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- FAO. FAO/INFOODS Database for Pulses on Dry Matter Basis Version 1.0—PulsesDM.1.0. 2017. Available online: https://www.fao.org/documents/card/en/c/ed8657c8-d5c8-4772-aaad-63405d253eb8/ (accessed on 7 March 2022).
- Beebe, S.G.V.; Rengifo, J. Research on trace minerals in the common bean. Food Nutr. Bull. 2000, 21, 387–391. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Graham, R. QTL analyses for seed iron and zinc concentrations in an intra-genepool population of Andean common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 122, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Boy, E.; Wirth, J.P.; Hurrell, R.F. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients 2015, 7, 1144–1173. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Grusak, M.A. Mineral accumulation in vegetative and reproductive tissues during seed development in Medicago truncatula. Front. Plant Sci. 2015, 6, 622. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.J.; Pate, J.S. Mobilization of Minerals to Developing Seeds of Legumes. Ann. Bot. 1977, 41, 1259–1278. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Butare, L. Breeding for abiotic stress tolerance in common bean: Present and future challenges. SABRAO J. Breed. Genet. 2009, 41, 11. [Google Scholar]
- Davies, S.L.; Turner, N.C.; Palta, J.A.; Siddique, K.H.M.; Plummer, J.A. Remobilisation of carbon and nitrogen supports seed filling in chickpea subjected to water deficit. Aust. J. Agric. Res. 2000, 51, 855–866. [Google Scholar] [CrossRef]
- Maillard, A.; Diquelou, S.; Billard, V.; Laine, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil. Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Tegeder, M.; Hammes, U.Z. The way out and in: Phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 2018, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Ricachenevsky, F.K.; Williams, L.E.; Vasconcelos, M.W.; Menguer, P.K. From soil to seed: Micronutrient movement into and within the plant. Front. Plant Sci. 2014, 5, 438. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.J.; Roberts, J.A.; Wagstaff, C. The role of the pod in seed development: Strategies for manipulating yield. New Phytol. 2011, 190, 838–853. [Google Scholar] [CrossRef]
- Pottier, M.; Masclaux-Daubresse, C.; Yoshimoto, K.; Thomine, S. Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds. Front. Plant Sci. 2014, 5, 11. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-Sink Relationships in Crop Plants and Their Influence on Yield Development and Nutritional Quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Behboudian, M.H.; Ma, Q.F.; Turner, N.C.; Palta, J.A. Reactions of chickpea to water stress: Yield and seed composition. J. Sci. Food Agric. 2001, 81, 1288–1291. [Google Scholar] [CrossRef]
- Cuellar-Ortiz, S.M.; Arrieta-Montiel, M.D.L.P.; Acosta-Gallegos, J.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409. [Google Scholar] [CrossRef]
- Dreccer, M.F.; van Herwaarden, A.F.; Chapman, S. Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration. Field Crop. Res. 2009, 112, 43–54. [Google Scholar] [CrossRef]
- Macfarlane, C.; Hansen, L.D.; Edwards, J.; White, D.A.; Adams, M. Growth efficiency increases as relative growth rate increases in shoots and roots of Eucalyptus globulus deprived of nitrogen or treated with salt. Tree Physiol. 2005, 25, 571–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gessler, A.; Rennenberg, H.; Keitel, C. Stable Isotope Composition of Organic Compounds Transported in the Phloem of European Beech—Evaluation of Different Methods of Phloem Sap Collection and Assessment of Gradients in Carbon Isotope Composition during Leaf-to-Stem Transport. Plant Biol. 2004, 6, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A. Developing Phloem δ13C and Sugar Composition as Indicators of Water Deficit in Lupinus angustifolius. HortScience 2012, 47, 691–696. [Google Scholar] [CrossRef]
- Merchant, A.; Richter, A.; Popp, M.; Adams, M. Targeted metabolite profiling provides a functional link among eucalypt taxonomy, physiology and evolution. Phytochemistry 2006, 67, 402–408. [Google Scholar] [CrossRef]
- Merchant, A.; Peuke, A.D.; Keitel, C.; Macfarlane, C.; Warren, C.; Adams, M.A. Phloem sap and leaf δ13C, carbohydrates and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. J. Exp. Bot. 2010, 61, 1785–1793. [Google Scholar] [CrossRef]
- Liu, Z.; Rochfort, S. A fast liquid chromatography–mass spectrometry (LC–MS) method for quantification of major polar metabolites in plants. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 912, 8–15. [Google Scholar] [CrossRef]
| Stage | Trt | A (μmol m−2 s−1) | gs (mmol m−2 s−1) | ci/ca (μmol m−1) | Leaf δ13C (‰) | Leaf N (%) |
|---|---|---|---|---|---|---|
| Flowering | Control (100%) | 8.52 ± 0.70 | 0.10 ± 0.01 | 0.60 ± 0.08 | −35.2 ± 0.3 | 4.2 ± 0.3 |
| Medium (50%) | 8.51 ± 0.48 | 0.11 ± 0.01 | 0.61 ± 0.10 | −35.2 ± 0.4 | 4.0 ± 0.3 | |
| Low (10%) | 8.56 ± 0.59 | 0.10 ± 0.01 | 0.56 ± 0.11 | −36.6 ± 1.1 | 3.3 ± 0.3 | |
| Mid Pod Fill | Control (100%) | 5.65 ± 0.52 | 0.06 ± 0.01 | 0.53 ± 0.07 | −33.3 ± 0.3 | 2.4 ± 0.3 |
| Medium (50%) | 4.99 ± 0.53 | 0.06 ± 0.01 | 0.51 ± 0.05 | −33.6 ± 0.6 | 1.9 ± 0.3 | |
| Low (10%) | 4.34 ± 0.44 | 0.05 ± 0.01 | 0.60 ± 0.06 | −33.8 ± 0.5 | 1.4 ± 0.1 | |
| Pod Maturity | Control (100%) | 3.18 ± 0.31a | 0.04 ± 0.01ab | 0.59 ± 0.08 | −32.6 ± 0.5 | 1.3 ± 0.2 |
| Medium (50%) | 3.06 ± 0.47a | 0.06 ± 0.01a | 0.73 ± 0.05 | −32.4 ± 0.5 | 1.1 ± 0.1 | |
| Low (10%) | 1.17 ± 0.20b | 0.03 ± 0.01b | 0.79 ± 0.06 | −31.7 ± 0.6 | 1.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, M.R.; Reis Hodecker, B.E.; Fuentes, D.; Merchant, A. Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean. Plants 2022, 11, 737. https://doi.org/10.3390/plants11060737
Smith MR, Reis Hodecker BE, Fuentes D, Merchant A. Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean. Plants. 2022; 11(6):737. https://doi.org/10.3390/plants11060737
Chicago/Turabian StyleSmith, Millicent R., Barbara Elias Reis Hodecker, David Fuentes, and Andrew Merchant. 2022. "Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean" Plants 11, no. 6: 737. https://doi.org/10.3390/plants11060737
APA StyleSmith, M. R., Reis Hodecker, B. E., Fuentes, D., & Merchant, A. (2022). Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean. Plants, 11(6), 737. https://doi.org/10.3390/plants11060737







