Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition †
Abstract
:1. Marine Algae as a Source of Bioactive Compounds
2. The Genus Asparagopsis
3. Biological Activities
3.1. Antioxidant Activity
3.2. Cytotoxic Activity
3.3. Antimicrobial Activity
3.4. Antiviral Activity
3.5. Enzyme Inhibition Activity
4. Chemical Characterization of Asparagopsis Species
4.1. General Chemical Composition
4.2. Secondary Metabolites Constituents
4.2.1. Sterols
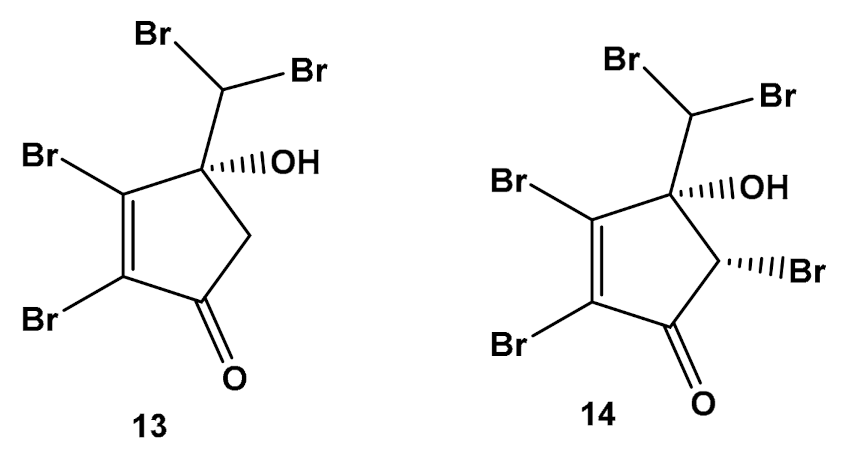
4.2.2. Halogenated Compounds
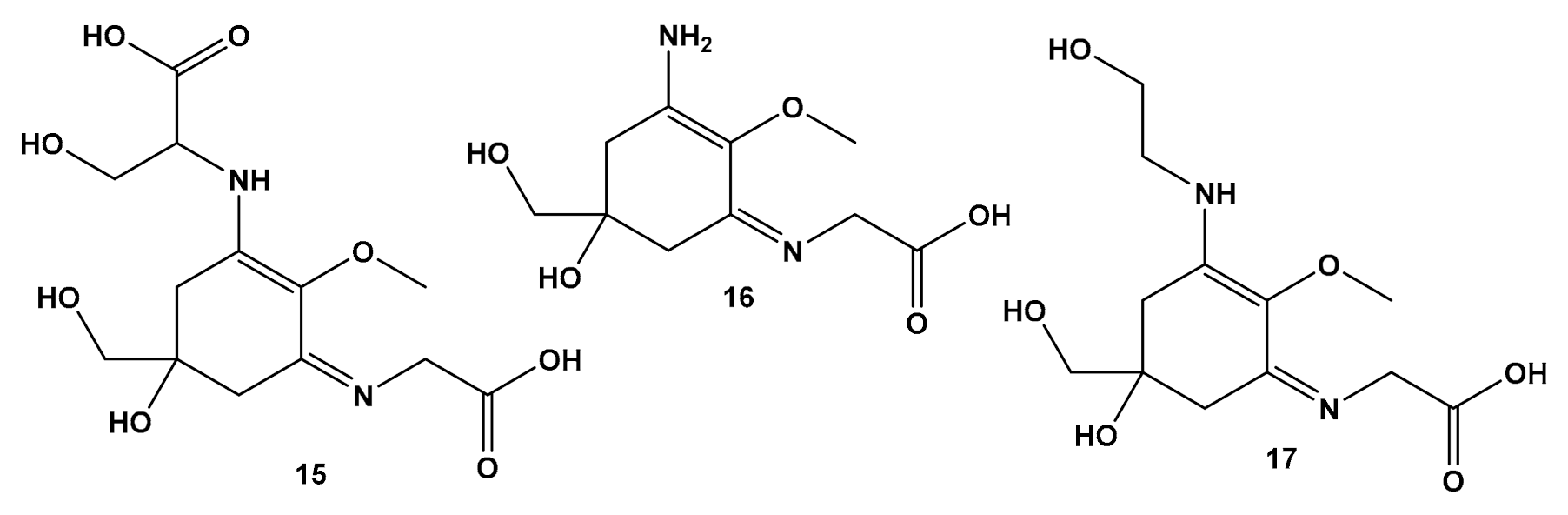
4.2.3. Other Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fusetani, N. Marine natural products: Chemical diversity. Nat. Prod. Rep. 2008, 2, 1–22. [Google Scholar]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Safe 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Anis, M.; Hasan, S.A. Algae as nutrition, medicine and cosmetic: The forgotten history, present status and future trends. World J. Pharm. Pharm. Sci. 2017, 6, 1934–1959. [Google Scholar]
- Dillehay, T.D.; Ramirez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The main seaweed foods in Japan. Hydrobiologia 1987, 151, 5–29. [Google Scholar] [CrossRef]
- Cannell, R.J. Algal biotechnology. Appl. Biochem. Biotechnol. 1990, 26, 85–105. [Google Scholar] [CrossRef]
- Schuenhoff, A.; Mata, L.; Santos, R. The tetrasporophyte of Asparagopsis armata as a novel seaweed biofilter. Aquaculture 2006, 252, 3–11. [Google Scholar] [CrossRef]
- Jacinto, M.L.J.; David, C.P.C.; Perez, T.R.; De Jesus, B.R. Comparative efficiency of algal biofilters in the removal of chromium and copper from wastewater. Ecol. Eng. 2009, 35, 856–860. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Hossain, A.S.; Salleh, A.; Boyce, A.N.; Chowdhury, P.; Naqiuddin, M. Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Ganzer, B.; Messerschmid, E. Integration of an algal photobioreactor into an environmental control and life support system of a space station. Acta Astronaut. 2009, 65, 248–261. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.; Silva, A. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Hirahara, T. Functional foods in Japan. Scand. J. Nutr. 2000, 44, 132–134. [Google Scholar]
- Hayes, M.; Tiwari, B.K. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef] [Green Version]
- Kraan, S.; Barrington, K.A. Commercial farming of Asparagopsis armata (Bonnemaisoniceae, Rhodophyta) in Ireland, maintenance of an introduced species? J. Appl. Phycol. 2005, 17, 103–110. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [Green Version]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K. Sargassum, Gracilaria and Ulva exhibit positive antimicrobial activity against human pathogens. Open Access Libr. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Karadeniz, F.; Karagozlu, M.Z.; Kim, S.K. Antiviral activities of marine algal extracts. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, pp. 371–380. [Google Scholar]
- Li, Y.X.; Li, Y.; Kim, S.K. Anticancer compounds from marine algae. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, pp. 267–276. [Google Scholar]
- Torres, F.A.; Passalacqua, T.G.; Velásquez, A.M.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A. New drugs with antiprotozoal activity from marine algae: A review. Rev. Bras. Farmacogn. 2014, 24, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet. Sci. 2013, 64, 193–209. [Google Scholar]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Kim, M.M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, S.; Pathak, J.; Ahmed, H.; Singh, V.; Singh, S.P.; Sinha, R.P. Mycosporine-like amino acids (MAAs) profile of two marine red macroalgae, Gelidium sp. and Ceramium sp. Int. J. Res. Appl. Sci. Biotechnol. 2017, 5, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef]
- AlgaeBase. Available online: http://www.algaebase.org (accessed on 20 November 2021).
- Chualáin, F.N.; Maggs, C.A.; Saunders, G.W.; Guiry, M.D. The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): Molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. J. Phycol. 2004, 40, 1112–1126. [Google Scholar] [CrossRef]
- Taylor, W.R. Pacific Marine Algae of the Allan Hancock expeditions to the Galapagos Islands, 1st ed.; University of Southern California Press: Los Angeles, CA, USA, 1945; pp. 149–150. [Google Scholar]
- Bonin, D.R.; Hawkes, M.W. Systematics and life histories of New Zealand Bonnemaisoniaceae (Bonnemaisoniales, Rhodophyta): I. The genus Asparagopsis. N. Z. J. Bot. 1987, 25, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, J.; Feldmann, G. Sur le développement des carpospores et l’alternance de générations de l’Asparagopsis armata Harvey. C. R. Acad. Sci. 1939, 208, 1420–1422. [Google Scholar]
- Feldmann, J. Recherches sur les Bonnemaisoniacées et leur alternance de générations. Ann. Sci. Nat. Bot. Ser. 1942, 11, 75–175. [Google Scholar]
- Chihara, M. Life cycle of the Bonnemaisoniaceous algae in Japan (2). Sci. Rep. Tokyo Kyoiku Daigaku. Sect. B 1962, 11, 27–33. [Google Scholar]
- Dixon, P.S. Asparagopsis in Europe. Nature 1964, 204, 902. [Google Scholar] [CrossRef]
- Zanolla, M.; Carmona, R.; Mata, L.; De la Rosa, J.; Sherwood, A.; Barranco, C.N.; Muñoz, A.R.; Altamirano, M. Concise review of the genus Asparagopsis Montagne, 1840. J. Appl. Phycol. 2022, 34, 1–17. [Google Scholar] [CrossRef]
- Huisman, J.M.; Walker, D.I. A catalogue of the marine plants of Rottnest Island, Western Australia, with notes on their distribution and biogeography. Kingia 1990, 1, 349–459. [Google Scholar]
- Sauvageau, C. Sur quelques algues Floridées renfermant de l’iode à l’état libre. Bull. Stn Biol. Arcachon. 1925, 22, 5–45. [Google Scholar]
- Womersley, H.B.S. The Marine Benthic Flora of Southern Australia. Rhodophyta. Part IIIB. Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales; Flora of Australia Supplementary Series Number 5; Australian Biological Resources Study; State Herbarium of South Australia: Canberra, Australia, 1996; pp. 325–331. [Google Scholar]
- Westbrook, M.A. Notes on the distribution of certain marine red algae. Lond. J. Bot. 1930, 68, 257–264. [Google Scholar]
- Svedelius, N. On the development of Asparagopsis armata Harv. and Bonnemaisonia asparagoides (Woodw.) Ag: A contribution to the cytology of the haplobiontic Rhodophyceae. Nova Acta Regiae Soc. Sci. Upsal. 1933, 9, 1–61. [Google Scholar]
- Guiry, M.D.; Dawes, C.J. Daylength, temperature and nutrient control of tetrasporogenesis in Asparagopsis armata (Rhodophyta). J. Exp. Mar. Biol. Ecol. 1992, 158, 197–217. [Google Scholar] [CrossRef]
- de Valera, M. A red algae new to Ireland: Asparagopsis armata Harv. on the west coast. Ir. Nat. J. 1942, 8, 30–33. [Google Scholar]
- Drew, K.M. Occurrence of Asparagopsis armata Harv. on the coast of Cornwall. Nature 1950, 166, 873–874. [Google Scholar] [CrossRef]
- Neto, A.I. Observations on the biology and ecology of selected macroalgae from the littoral of São Miguel (Azores). Bot. Mar. 2000, 43, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Zanolla, M.; Altamirano, M.; Carmona, R.; la Rosa, J.; Souza-Egipsy, V.; Sherwood, A.; Andreakis, N. Assessing global range expansion in a cryptic species complex: Insights from the red seaweed genus Asparagopsis (Florideophyceae). J. Phycol. 2018, 54, 12–24. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity–the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Sala, E.; Boudouresque, C.F. The role of fishes in the organization of a Mediterranean sublittoral community I: Algal communities. J. Exp. Mar. Biol. Ecol. 1997, 212, 25–44. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef] [Green Version]
- Guerra-García, J.M.; Ros, M.; Izquierdo, D.; Soler-Hurtado, M.M. The invasive Asparagopsis armata versus the native Corallina elongata: Differences in associated peracarid assemblages. J. Exp. Mar. Biol. Ecol. 2012, 416, 121–128. [Google Scholar] [CrossRef]
- Cebrian, E.; Linares, C.; Marschal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol. Invasions 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Svensson, J.R.; Nylund, G.M.; Cervin, G.; Toth, G.B.; Pavia, H. Novel chemical weapon of an exotic macroalga inhibits recruitment of native competitors in the invaded range. J. Ecol. 2013, 101, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Littler, M.M. Morphological form and photosynthetic performances of marine macroalgae: Tests of a functional/form hypothesis. Bot. Mar. 1980, 12, 161–165. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The evolution of thallus form and survival strategies in benthic marine macroalgae: Field and laboratory tests of a functional form. Am. Nat. 1980, 116, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Wallentinus, I. Comparisons of nutrient uptake rates for Baltic macroalgae with different thallus morphologies. Mar. Biol. 1984, 80, 215–225. [Google Scholar] [CrossRef]
- Çinar, M.E.; Arianoutsou, M.; Zenetos, A.; Golani, D. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar]
- Ribera, M.A.; Boudouresque, C.F. Introduced marine plants, with special reference to macroalgae: Mechanisms and impact. Prog. Phycol. Res. 1995, 11, 187–268. [Google Scholar]
- Abbott, I.A. The uses of seaweed as food in Hawaii. Econ. Bot. 1978, 32, 409–412. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Li, D.; Yang, Q.; Su, P.; Wang, H.; Heimann, K.; Zhang, W. Commercial cultivation, industrial application, and potential halocarbon biosynthesis pathway of Asparagopsis sp. Algal Res. 2021, 56, 102319. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetamides, but-3-en-2-ols, and isopropanols from Asparagopsis taxiformis (Delile) Trev. Tetrahedron 1976, 32, 2843–2846. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetic and acrylic acids from the red alga Asparagopsis taxiformis. Phytochemistry 1979, 18, 617–620. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Greff, S.; Zubia, M.; Genta-Jouve, G.; Massi, L.; Perez, T.; Thomas, O.P. Mahorones, highly brominated cyclopentenones from the red alga Asparagopsis taxiformis. J. Nat. Prod. 2014, 77, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos-Moreno, M.P.; Fries, G.R.; Gubert, C.; dos Santos, B.T.M.Q.; Fijtman, A.; Sartori, J.; Barbé-Tuana, F.M. Telomere length, oxidative stress, inflammation and BDNF levels in siblings of patients with bipolar disorder: Implications for accelerated cellular aging. Int. J. Neuropsychopharmacol. 2017, 20, 445–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Deslandes, E. Antioxidant and cytotoxic activities of some red algae (Rhodophyta) from Brittany coasts (France). Bot. Mar. 2009, 52, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Rhimou, B.; Hassane, R.; Nathalie, B. Antioxidant activity of Rhodophyceae extracts from Atlantic and Mediterranean coasts of Morocco. Afr. J. Plant Sci. 2013, 7, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Neethu, P.V.; Suthindhiran, K.; Jayasri, M.A. Antioxidant and antiproliferative activity of Asparagopsis taxiformis. Pharmacogn. Res. 2017, 9, 238. [Google Scholar]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.P. Nutraceutical potential of Asparagopsis taxiformis (Delile) Trevisan extracts and assessment of a downstream purification strategy. Heliyo 2018, 4, 00957. [Google Scholar] [CrossRef] [Green Version]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Ghenima, A.I.; Idir, M.; Nadjet, M.G.; Samia, M.A.; Mihoub, Z.M.; Karim, H. In vitro evaluation of biological activities of Pistacia lentiscus aqueous extract. Int. J. Pharm. 2015, 7, 133–139. [Google Scholar]
- Bhatti, M.Z.; Ali, A.; Ahmad, A.; Saeed, A.; Malik, S.A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res. Notes 2015, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40, 1–3. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Horta, A.; Pedrosa, R. High cytotoxicity and anti-proliferative activity of algae extracts on an in vitro model of human hepatocellular carcinoma. Springerplus. 2016, 5, 1339. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.; Pinteus, S.; Rodrigues, A.; Horta, A.; Pedrosa, R. Algae from Portuguese coast presented high cytotoxicity and antiproliferative effects on an in vitro model of human colorectal cancer. Pharmacogn. Res. 2018, 10, 24. [Google Scholar]
- Nunes, N.; Rosa, G.P.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.A. Fatty acid composition, TLC screening, ATR-FTIR analysis, anti-cholinesterase activity, and in vitro cytotoxicity to A549 tumor cell line of extracts of 3 macroalgae collected in Madeira. J. Appl. Phycol. 2020, 32, 759–771. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Pinteus, S.; Alves, C.; Monteiro, H.; Araújo, E.; Horta, A.; Pedrosa, R. Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J. Microbiol. Biotechnol. 2015, 31, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Littler, M.M.; Littler, D.S. Impact of CLOD pathogen on Pacific coral reefs. Science 1995, 267, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Kushmaro, A.; Rosenberg, E.; Fine, M.; Loya, Y. Bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 1997, 147, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Potin, P.; Bouarab, K.; Salaün, J.P.; Pohnert, G.; Kloareg, B. Biotic interactions of marine algae. Curr. Opin. Plant Biol. 2002, 5, 308–317. [Google Scholar] [CrossRef]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Correa, J.A.; Sánchez, P.A. Ecological aspects of algal infectious diseases. Hydrobiologia 1996, 326, 89–95. [Google Scholar] [CrossRef]
- McConnell, O.J.; Fenical, W. Antimicrobial agents from marine red algae of the family Bonnemaisoniaceae. In Marine Algae in Pharmaceutical Science; Hoppe, H.A., Levring, T., Tanaka, Y., Eds.; Walter de Gruyter: New York, NY, USA, 1979; Volume 1, pp. 403–427. [Google Scholar]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Bansemir, A.; Blume, M.; Schröder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Salvador, N.; Garreta, A.G.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Horta, A.G.C. Avaliação do Potencial Biotecnológico da Alga Asparagopsis armata e das Suas Bactérias Epífitas: Citotoxicidade e Atividade Antimicrobiana. Master’s Thesis, Instituto Politécnico de Leiria, Leiria, Portugal, 2013. [Google Scholar]
- Hornsey, I.S.; Hide, D. The production of antimicrobial compounds by British marine algae I. Antibiotic-producing marine algae. Br. Phycol. J. 1974, 9, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Oumaskour, K.; Boujaber, N.; Etahiri, S.; Assobhei, O. Anti-inflammatory and antimicrobial activities of twenty-three marine red algae from the coast of Sidi Bouzid (El Jadida-Morocco). Int. J. Pharm. Pharm. Sci. 2013, 5, 145–149. [Google Scholar]
- Pinteus, S.; Lemos, M.F.; Alves, C.; Silva, J.; Pedrosa, R. The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total Environ. 2021, 750, 141372. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef]
- Mata, L.; Wright, E.; Owens, L.; Paul, N.; de Nys, R. Water-soluble natural products from seaweed have limited potential in controlling bacterial pathogens in fish aquaculture. J. Appl. Phycol. 2013, 25, 1963–1973. [Google Scholar] [CrossRef]
- Jha, B.; Kavita, K.; Westphal, J.; Hartmann, A.; Schmitt-Kopplin, P. Quorum sensing inhibition by Asparagopsis taxiformis, a marine macroalga: Separation of the compound that interrupts bacterial communication. Mar. Drugs 2013, 11, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickschat, J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.M.; Ferreira, R.B.; Buckner, M.M.; Finlay, B.B. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [Green Version]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Singh, R.; Paul, D.; Jain, R.K. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef]
- Xu, Y.; He, H.; Schulz, S.; Liu, X.; Fusetani, N.; Xiong, H.; Qian, P.Y. Potent antifouling compounds produced by marine Streptomyces. Bioresour. Technol. 2010, 101, 1331–1336. [Google Scholar] [CrossRef]
- Viarengo, A. Heavy metals in marine invertebrates: Mechanisms of regulation and toxicity at the cellular level. Rev. Aquat. Sci. 1989, 1, 295–317. [Google Scholar]
- Elfwing, T.; Tedengren, M. Effects of copper on the metabolism of three species of tropical oysters, Saccostrea cucullata, Crassostrea lugubris and C. belcheri. Aquaculture 2002, 204, 157–166. [Google Scholar] [CrossRef]
- Givskov, M.; de Nys, R.; Manefield, M.; Gram, L.; Maximilien, R.I.A.; Eberl, L.E.O.; Kjelleberg, S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996, 178, 6618–6622. [Google Scholar] [CrossRef] [Green Version]
- Vedhagiri, K.; Manilal, A.; Valliyammai, T.; Shanmughapriya, S.; Sujith, S.; Selvin, J.; Natarajaseenivasan, K. Antimicrobial potential of a marine seaweed Asparagopsis taxiformis against Leptospira javanica isolates of rodent reservoirs. Ann. Microbiol. 2009, 59, 431–437. [Google Scholar] [CrossRef]
- Wiley, J.M.; Sherwood, L.M.; Woolverton, C.J. Prescott, Harley, and Klein’s Microbiology, 7th ed.; McGraw-Hill: New York, NY, USA, 2008; p. 840. [Google Scholar]
- Suttle, C. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Suttle, C. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, G.M.; Suttle, C. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J. 2018, 12, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Williamson, K.E.; Fuhrmann, J.J.; Wommack, K.E.; Radosevich, M. Viruses in soil ecosystems: An unknown quantity within an unexplored territory. Annu. Rev. Virol. 2017, 4, 201–219. [Google Scholar] [CrossRef]
- Microbiology by numbers. Nat. Rev. Microbiol. 2011, 9, 628. [CrossRef]
- Sanjuán, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef] [Green Version]
- Carroll, D.; Daszak, P.; Wolfe, N.D.; Gao, G.F.; Morel, C.M.; Morzaria, S.; Pablos-Mendez, A.; Tomori, O.; Mazet, J.A. The global virome project. Science 2018, 359, 872–874. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Antiviral activity of extract and purified compound from red macroalgae Asparagopsis taxiformis against H5N1. Univers. J. Pharm. Res. 2021, 6, 14–19. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; Nathalie, B. Antiviral activity of the extracts of Rhodophyceae from Morocco. Afr. J. Biotechnol. 2010, 9, 7968–7975. [Google Scholar]
- Haslin, C.; Lahaye, M.; Pellegrini, M.; Chermann, J.C. In vitro anti-HIV activity of sulfated cell-wall polysaccharides from gametic, carposporic and tetrasporic stages of the Mediterranean red alga Asparagopsis armata. Planta Med. 2001, 67, 301–305. [Google Scholar] [CrossRef]
- Pedersen, T.R.; Tobert, J.A. Simvastatin: A review. Expert Opin. Pharmacother. 2004, 5, 2583–2596. [Google Scholar] [CrossRef]
- Nichols, D.J.; Muirhead, G.J.; Harness, J.A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002, 53, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.H.; Caufield, C.E. Structure-based design approaches to cell wall biosynthesis inhibitors. Curr. Pharm. Des. 2003, 9, 857–866. [Google Scholar] [CrossRef]
- Silva, T.; Reis, J.; Teixeira, J.; Borges, F. Alzheimer’s disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 2014, 15, 116–145. [Google Scholar] [CrossRef]
- Gavrilova, S.I.; Alvarez, A. Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. Med. Res. Rev. 2021, 41, 2775–2803. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.M.L. Actividades Biológicas e Metabolitos Secundários de Espécies Marinhas Exóticas dos Açores. Master’s Thesis, University of Azores, Ponta Delgada, Portugal, 2015. Available online: http://hdl.handle.net/10400.3/3620 (accessed on 17 January 2022).
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, A.L.; Radvanyi, F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25, 1181–1188. [Google Scholar] [CrossRef]
- La Barre, S.; Longeon, A.; Barthélémy, M.; Guyot, M.; Le Caer, J.P.; Bargibant, G. Characterization of a novel elastase inhibitor from a fan coral. C. R. Acad. Sci. 1996, 319, 365–370. [Google Scholar]
- Félix, R.; Dias, P.; Félix, C.; Cerqueira, T.; Andrade, P.B.; Valentão, P.; Lemos, M.F.L. The biotechnological potential of Asparagopsis armata: What is known of its chemical composition, bioactivities and current market? Algal Res. 2021, 60, 102534. [Google Scholar] [CrossRef]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; de Carvalho, M.P. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Haslin, C.; Lahaye, M.; Pellegrini, M. Chemical composition and structure of sulphated water-soluble cell-wall polysaccharides from the gametic, carposporic and tetrasporic stages of Asparagopsis armata Harvey (Rhodophyta, Bonnemaisoniaceae). Bot. Mar. 2000, 43, 475–482. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [Green Version]
- El-Baroty, G.S.; Moussa, M.Y.; Shallan, M.A.; Ali, M.A.; Sabh, A.Z.; Shalaby, E.A. Contribution to the aroma, biological activities, minerals, protein, pigments and lipid contents of the red alga: Asparagopsis taxiformis (Delile) Trevisan. J. Appl. Sci. Res. 2007, 3, 1825–1834. [Google Scholar]
- Galindo, A.; Reis, D.; Rodríguez, I.; Pérez, J.; Abdul-Jalbar, B.; Zárate, R.; Nunes, N.; Pinheiro de Carvalho, M.; Acosta, N.; Rodríguez, C. Lipid characterization of 14 macroalgal species from Madeira Archipelago: Implications for animal and human nutrition. Bot. Mar. 2022, 65, 51–67. [Google Scholar] [CrossRef]
- Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Shakir, C.; Lipton, A.P. Antimicrobial potential of marine organisms collected from the southwest coast of India against multiresistant human and shrimp pathogens. Sci. Mar. 2010, 74, 287–296. [Google Scholar]
- Dai, Y.; Meng, Q.; Mu, W.; Zhang, T. Recent advances in the applications and biotechnological production of mannitol. J. Funct. Foods 2017, 36, 404–409. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Paul, N.A. Effects of a seaweed feed inclusion on different life stages of the mottled rabbitfish Siganus fuscescens. Aquac. Res. 2021, 52, 6626–6640. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnika, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Pollak, O.J. Reduction of blood cholesterol in man. Circulation 1953, 7, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009, 203, 18–31. [Google Scholar] [CrossRef]
- Jones, P.J.; AbuMweis, S.S. Phytosterols as functional food ingredients: Linkages to cardiovascular disease and cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 147–151. [Google Scholar] [CrossRef]
- Bouic, P.J.D. Sterols and sterolins: New drugs for the immune system? Drug Discov. Today 2002, 7, 775–778. [Google Scholar] [CrossRef]
- El Hattab, N.; Daghbouche, Y.; El Hattab, M.; Piovetti, L.; Garrigues, S.; de la Guardia, M. FTIR-determination of sterols from the red alga Asparagopsis armata: Comparative studies with HPLC. Talanta 2006, 68, 1230–1235. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Bernardo, J.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Mouga, T. Sterol profiles in 18 macroalgae of the portuguese coast. J. Phycol. 2011, 47, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Combaut, G.; Bruneau, Y.; Codomier, L.; Teste, J. Comparative sterols composition of the red alga Asparagopsis armata and its Tetrasporophyte Falkenbergia rufolanosa. J. Nat. Prod. 1979, 42, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.; Combaut, G.; Teste, J.; Tarchini, C.; Djerassi, C. Side chain hydroxylated sterols of the red alga Asparagopsis armata: Significant products or artifacts due to autoxidation? Steroids 1979, 34, 163–169. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae Rhodophyceae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Fenical, W. Polyhaloketones from the red seaweed Asparagopsis taxiformis. Tetrahedron Lett. 1974, 15, 4463–4466. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P. Haloforms in essential oil of alga Asparagopsis-taxiformis (Rhodophyta). Tetrahedron Lett. 1975, 16, 473–476. [Google Scholar] [CrossRef]
- Marshall, R.A.; Harper, D.B.; McRoberts, W.C.; Dring, M.J. Volatile bromocarbons produced by Falkenbergia stages of Asparagopsis spp. (Rhodophyta). Limnol. Oceanogr. 1999, 44, 1348–1352. [Google Scholar] [CrossRef]
- Marshall, R.A.; Hamilton, J.T.G.; Dring, M.J.; Harper, D.B. Do vesicle cells of the red alga Asparagopsis (Falkenbergia stage) play a role in bromocarbon production? Chemosphere 2003, 52, 471–475. [Google Scholar] [CrossRef]
- Paul, N.A.; Cole, L.; de Nys, R.; Steinberg, P.D. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae). J. Phycol. 2006, 42, 637–645. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Romanazzi, D.; Sanchez-Garcia, C.; Svenson, J.; Mata, L.; Pes, K.; Hayman, C.M.; Wheeler, T.T.; Magnusson, M. Rapid analytical method for the quantification of bromoform in the red seaweeds Asparagopsis armata and Asparagopsis taxiformis using gas chromatography−mass spectrometry. ACS Agric. Sci. Technol. 2021, 1, 436–442. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim. Prod. Sci. 2016, 58, 681–688. [Google Scholar] [CrossRef]
- Muizelaar, W.; Groot, M.; van Duinkerken, G.; Peters, R.; Dijkstra, J. Safety and transfer study: Transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 2021, 10, 584. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.M.V.M.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata exudate cocktail: The quest for the mechanisms of toxic action of an invasive seaweed on marine invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; de Carvalho, R.C.; Matos, A.R.; Fonseca, V.F.; Novais, S.C.; Lemos, M.F.L. Potential of Asparagopsis armata as a biopesticide for weed control under an invasive seaweed circular-economy framework. Biology 2021, 10, 1321. [Google Scholar] [CrossRef]
- Broadgate, W.J.; Malin, G.; Küpper, F.C.; Thompson, A.; Liss, P.S. Isoprene and other non-methane hydrocarbons from seaweeds: A source of reactive hydrocarbons to the atmosphere. Mar. Chem. 2004, 88, 61–73. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Calvert, J.; Fall, R.; Goldan, P.; Guenther, A.B.; Hewitt, C.N.; Zimmerman, P. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Glob. Biogeochem. Cycles 1992, 6, 389–430. [Google Scholar] [CrossRef]
- Shallcross, D.E.; Monks, P.S. New directions: A role for isoprene in biosphere–climate–chemistry feedbacks. Atmos. Environ. 2000, 34, 1659–1660. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of mycosporine-like amino acids in Asparagopsis armata grown in tanks with fishpond effluents of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2008, 39, 692–699. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Sahnouni, F.; Debib, A.; Saim, S.; Bouhadi, D.; Menadi, S. Phytochemical content, antioxidant and antibacterial activities of three red macroalgae from Algerian West Coast. Trop. J. Nat. Prod. Res. 2021, 5, 336–341. [Google Scholar]

| Species | Experimental Assay | Extract | Result | Units | Ref. |
|---|---|---|---|---|---|
| A. armata | DPPH | Dichloromethane-mehtanol 1:1 | 6.25 | EC50 mg/mL | [77] |
| BHA (positive control) | 0.04 | EC50 mg/mL | [77] | ||
| BHT (positive control) | 0.06 | EC50 mg/mL | [77] | ||
| Ascorbic acid (positive control) | 0.06 | EC50 mg/mL | [77] | ||
| α-tocopherol (positive control) | 0.014 | EC50 mg/mL | [77] | ||
| Methanol | 0.86 | EC50 mg/mL | [78] | ||
| BHA (positive control) | 0.008 | EC50 mg/mL | [78] | ||
| BHT (positive control) | 0.011 | EC50 mg/mL | [78] | ||
| Methanol | 23.9 ± 0.3 | % radical-scavenging activity at 10 mg/mL | [79] | ||
| Nitric oxide | Ethanol | 14.33 | EC25 mg dry algae/mL | [80] | |
| Deoxyribose test | Water | 68.76 | % inhibition at 1 mg/mL | [78] | |
| A. taxiformis | Hydrogen-peroxide scavenging | Chloroform | 11.07 ± 0.151 | % inhibition at 500 µg/mL | [81] |
| Petroleum ether | 10.88 ± 0.139 | % inhibition at 500 µg/mL | [81] | ||
| Methanol | 10.77 ± 0.131 | % inhibition at 500 µg/mL | [81] | ||
| Ethyl acetate | 10.25 ± 0.136 | % inhibition at 500 µg/mL | [81] | ||
| Ascorbic acid (positive control) | 17.59 ± 0.222 | % inhibition at 500 µg/mL | [81] | ||
| Superoxide-radical scavenging | Methanol | 85.00 ± 0.002 | % scavenging activity at 500 µg/mL | [81] | |
| Chloroform | 79.21 ± 0.006 | % scavenging activity at 500 µg/mL | [81] | ||
| Petroleum ether | 47.00 ± 0.018 | % scavenging activity at 500 µg/mL | [81] | ||
| Ethyl acetate | 45.00 ± 0.008 | % scavenging activity at 500 µg/mL | [81] | ||
| Ascorbic acid (positive control) | 87.11 ± 0.0005 | % scavenging activity at 500 µg/mL | [81] | ||
| FRAP (ferric-reducing antioxidant power) | Chloroform | 67.19 ± 0.0005 | % antioxidant activity at 500 µg/mL | [81] | |
| Methanol | 65.63 ± 0.001 | % antioxidant activity at 500 µg/mL | [81] | ||
| Petroleum ether | 64.06 ± 0.0005 | % antioxidant activity at 500 µg/mL | [81] | ||
| Ethyl acetate | 54.69 ± 0.0005 | % antioxidant activity at 500 µg/mL | [81] | ||
| Ascorbic acid (positive control) | 73.44 ± 0.002 | % antioxidant activity at 500 µg/mL | [81] | ||
| Reducing activity | Water (M1) | 233.15 ± 5.15 | mg AAE/100 g dw a | [82] | |
| Water (M2) | 174.38 ± 11.65 | mg AAE/100 g dw | [82] | ||
| Ethanol (M1) | 1908.44 ± 59.15 | mg AAE/100 g dw | [82] | ||
| Ethanol (M2) | 1156.86 ± 13.87 | mg AAE/100 g dw | [82] | ||
| Methanol (M1) | 584.46 ± 15.36 | mg AAE/100 g dw | [82] | ||
| Methanol (M2) | 1161.47 ± 14.43 | mg AAE/100 g dw | [82] | ||
| Ethyl acetate (M1) | 409.60 ± 10.84 | mg AAE/100 g dw | [82] | ||
| Ethyl acetate (M2) | 707.42 ± 98.78 | mg AAE/100 g dw | [82] | ||
| DPPH | Water (M1) | 4.65 ± 0.29 | IC50 mg/mL | [82] | |
| Water (M2) | 1.37 ± 0.03 | IC50 mg/mL | [82] | ||
| Ethanol (M1) | 1.54 ± 0.07 | IC50 mg/mL | [82] | ||
| Ethanol (M2) | 1.37 ± 0.04 | IC50 mg/mL | [82] | ||
| Methanol (M1) | 2.69 ± 0.03 | IC50 mg/mL | [82] | ||
| Methanol (M2) | 1.64 ± 0.01 | IC50 mg/mL | [82] | ||
| Ethyl acetate (M1) | 3.62 ± 0.04 | IC50 mg/mL | [82] | ||
| Ethyl acetate (M2) | 1.44 ± 0.08 | IC50 mg/mL | [82] | ||
| Ferrous-ion chelation | Water (M1) | 113.01 ± 10.62 | IC50 mg/mL | [82] | |
| Water (M2) | 74.00 ± 1.81 | IC50 mg/mL | [82] | ||
| Ethanol (M1) | 5.26 ± 0.27 | IC50 mg/mL | [82] | ||
| Ethanol (M2) | 10.49 ± 0.44 | IC50 mg/mL | [82] | ||
| Methanol (M1) | 8.36 ± 0.29 | IC50 mg/mL | [82] | ||
| Methanol (M2) | 10.07 ± 0.18 | IC50 mg/mL | [82] | ||
| Ethyl acetate (M1) | 1.57 ± 0.03 | IC50 mg/mL | [82] | ||
| Ethyl acetate (M2) | 5.88 ± 0.26 | IC50 mg/mL | [76] |
| Species | Experimental Assay | Extract/Control | Result | Units | Ref. |
|---|---|---|---|---|---|
| A. armata | Cytotoxicity (Daudi cells) | Dichloromethane–ethanol (1:1) | 32.52 ± 7.33 | % reduction of viable cells at 100 μg/mL after 24 h incubation | [77] |
| Cytotoxicity (Jurkat cells) | 30.07 ± 7.24 | % reduction of viable cells at 100 μg/mL after 24 h incubation | [77] | ||
| Cytotoxicity (HepG-2 cells) | Methanol | 567.9 | IC50 in μg/mL | [89] | |
| Dichloromethane | 473.1 | IC50 in μg/mL | [89] | ||
| Cisplatin (positive control) | 136.5 | IC50 in μg/mL | [89] | ||
| Cytotoxicity (Caco-2 cells) | Methanol | 823.0 | IC50 in μg/mL | [89] | |
| Dichloromethane | 531.6 | IC50 in μg/mL | [89] | ||
| Cisplatin (positive control) | 80.11 | IC50 in μg/mL | [89] | ||
| Antiproliferative activity (HepG-2 cells) | Methanol | 857.3 | IC50 in μg/mL | [89] | |
| Dichloromethane | 518.9 | IC50 in μg/mL | [89] | ||
| Cisplatin (positive control) | 22.63 | IC50 in μg/mL | [89] | ||
| Tamoxifen (positive control) | 16.97 | IC50 in μg/mL | [89] | ||
| Antiproliferative activity (Caco-2 cells) | Methanol | 508.1 | IC50 in μg/mL | [90] | |
| Dichloromethane | 271.5 | IC50 in μg/mL | [90] | ||
| Cisplatin (positive control) | 92.00 | IC50 in μg/mL | [90] | ||
| A. taxiformis | Cytotoxicity (A549 cells) | Chloroform-methanol (2:1) | 98.02 ± 0.23 | IC50 in μg/mL | [91] |
| Ethanol | >200 | IC50 in μg/mL | [91] | ||
| Colchicine (positive control) | 2.78 ± 0.71 | IC50 in μg/mL | [91] |
| Species | Experimental Assay | Extract/Compound | Target Species | Result | Units | Ref. |
|---|---|---|---|---|---|---|
| A. armata | Agar-diffusion method | Dichloromethane (2 mg/disk) | Vibrio anguillarum | 19.3 ± 1.3 | Inhibition zone diameter (mm) | [101] |
| Pseudomonas anguilliseptica | 26.9 ± 2.2 | Inhibition zone diameter (mm) | [101] | |||
| Aeromonas salmonicida | 17.0 ± 2.2 | Inhibition zone diameter (mm) | [101] | |||
| Aeromonas hydrophila | 14.9 ± 2.7 | Inhibition zone diameter (mm) | [101] | |||
| Yersinia ruckeri | 15.3 ± 1.7 | Inhibition zone diameter (mm) | [101] | |||
| Solid (fresh) (2 mg/well) | Bacillus subtilis | 29.4 | Inhibition zone diameter (mm) | [102] | ||
| Bacillus cereus | 30.2 | Inhibition zone diameter (mm) | [102] | |||
| Staphylococcus aureus | 22.2 | Inhibition zone diameter (mm) | [102] | |||
| Escherichia coli | 20.8 | Inhibition zone diameter (mm) | [102] | |||
| Pseudomonas aeruginosa | 25.5 | Inhibition zone diameter (mm) | [102] | |||
| Candida albicans | 32 | Inhibition zone diameter (mm) | [102] | |||
| Solid (lyophilized) (2 mg/well) | Bacillus subtilis | 38.9 | Inhibition zone diameter (mm) | [102] | ||
| Bacillus cereus | 51.1 | Inhibition zone diameter (mm) | [102] | |||
| Staphylococcus aureus | 35.1 | Inhibition zone diameter (mm) | [102] | |||
| Escherichia coli | 39.9 | Inhibition zone diameter (mm) | [102] | |||
| Pseudomonas aeruginosa | 27.3 | Inhibition zone diameter (mm) | [102] | |||
| Candida albicans | 53.2 | Inhibition zone diameter (mm) | [102] | |||
| Methanol (0.3 mg/disk) | Bacillus subtilis | 11 | Inhibition zone diameter (mm) | [93] | ||
| Dichloromethane (0.3 mg/disk) | Bacillus subtilis | 12 | Inhibition zone diameter (mm) | [93] | ||
| n-Hexane (0.3 mg/disk) | Bacillus subtilis | 8 | Inhibition zone diameter (mm) | [93] | ||
| Chloramphenicol | Bacillus subtilis | 30 | Inhibition zone diameter (mm) | [93] | ||
| Fungal growth measurement | Dichloromethane | Saccharomyces cerevisiae | 119.8 | IC50 in μg/mL | [93] | |
| n-hexane | Saccharomyces cerevisiae | 97.6 | IC50 in μg/mL | [93] | ||
| Amphotericin B (positive control) | Saccharomyces cerevisiae | 21.6 | IC50 in μg/mL | [93] | ||
| Broth microdilution | Dichloromethane—methanol (1:1) | Bacillus subtilis | 83.7 | IC50 in μg/mL | [103] | |
| Escherichia coli | 540.6 | IC50 in μg/mL | [103] | |||
| Pseudomonas aeruginosa | 374.3 | IC50 in μg/mL | [103] | |||
| Salmonella enteritidis | 613.8 | IC50 in μg/mL | [103] | |||
| Staphylococcus aureus | 528.6 | IC50 in μg/mL | [103] | |||
| Agar-diffusion method (using 3 cm thallus) | Fresh thallus | Bacillus subtilis | 11.0 | Inhibition zone diameter (mm) | [104] | |
| Escherichia coli | 9.0 | Inhibition zone diameter (mm) | [104] | |||
| Morganella morganii | 11.0 | Inhibition zone diameter (mm) | [104] | |||
| Staphylococcus aureus | 12.0 | Inhibition zone diameter (mm) | [104] | |||
| Streptococcus pyogenes | 12.0 | Inhibition zone diameter (mm) | [104] | |||
| Agar-diffusion method | Methanol (500 μg/disk) | Bacillus cereus | 10–15 | Inhibition zone diameter (mm) | [105] | |
| Clostridium sporogenes | 10–15 | Inhibition zone diameter (mm) | [105] | |||
| Acetone | Staphylococcus aureus ssp aureus | >15 | Inhibition zone diameter (mm) | [105] | ||
| Chloroform | Staphylococcus aureus ssp aureus | 10–15 | Inhibition zone diameter (mm) | [105] | ||
| Dichloromethane—methanol (1:1) | Bacillus cereus | 10–15 | Inhibition zone diameter (mm) | [105] | ||
| Bacterial growth measurement | Dichloromethane—methanol (1:1) | Aeromonas hydrophila | 5.7 ± 0.1 | % growth inhibition at 1 mg/mL | [106] | |
| Dichloromethane—methanol (1:1) Fraction 1 by VLC | Aeromonas hydrophila | 836.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 2 by VLC | Aeromonas hydrophila | 293.5 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 3 by VLC | Aeromonas hydrophila | 162.9 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 4 by VLC | Aeromonas hydrophila | 191.9 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 5 by VLC | Aeromonas hydrophila | 265.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 6 by VLC | Aeromonas hydrophila | 379.0 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) | Aeromonas aquariorum | 23.5 ± 0.6 | % growth inhibition at 1 mg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 1 by VLC | Aeromonas aquariorum | >1000 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 2 by VLC | Aeromonas aquariorum | 588.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 3 by VLC | Aeromonas aquariorum | 443.8 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 4 by VLC | Aeromonas aquariorum | 437 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 5 by VLC | Aeromonas aquariorum | 421.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 6 by VLC | Aeromonas aquariorum | 872.4 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) | Edwardsiella tarda | 86.0 ± 8.3 | % growth inhibition at 1 mg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 1 by VLC | Edwardsiella tarda | 414.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 2 by VLC | Edwardsiella tarda | 152.7 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 3 by VLC | Edwardsiella tarda | 54.4 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 4 by VLC | Edwardsiella tarda | 75.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 5 by VLC | Edwardsiella tarda | 83.8 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 6 by VLC | Edwardsiella tarda | 244.5 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) | Vibrio anguillarum | 35.1 ± 5.7 | % growth inhibition at 1 mg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 1 by VLC | Vibrio anguillarum | 41.1 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 2 by VLC | Vibrio anguillarum | 19.6 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 3 by VLC | Vibrio anguillarum | 41.7 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 4 by VLC | Vibrio anguillarum | 60.4 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 5 by VLC | Vibrio anguillarum | 52.5 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 6 by VLC | Vibrio anguillarum | 89.9 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) | Photobacterium damselae | 0.0 ± 4.1 | % growth inhibition at 1 mg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 1 by VLC | Photobacterium damselae | 18.9 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 2 by VLC | Photobacterium damselae | 14.5 | IC50 in μg/mL | [106] | ||
| Bacterial growth measurement | Dichloromethane—methanol (1:1) Fraction 3 by VLC | Photobacterium damselae | 13.6 | IC50 in μg/mL | [106] | |
| Dichloromethane—methanol (1:1) Fraction 4 by VLC | Photobacterium damselae | 20.0 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 5 by VLC | Photobacterium damselae | 11.7 | IC50 in μg/mL | [106] | ||
| Dichloromethane—methanol (1:1) Fraction 6 by VLC | Photobacterium damselae | 17.3 | IC50 in μg/mL | [106] | ||
| Alamar-blue assay [101] | Hexane | Leishmania donovani | >40 | IC50 in μg/mL | [107] | |
| Dichloromethane | Leishmania donovani | >40 | IC50 in μg/mL | [107] | ||
| Ethanol (VLC fraction eluted with hexane—ethyl acetate (1:1)) | Leishmania donovani | 10 | IC50 in μg/mL | [107] | ||
| Ethanol (VLC fraction eluted with ethyl acetate) | Leishmania donovani | 19 | IC50 in μg/mL | [107] | ||
| Pentamidin (positive control) | Leishmania donovani | 0.9–1 | IC50 in μg/mL | [107] | ||
| Amphotericin B (positive control) | Leishmania donovani | 0.18–0.19 | IC50 in μg/mL | [107] | ||
| A. taxiformis | Antibacterial/antifungal activity (growth measurement) | Mahorone | Acinetobacter baumannii | 8 | MIC80 in μg/mL | [69] |
| Escherichia coli (2884) | >32 | MIC80 in μg/mL | [69] | |||
| Escherichia coli (5746) | 16 | MIC80 in μg/mL | [69] | |||
| Pseudomonas aeruginosa | >10 | MIC80 in μg/mL | [69] | |||
| Staphylococcus aureus (MRSA) | 16 | MIC80 in μg/mL | [69] | |||
| Staphylococcus aureus (MSSA) | >32 | MIC80 in μg/mL | [69] | |||
| Aspergillus fumigatus | >32 | MIC80 in μg/mL | [69] | |||
| Candida albicans | >32 | MIC80 in μg/mL | [69] | |||
| 5-bromomahorone | Acinetobacter baumannii | 16 | MIC80 in μg/mL | [69] | ||
| Escherichia coli (2884) | >32 | MIC80 in μg/mL | [69] | |||
| Escherichia coli (5746) | 32 | MIC80 in μg/mL | [69] | |||
| Pseudomonas aeruginosa | >10 | MIC80 in μg/mL | [69] | |||
| Staphylococcus aureus (MRSA) | 16 | MIC80 in μg/mL | [69] | |||
| Staphylococcus aureus (MSSA) | >32 | MIC80 in μg/mL | [69] | |||
| Aspergillus fumigatus | >32 | MIC80 in μg/mL | [69] | |||
| Candida albicans | >32 | MIC80 in μg/mL | [69] | |||
| Rifampicin (positive control) | Acinetobacter baumannii | 1 | MIC80 in μg/mL | [69] | ||
| Novobiocin (positive control) | Escherichia coli (2884) | 0.3 | MIC80 in μg/mL | [69] | ||
| Novobiocin (positive control) | Escherichia coli (5746) | 0.1 | MIC80 in μg/mL | [69] | ||
| Ciprofloxacin (positive control) | Pseudomonas aeruginosa | 1.5 | MIC80 in μg/mL | [69] | ||
| Imipenem (positive control) | Staphylococcus aureus (MRSA) | 8 | MIC80 in μg/mL | [69] | ||
| Penicillin (positive control) | Staphylococcus aureus (MSSA) | 0.01 | MIC80 in μg/mL | [69] | ||
| Alamar-blue assay [108] | Hexane | Leishmania donovani | 17 | IC50 in μg/mL | [107] | |
| Dichloromethane | Leishmania donovani | 16 | IC50 in μg/mL | [107] | ||
| Ethanol (VLC fraction eluted with hexane–ethyl acetate (1:1)) | Leishmania donovani | 14 | IC50 in μg/mL | [107] | ||
| Ethanol (VLC fraction eluted with ethyl acetate) | Leishmania donovani | 20 | IC50 in μg/mL | [107] |
| Species | Experimental Assay | Extract | Target Virus | Result | Units | Ref. |
|---|---|---|---|---|---|---|
| A. taxiformis | Plaque infectivity reduction | Hexane (20 μg/mL) | H5N1 | 50 | % inhibition | [131] |
| Hexane (40 μg/mL) | H5N1 | 57 | % inhibition | [131] | ||
| Petroleum ether (20 μg/mL) | H5N1 | 73 | % inhibition | [131] | ||
| Petroleum ether (40 μg/mL) | H5N1 | >99.9 | % inhibition | [131] | ||
| Ethyl acetate (20 μg/mL) | H5N1 | 46 | % inhibition | [131] | ||
| Ethyl acetate (40 μg/mL) | H5N1 | 55 | % inhibition | [131] | ||
| Methylene chloride–ethanol (1:1) (20 μg/mL) | H5N1 | 0 | % inhibition | [131] | ||
| Methylene chloride–ethanol (1:1) (40 μg/mL) | H5N1 | 15 | % inhibition | [131] | ||
| Water (20 μg/mL) | H5N1 | >99.9 | % inhibition | [131] | ||
| Water (40 μg/mL) | H5N1 | >99.9 | % inhibition | [131] | ||
| A. armata | Antiviral assays based on cell viability | Methanol | HSV-1 | 7.7 | EC50 in μg/mL | [132] |
| Choloform–methanol (3:2) | HSV-1 | 40.9 | EC50 in μg/mL | [132] | ||
| Dichloromethane | HSV-1 | 22.8 | EC50 in μg/mL | [132] | ||
| Water | HSV-1 | <2.5 | EC50 in μg/mL | [132] |
| Species | Target Enzyme | Experimental Assay | Extract/Compound | Result | Units | Ref. |
|---|---|---|---|---|---|---|
| A. armata | Acetylcholinesterase | Ellman method [141] | Methanol | 58.4 ± 1.0 | % inhibition at 10 mg/mL | [79] |
| Galantamine (positive control) | 90.3 ± 0.6 | % inhibition at 1 mg/mL | [79] | |||
| Butyrylcholinesterase | Ellman method [141] | Methanol | 66.8 ± 1.3 | % inhibition at 10 mg/mL | [79] | |
| Galantamine (positive control) | 80.3 ± 0.1 | % inhibition at 1 mg/mL | [79] | |||
| Tyrosinase | Method reported by Nerya et al. [142] | Methanol | 81.4 ± 5.2 | % inhibition at 10 mg/mL | [79] | |
| Arbutin (positive control) | 78.0 ± 0.1 | % inhibition at 1 mg/mL | [79] | |||
| Phospholipase A2 | Colorimetric assay [143] | Dichloromethane-methanol (1:1) | 100 | % inhibition at 1 μg/mL | [105] | |
| Elastase | Colorimetric assay [144] | Dichloromethane-methanol (1:1) | 55 | % inhibition at 10 μg/mL | [105] | |
| A. taxiformis | Acetylcholinesterase | Ellman method [141] | Dichloromethane | 116.50 ± 10.94 | IC50 in μg/mL | [139] |
| Chloroform-methanol (2:1) | 8.92 ± 0.43 | IC50 in μg/mL | [91] | |||
| Ethanol | 46.33 ± 6.02 | IC50 in μg/mL | [91] | |||
| Donepezil (positive control) | 0.01 ± 0.00 | IC50 in μg/mL | [91] | |||
| Galantamine (positive control) | 0.43 ± 0.09 | IC50 in μg/mL | [91] | |||
| Butyrylcholinesterase | Ellman method [141] | Chloroform-methanol (2:1) | 13.96 ± 0.32 | IC50 in μg/mL | [91] | |
| Ethanol | 28.10 ± 0.93 | IC50 in μg/mL | [91] | |||
| Donepezil (positive control) | 55.62 ± 3.47 | IC50 in μg/mL | [91] | |||
| Galantamine (positive control) | >150 | IC50 in μg/mL | [91] |
| Constituents | A. armata | A. taxiformis |
|---|---|---|
| Water | 90.8–91.2 g/100 g fw [139,145] | 92.6 g/100 g fw [139] |
| Polysaccharides | Starch 1.26 g/100 g dw [145] | Starch 8.03 ± 0.38 g/100 g dw [146] |
| Other 72.0 g/100 g dw [145] | Other 32.47 ± 1.04 g/100 g dw [146] | |
| Protein | 10.9–14.0 g/100 g dw [145] | 17.55 ± 0.11 g/100 g dw [146] |
| Lipids | 2.51 ± 0.26 g/100 g dw [145] | 6.62 ± 0.54 g/100 g dw [146] |
| Ashes | 13.36 ± 0.68 g/100 g dw [145] | 23.76 ± 0.48 g/100 g dw [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponte, J.M.S.; Seca, A.M.L.; Barreto, M.C. Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition. Molecules 2022, 27, 1787. https://doi.org/10.3390/molecules27061787
Ponte JMS, Seca AML, Barreto MC. Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition. Molecules. 2022; 27(6):1787. https://doi.org/10.3390/molecules27061787
Chicago/Turabian StylePonte, José M. S., Ana M. L. Seca, and Maria Carmo Barreto. 2022. "Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition" Molecules 27, no. 6: 1787. https://doi.org/10.3390/molecules27061787
APA StylePonte, J. M. S., Seca, A. M. L., & Barreto, M. C. (2022). Asparagopsis Genus: What We Really Know About Its Biological Activities and Chemical Composition. Molecules, 27(6), 1787. https://doi.org/10.3390/molecules27061787








