Influence of Electronic Modulation of Phenanthroline-Derived Ligands on Separation of Lanthanides and Actinides
Abstract
:1. Introduction
2. Results and Discussion
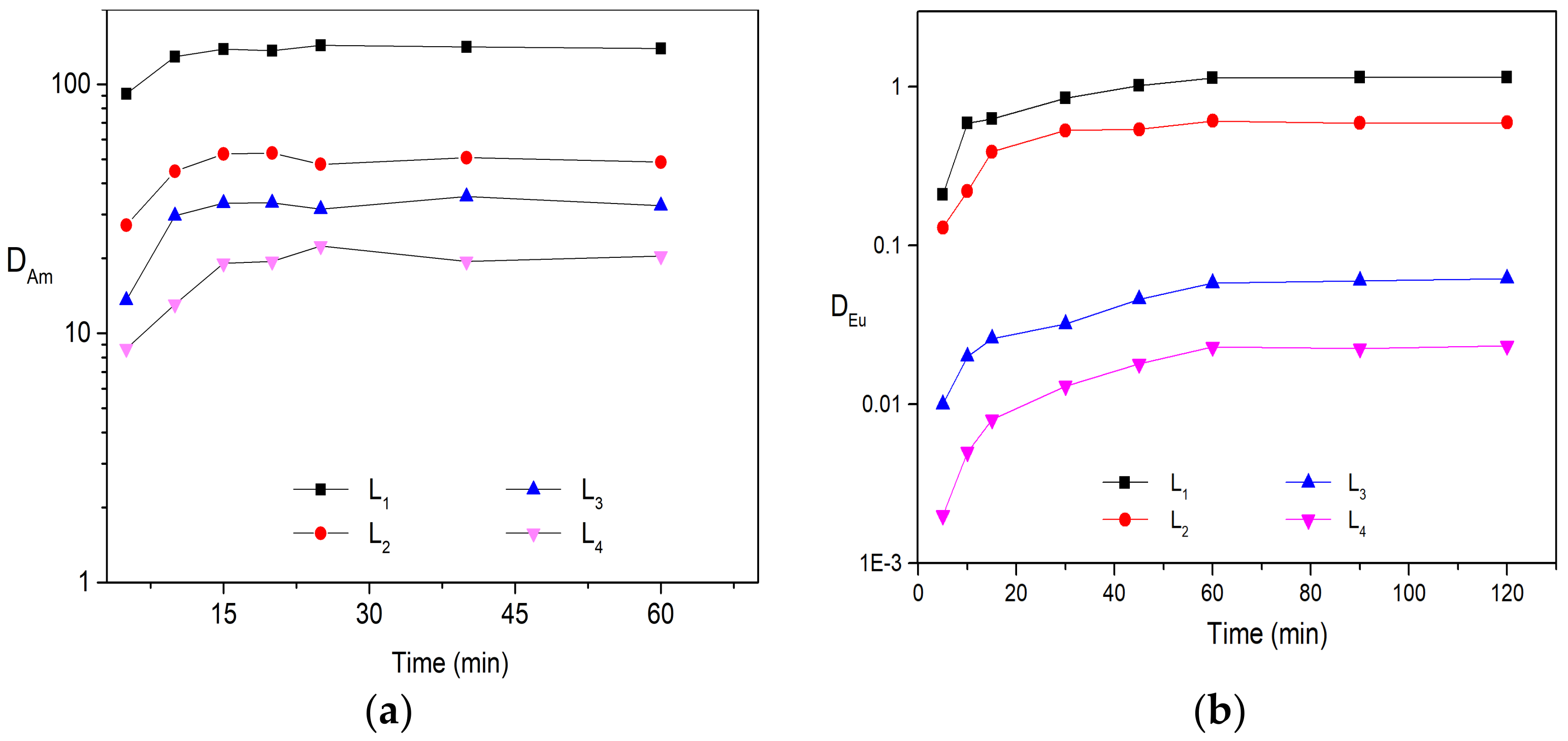
2.1. Solvent Extraction Study
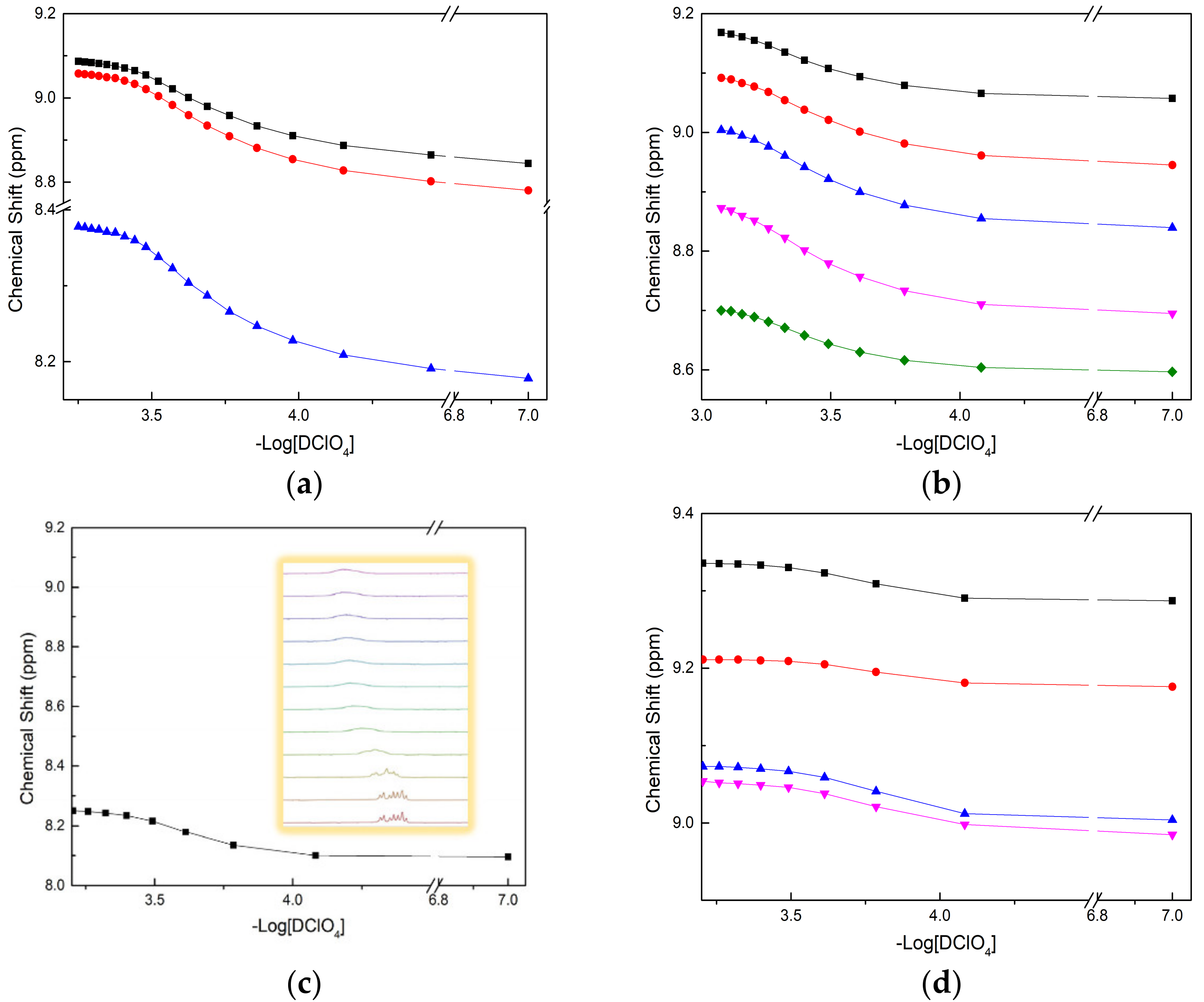
2.2. pKa Determination by NMR Titration
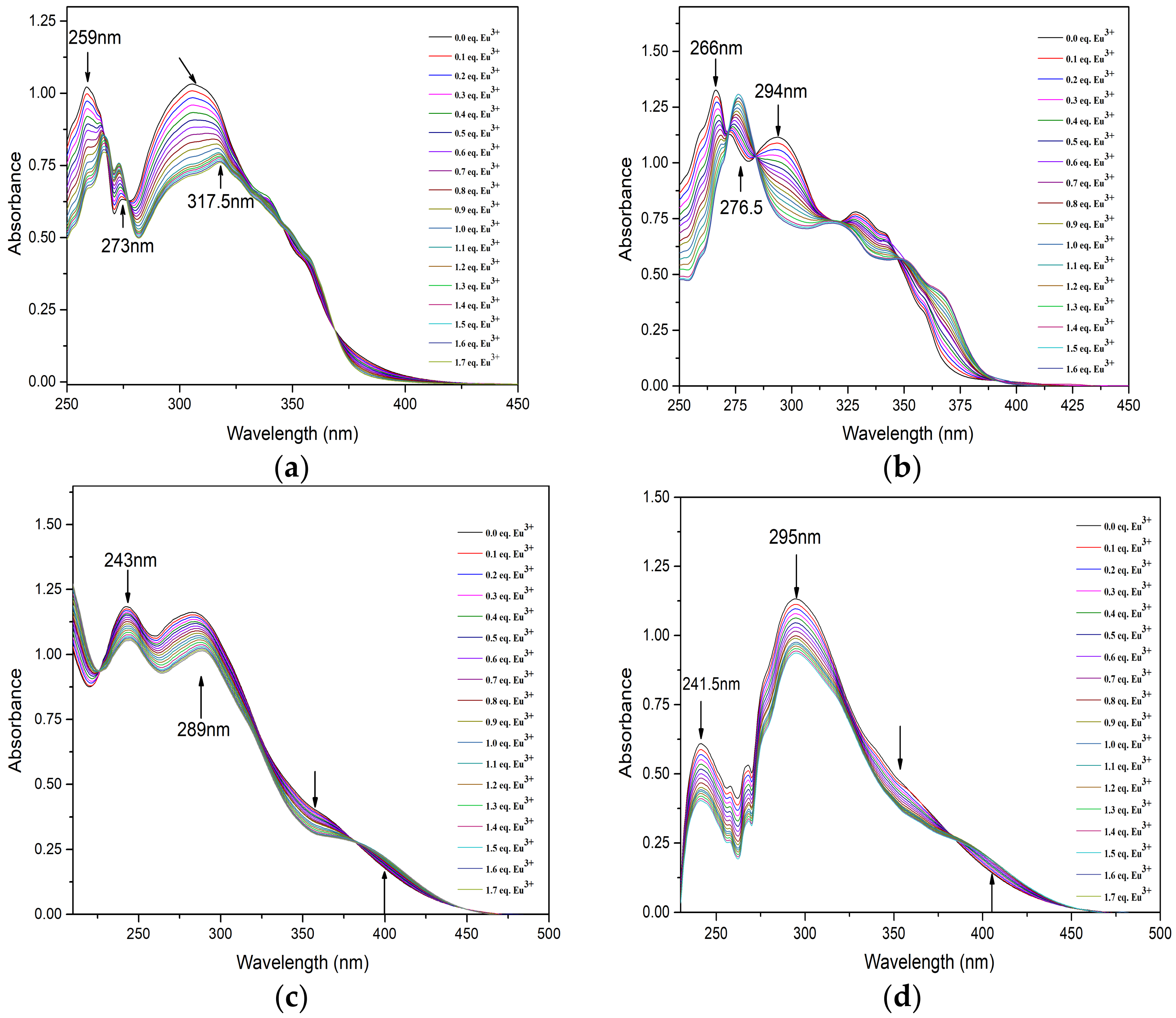
2.3. UV–vis Titration
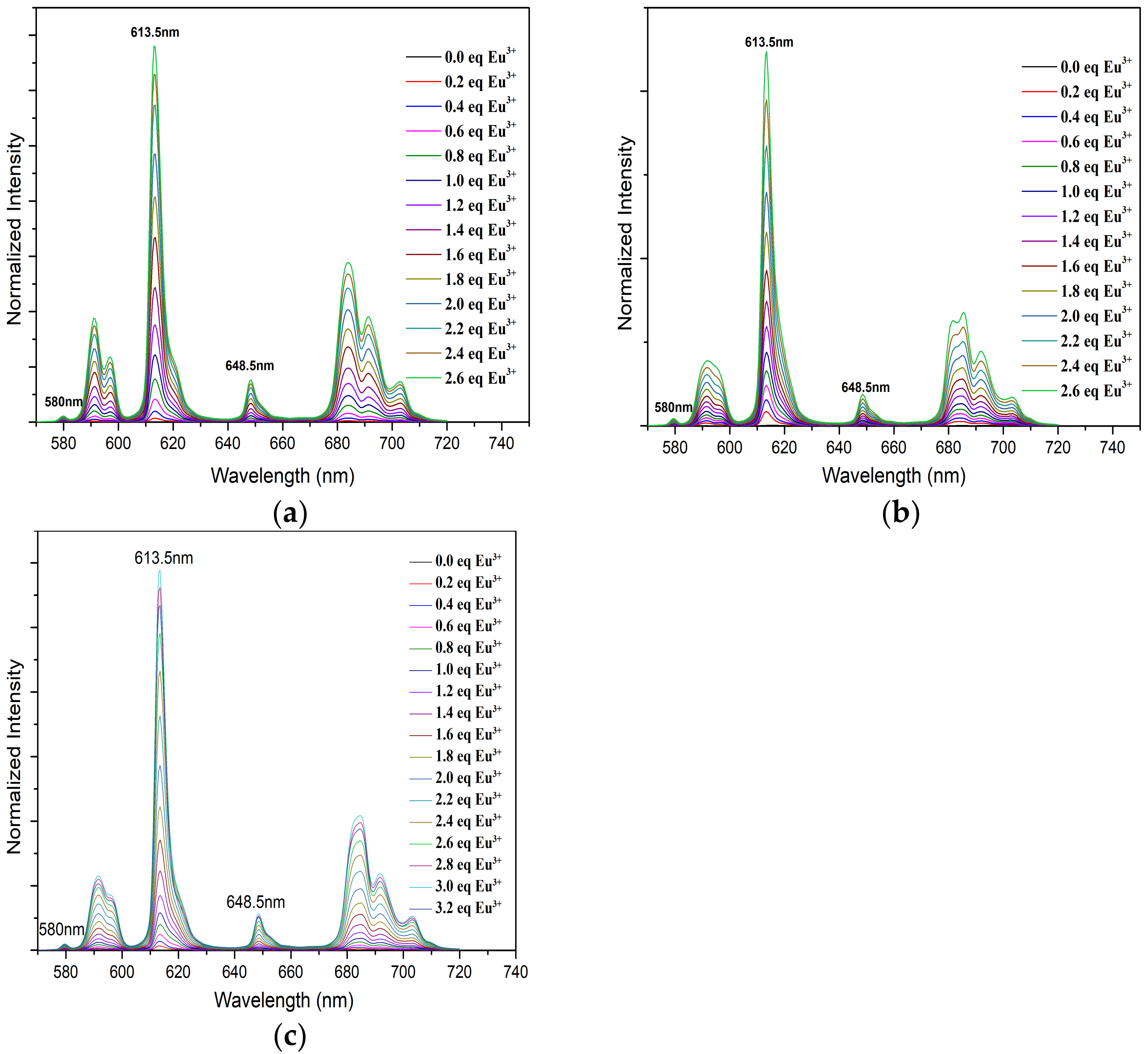
2.4. Fluorescence Spectroscopy
3. Materials and Methods
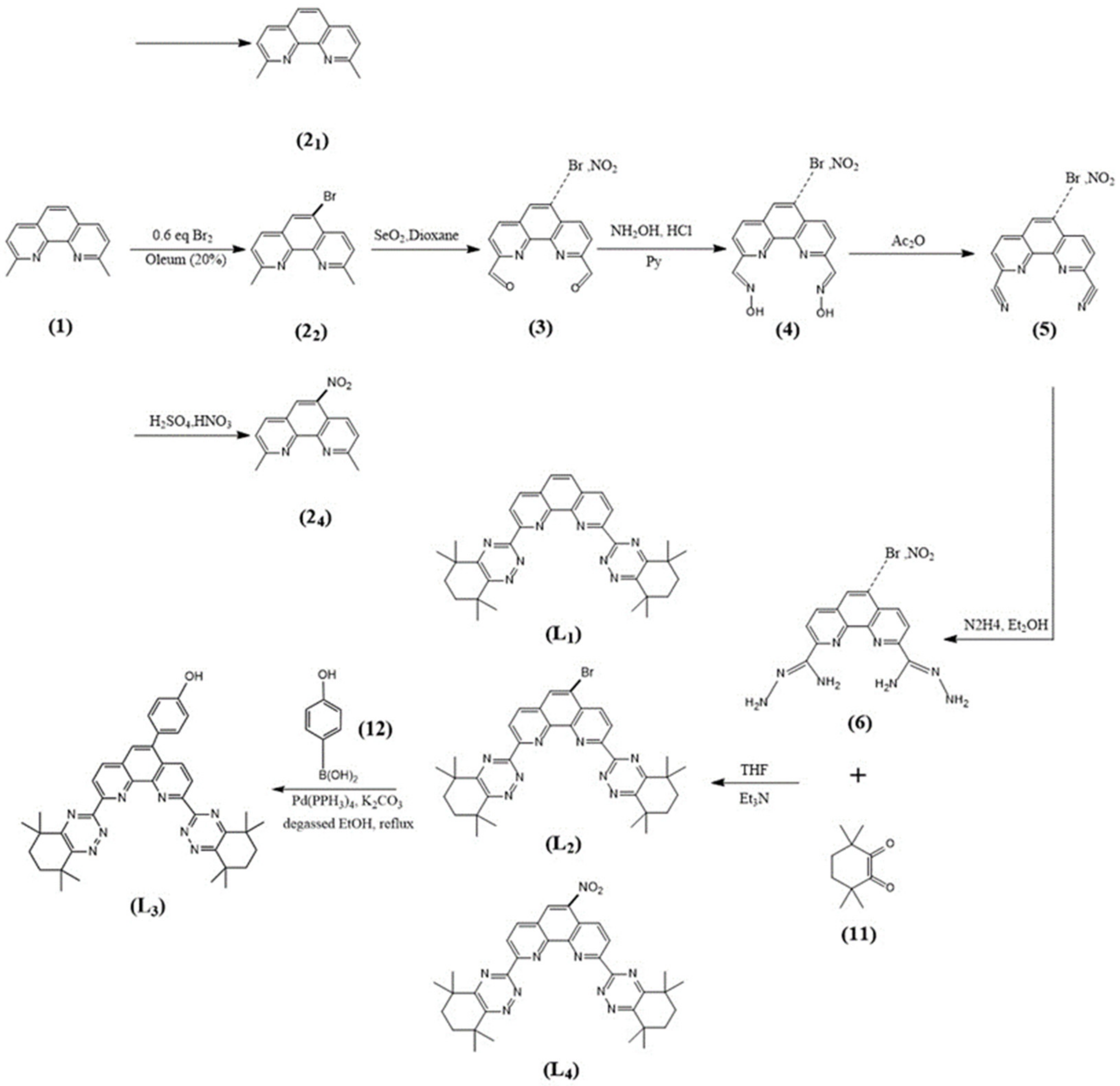
3.1. Syntheses
3.2. Solvent Extraction
3.3. pKa Determination
3.4. UV–vis Spectrometric Titration
3.5. Fluorescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- OECD—Nuclear Energy Agency (NEA). Status and Assessment Report on Actinide and Fission Product Partitioning and Transmutation; NEA: Paris, France, 1999. [Google Scholar]
- Magill, J.; Berthou, V.; Haas, D.; Galy, J.; Schenkel, R.; Wiese, H.W.; Heusener, G.; Tommasi, J.; Youinou, G. Impact limits of partitioning and transmutation scenarios on the radiotoxicity of actinides in radioactive waste. Nucl. Energy 2003, 42, 263–277. [Google Scholar] [CrossRef]
- Salvatores, M.; Palmiotti, G. Radioactive waste partitioning and transmutation within advanced fuel cycles: Achievements and challenges. Prog. Part. Nucl. Phys. 2011, 66, 144–166. [Google Scholar] [CrossRef]
- OECD—Nuclear Energy Agency (NEA). Potential Benefits and Impacts of Advanced Nuclear Fuel Cycles with Actinide Partitioning and Transmutation; Report NEA No. 6894; NEA: Paris, France, 2011. [Google Scholar]
- Sood, D.D.; Patil, S.K. Chemistry of Nuclear Fuel Reprocessing: Current Status. Radioanal. Nucl. Chem. 1996, 203, 547–573. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Gelis, A.V.; Vandegrift, G.F. Review: Solvent Systems Combining Neutral and Acidic Extractants for Separating Trivalent Lanthanides from the Transuranic Elements. Solvent Extr. Ion Exch. 2010, 28, 287–312. [Google Scholar] [CrossRef]
- Nash, K.L. A Review of the Basic Chemistry and Recent Developments in Trivalent f-Elements Separations. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Cotton, S. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2004; Volume 3, pp. 93–188. [Google Scholar]
- Katz, J.J.; Morss, L.R.; Edelstein, N.M.; Fuger, J. (Eds.) The Chemistry of the Actinide and Transactinide Elements; Springer: Dordrecht, The Netherlands, 2006; Volume 1, pp. 1–17. [Google Scholar]
- Afsar, A.; Laventine, D.M.; Harwood, L.M.; Hudson, M.J.; Geist, A. Utilizing electronic effects in the modulation of BTPhen ligands with respect to the partitioning of minor actinides from lanthanides. Chem. Commun. 2013, 49, 8534. [Google Scholar] [CrossRef]
- Lewis, F.W.; Harwood, L.M.; Hudson, M.J.; Drew, M.G.B.; Sypula, M.; Modolo, G.; Whittaker, D.; Sharrad, C.A.; Videva, V.; Hubscher-Bruder, V.; et al. Complexation of lanthanides, actinides and transition metal cations with a 6-(1,2,4-triazin-3-yl)-2,2′:6′,2″-terpyridine ligand: Implications for actinide(lll)/lanthanide(lll) partitioning. Dalton Trans. 2012, 41, 9209–9219. [Google Scholar] [CrossRef] [Green Version]
- Panak, P.J.; Geist, A. Complexation and Extraction of Trivalent Actinides and Lanthanides by Triazinylpyridine N-Donor Ligands. Chem. Rev. 2013, 113, 1199–1236. [Google Scholar] [CrossRef]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, C.Z.; Chai, Z.F.; Shi, W.Q. Excellent Selectivity for Actinides with a Tetradentate 2,9-Diamide-1,10-Phenanthroline Ligand in Highly Acidic Solution: A Hard–Soft Donor Combined Strategy. Inorg. Chem. 2014, 53, 1712–1720. [Google Scholar] [CrossRef]
- Kong, X.H.; Wu, Q.Y.; Lan, J.H. Theoretical Insights into Preorganized Pyridylpyrazole-Based Ligands toward the Separation of Am(III)/Eu(III). Inorg. Chem. 2018, 57, 14810–14820. [Google Scholar] [CrossRef] [PubMed]
- Trumm, S.; Geist, A.; Panak, P.J.; Fanghanel, T. An Improved Hydrolytically-Stable Bis-Triazinyl-Pyridine (BTP) for Selective Actinide Extraction. Solvent Extr. Ion Exch. 2011, 29, 213–229. [Google Scholar] [CrossRef]
- Kolarik, Z.; Mullich, U.; Gassner, F. Selective Extraction of Am(lll) over Eu(lll) by 2,6-ditriazolyl and 2,6-ditriazintlpridinies1. Solvent Extr. Ion Exch. 1999, 17, 23–32. [Google Scholar] [CrossRef]
- Kolarik, Z.; Mullich, U.; Gassner, F. Extraction of Am(lll) and Eu(lll) Nitrates by 2-6-di-(5,6-dipropyl-1,2,4-triazine-3-yl) pyridines1. Solvent Extr. Ion Exch. 1999, 17, 1155–1170. [Google Scholar] [CrossRef]
- Drew, M.G.B.; Foreman, M.R.S.J.; Hudson, M.J.; Madic, C. 6,6′-bis-(5,6-diethyl-[1,2,4]triazin-3-yl)-2,2′-bipyridyl the first example of a new class of quadridentate heterocyclic extraction reagents for the separation of americium(lll) and europium(lll). Inorg. Chem. Commun. 2005, 8, 239–241. [Google Scholar] [CrossRef]
- Geist, A.; Hill, C.; Modolo, G.; Foreman, M.R.S.; Weigl, M.; Gompper, K.; Hudson, M.J.; Madic, C. 6,6′-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl) [2,2′]bipyridine, an Effective Extracting Agent for the Separation of Americium(lll) and Curium(lll) from the Lanthanides. Solvent Extr. Ion Exch. 2006, 24, 463–483. [Google Scholar] [CrossRef]
- Foreman, M.R.S.; Hudson, M.J.; Drew, M.G.B. Complexes formed between the quadridentate, heterocyclic molecules 6,6′-bis-(5,6-dialkyl-1,2,4-triazin-3-yl)-2,2′-bipyridine (BTBP) and lanthanides(lll): Implications for the partitioning of actinides(lll) and lanthanides(lll). Dalton Trans. 2006, 1645–1653. [Google Scholar] [CrossRef]
- Foreman, M.R.S.; Hudson, M.J.; Geist, A.; Madic, C.; Weigl, M. An Investigation into the Extraction of Americium(lll), Lanthanides and D-Block Metals by 6,6′-Bis-(5,6-dipentyl-[1,2,4]triazin-3-yl)-[2,2′]bipyridinyl (C5-BTBP). Solvent Extr. Ion Exch. 2005, 23, 645–662. [Google Scholar] [CrossRef]
- Magnusson, D.; Christiansen, B.; Foreman, M.R.S.; Geist, A.; Glatz, J.P.; Malmbeck, R.; Modolo, G.; Serrano-Purroy, D.; Sorel, C. Demonstration of a SANEX Process in Centrifugal Contactors using the CyMe4-BTBP Molecule on a Genuine Fuel Solution. Solvent Extr. Ion Exch. 2009, 27, 97. [Google Scholar] [CrossRef]
- Lewis, F.W.; Harwood, L.M.; Hudson, M.J. Highly Efficient Separation of Actinides from Lanthanides by a Phenanthroline-Derived Bis-triazine Ligand. J. Am. Chem. Soc. 2011, 133, 13093–13102. [Google Scholar] [CrossRef] [Green Version]
- Afsar, A.; Harwood, L.M.; Westwood, J. Effective separation of the actinides Am(lll) and Cm(Ⅲ) by electronic modulation of bis-(1,2,4-triazin-3-yl) phenanthrolines. Chem. Commun. 2015, 51, 5860–5863. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.M.; Griffiths, T.L.; Helliwell, M. Lanthanide Speciation in Potential SANEX and GANEX Actinide/Lanthanide Separations Using Tetra-N-Donor Extractants. Inorg. Chem. 2013, 52, 3429–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenezer, C.; Solomon, R.V. Tailoring the selectivity of phenanthroline derivatives for the partitioning of trivalent Am/Eu ions—A relativistic DFT study. Inorg. Chem. Front. 2021, 8, 3012–3024. [Google Scholar] [CrossRef]
- Morgan, S.G.; Burstall, F.H. Dehydrogenation of pyridine by anhydrous ferric chloride. J. Chem. Soc. 1932, 20–30. [Google Scholar] [CrossRef]
- Morgan, S.G.; Burstall, F.H. Researches on residual affinity and coordination. Part XXXVII. Complex metallic salts containing 2:6-di-2′-pyridyl-pyridine (2:2′:2″-tripyridyl). J. Chem. Soc. 1937, 1649–1655. [Google Scholar] [CrossRef]
- Ackerman, J.J.H.; Soto, G.E.; Spees, W.M.; Zhu, Z.; Evelhoch, J.L. The NMR chemical shift pH measurement revisited: Analysis of error and modeling of a pH dependent reference. Magn. Reson. Med. 1996, 36, 674–683. [Google Scholar] [CrossRef]
- Grycova, L.; Dommisse, R.; Pieters, L.; Marek, R. NMR determination of pKa values of indoloquinoline alkaloids. Magn. Reson. Chem. 2009, 47, 977–981. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Ye, G. Unraveling the complexation mechanism of actinide(lll) and lanthanide(Ⅲ) with a new tetradentate phenanthroline-derived phosphonate ligand. Inorg. Chem. Front. 2020, 7, 1726–1740. [Google Scholar] [CrossRef]
- Kolarik, Z. Complexation and Separation of Lanthanides(lll) and Actinides(lll) by Heterocyclic N-Donors in Solutions. Chem. Rev. 2008, 108, 4208–4252. [Google Scholar] [CrossRef]
- Martin, R.B.; Lissfelt, J.A. The 2,2′,2″-Tripyridine System. J. Am. Chem. Soc. 1956, 78, 938–940. [Google Scholar] [CrossRef]
- Kou, F.; Yang, S.; Qian, H.; Zhang, L.; Beavers, C.M.; Teat, S.J.; Tian, G. A fluorescence study on the complexation of Sm(III), Eu(lll) and Tb(lll) with tetraalkyldiglycolamides (TRDGA) in aqueous solution, in solid state, and in solvent extraction. Dalton Trans. 2016, 45, 18484–18493. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fang, M.; Wang, X. Sorption Speciation of Lanthanides/Actinides on Minerals by TRLFS, EXAFS and DFT Studies: A Review. Molecules 2010, 15, 8431–8468. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.; Bauer, A.; Coppin, F.; Fanghanel, T.; Kim, J.I. Inner-sphere, outer-sphere and ternary surface complexes: A TRLFS study of the sorption process of Eu (lll) onto smectite and kaolinite. Radiochim. Acta 2002, 90, 345–349. [Google Scholar] [CrossRef]
- Bremer, A.; Whittaker, D.M.; Sharrad, C.A. Complexation of Cm(lll) and Eu(lll) with CyMe4-BTPhen and CyMe4-BTBP studied by time resolved laser fluorescence spectroscopy. Dalton Trans. 2014, 43, 2684–2694. [Google Scholar] [CrossRef]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatini, A.; Moruzzi, M.S.; Vacca, A. Nuclear magnetic resonance as a tool for determining protonation constants of natural polyprotic bases in solution. Anal Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]



| Ligands | Log pKa | Solvent | Methods | Reference |
|---|---|---|---|---|
| 1,10-phenanthroline | 4.92 | water | - | - |
| BTP | 3.3 | 76%Methanol | NMR | [33] |
| Terpy | 4.33 | Water, I = 0.1 M | UV/Vis | [34] |
| L1 | 3.1 ± 0.1 | Methanol | NMR | [24] |
| L1 | 3.1 ± 0.1 | Methanol | NMR | Present Work |
| L2 | 2.3 ± 0.2 | Methanol | NMR | Present Work |
| L3 | 0.9 ± 0.2 | Methanol | NMR | Present Work |
| L4 | 0.5 ± 0.1 | Methanol | NMR | Present Work |
| BTPhen Ligands | Logβ12 | Solvent | Methods | Reference |
|---|---|---|---|---|
| L1 | 12.6 ± 0.4 | Methanol (with 3.3 mol% water) | TRLFS | [38] |
| 15.6 ± 1.0 | Methanol (I = 0 M) | UV/vis | [26] | |
| 14.2 ± 0.5 | Methanol (I = 0.01 M) | UV/vis | Present Work | |
| 11.5 ± 0.6 | Methanol (I = 0.01 M) | TRLFS | Present Work | |
| L2 | 11.5 ± 0.3 | Methanol (I = 0.01 M) | UV/vis | Present Work |
| 12.1 ± 0.8 | Methanol (I = 0.01 M) | TRLFS | Present Work | |
| L3 | 8.3 ± 0.6 | Methanol (I = 0.01 M) | UV/vis | Present Work |
| L4 | 7.1 ± 0.4 | Methanol (I = 0.01 M) | UV/vis | Present Work |
| 8.6 ± 0.2 | Methanol (I = 0.01 M) | TRLFS | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-M.; Liu, X.-J.; Yang, Q.; Liu, Y.-K.; Nie, J.; Yang, S.-M.; Yang, Y.-Y.; Lou, F.-Y.; Xiao, S.-T.; Ouyang, Y.-G.; et al. Influence of Electronic Modulation of Phenanthroline-Derived Ligands on Separation of Lanthanides and Actinides. Molecules 2022, 27, 1786. https://doi.org/10.3390/molecules27061786
Li M-M, Liu X-J, Yang Q, Liu Y-K, Nie J, Yang S-M, Yang Y-Y, Lou F-Y, Xiao S-T, Ouyang Y-G, et al. Influence of Electronic Modulation of Phenanthroline-Derived Ligands on Separation of Lanthanides and Actinides. Molecules. 2022; 27(6):1786. https://doi.org/10.3390/molecules27061786
Chicago/Turabian StyleLi, Ming-Ming, Xiao-Juan Liu, Qi Yang, Yue-Kun Liu, Jiang Nie, Shu-Ming Yang, Ya-Ya Yang, Fu-Yan Lou, Song-Tao Xiao, Ying-Gen Ouyang, and et al. 2022. "Influence of Electronic Modulation of Phenanthroline-Derived Ligands on Separation of Lanthanides and Actinides" Molecules 27, no. 6: 1786. https://doi.org/10.3390/molecules27061786
APA StyleLi, M.-M., Liu, X.-J., Yang, Q., Liu, Y.-K., Nie, J., Yang, S.-M., Yang, Y.-Y., Lou, F.-Y., Xiao, S.-T., Ouyang, Y.-G., & Ye, G.-A. (2022). Influence of Electronic Modulation of Phenanthroline-Derived Ligands on Separation of Lanthanides and Actinides. Molecules, 27(6), 1786. https://doi.org/10.3390/molecules27061786






