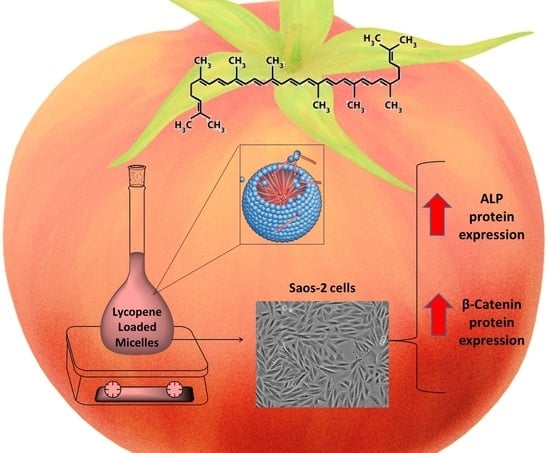
A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Extraction of Lycopene
2.3. Water Content Determination
2.4. Chemical Analyses
2.5. Lycopene Micellar Suspensions
2.6. Cell Cultures and Proliferation
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
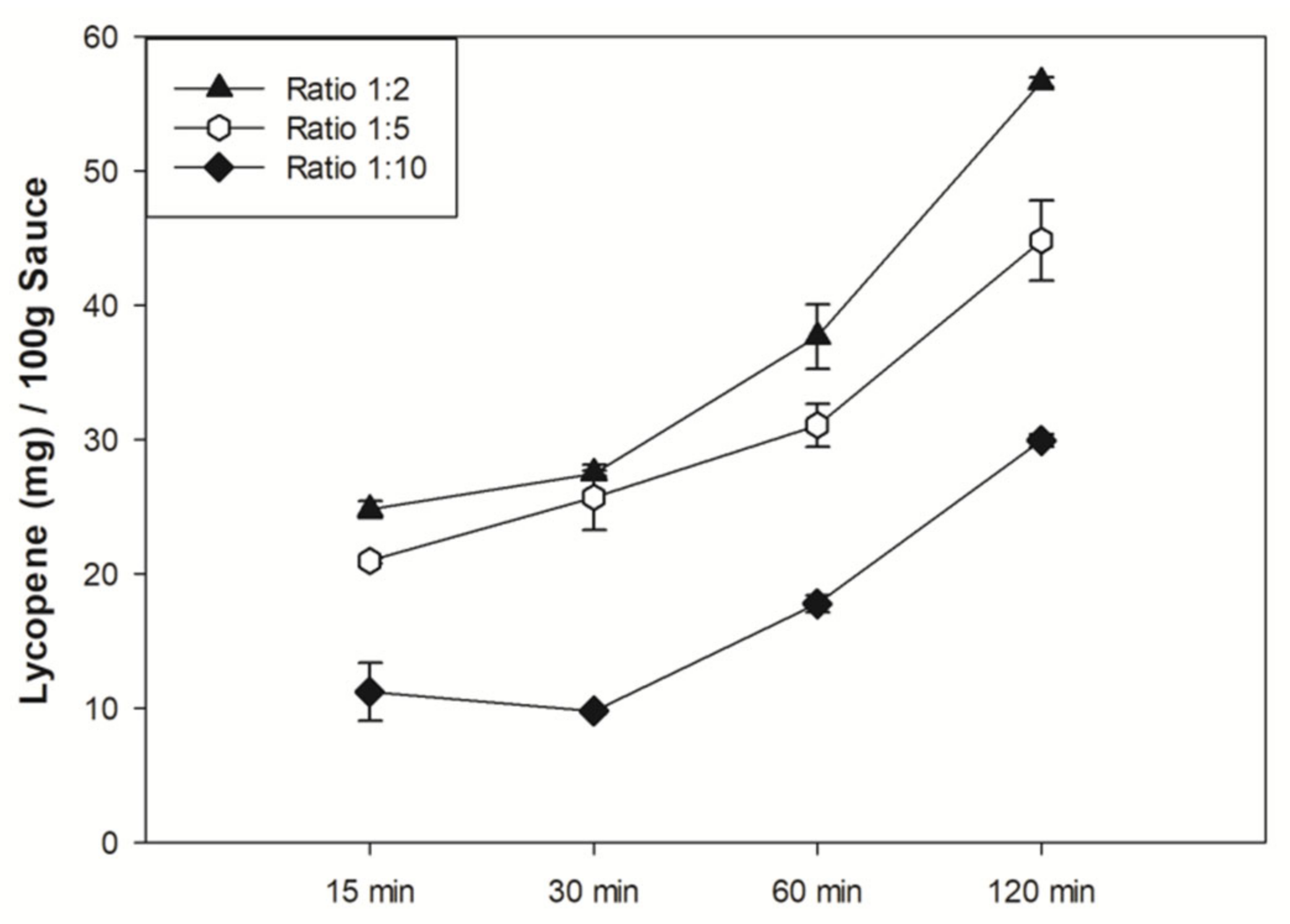
3.1. Lycopene Extraction and Quantification
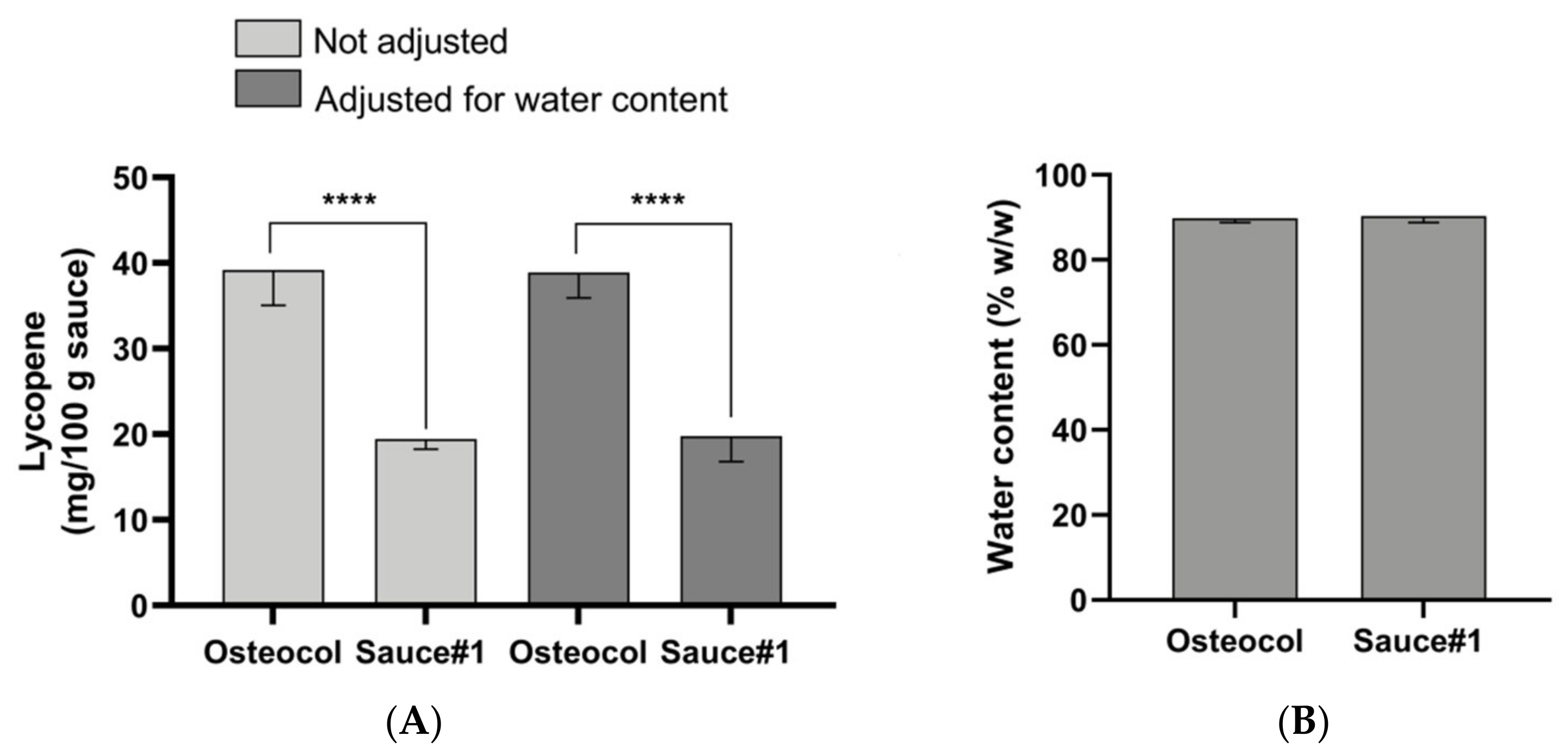
3.2. Sauces Comparison and Characterization
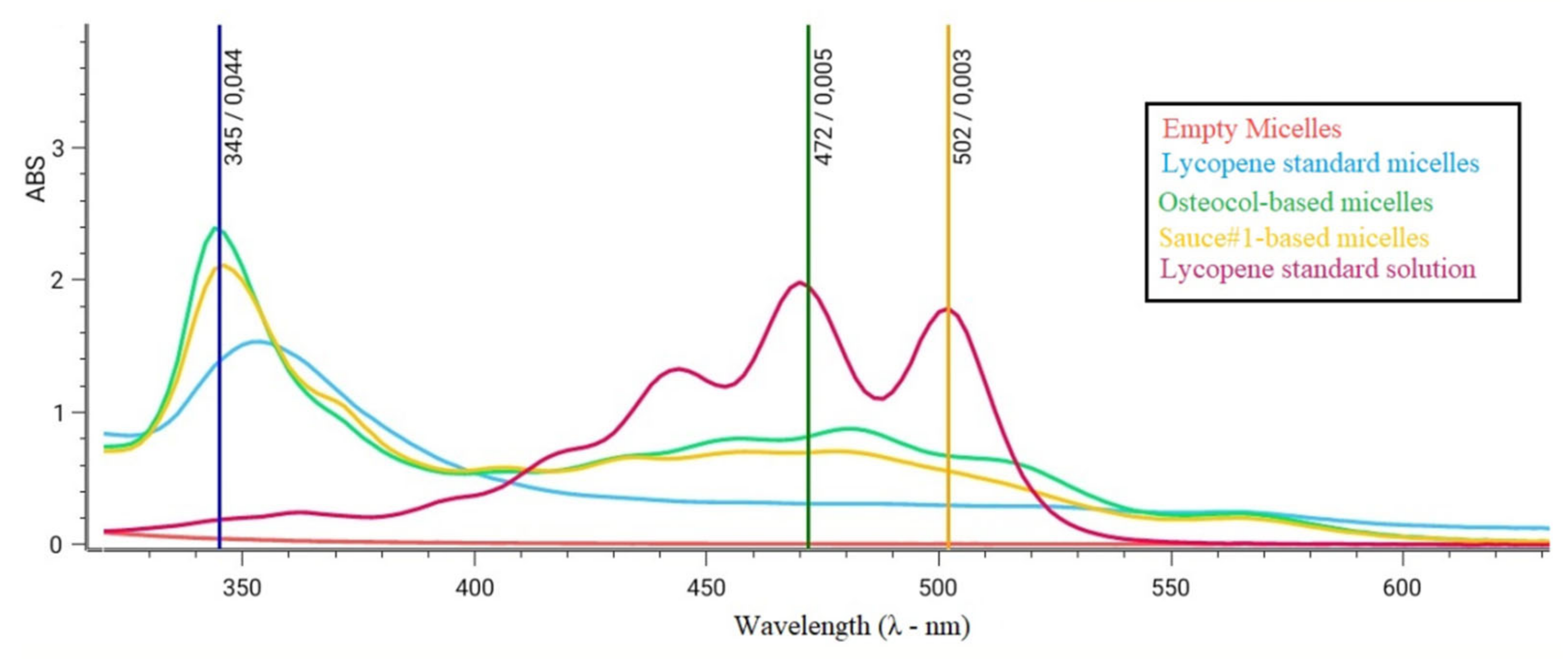
3.3. Lycopene Micelles Characterization
3.4. Lycopene-Based Micelles Do Not Effect Osteoblast Proliferation In Vitro
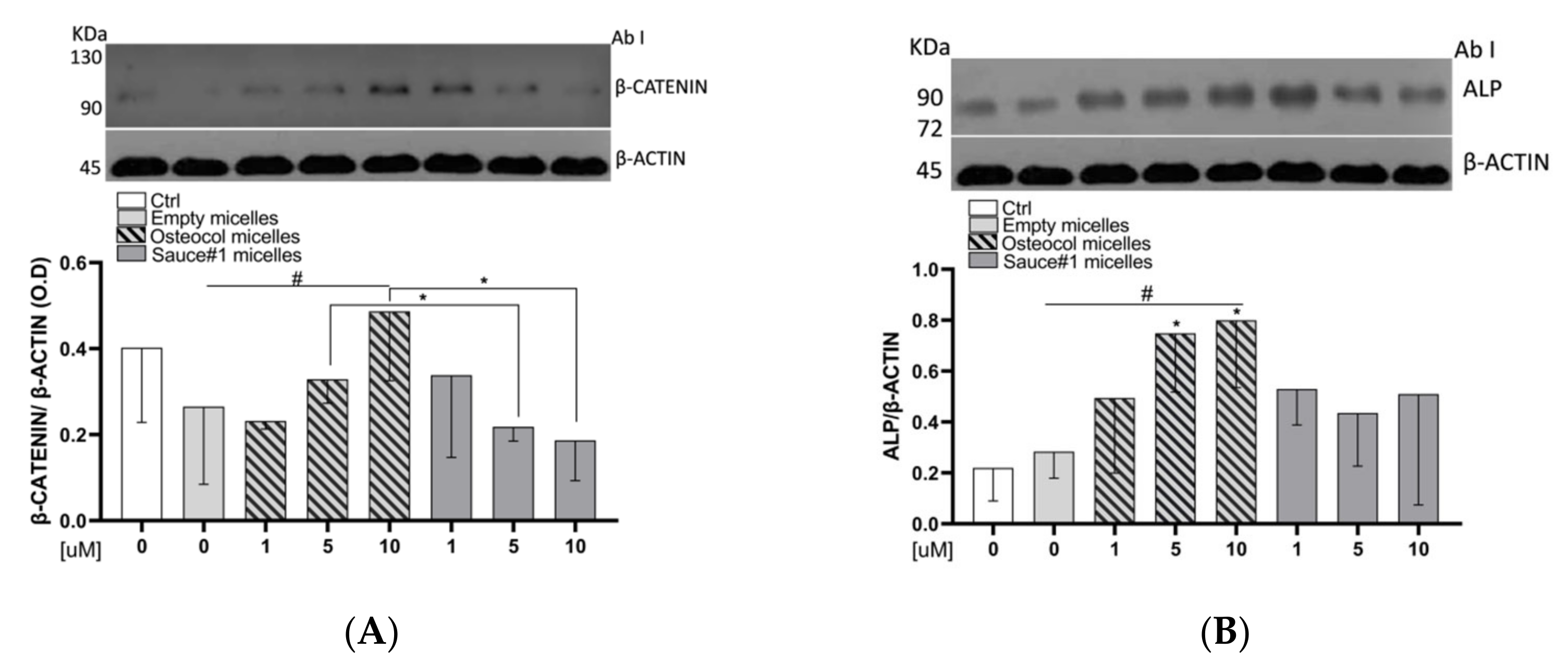
3.5. Osteocol-Based Micelles Increased β-Catenin Protein Expression Levels
3.6. Osteocol-Based Micelles Increased the ALP Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, S.; Montalcini, T.; Pujia, A. Mediterranean Diet in Healthy Aging. J. Nutr. Health Aging 2021, 25, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Mellert, W.; Deckardt, K.; Gembardt, C.; Schulte, S.; van Ravenzwaay, B.; Slesinski, R. Thirteen-week oral toxicity study of synthetic lycopene products in rats. Food Chem. Toxicol. 2002, 40, 1581–1588. [Google Scholar] [CrossRef]
- McClain, R.M.; Bausch, J. Summary of safety studies conducted with synthetic lycopene. Regul. Toxicol. Pharmacol. 2003, 37, 274–285. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agr. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Matos, H.R.; di Mascio, P.; Medeiros, M.H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys. 2000, 383, 56–59. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of Dietary and Supplemental Lycopene on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Sopian, M.M.; Abu Bakar, M.H.; Lee, L.K. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the Type II diabetes mellitus patients: A case-control study. Ann. Med. 2021, 53, 1058–1064. [Google Scholar] [CrossRef]
- Baykalir, B.G.; Aksit, D.; Dogru, M.S.; Yay, A.H.; Aksit, H.; Seyrek, K.; Atessahin, A. Lycopene Ameliorates Experimental Colitis in Rats via Reducing Apoptosis and Oxidative Stress. Int. J. Vitam. Nutr. Res. 2016, 86, 27–35. [Google Scholar] [CrossRef]
- Bailey, J.R. Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Nova Science: New York, NY, USA, 2015. [Google Scholar]
- Nakayama, M.; Okano, T. Drug delivery systems using nano-sized drug carriers. Gan To Kagaku Ryoho 2005, 32, 935–940. [Google Scholar]
- Barba, A.O.; Hurtado, M.C.; Mata, M.S.; Ruiz, V.F.; de Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Bunghez, I.; Raduly, M.; Doncea, S.; Aksahin, I.; Ion, R. Lycopene determination in tomatoes by different spectral techniques (UV-Vis, FTIR and HPLC). Dig. J. Nanomater. Biostruct. 2011, 6, 1349–1356. [Google Scholar]
- Rao, A.; Waseem, Z.; Agarwal, S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Maria, A.L.; Gogoasa, I.; Alda, S.; Diana, M.; Maria, B.D.; Maria, R.; Cristea, T.; Gergen, I. Monitoring the lycopene content in some fruits and vegetables. J. Hortic. For. Biotechnol. 2014, 18, 33–36. [Google Scholar]
- Ferro, Y.; Mazza, E.; Angotti, E.; Pujia, R.; Mirarchi, A.; Salvati, M.A.; Terracciano, R.; Savino, R.; Romeo, S.; Scuteri, A.; et al. Effect of a novel functional tomato sauce (OsteoCol) from vine-ripened tomatoes on serum lipids in individuals with common hypercholesterolemia: Tomato sauce and hypercholesterolemia. J. Transl. Med. 2021, 19, 19. [Google Scholar] [CrossRef]
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Analytical methods used in food chemical control. Rapp. ISTISAN 1996, 96, 34. [Google Scholar]
- Anthon, G.; Barrett, D.M. Standardization of a rapid spectrophotometric method for lycopene analysis. ISHS acta horticulturae. In Proceedings of the X International Symposium on the Processing Tomato, Beijing, China, 3–11 June 2007; Volume 758, pp. 111–128. [Google Scholar]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Law, C.L. Extraction of total phenolic content from garcinia mangostana linn. hull. I. Effects of solvents and UV-vis spectrophotometer absorbance method. Food Bioprocess Technol. 2012, 5, 2928–2933. [Google Scholar] [CrossRef]
- Büyüktunce, E.; Porgalı, E.; Çolak, C. Comparison of total phenolic content and total antioxidant activity in local red wines determined by spectrophotometric methods. Food Nutr. Sci. 2014, 5, 1660–1667. [Google Scholar]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Aruwa, C.; Amoo, S.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Doost, A.S.; Devlieghere, F.; Stevens, C.V.; Claeys, M.; van der Meeren, P. Self-assembly of Tween 80 micelles as nanocargos for oregano and trans-cinnamaldehyde plant-derived compounds. Food Chem. 2020, 327, 126970. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gupta, S.; Moulik, S. Characterisation of Tween 20 & Tween 80 Micelles in Aqueous Medium from Transport Studies. Indian J. Chem. 1985, 24A, 670–673. [Google Scholar]
- Chen, L.R.; Wesley, J.A.; Bhattachar, S.; Ruiz, B.; Bahash, K.; Babu, S.R. Dissolution behavior of a poorly water soluble compound in the presence of Tween 80. Pharm. Res. 2003, 20, 797–801. [Google Scholar] [CrossRef]
- Hayhoe, R.P.; Lentjes, M.A.; Mulligan, A.A.; Luben, R.N.; Khaw, K.-T.; Welch, A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iimura, Y.; Agata, U.; Takeda, S.; Kobayashi, Y.; Yoshida, S.; Ezawa, I.; Omi, N. Lycopene intake facilitates the increase of bone mineral density in growing female rats. J. Nutr. Sci. Vitaminol. 2014, 60, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Maguer, M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem. 2009, 112, 1038–1045. [Google Scholar] [CrossRef]
- Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J. Stability of lycopene during food processing and storage. J. Med. Food 2005, 8, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Sarah, M. Carotenoids preservation during sterilization of palm fruit using microwave irradiation. ARPN J. Eng. Appl. Sci. 2018, 13, 1009–1014. [Google Scholar]
- Taticchi, A.; Esposto, S.; Urbani, S.; Veneziani, G.; Selvaggini, R.; Sordini, B.; Servili, M. Effect of an olive phenolic extract added to the oily phase of a tomato sauce, on the preservation of phenols and carotenoids during domestic cooking. LWT 2017, 84, 572–578. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. The encapsulation of low viscosity omega-3 rich fish oil in polycaprolactone by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2017, 128, 227–234. [Google Scholar] [CrossRef]
- Esposto, B.S.; Jauregi, P.; Tapia-Blacido, D.R.; Martelli-Tosi, M. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Q.; Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020, 30, 37–44. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B. PLGA-PEG Nanoparticles Facilitate In Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef]
- Xiao, Y.; Nie, M.; Zhao, H.; Li, D.; Gao, R.; Zhou, C.; Xu, Y.; Dai, Z.; Zhang, Z. Citrus flavanones enhance the bioaccessibility of β-carotene by improving lipid lipolysis and incorporation into mixed micelles. J. Funct. Foods 2021, 87, 104792. [Google Scholar] [CrossRef]
- Kim, L.; Rao, A.V.; Rao, L.G. Lycopene II—Effect on osteoblasts: The carotenoid lycopene stimulates cell proliferation and alkaline phosphatase activity of SaOS-2 cells. J. Med. Food 2003, 6, 79–86. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Park, C.-K.; Ishimi, Y.; Ohmura, M.; Yamaguchi, M.; Ikegami, S. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J. Nutr. Sci. Vitaminol. 1997, 43, 281–296. [Google Scholar] [CrossRef]
- Liu, F.; Liu, D. Long-circulating emulsions (oil-in-water) as carriers for lipophilic drugs. Pharm. Res. 1995, 12, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kita, Y. Protection of bovine serum albumin from aggregation by Tween 80. J. Pharm. Sci. 2000, 89, 646–651. [Google Scholar] [CrossRef]
- Park, B.; Lim, J.W.; Kim, H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 2019, 70, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Preet, R.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis 2013, 34, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etherington, J.; Keeling, J.; Bramley, R.; Swaminathan, R.; McCurdie, I.; Spector, T. The effects of 10 weeks military training on heel ultrasound and bone turnover. Calcif. Tissue Int. 1999, 64, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M. Retinoid roles and action in skeletal development and growth provide the rationale for an ongoing heterotopic ossification prevention trial. Bone 2018, 109, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Cao, X.M.; Wang, H.Y.; Liao, X.J. Changes of tomato powder qualities during storage. Powder Technol. 2010, 204, 159–166. [Google Scholar] [CrossRef]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A regular lycopene enriched tomato sauce consumption influences antioxidant status of healthy young-subjects: A crossover study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
| UV/VIS Spectrophotometer Data | |||||
| Medium | Extract Conc. (M) | λ472 nm | Lycopene Concentration (M) | Lycopene (g) | Lycopene % (w/w) |
| DCM | 9.31 × 10−4 | 1.65 | 1.11 × 10−5 | 5.97 × 10−6 | 1.20 |
| 2-Me-THF | 1.86 × 10−4 | 1.45 | 9.79 × 10−6 | 5.26 × 10−6 | 5.25 |
| He | 4.66 × 10−5 | 1.19 | 8.08 × 10−6 | 4.34 × 10−6 | 17.35 |
| He/Ac | 1.86 × 10−4 | 1.70 | 1.15 × 10−6 | 6.15 × 10−6 | 6.15 |
| HPLC data | |||||
| Medium | Extract Injected (mg/mL) | Ritention Time (min.) | All trans-Lycopene (mg/mL) | Lycopene (g) | All trans-Lycopene (% w/w) |
| DCM | 2 | 38 | 0.016 | 3.12 × 10−5 | 0.78 |
| 2-Me-THF | 2 | 38.1 | 0.083 | 1.65 × 10−4 | 4.16 |
| He | 2 | 37.7 | 0.325 | 6.5 × 10−4 | 16.26 |
| He/Ac | 2 | 36 | 0.138 | 2.76 × 10−4 | 6.89 |
| Sample | Extract Injected (mg/mL) | All-Trans Lycopene (mg/mL) | All-Trans Lycopene (% w/w) |
|---|---|---|---|
| Osteocol® | 2 | 0.325 | 16.26 |
| Sauce #1 | 2 | 0.329 | 16.44 |
| Sauce #2 | 2 | 0.318 | 15.89 |
| Sauce #3 | 2 | 0.295 | 14.75 |
| Sauce #4 | 2 | 0.254 | 12.7 |
| Sauce #5 | 2 | 0.246 | 12.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mare, R.; Maurotti, S.; Ferro, Y.; Galluccio, A.; Arturi, F.; Romeo, S.; Procopio, A.; Musolino, V.; Mollace, V.; Montalcini, T.; et al. A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells. Nutrients 2022, 14, 717. https://doi.org/10.3390/nu14030717
Mare R, Maurotti S, Ferro Y, Galluccio A, Arturi F, Romeo S, Procopio A, Musolino V, Mollace V, Montalcini T, et al. A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells. Nutrients. 2022; 14(3):717. https://doi.org/10.3390/nu14030717
Chicago/Turabian StyleMare, Rosario, Samantha Maurotti, Yvelise Ferro, Angelo Galluccio, Franco Arturi, Stefano Romeo, Antonio Procopio, Vincenzo Musolino, Vincenzo Mollace, Tiziana Montalcini, and et al. 2022. "A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells" Nutrients 14, no. 3: 717. https://doi.org/10.3390/nu14030717
APA StyleMare, R., Maurotti, S., Ferro, Y., Galluccio, A., Arturi, F., Romeo, S., Procopio, A., Musolino, V., Mollace, V., Montalcini, T., & Pujia, A. (2022). A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells. Nutrients, 14(3), 717. https://doi.org/10.3390/nu14030717