Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Biochemical Indexes Measurements
2.3. Assessment of Atherosclerotic Plaques by Oil Red O Staining
2.4. Immunohistochemistry Staining
2.5. Measurements of VSMCs Proliferation and Migration
2.6. Western Blot
2.7. Real-Time qPCR
2.8. Statistical Analyses
3. Results
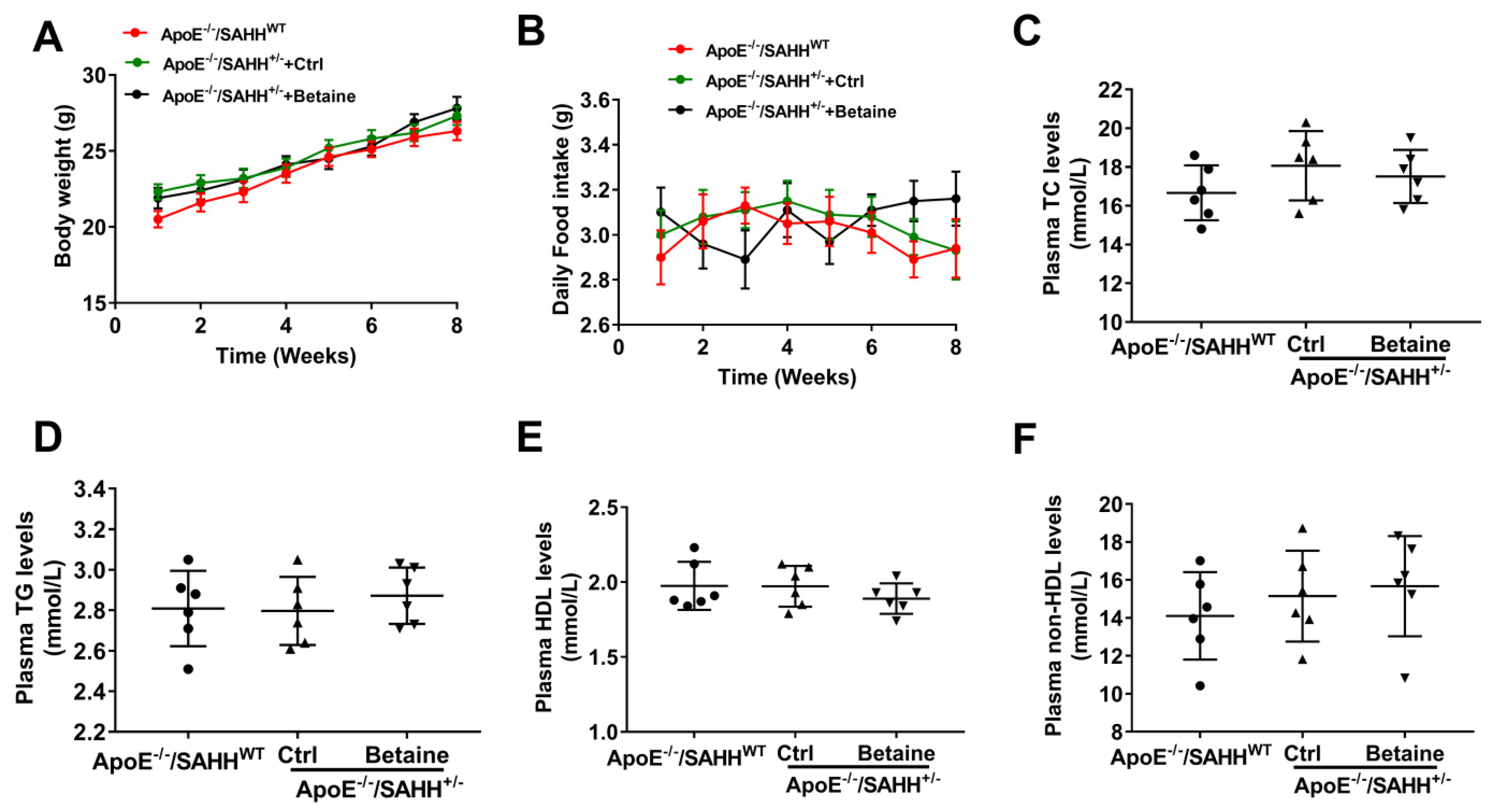
3.1. The Body Weight and Intake of Daily Food and Plasma Lipids Levels among the Three Groups
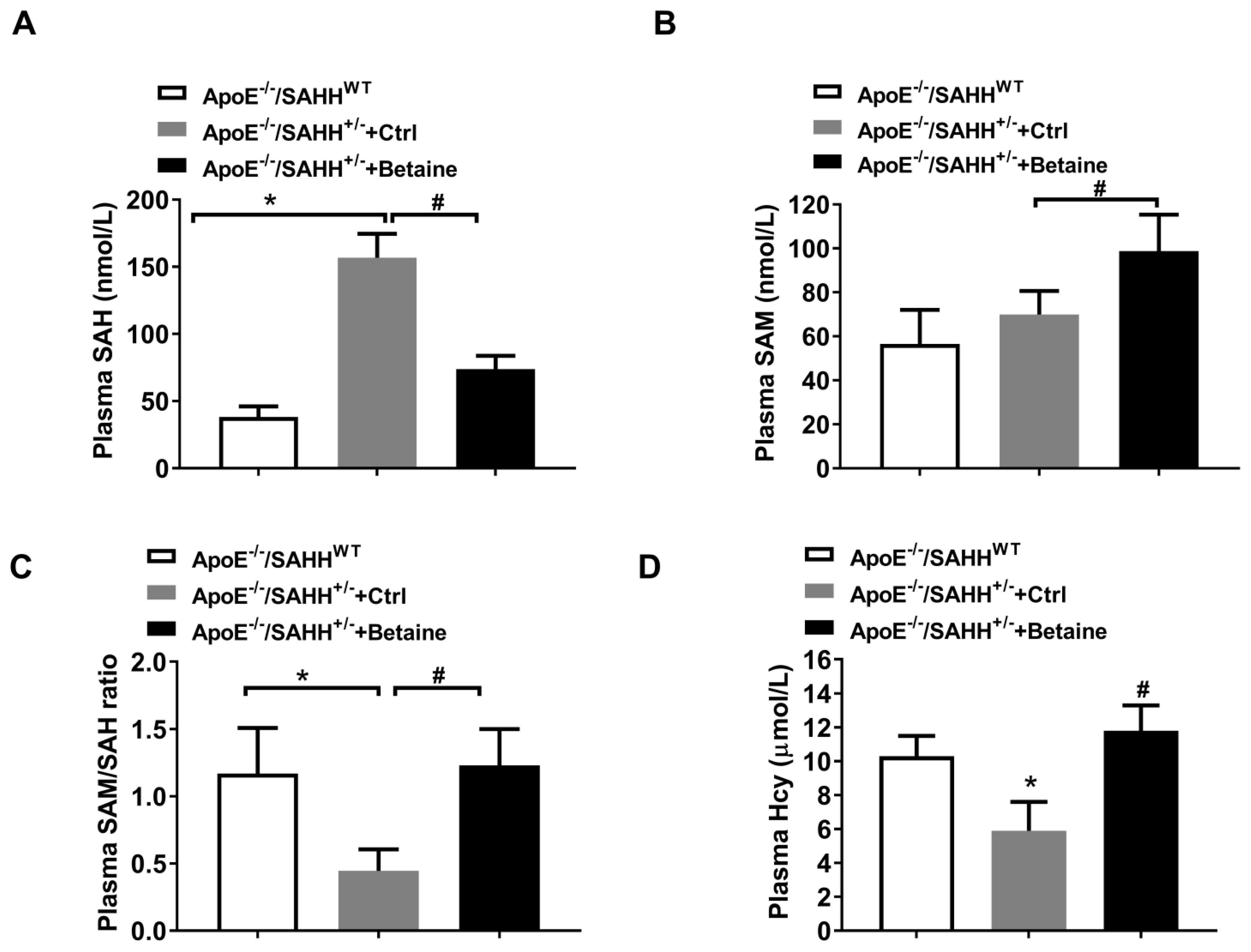
3.2. Betaine Supplementation Lowered SAHH-Deficiency-Accumulated SAH in ApoE−/−/SAHH+/− Mice
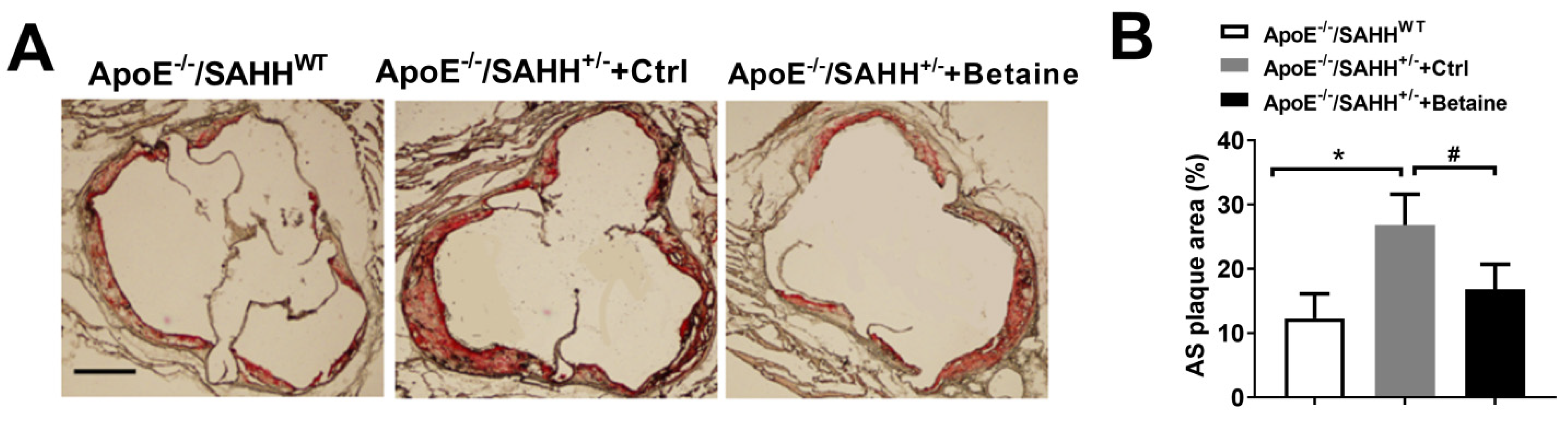
3.3. Betaine Supplementation Alleviated Atherosclerotic Lesions in ApoE−/−/SAHH+/− Mice
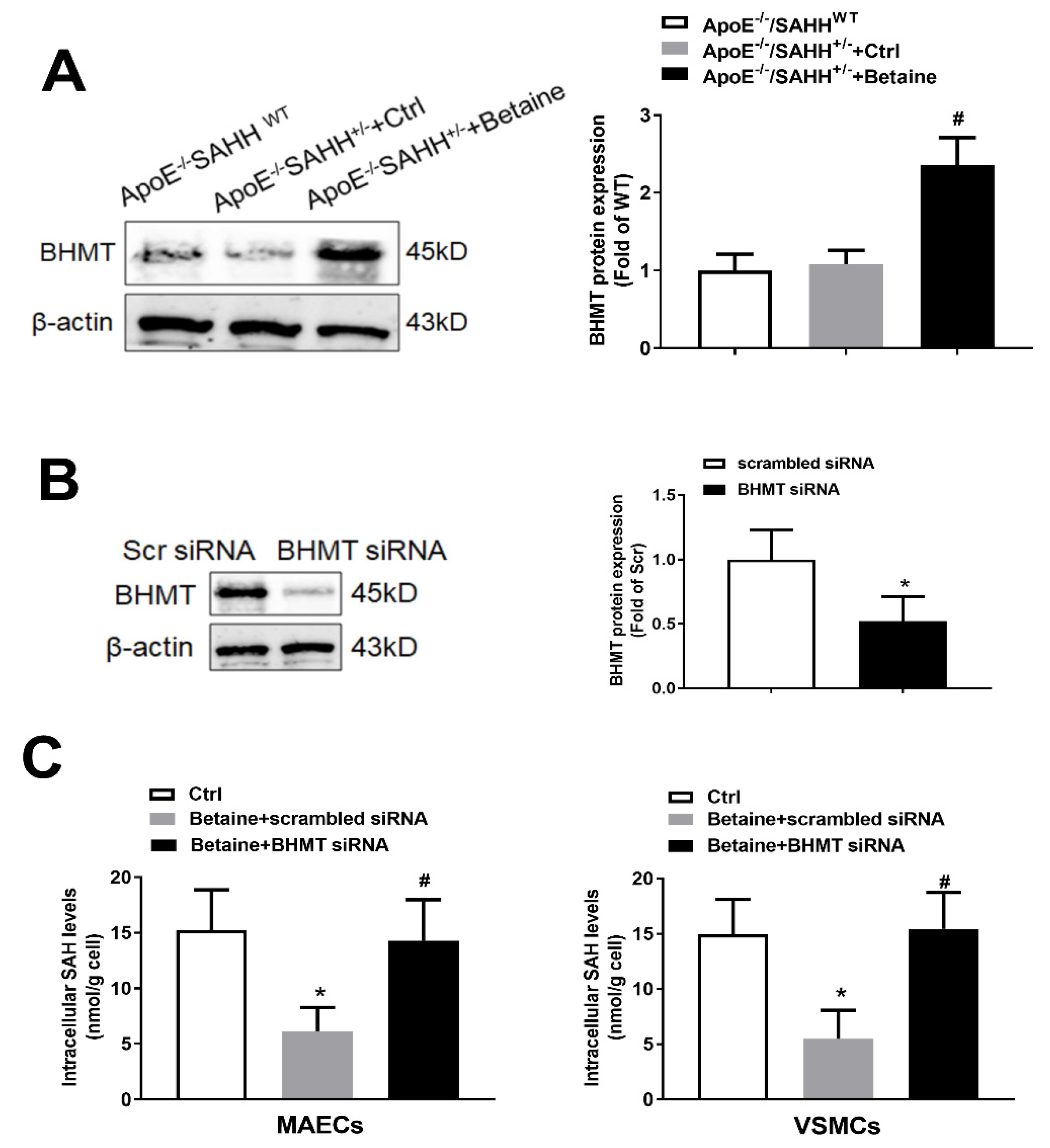
3.4. Betaine Supplementation Lowered SAHH-Deficiency-Accumulated SAH through Increasing Expression of BHMT
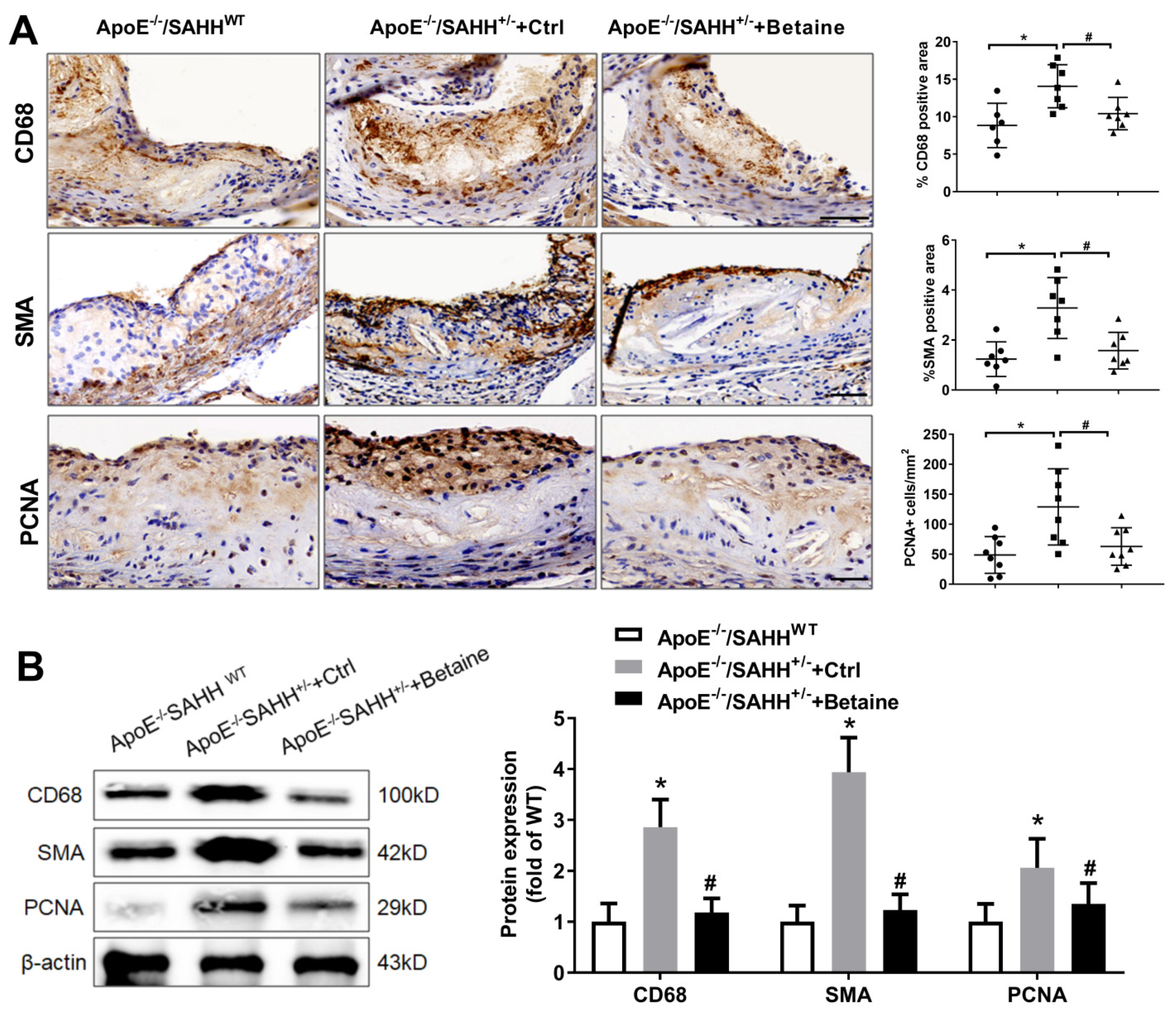
3.5. Quantitative and Phenotypic Differences in Atherosclerotic Plaque in ApoE−/−/SAHH+/− Mice with or without Betaine Supplementation
3.6. Betaine Supplementation Attenuated the Levels of Inflammatory Markers in ApoE−/−/SAHH+/− Mice
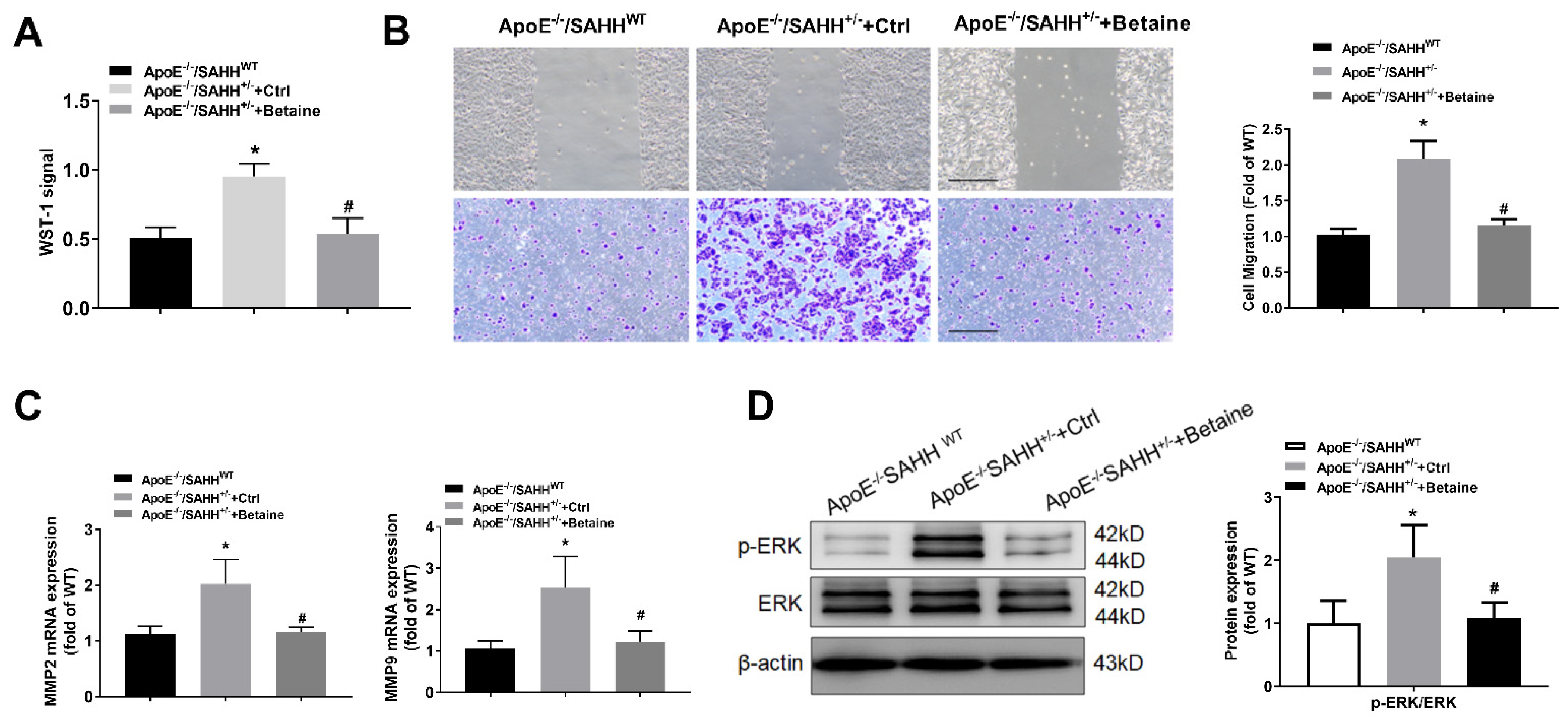
3.7. Betaine Supplementation Inhibited the Proliferation and Migration of VSMCs Isolated from Aortas of ApoE−/−/SAHH+/− Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Evison, B.J.; Actis, M.L.; Wu, S.Z.; Shao, Y.; Heath, R.J.; Yang, L.; Fujii, N. A site-selective, irreversible inhibitor of the DNA replication auxiliary factor proliferating cell nuclear antigen (PCNA). Bioorg. Med. Chem. 2014, 22, 6333–6343. [Google Scholar] [CrossRef]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xiang, Y.; Zhao, X.; Cai, C.; Chen, H.; Jiang, W.; Wang, Y.; Zeng, C. Thymoquinone suppresses platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferation, migration and neointimal formation. J. Cell Mol. Med. 2019, 23, 8482–8492. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Liu, J.; Zhao, J.; Mao, J.; Zhang, X.; Feng, L.; Han, C.; Li, M.; Wang, S.; Wu, D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014, 236, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Wang, H.M.; Lv, Y.Z.; Wang, Z.Z.; Xiao, W. Anti-atherosclerotic effect of Longxuetongluo Capsule in high cholesterol diet induced atherosclerosis model rats. Biomed. Pharmacother. 2018, 97, 793–801. [Google Scholar] [CrossRef]
- Loehrer, F.M.; Tschopl, M.; Angst, C.P.; Litynski, P.; Jager, K.; Fowler, B.; Haefeli, W.E. Disturbed ratio of erythrocyte and plasma S-adenosylmethionine/S-adenosylhomocysteine in peripheral arterial occlusive disease. Atherosclerosis 2001, 154, 147–154. [Google Scholar] [CrossRef]
- Sipkens, J.A.; Hahn, N.E.; Blom, H.J.; Lougheed, S.M.; Stehouwer, C.D.; Rauwerda, J.A.; Krijnen, P.A.; van Hinsbergh, V.W.; Niessen, H.W. S-Adenosylhomocysteine induces apoptosis and phosphatidylserine exposure in endothelial cells independent of homocysteine. Atherosclerosis 2012, 221, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Hummel, B.; Berg, J.T.; Friedrich, A.; Roth, H.J.; Obeid, R.; Geisel, J.; Fliser, D.; Heine, G.H. S-adenosylhomocysteine is associated with subclinical atherosclerosis and renal function in a cardiovascular low-risk population. Atherosclerosis 2014, 234, 17–22. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; Tavares de Almeida, I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Q.; Guo, H.; Xia, M.; Yuan, Q.; Hu, Y.; Zhu, H.; Hou, M.; Ma, J.; Tang, Z.; et al. Plasma S-adenosylhomocysteine is a better biomarker of atherosclerosis than homocysteine in apolipoprotein E-deficient mice fed high dietary methionine. J. Nutr. 2008, 138, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Homocysteine trials--clear outcomes for complex reasons. N. Engl. J. Med. 2006, 354, 1629–1632. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [PubMed]
- Ebbing, M.; Bleie, O.; Ueland, P.M.; Nordrehaug, J.E.; Nilsen, D.W.; Vollset, S.E.; Refsum, H.; Pedersen, E.K.; Nygard, O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: A randomized controlled trial. JAMA 2008, 300, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Smulders, Y.M.; Teerlink, T.; Struys, E.A.; de Meer, K.; Kostense, P.J.; Jakobs, C.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; et al. S-adenosylhomocysteine and the ratio of S-adenosylmethionine to S-adenosylhomocysteine are not related to folate, cobalamin and vitamin B6 concentrations. Eur. J. Clin. Invest. 2003, 33, 17–25. [Google Scholar] [CrossRef]
- Green, T.J.; Skeaff, C.M.; McMahon, J.A.; Venn, B.J.; Williams, S.M.; Devlin, A.M.; Innis, S.M. Homocysteine-lowering vitamins do not lower plasma S-adenosylhomocysteine in older people with elevated homocysteine concentrations. Br. J. Nutr. 2010, 103, 1629–1634. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Rosas-Rodriguez, J.A.; Valenzuela-Soto, E.M. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: Insights into disease and dysfunction networks. Life Sci. 2021, 285, 119943. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Mailliard, M.E.; Kharbanda, K.K.; Tuma, D.J. Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J. Nutr. 2003, 133, 2845–2848. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Fan, R.; Du, Y.; Hou, M.; Tang, Z.; Ling, W.; Zhu, H. Betaine supplementation attenuates atherosclerotic lesion in apolipoprotein E-deficient mice. Eur. J. Nutr. 2009, 48, 205–212. [Google Scholar] [CrossRef] [PubMed]
- van Lee, L.; Tint, M.T.; Aris, I.M.; Quah, P.L.; Fortier, M.V.; Lee, Y.S.; Yap, F.K.; Saw, S.M.; Godfrey, K.M.; Gluckman, P.D.; et al. Prospective associations of maternal betaine status with offspring weight and body composition at birth: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am. J. Clin. Nutr. 2016, 104, 1327–1333. [Google Scholar] [CrossRef]
- Joselit, Y.; Nanobashvili, K.; Jack-Roberts, C.; Greenwald, E.; Malysheva, O.V.; Caudill, M.A.; Saxena, A.; Jiang, X. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr. Diabetes 2018, 8, 41. [Google Scholar] [CrossRef]
- Ribo, S.; Sanchez-Infantes, D.; Martinez-Guino, L.; Garcia-Mantrana, I.; Ramon-Krauel, M.; Tondo, M.; Arning, E.; Nofrarias, M.; Osorio-Conles, O.; Fernandez-Perez, A.; et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci. Transl. Med. 2021, 13, eabb0322. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, J.; Cheng, J.; Huang, H.; Zhou, Y.; Yang, X.; Su, X.; Ke, Y.; Ling, W. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Dysfunction via Epigenetic Regulation of p66shc-Mediated Oxidative Stress Pathway. Circulation 2019, 139, 2260–2277. [Google Scholar] [CrossRef] [PubMed]
- Ubbink, J.B.; Hayward Vermaak, W.J.; Bissbort, S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J. Chromatogr. 1991, 565, 441–446. [Google Scholar] [CrossRef]
- Gellekink, H.; van Oppenraaij-Emmerzaal, D.; van Rooij, A.; Struys, E.A.; den Heijer, M.; Blom, H.J. Stable-isotope dilution liquid chromatography-electrospray injection tandem mass spectrometry method for fast, selective measurement of S-adenosylmethionine and S-adenosylhomocysteine in plasma. Clin. Chem. 2005, 51, 1487–1492. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Wang, M.; Li, X.; Su, D.; Qiu, J.; Li, D.; Yang, Y.; Xia, M.; Ling, W. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: A cohort study. Am. J. Clin. Nutr. 2013, 98, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, W.; Zhang, J.; Peng, C.; Xia, M.; Ling, W. Increased Plasma S-Adenosylhomocysteine-Accelerated Atherosclerosis Is Associated With Epigenetic Regulation of Endoplasmic Reticulum Stress in apoE−/− Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiao, Y.; Song, F.; Yang, Y.; Xia, M.; Ling, W. Increased plasma S-adenosyl-homocysteine levels induce the proliferation and migration of VSMCs through an oxidative stress-ERK1/2 pathway in apoE(−/−) mice. Cardiovasc. Res. 2012, 95, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, R.; Dai, X.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Association between plasma S-adenosylmethionine and risk of mortality in patients with coronary artery disease: A cohort study. Am. J. Clin. Nutr. 2021, 114, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Kerins, D.M.; Koury, M.J.; Capdevila, A.; Rana, S.; Wagner, C. Plasma S-adenosylhomocysteine is a more sensitive indicator of cardiovascular disease than plasma homocysteine. Am. J. Clin. Nutr. 2001, 74, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Wong, P.W.K.; Malinow, M.R. Hyperhomocyst(e)inemia as a Risk Factor for Occlusive Vascular Disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Tapani, K.; Nissinen, K.; Saarela, J.; Aro, A. The effect of low doses of betaine on plasma homocysteine in healthy volunteers. Br. J. Nutr. 2004, 92, 665–669. [Google Scholar] [CrossRef][Green Version]
- Schwab, U.; Alfthan, G.; Aro, A.; Uusitupa, M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur. J. Clin. Nutr. 2011, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef]
- Clarke, R.; Daly, L.; Robinson, K.; Naughten, E.; Cahalane, S.; Fowler, B.; Graham, I. Hyperhomocysteinemia: An independent risk factor for vascular disease. N. Engl. J. Med. 1991, 324, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.M.; Daly, L.E.; Refsum, H.M.; Robinson, K.; Brattstrom, L.E.; Ueland, P.M.; Palma-Reis, R.J.; Boers, G.H.; Sheahan, R.G.; Israelsson, B.; et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA 1997, 277, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine and vascular disease. Nat. Med. 1996, 2, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Moon, M.K.; Shin, H.; Kim, T.H.; Cho, B.J.; Kim, M.; Park, H.S.; Choi, S.H.; Ko, S.H.; Chung, M.H.; et al. Effect of S-adenosylmethionine on neointimal formation after balloon injury in obese diabetic rats. Cardiovasc. Res. 2011, 90, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, S.W.; Kim, M.O.; Kim, H.S.; Jang, J.E.; Leem, J.; Park, I.S.; Lee, K.U.; Koh, E.H. S-adenosyl methionine prevents endothelial dysfunction by inducing heme oxygenase-1 in vascular endothelial cells. Mol. Cells 2013, 36, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Kao, D.; Blom, H.J.; Tavares de Almeida, I.; Castro, R.; Loscalzo, J.; Handy, D.E. S-adenosylhomocysteine induces inflammation through NFkB: A possible role for EZH2 in endothelial cell activation. Biochim. Biophys. Acta 2016, 1862, 82–92. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | ApoE−/−/SAHHWT a (n = 10) | ApoE−/−/SAHH+/− b (n = 10) | ApoE−/−/SAHH+/− + Betaine (n = 10) |
|---|---|---|---|
| Cornstarch | 397.486 | 397.486 | 397.486 |
| Casein | 200 | 200 | 200 |
| Dextrinized cornstarch | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 |
| Soybean oil | 70 | 70 | 70 |
| Fiber | 50 | 50 | 50 |
| Mineral mix (AIN-93G-MX) * | 35 | 35 | 35 |
| Vitamin mix (AIN-93G-VX) * | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Betaine | — | — | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice. Nutrients 2022, 14, 718. https://doi.org/10.3390/nu14030718
Dai X, Liu S, Cheng L, Huang T, Guo H, Wang D, Xia M, Ling W, Xiao Y. Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice. Nutrients. 2022; 14(3):718. https://doi.org/10.3390/nu14030718
Chicago/Turabian StyleDai, Xin, Si Liu, Lokyu Cheng, Ting Huang, Honghui Guo, Dongliang Wang, Min Xia, Wenhua Ling, and Yunjun Xiao. 2022. "Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice" Nutrients 14, no. 3: 718. https://doi.org/10.3390/nu14030718
APA StyleDai, X., Liu, S., Cheng, L., Huang, T., Guo, H., Wang, D., Xia, M., Ling, W., & Xiao, Y. (2022). Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice. Nutrients, 14(3), 718. https://doi.org/10.3390/nu14030718








