Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production
Abstract
:1. Introduction
1.1. Algae Biomass-Based Biohydrogen Production
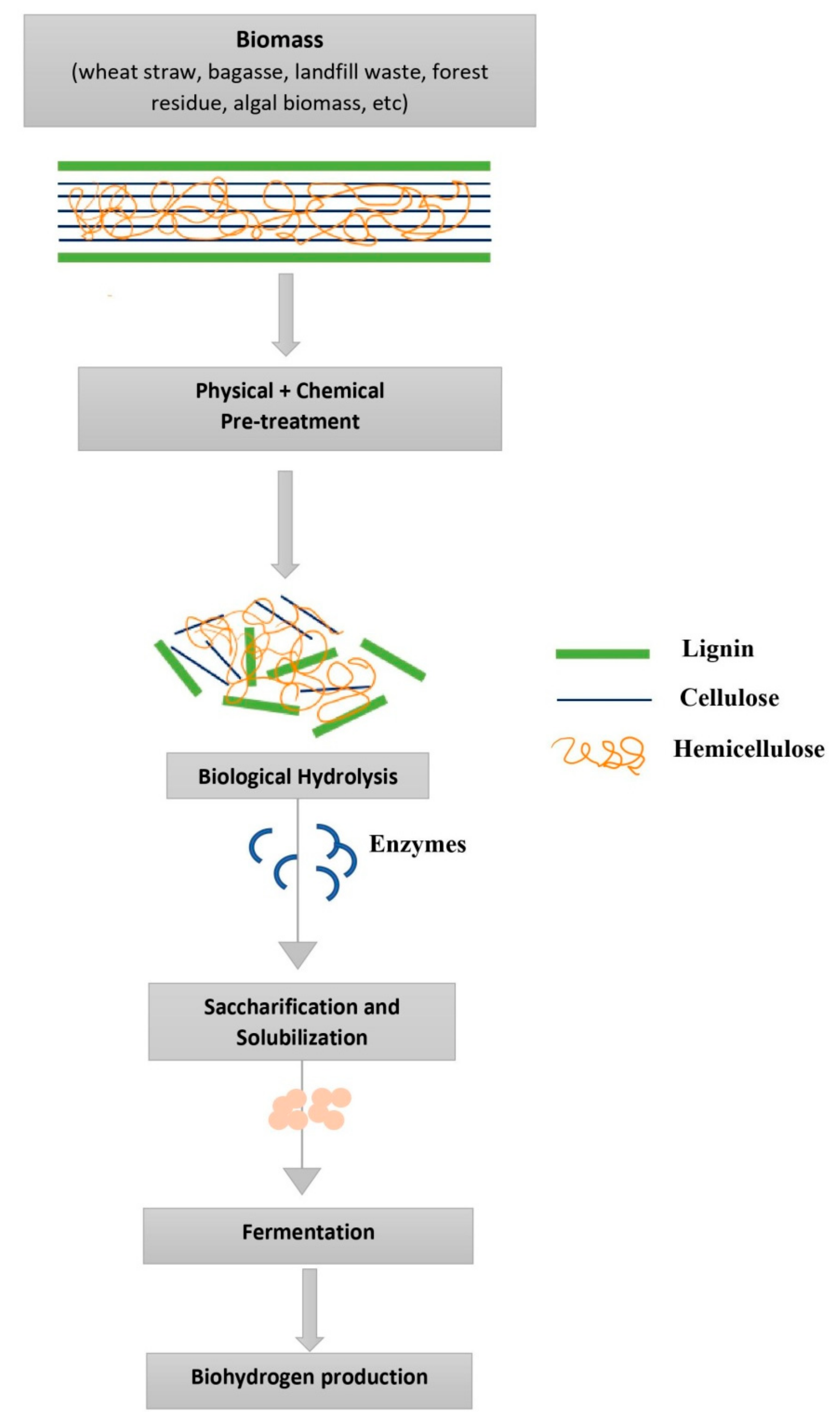
1.2. Substrates for Biohydrogen Production and Their Pretreatment
1.3. Inoculum Pretreatment
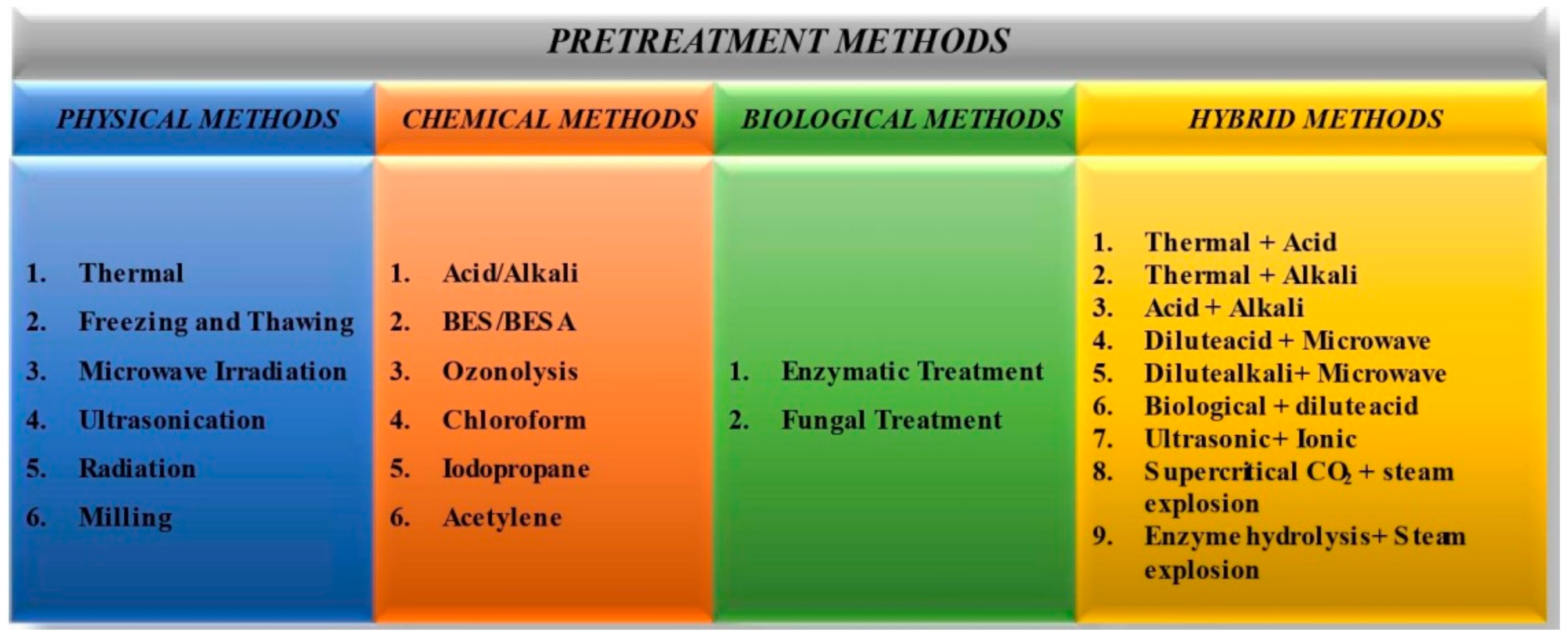
2. Pretreatment Technologies
2.1. Physical
2.1.1. Thermal
2.1.2. Freezing and Thawing
2.1.3. Microwave Radiation
2.1.4. Ultrasonication
2.1.5. Radiation
2.1.6. Milling
2.2. Chemical Pretreatment Technologies
2.2.1. Acid and Alkali Pretreatment
2.2.2. BES and BESA
2.2.3. Ozonolysis
2.2.4. Chloroform
2.2.5. Iodopropane
2.2.6. Acetylene
2.3. Biological Technologies
2.3.1. Hybrid Technologies
2.3.2. Combined Technologies for Substrates and Inoculum
2.4. Other Advanced Pretreatment Methods
2.4.1. Hydrothermal Carbonization (HTC)
2.4.2. Supercritical Fluid
2.4.3. Ammonia Fiber Explosion and Ammonia Pretreatment
2.4.4. Ionic Liquid-Based Pretreatment
2.4.5. Low-Temperature Steep Delignification (LTSD)
2.4.6. Co-Solvent Enhanced Lignocellulosic Fractionation (CELF)
3. New Approaches to Biomass-Based Biohydrogen Production
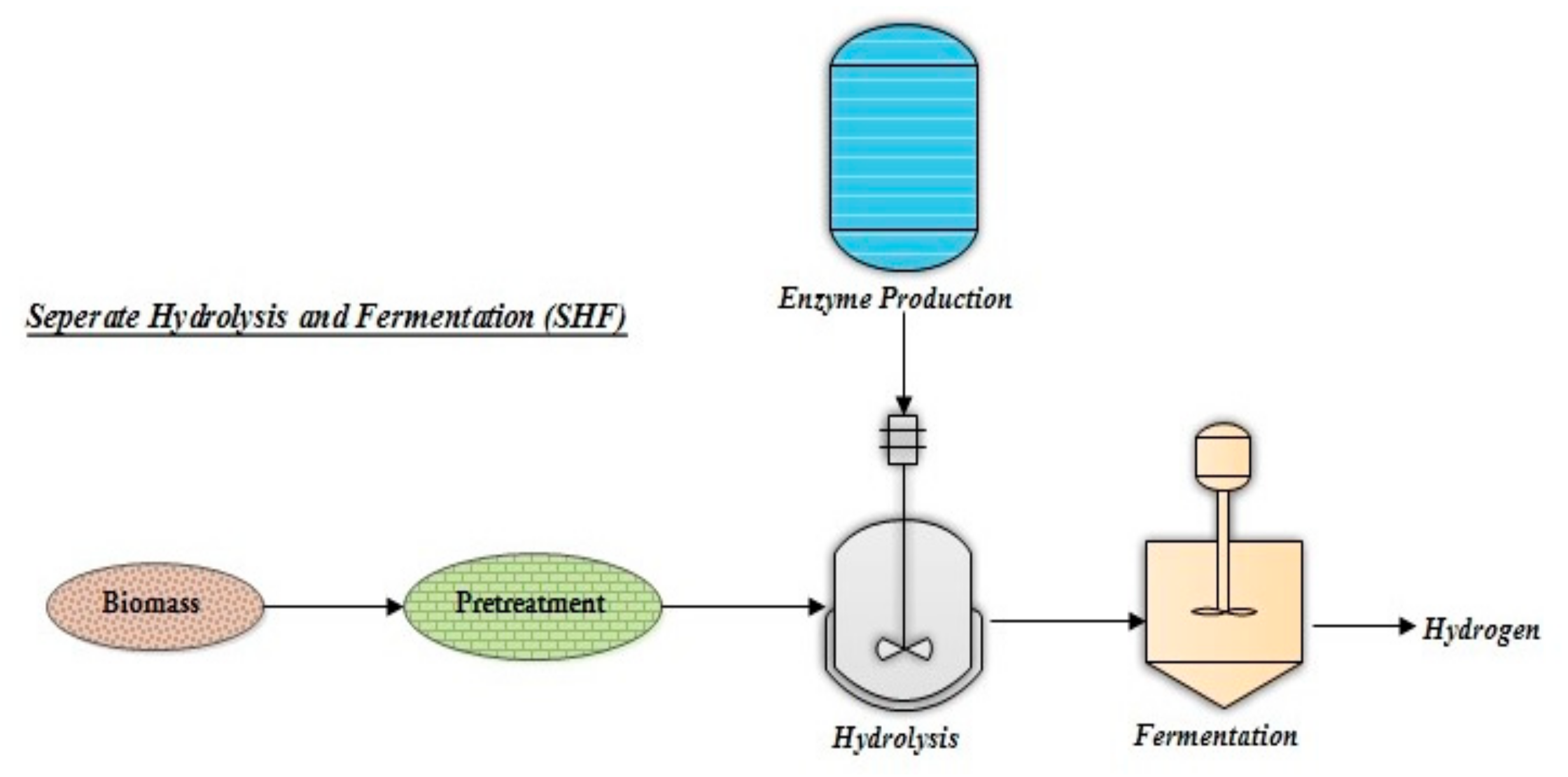
3.1. Separate Hydrolysis and Fermentation (SHF)
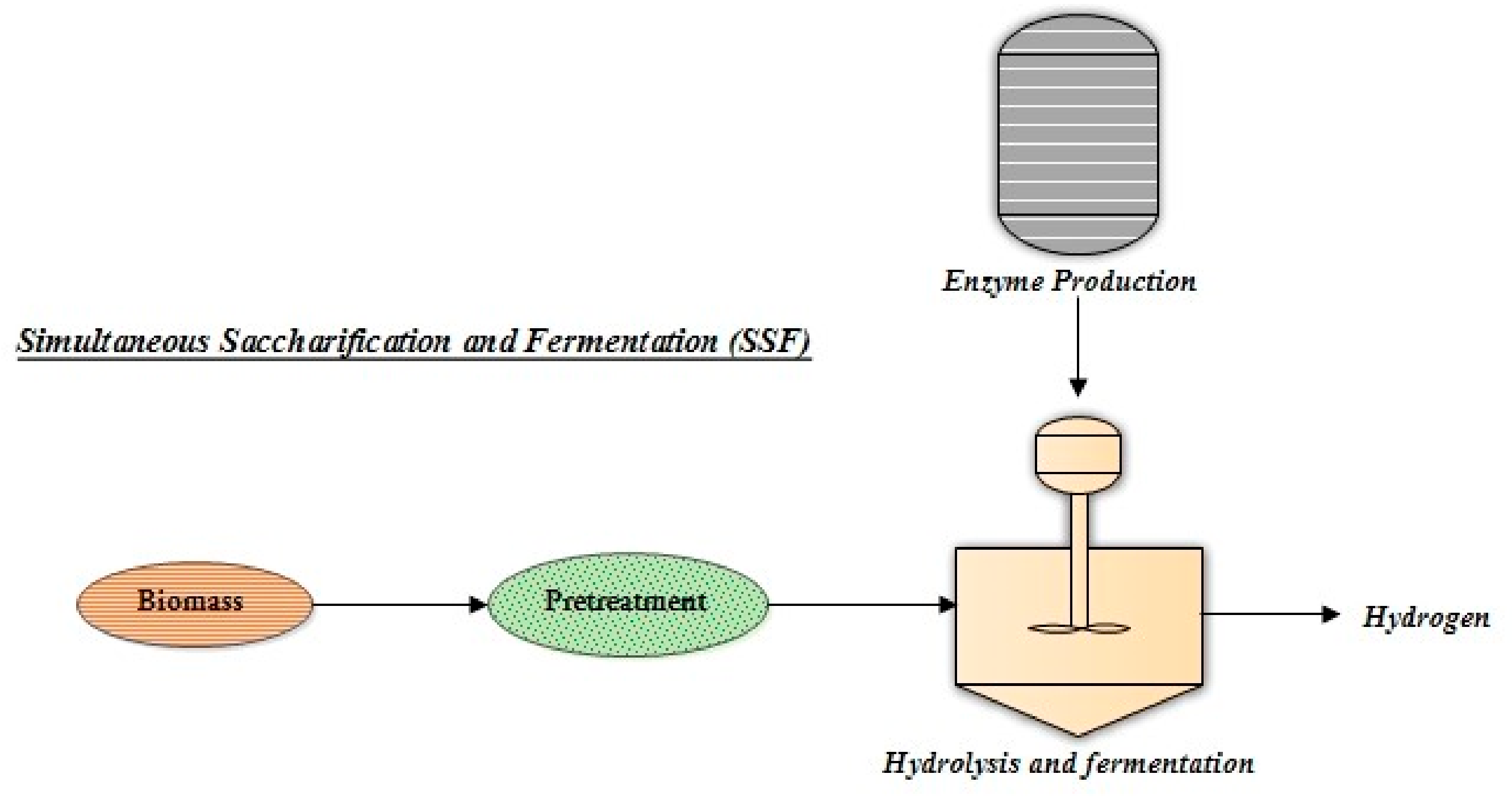
3.2. Simultaneous Saccharification and Fermentation (SSF)
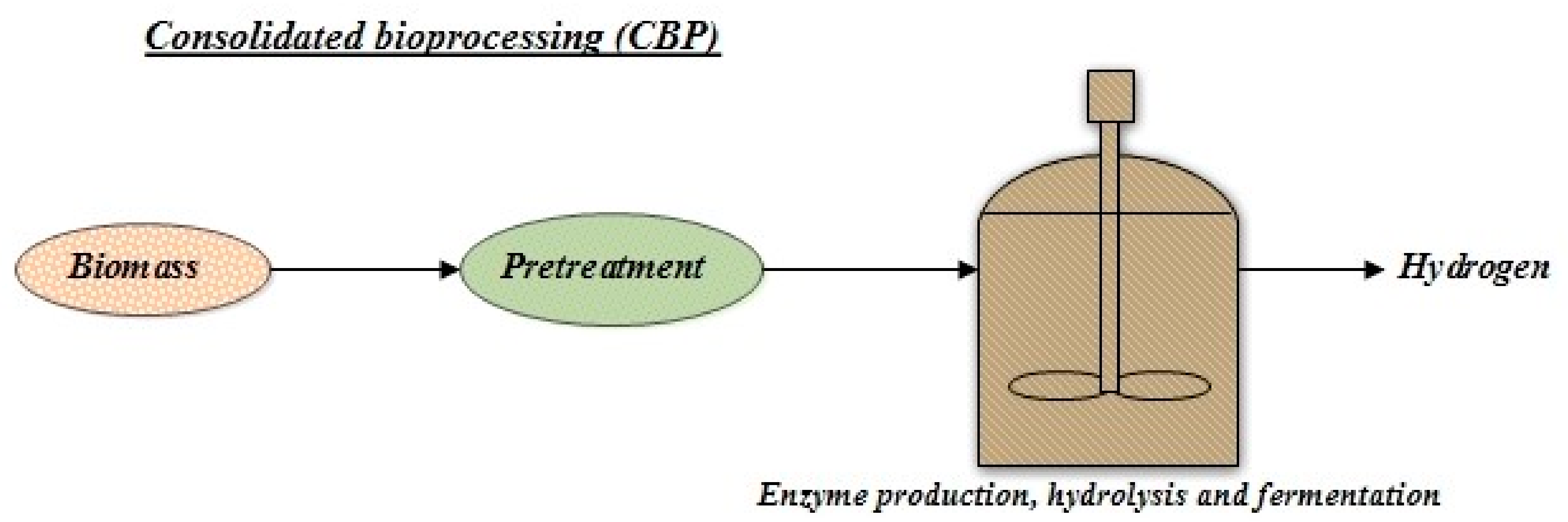
3.3. Consolidated Bioprocessing (CBP)
3.4. Integrated Strategy for Alternative Bioenergy Resources in Addition to H2
3.4.1. Co-Production of Bio Alcohols and VFAs
3.4.2. Co-Production of Methane and Hydrogen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rai, P.K.; Singh, H.; Mueed, Z.; Kumar, M.; Kumar, A. A Status Report on Biohydrogen Production from Algae. Invertis J. Sci. Technol. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Trchounian, K.; Trchounian, A. Hydrogen production from glycerol by Escherichia coli and other bacteria: An overview and perspectives. Appl. Energy 2015, 156, 174–184. [Google Scholar] [CrossRef]
- Rai, P.K. Hydrogen Production from Dairy and Agro Wastes by Integrating Dark-and Photo-Fermentation. Doctoral Dissertation, Banaras Hindu University, Varanasi, India, 2013. [Google Scholar]
- Eroglu, E.; Gunduz, U.; Yucel, M.; Turker, L.; Eroglu, I. Photobiological hydrogen production from olive mill wastewater as sole substrate sources. Int. J. Hydrogen Energy 2004, 29, 163–171. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Kim, K.R.; Nguyen, M.T.; Kim, M.S.; Kim, D.; Sim, S.J. Enhancement of fermentative hydrogen production from green algal biomass of Thermotoganeapolitanaby various pretreatment methods. Int. J. Hydrogen Energy 2010, 35, 13035–13040. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Hydrogen generation in photobiological process from dairy waste water. Int. J. Hydrogen Energy 2010, 35, 9624–9629. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, A.; Liu, Y.; Lin, R.; Zhou, J.; Cen, K. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrogen Energy 2012, 37, 13330–13337. [Google Scholar] [CrossRef]
- Ho, K.-L.; Lee, D.-J.; Su, A.; Chang, J.-S. Biohydrogen from lignocellulosic feedstock via one-step process. Int. J. Hydrogen Energy 2012, 37, 15569–15574. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K. Biohydrogen Production from Cheese Whey Wastewater in a Two-Step Anaerobic Process. Appl. Biochem. Biotechnol. 2012, 167, 1540–1549. [Google Scholar] [CrossRef]
- Rai, P.K.; Asthana, R.K.; Singh, S.P. Optimization of photo-hydrogen production based on cheese whey spent medium. Int. J. Hydrogen Energy 2014, 39, 7597–7603. [Google Scholar] [CrossRef]
- Chaitanya, N.; Sivaramakrishna, D.; Kumar, B.S.; Himabindu, V.; Lakshminarasu, M.; Vishwanadham, M. Selection of pretreatment method for enriching hydrogen-producing bacteria using anaerobic sewage sludge with three different substrates. Biofuels 2016, 7, 163–171. [Google Scholar] [CrossRef]
- Rai, P.K.; Kadier, A.; Kumar, M.; Singh, S.P. Utilization of Microalgal Biomass as a Source of Bioenergy. In Role of Photosynthetic Microbes in Agriculture and Industry; Tripathi, K., Kumar, N., Abraham, G., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018; ISBN 978-153-614-033-0. [Google Scholar]
- Rashidi, B.; Dechesne, A.; Rydahl, M.G.; Jorgensen, B.; Trindade, L.M. Neochlorisoleoabundans cell walls have an altered composition when cultivated under different growing conditions. Algal Res. 2019, 40, 101482. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, C.; Sun, Y.; Chen, W.; Tan, H.; Cao, X.; Xue, S.; Yin, H. Production and structural characterization of a new type of polysaccharide from nitrogen-limited Arthrospira platensis cultivated in outdoor industrial-scale open raceway ponds. Biotechnol. Biofuels 2019, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E. Biomass ethanol: Technical progress, opportunities, and commercial challenges. Ann. Rev. Energy Environ. 1999, 24, 189–226. [Google Scholar] [CrossRef] [Green Version]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Rai, P.K. Recent Advances in substrate utilization for fermentative hydrogen Production. J. Appl. Biol. Biotechnol. 2016, 4, 59–67. [Google Scholar] [CrossRef]
- Mohan, S.V.; Babu, V.L.; Sarma, P.N. Anaerobic biohydrogen production from dairy waste water treatment in sequencing batch reactor (AnSBR): Effect of organic loading rate. Enzyme Microb. Technol. 2007, 41, 506–515. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K. Prospects of utilizing dairy waste for biohydrogen production. Int. J. Biotech. Biosci. 2011, 1, 263–270. [Google Scholar]
- Rossi, D.M.; Da Costa, J.B.; De Souza, E.A.; Peralba, M.d.C.R.; Samios, D.; Ayub, M.A.Z. Comparison of different pre-treatment methods for hydrogen production using environmental microbial consortia on residual glycerol from biodiesel. Int. J. Hydrogen Energy 2011, 36, 4814–4819. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Venkata Mohan, S. Fermentative hydrogen production with simultaneous wastewater treatment: Influence of pretreatment and system operating conditions. J. Sci. Ind. Res. 2008, 67, 950–961. [Google Scholar]
- Yetis, M.; Gunduz, U.; Eroglu, I.; Yucel, M.; Turker, L. Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides OU 001. Int. J. Hydrogen Energy. 2000, 25, 1035–1041. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P. Integrated dark- and photo-fermentation: Recent advances and provisions for improvement. Int. J. Hydrogen Energy 2016, 41, 19957–19971. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P. Biological production of clean energy: Hydrogen. In Advances in Microbiology; Tiwari, S.P., Rajesh Sharm, R., Rajeeva Gaur, R., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2013; Volume 2, pp. 55–83. ISBN 978-1-62808-633-1. [Google Scholar]
- Wang, Y.Y.; Zhang, Y.L.; Wang, J.B.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Xie, B.F.; Cheng, J.; Zhou, J.H.; Song, W.L.; Liu, J.Z.; Cen, K.F. Production of hydrogen and methane from potatoes by two-phase anaerobic fermentation. Bioresour. Technol. 2007, 99, 5942–5946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Beland, M. Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int. J. Hydrogen Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S. Pretreatment of methanogenic granules for immobilized hydrogen fermentation. Int. J. Hydrogen Energy 2007, 32, 3266–3273. [Google Scholar] [CrossRef]
- Luste, S.; Luostarinen, S.; Sillanpää, M. Effect of pre-treatments on hydrolysis and methane production potentials of by-products from meat-processing industry. Hazard. Mater. 2009, 164, 247–255. [Google Scholar] [CrossRef]
- Haridoss, S. Studies on biohydrogen production from rice mill waste water using Enterobacter aerogenes MTCC 2822 by Dark Fermentation Process. J. Pet Environ. Biotechnol. 2016, 7, 312. [Google Scholar]
- Ozkan, L.; Erguder, T.H.; Demirer, G.N. Effects of pretreatment methods on solubilization of beet-pulp and bio-hydrogen production yield. Int. J. Hydrogen Energy 2011, 36, 382–389. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.M.; Abdul, P.M. Pretreatment conditions of palm oil mill effluent (POME) for thermophilic biohydrogen production by mixed culture. Int. J. Hydrogen Energy 2017, 42, 27512–27522. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Ozone pretreatment of biomethanated distillery wastewater in a semi batch reactor: Mapping pretreatment efficiency in terms of COD, color, toxicity and biohydrogen generation. Biofuels 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Leano, E.P.; Babel, S. Effects of pretreatment methods on cassava wastewater for biohydrogen production optimization. Renew. Energy 2012, 39, 339–346. [Google Scholar] [CrossRef]
- Ramprakash, B.; Muthukumar, K. Comparative study on the performance of various pretreatment and hydrolysis methods for the production of biohydrogen using Enterobacter aerogenes RM 08 from rice mill wastewater. Int. J. Hydrogen Energy 2015, 40, 9106–9112. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lay, C.-H.; Sen, B.; Chu, C.-Y.; Kumar, G.; Chen, C.-C.; Chang, J.-S. Fermentative hydrogen production from wastewaters: A review and prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Chaganti, S.R.; Kim, D.-H.; Lalman, J.A. Dark fermentative hydrogen production by mixed anaerobic cultures: Effect of inoculum treatment methods on hydrogen yield. Renew. Energy 2012, 48, 117–121. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestothy, N.; Simon, V.; Bélafi-Bakó, K. Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew. Sustain. Energy Rev. 2014, 40, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Sivagurunathan, P.; Anburajan, P.; Kumar, G.; Arivalagan, P.; Bakonyi, P.; Kim, S.-H. Improvement of hydrogen fermentation of galactose by combined inoculation strategy. J. Biosci. Bioeng. 2017, 123, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gong, Y.; Liu, S.; Wang, D.; Liu, R.; Zhou, X.; Nghiem, L.D.; Zhao, Y. Free Ammonia Pretreatment To Improve Bio-hydrogen Production from Anaerobic Dark Fermentation of Microalgae. ACS Sustain. Chem. Eng. 2019, 7, 1642–1647. [Google Scholar] [CrossRef]
- Dixon, C.; Wilken, L.R. Green microalgae biomolecule separations and recovery. Bioresour. Bioprocess. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Kalichevsky, M.T.; Knorr, D.; Lillford, P.J. Potential food applications of high-pressure effects on ice-water transitions. Trends Food Sci. Technol. 1995, 6, 253–258. [Google Scholar] [CrossRef]
- Martino, M.N.; Otero, L.; Sanz, P.D.; Zaritzky, N.E. Size and location of ice crystals in pork frozen by high-pressure-assisted freezing as compared to classical methods. Meat Sci. 1998, 50, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Otero, L.; Martino, M.; Zaritzky, N.; Solas, M.; Sanz, P. Preservation of Microstructure in Peach and Mango during High-pressure-shift Freezing. J. Food Sci. 2000, 65, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Makita, T. Application of high pressure and thermophysical properties of water to biotechnology. Fluid Phase Equilibria 1992, 76, 87–95. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fores, R.A.; Olson, D.G. The action of high hydrostatic pressure on the thawing of frozen meat. In Proceedings of the Annual Meeting of Institute of Food Technologist, New Orleans, LA, USA, June 1996; pp. 22–26. [Google Scholar]
- Jan, T.-W.; Adav, S.S.; Lee, D.J.; Wu, R.M.; Su, A.; Tay, J.-H. Hydrogen Fermentation and Methane Production from Sludge with Pretreatments. Energy Fuels 2007, 22, 98–102. [Google Scholar] [CrossRef]
- Hu, Z.; Wan, Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Hong, S.M.; Park, J.K.; Lee, Y. Mechanisms of microwave irradiation involved in the destruction of fecal coliforms from biosolids. Water Res. 2004, 38, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Thungklin, P.; Reungsang, A.; Sittijunda, S. Hydrogen production from sludge treatment strategy for enhancement of biohydrogen production from complex ultrasonically treated waste-activated sludge. Water Res. 2011, 35, 1038–1046. [Google Scholar]
- Chu, C.; Chang, B.-V.; Liao, G.; Jean, D.; Lee, D. Observations on changes in ultrasonically treated waste-activated sludge. Water Res. 2001, 35, 1038–1046. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Evaluation of ultrasonication as a treatment strategy for enhancement of biohydrogen production from complex distillery wastewater and process optimization. Int. J. Hydrogen Energy 2014, 39, 10041–10050. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Wang, J. Enriching hydrogen-producing bacteria from digested sludge by different pretreatment methods. Int. J. Hydrogen Energy 2014, 39, 13550–13556. [Google Scholar] [CrossRef]
- Fan, Y.-T.; Zhang, Y.-H.; Zhang, S.-F.; Hou, H.-W.; Ren, B. Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Bioresour. Technol. 2006, 97, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Chang, V.S.; Burr, B.; Holtzapple, M.T. Lime pretreatment of switchgrass. Appl. Biochem. Biotechnol. 1997, 63–65, 3–19. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, L.; Chen, H.; Zhao, Y. Co-inhibition of methanogens for methane mitigation in biodegradable wastes. J. Environ. Sci. 2009, 21, 827–833. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, J.H.; Jeong, B.Y.; Lee, J.H. Comparison of milling modes as a pretreatment method for cellulosic biofuel production. J. Clean Energy Technol. 2013, 1, 45–48. [Google Scholar] [CrossRef]
- Zeng, M.; Mosier, N.S.; Huang, C.-P.; Sherman, D.M.; Ladisch, M.R. Microscopic examination of changes of plant cell structure in corn stover due to hot water pretreatment and enzymatic hydrolysis. Biotechnol. Bioeng. 2007, 97, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef]
- Mu, Y.; Yu, H.-Q.; Wang, G. Evaluation of three methods for enriching H2-producing cultures from anaerobic sludge. Enzyme Microb. Technol. 2007, 40, 947–953. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M.; Law, S. Effects of different pretreatment methods on anaerobic mixed microflora for hydrogen production and COD reduction from palm oil mill effluent. J. Clean. Prod. 2011, 19, 1654–1658. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M. Comparative study on the effect of various pretreatment methods on the enrichment of hydrogen producing bacteria in anaerobic granulated sludge from brewery wastewater. Korean J. Chem. Eng. 2012, 29, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Song, J.-H.; Hwang, S.-J. Effects of acid pre-treatment on bio-hydrogen production and microbial communities during dark fermentation. Bioresour. Technol. 2009, 100, 1491–1493. [Google Scholar] [CrossRef]
- Pita, F.; Perez, M. Pretreatment Application of Mixed Sewage Sludge on Self fermentation for Biohydrogen Production; University of Cadiz: Cádiz, Spain, 2011. [Google Scholar]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pre-treatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pre-treatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, C.; Yang, M.; Zang, L. Comparison of Calcium Oxide and Calcium Peroxide Pre-treatments of Wheat Straw for Improving Biohydrogen Production. ACS Omega 2020, 5, 9151–9161. [Google Scholar] [CrossRef] [PubMed]
- Rorke, D.; Gueguim Kana, E.B. Biohydrogen process development on waste sorghum (Sorghum bicolor) leaves: Optimization of saccharification, hydrogen production and preliminary scale up. Int. J. Hydrogen Energy 2016, 41, 12941–12952. [Google Scholar] [CrossRef]
- Bouwer, E.J.; McCarty, P.L. Effects of 2-Bromoethanesulfonic Acid and 2-Chloroethanesulfonic Acid on Acetate Utilization in a Continuous-Flow Methanogenic Fixed-Film Column. Appl. Environ. Microbiol. 1983, 45, 1408–1410. [Google Scholar] [CrossRef] [Green Version]
- Sparling, R.; Risbey, D.; Poggi-Varaldo, H.M. Hydrogen production from inhibited anaerobic composters. Int. J. Hydrogen Energy 1997, 22, 563–566. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chang, C.-W.; Chu, C.-P.; Lee, D.-J.; Chang, B.-V. Sequential production of hydrogen and methane from wastewater sludge using anaerobic fermentation. J. Chin. Inst. Chem. Eng. 2003, 34, 683–687. [Google Scholar]
- Kumar, G.; Zhen, G.; Sivagurunathan, P.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K.; Kobayashi, T.; Xu, K.-Q. Biogenic H2 production from mixed microalgae biomass: Impact of pH control and methanogenic inhibitor (BESA) addition. Biofuel Res. J. 2016, 3, 470–474. [Google Scholar] [CrossRef]
- Bule, M.V.; Gao, A.H.; Hiscox, B.; Chen, S. Structural Modification of Lignin and Characterization of Pretreated Wheat Straw by Ozonation. J. Agric. Food Chem. 2013, 61, 3916–3925. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Rubio, M.; Gómez, D. Ozonation of Lignin Rich Solid Fractions from Corn Stalks. J. Wood Chem. Technol. 1999, 19, 115–137. [Google Scholar] [CrossRef]
- Bellido, C.; Bolado, S.; Coca, M.; Lucas, S.; Gonzalez-Benito, G.G.; García-Cubero, M.T. Effect of inhibitors formed during wheat straw pretreatment on ethanol fermentation by Pichia stipitis. Bioresour. Technol. 2011, 102, 10868–10874. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Khanal, S.K. Biohydrogen production: Fundamentals, challenges, and operating strategies for enhanced yield. In Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Khanal, S.K., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 189–220. [Google Scholar]
- Ueki, K.; Ueki, A.; Simogoh, Y. Terminal steps in the anaerobic digestion of municipal sewage sludge: Effects of inhibitors of methanogenesis and sulfate reduction. J. Gen. Appl. Microbiol. 1988, 34, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Argun, H.; Kargi, F. Effects of sludge pre-treatment method on bio-hydrogen production by dark fermentation of waste ground wheat. Int. J. Hydrogen Energy 2009, 34, 8543–8548. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S. Biological Hydrogen Production Using Chloroform-treated Methanogenic Granules. Appl. Biochem. Biotechnol. 2008, 148, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chidthaisong, A.; Conrad, R. Specificity of chloroform, 2-bromoethanesulfonate and fluoroacetate to inhibit methanogenesis and other anaerobic processes in anoxic rice field soil. Soil Biol. Biochem. 2000, 32, 977–988. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrogen Energy 2008, 33, 2934–2941. [Google Scholar] [CrossRef]
- Luo, G.; Xie, L.; Zou, Z.; Wang, W.; Zhou, Q. Evaluation of pretreatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. Bioresour. Technol. 2010, 101, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, W.; Zeikus, J.G. Influence of corrinoid antagonists on methanogen metabolism. J. Bacteriol. 1981, 146, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Valdez-Vazquez, I.; Rios-Leal, E.; Munoz-Paez, K.M.; Carmona-Martinez, A.; Poggi-Varaldo, H.M. Effect of inhibition treatment, type of Inocula, and incubation temperature on batch H2 production from organic solid waste. Biotechnol. Bioeng. 2006, 95, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Vazquez, I.; Sparling, R.; Rinderknecht-Seijas, N.; Risbey, D.; Poggi-Varaldo, H.M. Hydrogen from the anaerobic fermentation of industrial solid waste. Biores. Technol. 2005, 96, 1907–1913. [Google Scholar] [CrossRef]
- Vats, S.; Maurya, D.P.; Shaimoon, M.; Agarwal, A.; Negi, S. Develpoment of a microbial consortium for production of blend of enzymes for hydrolysis of agricultural wastes into sugars. J. Sci. Ind. Res. 2013, 72, 585–590. [Google Scholar]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Cui, M.; Yuan, Z.; Zhi, X.; Wei, L.; Shen, J. Biohydrogen production from poplar leaves pretreated by different methods using anaerobic mixed bacteria. Int. J. Hydrogen Energy 2010, 35, 4041–4047. [Google Scholar] [CrossRef]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- Liu, C.-Z.; Cheng, X.-Y. Improved hydrogen production via thermophilic fermentation of corn stover by microwave-assisted acid pretreatment. Int. J. Hydrogen Energy 2010, 35, 8945–8952. [Google Scholar] [CrossRef]
- Assawamongkholsiri, T.; Reungsang, A.; Pattra, S. Effect of acid, heat and combined acid-heat pretreatments of anaerobic sludge on hydrogen production by anaerobic mixed cultures. Int. J. Hydrogen Energy 2013, 38, 6146–6153. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Yang, S.-S.; Guo, W.-Q.; Cao, G.-L.; Zheng, H.-S.; Ren, N.-Q. Simultaneous waste activated sludge disintegration and biological hydrogen production using an ozone/ultrasound pretreatment. Bioresour. Technol. 2012, 124, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zhou, S.; Shang, H.; Luo, J.; Tsang, D.C. Chapter 15-hydrothermal carbonization for hydrochar production and its application. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–294. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F.; DE LA Rubia, M.A. Valorization of microalgal biomass by hydrothermal carbonization and anaerobic digestion. Bioresour. Technol. 2019, 274, 395–402. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Román, S.; Ledesma, B.; Álvarez, A.; Coronella, C.; Qaramaleki, S.V. Suitability of hydrothermal carbonization to convert water hyacinth to added-value products. Renew. Energy 2020, 146, 1649–1658. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Kumar, J.; Bhaskar, T. Advanced hydrothermal liquefaction of biomass for bio-oil production. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Academic Press: Cambridge, MA, USA, 2019; pp. 245–266. [Google Scholar]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrol. 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Daza Serna, L.V.; OrregoAlzate, C.E.; Cardona Alzate, C.A. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Dias, A.L.B.; dos Santos, P.; Martínez, J. Supercritical CO2 technology applied to the production of flavor ester compounds through lipase-catalyzed reaction: A review. J. CO2 Util. 2018, 23, 159–178. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Liu, D.; Zhao, X. Pretreatment of lignocellulosic biomass for efficient enzymatic saccharification of cellulose. In Lignocellulosic Biomass to Liquid Biofuels; Academic Press: Cambridge, MA, USA, 2019; pp. 17–65. [Google Scholar] [CrossRef]
- Ferreira Santos, A.L.; Fausta Kawase, K.Y.; Vieira Coelho, G.L. Enzymatic saccharification of lignocellulosic materials after treatment with supercritical carbon dioxide. J. Supercrit. Fluids 2011, 56, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Rijal, B.; Biersbach, G.; Gibbons, W.R.; Pryor, S.W. Effect of initial particle size and densification on AFEX-pretreated biomass for ethanol production. Appl. Biochem. Biotechnol. 2014, 174, 845–854. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, S.; Gao, L.; Zhou, X.; Wang, D.; Song, K.; Nghiem, L.D. Free ammonia pretreatment improves anaerobic methane generation from algae. Water Res. 2019, 162, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Mokomele, T.; Da CostaSousa, L.; Balan, V.; van Rensburg, E.; Dale, B.E.; Görgens, J.F. Incorporating anaerobic co-digestion of steam exploded or ammonia fiber expansion pretreated sugarcane residues with manure into a sugarcane-based bioenergy-livestock nexus. Bioresour. Technol. 2019, 272, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar]
- Ozgur, E.; Mars, A.E.; Peksel, B.; Louwerse, A.; Yücel, M.; Gündüz, U.; Classen, P.A.M.; Eroğlu, I. Biohydrogen production from beet molasses by sequential dark and photofermentation. Int. J. Hydrogen Energy 2010, 35, 511–517. [Google Scholar] [CrossRef]
- Lay, C.-H.; Sen, B.; Chen, C.-C.; Wu, J.-H.; Lee, S.-C.; Lin, C.-Y. Co-fermentation of water hyacinth and beverage wastewater in powder and pellet form for hydrogen production. Bioresour. Technol. 2013, 135, 610–615. [Google Scholar] [CrossRef]
- Lari, Z.; Ahmadzadeh, H.; Hosseini, M. Chapter 2—Cell wall disruption: A critical upstream process for biofuel production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; Hosseini, M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 21–35. [Google Scholar]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Shi, J.; Murthy Konda, N.V.S.N.; Campos, D.; Liu, D.; Nemser, S.; Shamshina, J.; Dutta, T.; Berton, P.; Gurau, G.; et al. Efficient dehydration and recovery of ionic liquid after lignocellulosic processing using pervaporation. Biotechnol. Biofuels 2017, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Socha, A.M.; Parthasarathi, R.; Shi, J.; Pattathil, S.; Whyte, D.; Bergeron, M.; George, A.; Tran, K.; Stavila, V.; Venkatachalam, S.; et al. Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc. Natl. Acad. Sci. USA 2014, 111, E3587–E3595. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Shin, H.; Yoo, S.; Zoppe, J.O.; Park, S. Delignification of lignocellulosic biomass and its effect on subsequent enzymatic hydrolysis. BioResources 2015, 10, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Parikh, A.; Seemala, B.; Kumar, R.; Pu, Y.; Christopher, P.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Chemical Transformations of Poplar Lignin during Cosolvent Enhanced Lignocellulosic Fractionation Process. ACS Sustain. Chem. Eng. 2018, 6, 8711–8718. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Ren, N.-Q.; Zhao, L.; Chen, C.; Guo, W.-Q.; Cao, G.-L. A review on bioconversion of lignocellulosic biomass to H2: Key challenges and new insights. Bioresour. Technol. 2016, 215, 92–99. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.-L.; Wang, A.-J.; Ren, H.-Y.; Xu, C.-J.; Ren, N.-Q. Enzymatic saccharification of cornstalk by onsite cellulases produced by Trichoderma viride for enhanced biohydrogen production. GCB Bioenergy 2013, 5, 591–598. [Google Scholar] [CrossRef]
- Reginatto, V.; Antônio, R.V. Fermentative hydrogen production from agroindustrial lignocellulosic substrates. Braz. J. Microbiol. 2015, 46, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.F.; Abd-Aziz, S.; Yusoff, M.E.M.; Phang, L.Y.; Hassan, M.A. Simultaneous enzymatic saccharification and ABE fermentation using pretreated oil palm empty fruit bunch as substrate to produce butanol and hydrogen as biofuel. Renew. Energy 2015, 77, 447–455. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Recent insights into consolidated bioprocessing for lignocellulosic biohydrogen production. Int. J. Hydrogen Energy 2019, 44, 14362–14379. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Z.-Y.; Hao, M.; Zhang, Y.-F.; Qi, Q.-S. An isolated cellulolytic Escherichia coli from bovine rumen produces ethanol and hydrogen from corn straw. Biotechnol. Biofuels 2017, 10, 165. [Google Scholar] [CrossRef]
- Islam, S.; Guo, C.; Liu, C.-Z. Enhanced hydrogen and volatile fatty acid production from sweet sorghum stalks by two-steps dark fermentation with dilute acid treatment in between. Int. J. Hydrogen Energy 2018, 43, 659–666. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K.; Singh, S. Biohydrogen production from sugarcane bagasse by integrating dark- and photo-fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.-Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef] [PubMed]


| Substrates | H2 Yield | References |
|---|---|---|
| Olive mill wastewater | 13.9 L H2/L OMW | [4] |
| Chlamydomonas reinhardtii | 311.1 mL H2/g monosaccharides | [5] |
| Dairy Wastewater | 3.6 L H2/L dairy wastewater | [6] |
| Arthrospira platensis | 337.0 mL H2/g DW | [7] |
| Microalgae | 25.1 mL H2/g dry biomass powder | [8] |
| Cheese whey | 2.04 mol H2/mol lactose | [9] |
| Sugarcane bagasse | 1753 mL H2/L | [10] |
| Substrate | Pretreatment | Pretreatment Conditions | Hydrogen Yield | Increase in H2 Yield (%) | References |
|---|---|---|---|---|---|
| Rice mill wastewater | Thermal | 121 °C temp Time: 15 min | Control: 90 mL of hydrogen Pretreated: 200 mL of hydrogen | 55 | [32] |
| Beetroot pulp | Microwave | 700 W; 170 °C temp Time: 30 min | Control: 95.7 mL of hydrogen Pretreated: 108.7 mL of hydrogen | 12 | [33] |
| Palm oil mill effluent (POME) | Acid | 0.8% (w/v) phosphoric acid and 1% (w/v) nitric acid. Time: 10 min | Control: 0.64 mol H2/mol Pretreated: 1.24 mol H2/mol | 48.4 | [34] |
| Bio-methenated distillery wastewater | Ozone | Ozone dose: 4.6 g/h Time: 160 min | Control: 0.37 mL/h of hydrogen yield with 44.19 mL/g COD Pretreated: 1.18 mL/h of H2 yield with 185.5 mL/g COD | 68.6 | [35] |
| Cassava wastewater | Enzymatic | α-amylase (Incubation temperature: 37 °C) OPTIMASH BG® (Incubation temperature: 60 °C) Incubation period: 10 day | Control: 2.06 mol/g COD Pretreated: 5.02 mol/g COD (α-amylase) 4.24 mol/g COD (OPTIMASH BG®) | 58.9 51.4 | [36] |
| Rice mill wastewater | Combined (acid followed by enzymatic) | Acid −1.5% H2SO4 Reaction time: 60 min Enzyme: A. niger Incubation temperature: 29 °C | Control: 0.128 mol/L of hydrogen Pretreated: 5.34 mol/L of hydrogen. | 97.6 | [37] |
| Textile desizing wastewater | Combined pretreatment (flocculation and coagulation) | Coagulant GGEFloc-653 −1 g/L Rapid mixing 100 rpm (3 min), Slow mixing 30 rpm (20 min), Sludge settling time: 1 h | Control: 0.88 L/L-d H2 production rate Pretreated: 3.8 L/L-d H2 production rate | 76.8 | [38] |
| Mechanical Methods | |||
|---|---|---|---|
| Pretreatment | Cell Disruption Method | Advantages | Disadvantages |
| Bead milling | Cell wall grinding with spinning solid beads. | Applicable for wet microalgal pastes and components that can be easily separated. Reduced cellulose crystallinity. | High energy required. Heat generated may cause thermal degeneration. High installation cost. Beads should be replaced periodically. |
| Ultrasonication | Cavitation effect and acoustic streaming from ultrasonic waves create high pressure and heat for disrupting cell | High disruption efficiency. Brief pretreatment time. Components such as solvent/beads are not required. Minimum maintenance costs. | High energy required. Cooling required for the excess heat generated. Oxidative free radicals formed cause damage. Not applicable to all algae. |
| Microwaves | Interaction between high-frequency shock waves and charged molecules creates high-pressure and heat to disrupt cells. | High disruption efficiency. Short pretreatment time. Low energy demand. Easy to scale up. | Component structure may change due to changes in non-covalent interactions. High maintenance cost. Cooling required for excess heat generated. |
| Thermal Methods | |||
| Steam explosion | Biomass is exposed to steam at high T and P followed by depressurization that causes an explosion to rupture the cell membrane. | High cell disruption efficiency. Low maintenance. Resistant to corrosion. | High temperature (180–240 °C) and pressure (1.03–3.45 MPa). Disruption efficiency varies with species. |
| Freezing and thawing | Biomass freezing to form large ice crystals that expand and disrupt the cell wall. Excess disruption with repeated freeze-thaw cycles. | Mild operating conditions, suitable for extracting sensitive components. Biomass blending is not needed. | Time-consuming. Energy and cost-intensive. |
| Chemical methods | |||
| Acid/alkali treatment | Acid- hydrolysis of cell wall polymers. Alkali- saponification of cell wall lipids for cell disruption. | Low energy demand Low temperature. Short reaction time. Easy to scale up. | Highly prone to corrosion. Formation of fermentation inhibitors (furfurals). Protein denaturation |
| Biological methods | |||
| Enzymatic hydrolysis/Fungal Treatment | Hydrolysis of the cell wall by enzymes from bacteria and fungi destroys cell wall polymers to access intracellular glucans. | Mild reaction conditions. High product quality and purity. Low energy demand. No chemicals required. | Enzyme is costly. Enzyme specificity requires a cocktail of enzymes. Long reaction time (2–4 weeks). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. https://doi.org/10.3390/en15030999
Singh H, Tomar S, Qureshi KA, Jaremko M, Rai PK. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies. 2022; 15(3):999. https://doi.org/10.3390/en15030999
Chicago/Turabian StyleSingh, Harshita, Sakshi Tomar, Kamal A. Qureshi, Mariusz Jaremko, and Pankaj K. Rai. 2022. "Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production" Energies 15, no. 3: 999. https://doi.org/10.3390/en15030999
APA StyleSingh, H., Tomar, S., Qureshi, K. A., Jaremko, M., & Rai, P. K. (2022). Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies, 15(3), 999. https://doi.org/10.3390/en15030999







