Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations
Abstract
1. Introduction
1.1. Overview of Curcumin: Sources and Structure
1.2. Historical Use in Medicine and Pharmacology
2. Curcumin’s Biological Mechanisms
2.1. The Wnt/β-Catenin Pathway: A Key Driver in Cancer Progression
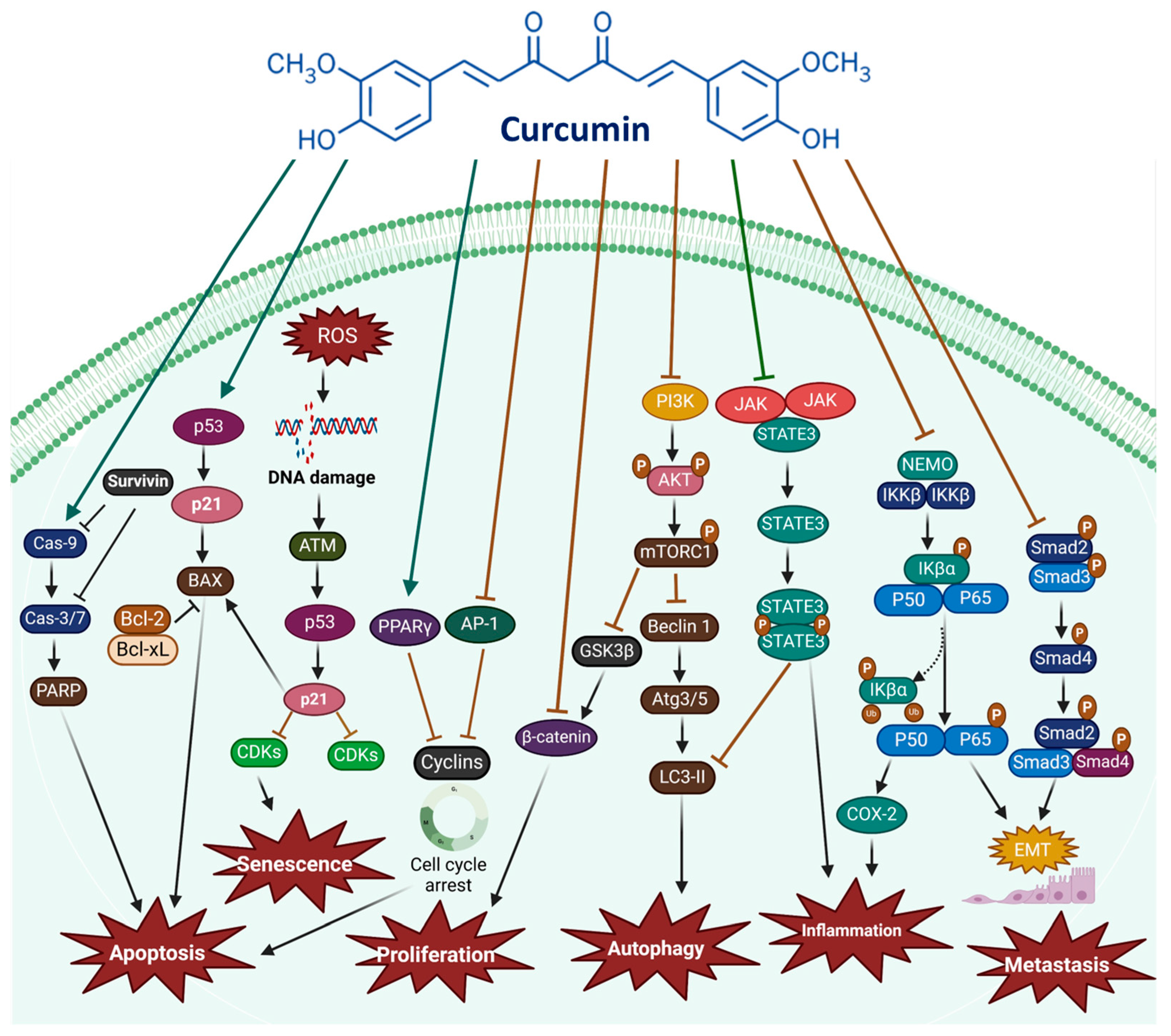

2.2. The PI3K/Akt/mTOR Pathway
2.3. The JAK/STAT Signaling Pathway
2.4. The MAPK Signaling Pathway
2.5. The p53 Signaling Pathway
2.6. Intrinsic and Extrinsic Pathways
2.7. Curcumin Inhibits Cancer Cell Proliferation and Induces Cell Cycle Arrest
2.8. Apoptosis Induction by Curcumin
2.9. Ferroptosis Induction by Curcumin
2.10. Curcumin Inhibits Angiogenesis, Invasion, and Metastasis in Cancer Cells
| Type | Cell Lines | Concentration | Target Pathway | Main Finding | Ref. |
|---|---|---|---|---|---|
| Breast cancer | 4T1 (in vivo) | 0.2 mL solution | Ubiquitin-proteasome pathway |
| [131] |
| MCF-7 (in vitro) | 7 μM | miR-15a-5p |
| [132] | |
| T47D, MCF7, MDA-MB-415, MDA-MB-231, SK-BR-3, MDA-MB-468, BT-20 (in vitro) | 10 or 30 μΜ | Akt/mTOR signaling pathway |
| [133] | |
| 4T1 (in vitro and in vivo) | 50 μg/mL | - |
| [134] | |
| Colorectal cancer | HCT-116 (in vitro) | 10, 20, 40 μΜ | CDCA3/CDK1 signaling inhibition |
| [135] |
| SW620, HT-29 (in vitro) | 5, 10, 20, 40, 80 μΜ | ATF6-mediated endoplasmic reticulum stress |
| [136] | |
| SW620, LoVo (in vitro and in vivo) | 10, 20, 40 μΜ | p53 and SLC7A11/glutathione/GPX4 axis signaling activation |
| [106] | |
| LoVo (in vitro) | 40, 80, 122 μΜ | PI3K/Akt pathway inhibition |
| [137] | |
| HCT-116 (in vitro and in vivo) | 10, 20, 30 μΜ | USP4/LAMP3 signaling pathway inhibition |
| [138] | |
| HCT-8 (in vitro) | 20 μΜ | PI3K/mTOR signaling inhibition |
| [120] | |
| SW480 (in vitro) | 6.29 μM | JAK2/STAT3 pathway inactivation |
| [139] | |
| Lung cancer | H446, SBC-2, H1299 (in vitro and in vivo) | 6.47 μM | JNK/c-Jun signaling pathway activation |
| [78] |
| A549, H1299 (in vitro and in vivo) | 40 µM | ATOX1-mediated copper pathway |
| [140] | |
| LK-2, H1650 (in vitro and in vivo) | 6.25, 12.5, 25, 50 and 100 μmol/L | DMRT3/SLC7A11 Axis |
| [141] | |
| A549 (in vitro) | 10, 20, 40, 80 μM | GSH-GPX4 inhibition |
| [142] | |
| H1975 and PC9 (in vitro and in vivo) | 20, 30, 40 μM | miR-760/RAB3D axis |
| [100] | |
| DOC/A549- and VCR/A549-resistant cells (in vitro) | 20, 30, 40 μM | ROS-regulated p38 MAPK Phosphorylation |
| [115] | |
| A549 (in vitro) | 20 μM | EMT signaling pathway |
| [143] | |
| A549 (in vitro) | 5, 25, 125 nM | Nuclear-cytoplasm translocation of TAZ signaling pathway activation Hippo signaling pathway activation |
| [134] | |
| Prostate cancer | LNCaP (in vitro) | 20, 30, 40 μM | miR-483-3p signaling pathway activation UBE2C signaling pathway inhibition |
| [144] |
| PC-4, DU145 (in vitro) | 10 μM | m6A-modified circ0030568-FMR1 signaling pathway |
| [145] | |
| LNCaP, C4-2, PC3, DU145, C42R (in vitro and in vivo) | 4, 8, 12 μM | JARID1D demethylation by regulating EMT and AR signaling pathway |
| [146] | |
| PC3, LNCaP (in vitro) | 10, 15 μM | - |
| [147] | |
| DU145, PC3 (in vitro) | 2.5, 5 μg/mL | Akt signaling pathway inhibition |
| [148] | |
| Ovarian cancer | SKOV3 (in vitro) | 20, 40 μM | NF-κB pathway |
| [148] |
| PA1 and A2780 (in vitro) | 5, 10 μM | PI3K-AKT pathways |
| [149] | |
| Anglne, HO8910PM (in vitro and in vivo) | 4, 6 μM | HCAR1-AMPK-SREBP1 signaling pathway |
| [107] | |
| SKOV3, A2780 (in vitro) | 5 μM | miR-9-5p/BRCA1 signaling pathway |
| [104] | |
| SKOV3 (in vitro) | 10, 20 μM | NFκB pathway |
| [150] | |
| Liver cancer | HepG2 (in vitro and in vivo) | 10 μM | VEGF/AKT/PI3K signaling pathway inhibition |
| [105] |
| HepG2 (in vitro and in vivo) | 2.5, 5, 10 μg/mL | - |
| [151] | |
| HepG2 (in vitro) | 100 μmol/L | - |
| [152] | |
| Pancreatic cancer | PANC-1 (in vitro) | 10, 20, 30, and 40 μM | p53 signaling pathway |
| [84] |
| MiaPaCa-2, Panc-1 (in vitro) | 5 μM | - |
| [153] | |
| PANC-1, SW1990 (in vitro) | 20, 40, and 60 µM | Beclin1 signaling pathway |
| [154] | |
| BxPC3, SW1990, and PANC-1 (in vitro) | 25, 50, and 100 µM | JNK-mediated Inflammation |
| [155] | |
| Cervical cancer | HeLa, CaSki (in vitro) | 20, 40 µM | E6 signaling pathway |
| [156] |
| SiHa, HeLa (in vitro) | 25 µmol/L | ATG3-dependent autophagy |
| [157] | |
| Hela (in vitro) | 15 μM | E6, E7, P53, and Rb pathway |
| [158] |
3. Preclinical and Clinical Evidence of Curcumin in Cancer Therapy
Anti-Inflammatory and Immunomodulatory Effects of Curcumin
4. The Significance of Curcumin in Cancer Prevention
5. Combination Therapy: Synergistic Effects of Curcumin with Chemotherapy and Nanoparticle-Based Drug Delivery Systems
6. Curcumin with Combined Treatments in Clinical Trials in Cancer
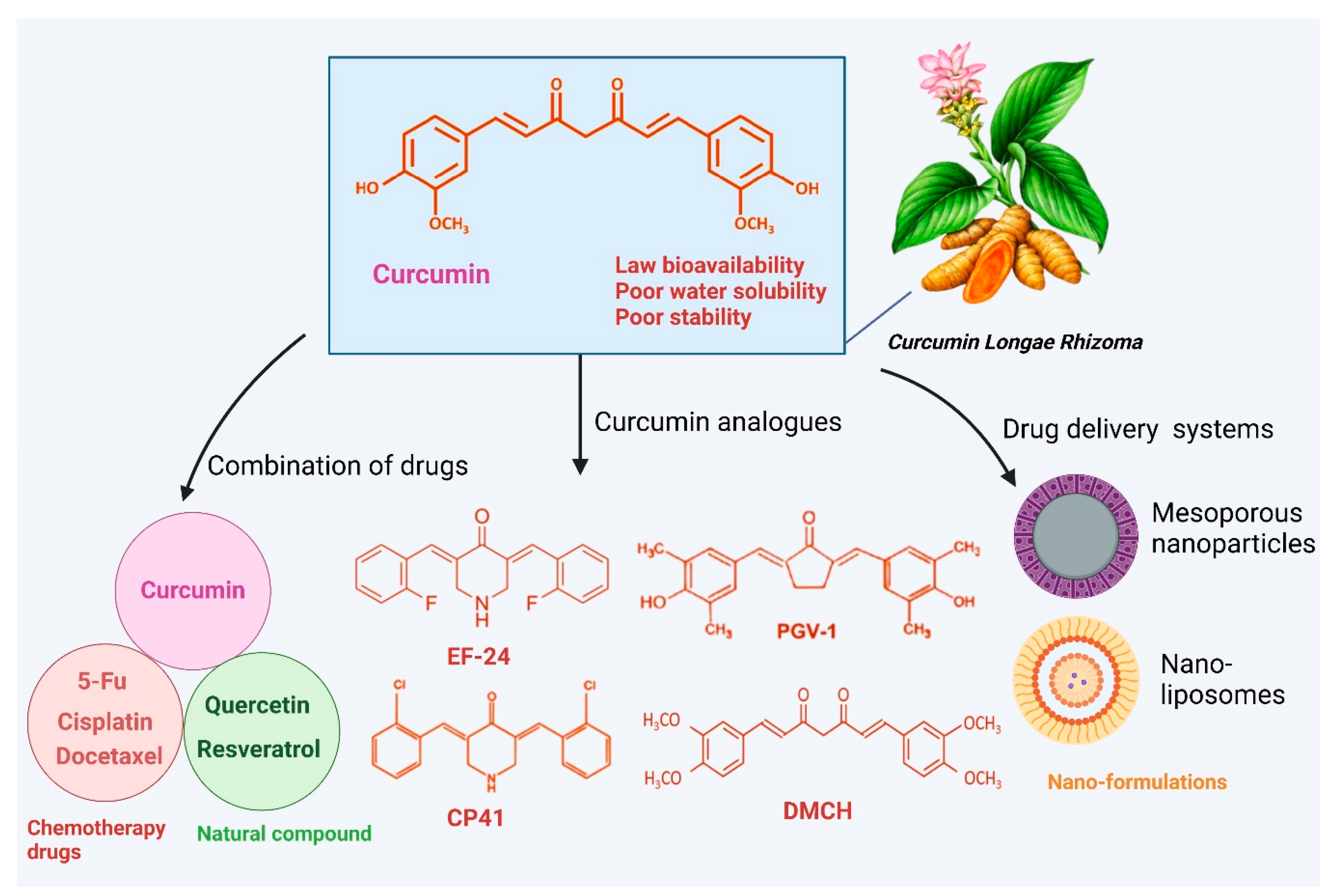
Bioavailability and Delivery Challenges
7. Enhancing Curcumin’s Efficacy Through Formulation and Nanotechnology in Cancer
| Curcumin Nanoformulation | Material Used | Target | Main Results | Ref. |
|---|---|---|---|---|
| Liposomes | Folic acid | Breast cancer |
| [252] |
| Polyethylene glycol (PEG) | Lung cancer |
| [253] | |
| Glycyrrhetinic acid | Hepatocellular carcinoma |
| [254] | |
| Chitosan | Hepatocellular carcinoma |
| [255] | |
| Glycyrrhetinic acid (GA) | Hepatocellular cancer |
| [256] | |
| Polymeric nanoparticles | PEG-PLGA | Breast cancer |
| [257] |
| Chitosan | Breast cancer |
| [258] | |
| Dextran | Lung cancer |
| [259] | |
| Solid lipid nanoparticles (SLNs) | Stearic acid | Lung cancer |
| [260] |
| Glyceryl monostearate, stearic acid, triglycerides | Liver cancer |
| [261] | |
| Surfactant | Lung cancer |
| [262] | |
| Stearic acid, glyceryl, monostearate, tristearin, Precirol ATO 5 | Lung cancer |
| [263] | |
| Nanomicelles | Amphiphilic block copolymers, surfactant | Breast cancer |
| [264] |
| CZL polymer | Liver cancer |
| [265] | |
| Silica nanoparticles | Tetraethyl orthosilicate, surfactants, 3-aminopropyltriethoxysilane | Breast cancer |
| [266] |
| Alginate oligosaccharide, amination | Colon cancer |
| [267] | |
| Protein nanoparticle (human serum albumin) | Folic acid | Esophageal cancer |
| [268] |
| Dendrimers | Poly (amidoamine) dendrimers | Breast cancer |
| [269] |
| Poly amidoamine dendrimer-peptide, cholesterol | Skin cancer |
| [270] | |
| Glucan nanoparticles | β-Glucan | Hepatic cancer |
| [271] |
| Carbon nanotubes (CNTs) | Carbon nanotubes | Melanoma cancer |
| [272] |
| Folic acid | Ovarian cancer |
| [273] | |
| Metal–organic frameworks (MOFs) | Metal nodes, organic linkers | Colorectal cancer |
| [274] |
| Zirconium, terephthalic acid | Breast cancer |
| [275] |
7.1. Solid Lipid-Based Curcumin Nanoparticles
7.2. Polymeric-Based Nanoparticles
Polydopamine (PDA)-Based Nanoparticles
7.3. Liposome-Based Nanoparticles
7.4. Metalloid Nanoparticles
Manganese (Mn)-Based Nanoparticles
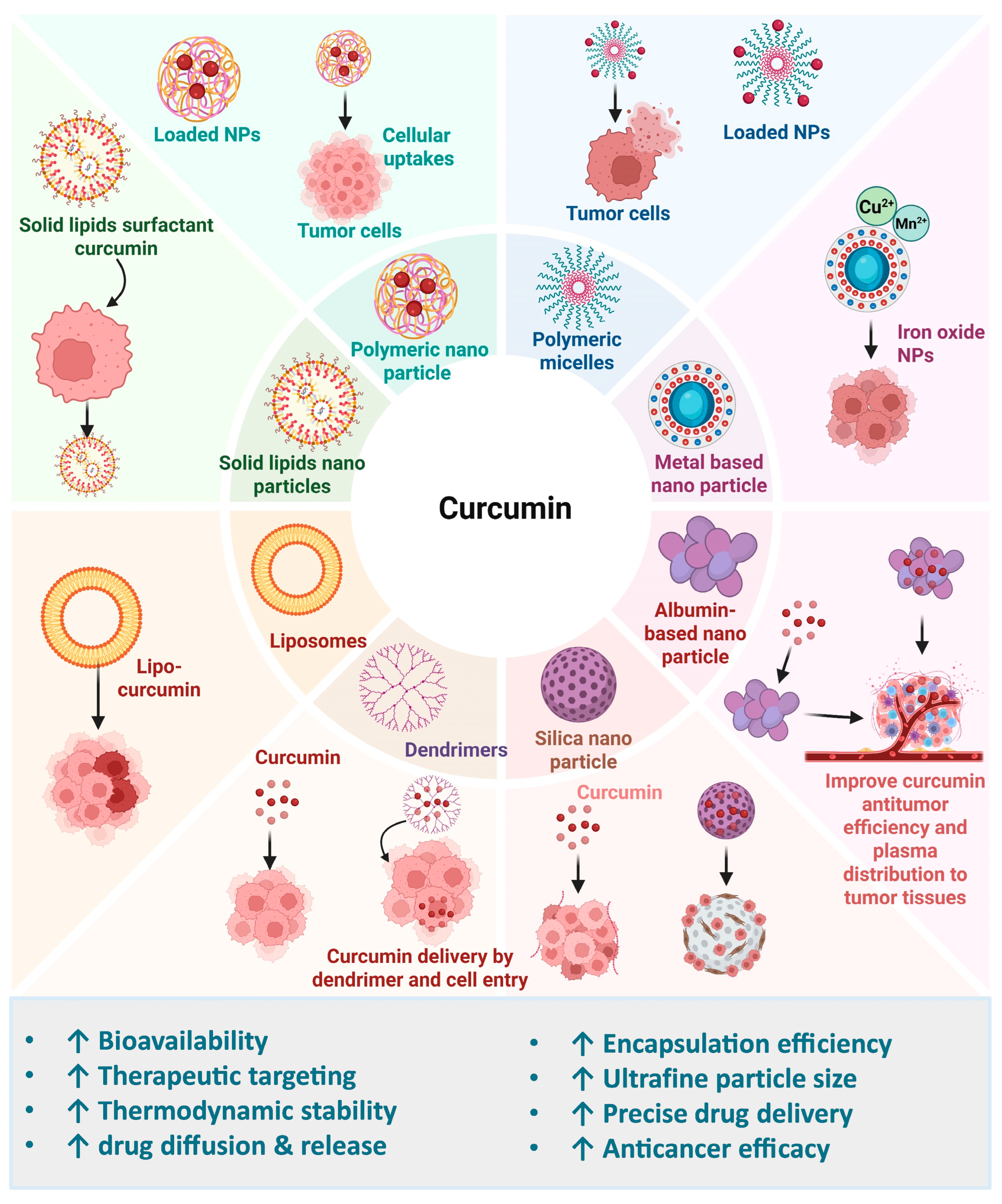
7.5. Protein-Based Nanoparticles
7.6. Polymeric Nanomicelle-Based Nanoparticles
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| TCM | Traditional Chinese Medicine |
| EMT | Epithelial–mesenchymal transition |
| MMP | Matrix metalloproteinase |
| GSK3β | Glycogen synthase kinase 3β |
| CK1α | Casein kinase 1α |
| FZD | Frizzled |
| TGF-β1 | Transforming growth factor-beta 1 |
| DAC | 5-aza-2′-deoxycytidine |
| CRCs | Colorectal cancer cells |
| MDA | Malondialdehyde |
| 5-FU | 5-fluorouracil |
| SLNs | Solid lipid nanoparticles |
| Cur-MPKEs | Curcumin-loaded nanoparticles |
| CUR-NMs | Curcumin-encapsulated nanomicelles |
| CU1-LSLNs | Curcumin encapsulated in long-circulating solid lipid nanoparticles |
| PNPs | Polymeric nanoparticles |
References
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, Purification and Applications of Curcumin from Plant Materials-A Comprehensive Review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 297–318. [Google Scholar]
- Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akaao, N.; Kondo, K.; Tsuda, Y. Nematocidal Activity of Turmeric: Synergistic Action of Curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N. Impact of Nonthermal Plasma on Human Metapneumovirus (HMPV): Mechanisms of Viral Inactivation and Replication Inhibition. Contrib. Plasma Phys. 2025, e70044. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, A. Turmeric (Curcuma longa): MiRNAs and Their Regulating Targets Are Involved in Development and Secondary Metabolite Pathways. C. R. Biol. 2017, 340, 481–491. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Kumar, N.V.A.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Curcumin: Historical Background, Chemistry, Pharmacological Action, and Potential Therapeutic Value. In Curcumin for Neurological and Psychiatric Disorders; Academic Press: Cambridge, MA, USA, 2019; pp. 23–44. [Google Scholar]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef] [PubMed]
- Lodi, R.S.; Jia, X.; Yang, P.; Peng, C.; Dong, X.; Han, J.; Liu, X.; Wan, L.; Peng, L. Whole Genome Sequencing and Annotations of Trametes Sanguinea ZHSJ. Sci. Data 2025, 12, 1460. [Google Scholar] [CrossRef]
- Tudu, H.; Pradhan, S.; Das, T.; Das, B.K. Turmeric Cultivation and Medicinal Benefits: Special Reference to Kandhamal District of Odisha, India. Asian J. Agric. Hortic. Res. 2024, 11, 75–90. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Tan, F.; He, J.; Liu, J.; Lu, M.; Hu, Z.; Zhuo, Y.; Liu, J.; Tang, X.; Jiang, Z.; et al. Curcumin-Primed Olfactory Mucosa-Derived Mesenchymal Stem Cells Mitigate Cerebral Ischemia/Reperfusion Injury-Induced Neuronal PANoptosis by Modulating Microglial Polarization. Phytomedicine 2024, 129, 155635. [Google Scholar] [CrossRef]
- Prieto, J.M.; Schinella, G.R. Anti-Inflammatory and Antioxidant Chinese Herbal Medicines: Links between Traditional Characters and the Skin Lipoperoxidation “Western” Model. Antioxidants 2022, 11, 611. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. In Studies on Biomarkers and New Targets in Aging Research in Iran; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–39. [Google Scholar]
- Rahman, M.; Akter, K.; Ahmed, K.R.; Fahim, M.M.H.; Aktary, N.; Park, M.N.; Shin, S.-W.; Kim, B. Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies. Cancers 2024, 16, 2777. [Google Scholar] [CrossRef]
- Aktary, N.; Jeong, Y.; Oh, S.; Shin, Y.; Sung, Y.; Rahman, M.; Ramos Santiago, L.; Choi, J.; Song, H.G.; Nurkolis, F.; et al. Unveiling the Therapeutic Potential of Natural Products in Alzheimer’s Disease: Insights from in Vitro, in Vivo, and Clinical Studies. Front. Pharmacol. 2025, 16, 1601712. [Google Scholar] [CrossRef]
- Ahn, C.-H.; Myong, J.S.; Ahmed, K.R.; Rahman, M.A.; Fahim, M.M.H.; Choi, M.; Rahman, M.; Choi, J.; Kim, K.; Moon, S.; et al. A Pharmacoinformatic Approach for Studying Atractylodes Lancea DC’s Anticancer Potential and Control ROS-Mediated Apoptosis against Prostate Cancer Cells. Front. Oncol. 2025, 15, 1471110. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of Cancer Metabolism: The Therapeutic Potential of Cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPARγ-Dependent NF-ΚB Signaling Pathway in Vivo and in Vitro. Mediators Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef]
- Rahimi, K.; Ahmadi, A.; Hassanzadeh, K.; Soleimani, Z.; Sathyapalan, T.; Mohammadi, A.; Sahebkar, A. Targeting the Balance of T Helper Cell Responses by Curcumin in Inflammatory and Autoimmune States. Autoimmun. Rev. 2019, 18, 738–748. [Google Scholar] [CrossRef]
- Adnan, M.; Ali, S.; Sheikh, K.; Amber, R. Review on Antibacterial Activity of Himalayan Medicinal Plants Traditionally Used to Treat Pneumonia and Tuberculosis. J. Pharm. Pharmacol. 2019, 71, 1599–1625. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, M.; Guo, Y.; Liu, J.; Chen, W.; Guan, M.; Wang, Y.; Zhao, X.; Wang, X.; Li, H.; et al. Prevention and Treatment of Infectious Diseases by Traditional Chinese Medicine: A Commentary. Apmis 2019, 127, 372–384. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Y.; Zhang, X.; Wang, Y.; Chen, Q.; Zhang, M.; Sheng, H.; Zhang, Y.; Guo, J.; Li, J.; et al. Short-Chain Acyl Post-Translational Modifications in Cancers: Mechanisms, Roles, and Therapeutic Implications. Cancer Commun. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiao, W.; Zeng, Q. Curcumin Inhibits Bladder Cancer by Inhibiting Invasion via AKT/MMP14 Pathway. Discov. Med. 2024, 36, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.; Luo, H.; Meng, X.; Chen, M.; Zhu, D. Wnt Signaling Pathway in Cancer Immunotherapy. Cancer Lett. 2022, 525, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Le, P.N.; Keysar, S.B.; Miller, B.; Eagles, J.R.; Chimed, T.-S.; Reisinger, J.; Gomez, K.E.; Nieto, C.; Jackson, B.C.; Somerset, H.L.; et al. Wnt Signaling Dynamics in Head and Neck Squamous Cell Cancer Tumor-Stroma Interactions. Mol. Carcinog. 2019, 58, 398–410. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Qu, Y. Targeting the β-Catenin Signaling for Cancer Therapy. Pharmacol. Res. 2020, 160, 104794. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Zhu, M.-X.; Yang, Y.-W.; Zhang, P.-F.; Yang, X.; Peng, R.; Gao, C.; Lu, J.-C.; Wang, L.; Deng, X.-Y.; et al. Downregulation of RNF128 Activates Wnt/β-Catenin Signaling to Induce Cellular EMT and Stemness via CD44 and CTTN Ubiquitination in Melanoma. J. Hematol. Oncol. 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Wiese, K.E.; Nusse, R.; van Amerongen, R. Wnt Signalling: Conquering Complexity. Development 2018, 145, dev165902. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, P.; Li, M.; Zhang, Q.; He, T.; Gan, L. Curcumin Suppresses Metastasis of Triple-Negative Breast Cancer Cells by Modulating EMT Signaling Pathways: An Integrated Study of Bioinformatics Analysis. Medicine 2024, 103, e37264. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The Role of Curcumin in Cancer: A Focus on the PI3K/Akt Pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Chen, T.; Cheng, X.; Xiao, H.; Meng, X.; Jiang, Y. Inhibition and Potential Treatment of Colorectal Cancer by Natural Compounds via Various Signaling Pathways. Front. Oncol. 2022, 12, 956793. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Gao, C.; Li, H.; Liu, C.; Wang, L.; Li, Y.; Liu, R.; Sun, C.; Zhuang, J. Natural Compounds: Wnt Pathway Inhibitors with Therapeutic Potential in Lung Cancer. Front. Pharmacol. 2023, 14, 1250893. [Google Scholar] [CrossRef]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and Quercetin Synergistically Inhibit Cancer Cell Proliferation in Multiple Cancer Cells and Modulate Wnt/β-Catenin Signaling and Apoptotic Pathways in A375 Cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Xu, J.-H.; Yang, H.-P.; Zhou, X.-D.; Wang, H.-J.; Gong, L.; Tang, C.-L. Role of Wnt Inhibitory Factor-1 in Inhibition of Bisdemethoxycurcumin Mediated Epithelial-to-Mesenchymal Transition in Highly Metastatic Lung Cancer 95D Cells. Chin. Med. J. (Engl.) 2015, 128, 1376–1383. [Google Scholar] [CrossRef]
- Salehi, B.; Jornet, P.L.; Lopez, E.P.-F.; Calina, D.; Sharifi-Rad, M.; Ramirez-Alarcon, K.; Forman, K.; Fernandez, M.; Martorell, M.; Setzer, W.N.; et al. Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe Vera, Green Tea and Coffee Properties. Biomolecules 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Vallee, A.; Guillevin, R.; Vallee, J.-N. Vasculogenesis and Angiogenesis Initiation under Normoxic Conditions through Wnt/β-Catenin Pathway in Gliomas. Rev. Neurosci. 2017, 29, 71–91. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y. Crosstalk between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress during Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Tsao, C.-W.; Lin, Y.-W.; Kuo, C.-C.; Tsao, C.-H.; Liu, C.-Y. Regulation of Carcinogenesis and Modulation through Wnt/β-Catenin Signaling by Curcumin in an Ovarian Cancer Cell Line. Sci. Rep. 2019, 9, 17267. [Google Scholar] [CrossRef]
- Bian, L.; Yan, H.; Zhu, B.; Xin, P.; Liao, Q. Curcumin Promotes Apoptosis of Liver Cancer Cells by Down-Regulating WNT/β-Catenin. Mater. Express 2023, 13, 1326–1331. [Google Scholar] [CrossRef]
- Huang, L.; He, X.; Zuo, X. The Effect and Mechanism of Curcumin Combined With Carboplatin Chemotherapy Promoting on Apoptosis of Lung Cancer HCC827 Cells. J. Immunol. Res. 2022, 2022, 1932692. [Google Scholar] [CrossRef]
- Hao, J.; Dai, X.; Gao, J.; Li, Y.; Hou, Z.; Chang, Z.; Wang, Y. Curcumin Suppresses Colorectal Tumorigenesis via the Wnt/β-Catenin Signaling Pathway by Downregulating Axin2. Oncol. Lett. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/MTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/MTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef]
- Safaroghli-Azar, A.; Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Phosphoinositide 3-Kinase (PI3K) Classes: From Cell Signaling to Endocytic Recycling and Autophagy. Eur. J. Pharmacol. 2023, 953, 175827. [Google Scholar] [CrossRef]
- Ameer, S.F.; Mohamed, M.Y.; Elzubair, Q.A.; Sharif, E.A.M.; Ibrahim, W.N. Curcumin as a Novel Therapeutic Candidate for Cancer: Can This Natural Compound Revolutionize Cancer Treatment? Front. Oncol. 2024, 14, 1438040. [Google Scholar] [CrossRef] [PubMed]
- Tamaddoni, A.; Mohammadi, E.; Sedaghat, F.; Qujeq, D.; As’Habi, A. The Anticancer Effects of Curcumin via Targeting the Mammalian Target of Rapamycin Complex 1 (MTORC1) Signaling Pathway. Pharmacol. Res. 2020, 156, 104798. [Google Scholar] [CrossRef]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Antitumor Activity of Curcumin by Modulation of Apoptosis and Autophagy in Human Lung Cancer A549 Cells through Inhibiting PI3K/Akt/MTOR Pathway. Oncol. Rep. 2018, 39, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Scott, J.; Sengupta, D.; Al-Gharaibeh, A.; Dunbar, G.L. Curcumin and Solid Lipid Curcumin Particles Induce Autophagy, but Inhibit Mitophagy and the PI3K-Akt/MTOR Pathway in Cultured Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 399. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, L.; Li, W.; Liang, Q.; Li, Z. Curcumin Induces Apoptosis and Autophagy Inhuman Renal Cell Carcinoma Cells via Akt/MTOR Suppression. Bioengineered 2021, 12, 5017–5027. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A.; Xu, P. Molecular Regulation of Autophagy Machinery by MTOR-Dependent and-Independent Pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced Effect of Curcumin and Resveratrol on Mitigating Hepatic Steatosis in Metabolic Associated Fatty Liver Disease via the PI3K/AKT/MTOR and HIF-1/VEGF Cascade. Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, K.; Wang, M.; He, Z.; Yu, B.; Wang, X.; Pan, X.; Luo, Y.; Xu, S.; Lau, J.T.Y.; et al. VEGF-FGF Signaling Activates Quiescent CD63+ Liver Stem Cells to Proliferate and Differentiate. Adv. Sci. 2024, 11, 2308711. [Google Scholar] [CrossRef]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M.; et al. Curcumin Induces Apoptotic Cell Death via Inhibition of PI3-Kinase/AKT Pathway in B-Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef]
- Luo, N.; Balko, J.M. Role of JAK-STAT Pathway in Cancer Signaling. In Predictive Biomarkers in Oncology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 311–319. [Google Scholar]
- Maji, L.; Sengupta, S.; Purawarga Matada, G.S.; Teli, G.; Biswas, G.; Das, P.K.; Panduranga Mudgal, M. Medicinal Chemistry Perspective of JAK Inhibitors: Synthesis, Biological Profile, Selectivity, and Structure Activity Relationship. Mol. Divers. 2024, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The Role of JAK/STAT Signaling Pathway and Its Inhibitors in Diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Petiti, J.; Rosso, V.; Lo Iacono, M.; Panuzzo, C.; Calabrese, C.; Signorino, E.; Pironi, L.; Cartellà, A.; Bracco, E.; Pergolizzi, B.; et al. Curcumin Induces Apoptosis in JAK2-Mutated Cells by the Inhibition of JAK2/STAT and MTORC1 Pathways. J. Cell. Mol. Med. 2019, 23, 4349–4357. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Zamanian, M.Y.; Al-Ani, A.M.; Jabbar, T.L.; Kareem, A.K.; Aghaei, Z.H.; Tahernia, H.; Hjazi, A.; Jissir, S.A.; Hakimizadeh, E. Targeting STAT3 Signaling Pathway by Curcumin and Its Analogues for Breast Cancer: A Narrative Review. Anim. Model. Exp. Med. 2024. [Google Scholar] [CrossRef]
- Wang, R.; Yu, H.; Chen, P.; Yuan, T.; Zhang, J. Integrated Transcriptome and Molecular Docking to Identify the Hub Superimposed Attenuation Targets of Curcumin in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 12479. [Google Scholar] [CrossRef] [PubMed]
- Afshari, H.; Noori, S.; Zarghi, A. Curcumin Potentiates the Anti-Inflammatory Effects of Tehranolide by Modulating the STAT3/NF-$κ$B Signaling Pathway in Breast and Ovarian Cancer Cell Lines. Inflammopharmacology 2023, 31, 2541–2555. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, G.; Shi, Y.; Lin, Y.; Ou, J.; Zhu, H.; Wu, J.; Li, G.; Lv, L. Curcumin and Its Nano-Formulations: Defining Triple-Negative Breast Cancer Targets through Network Pharmacology, Molecular Docking, and Experimental Verification. Front. Pharmacol. 2022, 13, 920514. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.; Fathima, H.; Prabhu, K.S.; Siveen, K.S.; Kulinski, M.; Azizi, F.; Dermime, S.; Ahmad, A.; et al. Curcumin-Mediated Apoptotic Cell Death in Papillary Thyroid Cancer and Cancer Stem-like Cells through Targeting of the JAK/STAT3 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 438. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Li, S.; Ma, J.; Dai, X.; Lu, J. Deciphering JAK/STAT Signaling Pathway: A Multifaceted Approach to Tumorigenesis, Progression and Therapeutic Interventions. Int. Immunopharmacol. 2024, 131, 111846. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Afshar, E.G.; Farkhondeh, T.; Samarghandian, S. Potential Therapeutic Effects of Curcumin Mediated by JAK/STAT Signaling Pathway: A Review. Phyther. Res. 2020, 34, 1745–1760. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Signaling Pathways and Effectors of Aging. Growth 2021, 3, 53. [Google Scholar] [CrossRef]
- Lin, X.; Liao, Y.; Chen, X.; Long, D.; Yu, T.; Shen, F. Regulation of Oncoprotein 18/Stathmin Signaling by ERK Concerns the Resistance to Taxol in Nonsmall Cell Lung Cancer Cells. Cancer Biother. Radiopharm. 2016, 31, 37–43. [Google Scholar] [CrossRef]
- Kwak, A.-W.; Lee, M.-J.; Lee, M.-H.; Yoon, G.; Cho, S.-S.; Chae, J.-I.; Shim, J.-H. The 3-Deoxysappanchalcone Induces ROS-Mediated Apoptosis and Cell Cycle Arrest via JNK/P38 MAPKs Signaling Pathway in Human Esophageal Cancer Cells. Phytomedicine 2021, 86, 153564. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hao, X.; Li, X.; Yan, Y.; Tian, W.; Xiao, L.; Wang, Z.; Dong, J. Curcumin Inhibits Adverse Psychological Stress-Induced Proliferation and Invasion of Glioma Cells via down-Regulating the ERK/MAPK Pathway. J. Cell. Mol. Med. 2021, 25, 7190–7203. [Google Scholar] [CrossRef]
- Lin, X.; Yu, T.; Zhang, L.; Chen, S.; Chen, X.; Liao, Y.; Long, D.; Shen, F. Silencing Op18/Stathmin by RNA Interference Promotes the Sensitivity of Nasopharyngeal Carcinoma Cells to Taxol and High-Grade Differentiation of Xenografted Tumours in Nude Mice. Basic Clin. Pharmacol. Toxicol. 2016, 119, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, S.; Zeng, J. TGF-β Signaling: A Complex Role in Tumorigenesis. Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hui, M.; Chen, G.; Huang, H.; Wang, S.; Ye, Y.; Wang, Y.; Wang, M.; Zhang, S.; Huang, L.; et al. Curcumin-Piperlongumine Hybrid Molecule Increases Cell Cycle Arrest and Apoptosis in Lung Cancer through JNK/c-Jun Signaling Pathway. J. Agric. Food Chem. 2024, 72, 7244–7255. [Google Scholar] [CrossRef]
- Asl, E.R.; Rostamzadeh, D.; Duijf, P.H.G.; Mafi, S.; Mansoori, B.; Barati, S.; Cho, W.C.; Mansoori, B. Mutant P53 in the Formation and Progression of the Tumor Microenvironment: Friend or Foe. Life Sci. 2023, 315, 121361. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Yang, L.V.; Abrams, S.L.; Steelman, L.S.; Follo, M.Y.; Cocco, L.; Ratti, S.; Martelli, A.M.; Augello, G.; Cervello, M. Effects of TP53 Mutations and MiRs on Immune Responses in the Tumor Microenvironment Important in Pancreatic Cancer Progression. Cells 2022, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Rehman, B. Roles of Mutant TP53 Gene in Cancer Development and Progression. Proc. Anticancer Res. 2024, 8, 165–181. [Google Scholar] [CrossRef]
- Canale, M.; Andrikou, K.; Priano, I.; Cravero, P.; Pasini, L.; Urbini, M.; Delmonte, A.; Crinò, L.; Bronte, G.; Ulivi, P. The Role of TP53 Mutations in EGFR-Mutated Non-Small-Cell Lung Cancer: Clinical Significance and Implications for Therapy. Cancers 2022, 14, 1143. [Google Scholar] [CrossRef]
- Oak, S.; Karajgikar, O.; Teni, T. Curcumin Mediates Selective Aggregation of Mutant P53 in Cancer Cells: A Promising Therapeutic Strategy. Biochem. Biophys. Res. Commun. 2023, 677, 141–148. [Google Scholar] [CrossRef]
- Demirci, Z.; Islek, Z.; Siginc, H.I.; Sahin, F.; Ucisik, M.H.; Bolat, Z.B. Curcumin-Loaded Emulsome Nanoparticles Induces Apoptosis through P53 Signaling Pathway in Pancreatic Cancer Cell Line PANC-1. Toxicol. Vitr. 2025, 102, 105958. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M. Preparation, Optimization and in-Vitro Evaluation of Curcumin-Loaded Niosome@ Calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef]
- Wei, L.; Li, S.; Ma, Y.; Ye, S.; Yuan, Y.; Zeng, Y.; Raza, T.; Xiao, F. Curcumin Attenuates Diphenyl Phosphate-Induced Apoptosis in GC-2spd (Ts) Cells through Activated Autophagy via the Nrf2/P53 Pathway. Environ. Toxicol. 2024, 39, 2032–2042. [Google Scholar] [CrossRef]
- Patiño-Morales, C.C.; Soto-Reyes, E.; Arechaga-Ocampo, E.; Ortiz-Sánchez, E.; Antonio-Véjar, V.; Pedraza-Chaverri, J.; García-Carrancá, A. Garcia-Carranca Curcumin Stabilizes P53 by Interaction with NAD (P) H: Quinone Oxidoreductase 1 in Tumor-Derived Cell Lines. Redox Biol. 2020, 28, 101320. [Google Scholar] [CrossRef]
- Ponomarev, A.; Gilazieva, Z.; Solovyeva, V.; Allegrucci, C.; Rizvanov, A. Intrinsic and Extrinsic Factors Impacting Cancer Stemness and Tumor Progression. Cancers 2022, 14, 970. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Chapter Four—Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. In Apoptosis in Health and Disease—Part A; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 125, pp. 73–120. ISBN 1876-1623. [Google Scholar]
- Liu, L.; Pang, Y.; Zhao, X.; Li, R.; Jin, C.; Xue, J.; Dong, R.; Liu, P. Curcumin Induces Apoptotic Cell Death and Protective Autophagy by Inhibiting AKT/MTOR/P70S6K Pathway in Human Ovarian Cancer Cells. Arch. Gynecol. Obstet. 2019, 299, 1627–1639. [Google Scholar] [CrossRef]
- Coker-Gurkan, A.; Celik, M.; Ugur, M.; Arisan, E.-D.; Obakan-Yerlikaya, P.; Durdu, Z.B.; Palavan-Unsal, N. Curcumin Inhibits Autocrine Growth Hormone-Mediated Invasion and Metastasis by Targeting NF-KappaB Signaling and Polyamine Metabolism in Breast Cancer Cells. Amino Acids 2018, 50, 1045–1069. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, D.; Zheng, R.; Chen, H.; Xu, T.; Wang, C.; Zhu, S.; Gao, X.; Zhang, J.; Li, D.; et al. Curcumin Induced G2/M Cycle Arrest in SK-N-SH Neuroblastoma Cells through the ROS-Mediated P53 Signaling Pathway. J. Food Biochem. 2021, 45, e13888. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [PubMed]
- Yavuz Turel, G.; Sahin Calapoglu, N.; Bayram, D.; Ozgocmen, M.; Togay, V.A.; Evgen Tuluceoglu, E. Curcumin Induces Apoptosis through Caspase Dependent Pathway in Human Colon Carcinoma Cells. Mol. Biol. Rep. 2022, 49, 1351–1360. [Google Scholar] [CrossRef]
- Visitnonthachai, D.; Nakareangrit, W.; Suntararuks, S.; Chaiyot, K.; Watcharasit, P.; Satayavivad, J. Potentiation of TRAIL-Induced Apoptosis in TRAIL-Resistant Cholangiocarcinoma Cells by Curcumin through the Induction of DR5 Membrane Localization and Disruption of the Anti-Apoptotic Complex DR5/DDX3/GSK3β. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 425. [Google Scholar] [CrossRef]
- Brockmueller, A.; de Porras, V.; Shakibaei, M. Curcumin and Its Anti-Colorectal Cancer Potential: From Mechanisms of Action to Autophagy. Phyther. Res. 2024. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.-C. Curcumin in Cancer Therapy: Exploring Molecular Mechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 216332. [Google Scholar] [CrossRef]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin Selectively Induces Colon Cancer Cell Apoptosis and S Cell Cycle Arrest by Regulates Rb/E2F/P53 Pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Wu, X.; Chen, H.; Liu, N.; Liu, S.; Lin, G. Curcumin Suppresses Lung Cancer Progression via CircRUNX1 Mediated MiR-760/RAB3D Axis. Thorac. Cancer 2023, 14, 506–516. [Google Scholar] [CrossRef]
- Patra, D.; Bhavya, K.; Ramprasad, P.; Kalia, M.; Pal, D. Anti-Cancer Drug Molecules Targeting Cancer Cell Cycle and Proliferation. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2023; Volume 135, pp. 343–395. [Google Scholar]
- Selvaraj, C. Therapeutic Targets in Cancer Treatment: Cell Cycle Proteins. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2023; Volume 135, pp. 313–342. [Google Scholar]
- Wang, T.; Wu, X.; Song, Y.; Cheng, H. Curcumin Induces G2/M Arrest and Triggers Autophagy, ROS Generation and Cell Senescence in Cervical Cancer Cells. J. Cancer 2020, 11, 6704. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Zhu, T.; Lu, W.; Fu, Y. Curcumin Enhances the Anti-Cancer Efficacy of Paclitaxel in Ovarian Cancer by Regulating the MiR-9-5p/BRCA1 Axis. Front. Pharmacol. 2023, 13, 1014933. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Liu, W.; Bi, J.; Bai, J.; Wu, Q.; Hu, B.; Hu, J.; Ma, L. Smart Mesoporous Silica Nanoparticles Loading Curcumin Inhibit Liver Cancer. J. Agric. Food Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Lei, J.; Peng, Y.; Wang, M.; Liang, Y.; Tang, S.; Tao, Q.; Wang, M.; Tang, X.; He, Z.; et al. Curcumin Suppresses Colorectal Cancer by Induction of Ferroptosis via Regulation of P53 and Solute Carrier Family 7 Member 11/Glutathione/Glutathione Peroxidase 4 Signaling Axis. Phyther. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, M.-J.; Yu, Y.; Ou, R.; Wang, Y.; Li, H.; Ge, R.-S. Curcumin Derivative NL01 Induces Ferroptosis in Ovarian Cancer Cells via HCAR1/MCT1 Signaling. Cell. Signal. 2023, 109, 110791. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The Nuclear Envelope: Target and Mediator of the Apoptotic Process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef]
- Roy, A.; Chatterjee, O.; Banerjee, N.; Roychowdhury, T.; Dhar, G.; Mukherjee, G.; Chatterjee, S. Curcumin Arrests G-Quadruplex in the Nuclear Hyper-Sensitive III1 Element of c-MYC Oncogene Leading to Apoptosis in Metastatic Breast Cancer Cells. J. Biomol. Struct. Dyn. 2022, 40, 10203–10219. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Li, L.; Xie, S. Curcumin Inhibits Papillary Thyroid Cancer Cell Proliferation by Regulating LncRNA LINC00691. Anal. Cell. Pathol. 2022, 2022, 5946670. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fang, H. Curcumin Inhibits Ovarian Cancer Progression by Regulating Circ-PLEKHM3/MiR-320a/SMG1 Axis. J. Ovarian Res. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Deng, B.; Akbari, A.; Liu, Q.; Zhu, B. The Ketogenic Diet Could Improve the Efficacy of Curcumin and Oldenlandia Diffusa Extract in the Treatment of Gastric Cancer by Increasing MiR340 Expression and Apoptosis Mediated by Autophagy, Oxidative Stress, and Angiogenesis. J. Food Biochem. 2022, 46, e14407. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-T.; Koh, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. A Natural Degradant of Curcumin, Feruloylacetone Inhibits Cell Proliferation via Inducing Cell Cycle Arrest and a Mitochondrial Apoptotic Pathway in HCT116 Colon Cancer Cells. Molecules 2021, 26, 4884. [Google Scholar] [CrossRef]
- Wu, M.-F.; Huang, Y.-H.; Chiu, L.-Y.; Cherng, S.-H.; Sheu, G.-T.; Yang, T.-Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated P38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef]
- Malhotra, L.; Goyal, H.K.V.; Jhuria, S.; Dev, K.; Kumar, S.; Kumar, M.; Kaur, P.; Ethayathulla, A.S. Curcumin Rescue P53Y220C in BxPC-3 Pancreatic Adenocarcinomas Cell Line: Evidence-Based on Computational, Biophysical, and in Vivo Studies. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129807. [Google Scholar] [CrossRef]
- Wang, G.; Duan, P.; Wei, Z.; Liu, F. Curcumin Sensitizes Carboplatin Treatment in Triple Negative Breast Cancer through Reactive Oxygen Species Induced DNA Repair Pathway. Mol. Biol. Rep. 2022, 49, 3259–3270. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Peng, C.; Peng, F. Potential Roles and Mechanisms of Curcumin and Its Derivatives in the Regulation of Ferroptosis. Int. J. Biol. Sci. 2024, 20, 4838. [Google Scholar] [CrossRef]
- Wu, L.; Xu, G.; Li, N.; Zhu, L.; Shao, G. Curcumin Analog, HO-3867, Induces Both Apoptosis and Ferroptosis via Multiple Mechanisms in NSCLC Cells with Wild-Type P53. Evid.-Based Complement. Altern. Med. 2023, 2023, 8378581. [Google Scholar] [CrossRef]
- Chen, M.; Tan, A.; Li, J. Curcumin Represses Colorectal Cancer Cell Proliferation by Triggering Ferroptosis via PI3K/Akt/MTOR Signaling. Nutr. Cancer 2023, 75, 726–733. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Lai, Z. The Role of Ferroptosis and Cuproptosis in Curcumin against Hepatocellular Carcinoma. Molecules 2023, 28, 1623. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, S.C.; Kipp, A.P.; Stuppner, H.; Koeberle, A. Ferroptosis-Modulating Small Molecules for Targeting Drug-Resistant Cancer: Challenges and Opportunities in Manipulating Redox Signaling. Med. Res. Rev. 2023, 43, 614–682. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin Suppresses Tumorigenesis by Ferroptosis in Breast Cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, F.; Zhao, J.; Zhang, W.; Chen, L.; Wang, X.; Yang, P.; Tang, J.; Chi, Y. Unraveling mitochondria-targeting reactive oxygen species modulation and their implementations in cancer therapy by nanomaterials. Exploration 2023, 3, 20220115. [Google Scholar] [CrossRef]
- Khatun, J.; Gelles, J.D.; Chipuk, J.E. Dynamic Death Decisions: How Mitochondrial Dynamics Shape Cellular Commitment to Apoptosis and Ferroptosis. Dev. Cell 2024, 59, 2549–2565. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, Y. Curcumin Activates the JNK Signaling Pathway to Promote Ferroptosis in Colon Cancer Cells. Chem. Biol. Drug Des. 2024, 103, e14468. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Huang, H.; Huang, F.; Liang, X.; Fu, Y.; Cheng, Z.; Huang, Y.; Chen, Z.; Duan, Y.; Chen, Y. Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma. Pharmaceuticals 2023, 16, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, J.; Zhu, N.; Ge, H.; Sheng, X.; Deng, S.; Chen, J.; Yu, L.; Zhou, Y.; Shen, J. Curcumin in Combination with Omacetaxine Suppress Lymphoma Cell Growth, Migration, Invasion, and Angiogenesis via Inhibition of VEGF/Akt Signaling Pathway. Front. Oncol. 2021, 11, 656045. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, K.; Francis, A.P.; Pajaniradje, S.; Rajagopalan, R. Synthetic Curcumin Analog: Inhibiting the Invasion, Angiogenesis, and Metastasis in Human Laryngeal Carcinoma Cells via NF-KB Pathway. Mol. Biol. Rep. 2021, 48, 6065–6074. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.; Chen, H.; Li, X.; Ye, C.; Zhang, F.; Zhang, Z.; Yao, Q.; Guo, Y. Curcumin Targeting NF-ΚB/Ubiquitin-Proteasome-System Axis Ameliorates Muscle Atrophy in Triple-Negative Breast Cancer Cachexia Mice. Mediat. Inflamm. 2022, 2022, 2567150. [Google Scholar] [CrossRef]
- Suer, I.; Abuaisha, A.; Kaya, M.; Abanoz, F.; Cefle, K.; Palanduz, S.; Ozturk, S. Curcumin Suppresses Cell Viability in Breast Cancer Cell Line by Affecting the Expression of MiR-15a-5p. Turkish J. Biochem. 2024, 49, 656–665. [Google Scholar] [CrossRef]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. Curcumin Suppresses the Stemness of Non-Small Cell Lung Cancer Cells via Promoting the Nuclear-Cytoplasm Translocation of TAZ. Environ. Toxicol. 2021, 36, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, C.; Ma, H.; Yang, Q. Curcumin Targets MiR-134-5p to Suppress the Progression of Colorectal Cancer through Regulating the CDCA3/CDK1 Pathway. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 109–122. [Google Scholar] [CrossRef]
- Xu, W.; Shen, Y. Curcumin Affects Apoptosis of Colorectal Cancer Cells through ATF6-Mediated Endoplasmic Reticulum Stress. Chem. Biol. Drug Des. 2024, 103, e14433. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Li, T.Y.; Liu, D.N.; Zhang, H.T. PI3K/Akt Pathway Involving into Apoptosis and Invasion in Human Colon Cancer Cells LoVo. Mol. Biol. Rep. 2014, 41, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, X.; Liu, F.; Li, Y.; Luo, B.; Huang, X.; Chen, H.; Wen, B.; Ma, P. Curcumin Inhibits the Development of Colorectal Cancer via Regulating the USP4/LAMP3 Pathway. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 397, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Reihani, R.Z.; Doustvandi, M.A.; Amini, M.; Zargari, F.; Baradaran, B.; Yari, A.; Hashemi, M.; Tohidast, M.; Mokhtarzadeh, A. Synergistic Anticancer Effects of Curcumin and Crocin on Human Colorectal Cancer Cells. Mol. Biol. Rep. 2022, 49, 8741–8752. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, P.; Liang, H.; Si, W. Curcumin Suppresses Copper Accumulation in Non-Small Cell Lung Cancer by Binding ATOX1. BMC Pharmacol. Toxicol. 2024, 25, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation Treatment of Primary Lung Cancer Using Liposomal Curcumin Dry Powder Inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Yan, J.; Hou, A.; Sui, W.; Sun, M. Curcumin Induces Ferroptosis in A549 CD133+ Cells through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discov. Med. 2023, 35, 251–263. [Google Scholar] [CrossRef]
- Shao, L.; Zhu, Y.; Liao, B.; Wang, G.; Huang, L.; Yu, L.; Bai, D. Effects of Curcumin-Mediated Photodynamic Therapy on Autophagy and Epithelial-Mesenchymal Transition of Lung Cancer Cells. Photodiagnosis Photodyn. Ther. 2022, 38, 102849. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Wang, X.; Xu, W.; Liu, F.; Hu, R.; Li, S. Curcumin Inhibits Prostate Cancer by Upregulating MiR-483-3p and Inhibiting UBE2C. J. Biochem. Mol. Toxicol. 2024, 38, e23645. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liu, L.; Shen, W.; Zheng, F.; Liu, M.; Sun, C. Anti-Cancer Role of Curcumin in Prostate Cancer Cells via Regulation of M6A-Modified Circ0030568-FMR1 Signaling Pathway. Transl. Androl. Urol. 2024, 13, 2358. [Google Scholar] [CrossRef]
- Xie, Q.; Hu, Y.; Zhang, C.; Zhang, C.; Qin, J.; Zhao, Y.; An, Q.; Zheng, J.; Shi, C. Curcumin Blunts Epithelial-Mesenchymal Transition to Alleviate Invasion and Metastasis of Prostate Cancer through the JARID1D Demethylation. Cancer Cell Int. 2024, 24, 303. [Google Scholar] [CrossRef]
- Mirzaei, A.; Jahanshahi, F.; Khatami, F.; Reis, L.O.; Aghamir, S.M.K. Human Prostate Cancer Cell Epithelial-to-Mesenchymal Transition as a Novel Target of Arsenic Trioxide and Curcumin Therapeutic Approach. Tissue Cell 2022, 76, 101805. [Google Scholar] [CrossRef]
- Pellegrino, M.; Bevacqua, E.; Frattaruolo, L.; Cappello, A.R.; Aquaro, S.; Tucci, P. Enhancing the Anticancer and Anti-Inflammatory Properties of Curcumin in Combination with Quercetin, for the Prevention and Treatment of Prostate Cancer. Biomedicines 2023, 11, 2023. [Google Scholar] [CrossRef]
- Ravindran, F.; Mhatre, A.; Koroth, J.; Narayan, S.; Choudhary, B. Curcumin Modulates Cell Type-Specific MiRNA Networks to Induce Cytotoxicity in Ovarian Cancer Cells. Life Sci. 2023, 334, 122224. [Google Scholar] [CrossRef]
- Huang, S.-L.; Chang, T.-C.; Sun, N.-K. Curcumin Reduces Paclitaxel Resistance in Ovarian Carcinoma Cells by Upregulating SNIP1 and Inhibiting NF$κ$B Activity. Biochem. Pharmacol. 2023, 212, 115581. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Saber, S.H.; Al-Raddadi, R.; Alamri, T.; Al-Jaouni, S.; Qari, M.; Qari, Y.; Haque, S.; Zawawi, A.; Ali, S.S.; et al. Novel Curcumin Nanoformulation Induces Apoptosis, and Reduces Migration and Angiogenesis in Liver Cancer Cells. Artif. Cells Nanomed. Biotechnol. 2023, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Moawad, M.; Nasr, G.M.; Osman, A.S.; Shaker, E.S. Curcumin Nanocapsules Effect in Apoptotic Processes, Gene Expression, and Cell Cycle on Hep-G2 Cell Lines. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231176396. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, H.; Covarrubias-Zambrano, O.; Jain, K.; Swamy, K.V.; Kasi, A.; Hamza, A.; Anant, S.; VanSaun, M.; Weir, S.J.; et al. Anticancer Activity of Novel Difluorinated Curcumin Analog and Its Inclusion Complex with 2-Hydroxypropyl-β-Cyclodextrin against Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 6336. [Google Scholar] [CrossRef]
- Guo, W.; Ding, Y.; Pu, C.; Wang, Z.; Deng, W.; Jin, X. Curcumin Inhibits Pancreatic Cancer Cell Proliferation by Regulating Beclin1 Expression and Inhibiting the Hypoxia-Inducible Factor-1α-Mediated Glycolytic Pathway. J. Gastrointest. Oncol. 2022, 13, 3254. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Y.; Liu, R.; Deng, J.; Chen, Q.; Chen, L.; Liang, G.; Chen, X.; Xu, Z. Curcumin Derivative C66 Suppresses Pancreatic Cancer Progression through the Inhibition of JNK-Mediated Inflammation. Molecules 2022, 27, 3076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, R.; Song, Z.; Yang, K.; He, H.; Jin, L.; Zhang, W. Curcumin Suppressed the Proliferation and Apoptosis of HPV-Positive Cervical Cancer Cells by Directly Targeting the E6 Protein. Phyther. Res. 2024, 38, 4967–4981. [Google Scholar] [CrossRef]
- Zheng, F.; Lu, J.; Wang, C.; Yu, H.; Fu, Y.; Ma, D. Curcumin Enhances ATG3-Dependent Autophagy and Inhibits Metastasis in Cervical Carcinoma. Cell Div. 2024, 19, 33. [Google Scholar] [CrossRef]
- Sadeghi, R.V.; Parsania, M.; Sadeghizadeh, M.; Haghighat, S. Investigation of Curcumin-Loaded OA400 Nanoparticle’s Effect on the Expression of E6 and E7 Human Papilloma-Virus Oncogenes and P53 and Rb Factors in HeLa Cell Line. Iran. J. Pharm. Res. IJPR 2022, 21. [Google Scholar]
- Wang, S.; Zhang, F.; Chen, J. Application and Potential Value of Curcumin in Prostate Cancer: A Meta-Analysis Based on Animal Models. Front. Pharmacol. 2024, 15, 1379389. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Invest. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Lv, Z.-D.; Liu, X.-P.; Zhao, W.-J.; Dong, Q.; Li, F.-N.; Wang, H.-B.; Kong, B. Curcumin Induces Apoptosis in Breast Cancer Cells and Inhibits Tumor Growth in Vitro and in Vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818. [Google Scholar]
- Shen, H.; Shen, J.; Pan, H.; Xu, L.; Sheng, H.; Liu, B.; Yao, M. Curcumin Analog B14 Has High Bioavailability and Enhances the Effect of Anti-Breast Cancer Cells in Vitro and in Vivo. Cancer Sci. 2021, 112, 815–827. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Kuzhuvelil, H.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin Sensitizes Human Colorectal Cancer to Capecitabine by Modulation of Cyclin D1, COX-2, MMP-9, VEGF and CXCR4 Expression in an Orthotopic Mouse Model. Int. J. Cancer 2009, 125, 2187–2197, Erratum in Int. J. Cancer 2010, 126, 799. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, R.; Xie, W.; Geng, H.; Zhao, L.; Xie, C.; Wu, J.; Geng, S.; Li, X.; Zhu, M.; et al. Curcumin Suppresses MAPK Pathways to Reverse Tobacco Smoke-Induced Gastric Epithelial--Mesenchymal Transition in Mice. Phyther. Res. 2015, 29, 1665–1671. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I Dose Escalation Trial of Docetaxel plus Curcumin in Patients with Advanced and Metastatic Breast Cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Weber, D.M.; Wang, M.; Giralt, S.A.; Thomas, S.K.; Alexanian, R.; Zhou, X.; Patel, P.; Bueso-Ramos, C.E.; Newman, R.A.; et al. Curcumin Downregulates NF-KB and Related Genes in Patients with Multiple Myeloma: Results of a Phase I/II Study. Blood 2007, 110, 1177. [Google Scholar] [CrossRef]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Woltz, M. Safety, Tolerability and Pharmacokinetics of Liposomal Curcumin (LipocurcTM) in Healthy Humans. Int. J. Clin. Pharmacol. Ther 2015, 53, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.B.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.L.; Scott, E.N.; Berry, D.P.; et al. Prolonged Biologically Active Colonic Tissue Levels of Curcumin Achieved after Oral Administration—a Clinical Pilot Study Including Assessment of Patient Acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens Jr, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364, Erratum in Cancer Prev. Res. 2012, 5, 1407. [Google Scholar] [CrossRef]
- Irving, G.R.B.; Iwuji, C.O.O.; Morgan, B.; Berry, D.P.; Steward, W.P.; Thomas, A.; Brown, K.; Howells, L.M. Combining Curcumin (C3-Complex, Sabinsa) with Standard Care FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer (CUFOX): Study Protocol for a Randomised Control Trial. Trials 2015, 16, 1–10. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Li, M.; Huang, Z.; Najafi, M. Targeting of the Tumor Microenvironment by Curcumin. Biofactors 2021, 47, 914–932. [Google Scholar] [CrossRef]
- Pereira, J.F.S.; Jordan, P.; Matos, P. A Signaling View into the Inflammatory Tumor Microenvironment. Immuno 2021, 1, 91–118. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Immunosuppressive Cells in Tumor Immune Escape and Metastasis. J. Mol. Med. 2016, 94, 509–522. [Google Scholar] [CrossRef]
- Cao, J.-F.; Hang, K.; Zhang, H.; Xia, Q.; Zhang, X.; Men, J.; Tian, J.; Xia, Z.; Liao, D.; Li, K. Mechanistic Insights Curcumin’s Anti-Inflammatory in Pancreatic Cancer: Experimental and Computational Evidence Implicating IL1B Interference via IL10RA Upregulation and NLRP3/TLR3 Downregulation. Front. Cell Dev. Biol. 2025, 13, 1601908. [Google Scholar] [CrossRef]
- Boroumand, N.; Samarghandian, S.; Hashemy, S.I. Immunomodulatory, Anti-Inflammatory, and Antioxidant Effects of Curcumin. J. Herbmed Pharmacol. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Prasad, S.; Saha, P.; Chatterjee, B.; Chaudhary, A.A.; Lall, R.; Srivastava, A.K. Complexity of Tumor Microenvironment: Therapeutic Role of Curcumin and Its Metabolites. Nutr. Cancer 2022, 75, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The Roles of Curcumin in Regulating the Tumor Immunosuppressive Microenvironment. Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Tang, J.; Gu, Z.; Wang, Y.; Yang, Y.; Yang, Y.; Yu, C. Eliciting Immunogenic Cell Death via a Unitized Nanoinducer. Nano Lett. 2020, 20, 6246–6254. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Westbom, C.M.; Sayan, M.; Shukla, A. Curcumin: A Double Hit on Malignant Mesothelioma. Cancer Prev. Res. 2014, 7, 330–340. [Google Scholar] [CrossRef]
- Reuter, S.; Charlet, J.; Juncker, T.; Teiten, M.-H.; Dicato, M.; Diederich, M. Effect of Curcumin on Nuclear Factor ΚB Signaling Pathways in Human Chronic Myelogenous K562 Leukemia Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 436–447. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. Phyther. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H.; et al. A Randomized, Phase 1, Placebo-Controlled Trial of APG-157 in Oral Cancer Demonstrates Systemic Absorption and an Inhibitory Effect on Cytokines and Tumor-Associated Microbes. Cancer 2020, 126, 1668–1682. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular Mechanism of Curcumin Action in Signaling Pathways: Review of the Latest Research. Phyther. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef]
- Chang, C.-S.; Chen, W.-N.; Lin, H.-H.; Wu, C.-C.; Wang, C.-J. Increased Oxidative DNA Damage, Inducible Nitric Oxide Synthase, Nuclear Factor $κ$B Expression and Enhanced Antiapoptosis-Related Proteins in Helicobacter Pylori-Infected Non-Cardiac Gastric Adenocarcinoma. World J. Gastroenterol. 2004, 10, 2232. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Evidence That Calebin A, a Component of Curcuma longa Suppresses NF-ΚB Mediated Proliferation, Invasion and Metastasis of Human Colorectal Cancer Induced by TNF-β (Lymphotoxin). Nutrients 2019, 11, 2904. [Google Scholar] [CrossRef]
- Tong, W.; Wang, Q.; Sun, D.; Suo, J. Curcumin Suppresses Colon Cancer Cell Invasion via AMPK-Induced Inhibition of NF-ΚB, UPA Activator and MMP9. Oncol. Lett. 2016, 12, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary Curcumin Modulates Transcriptional Regulators of Phase I and Phase II Enzymes in Benzo [a] Pyrene-Treated Mice: Mechanism of Its Anti-Initiating Action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [CrossRef]
- Barone, D.; Cito, L.; Tommonaro, G.; Abate, A.A.; Penon, D.; De Prisco, R.; Penon, A.; Forte, I.M.; Benedetti, E.; Cimini, A.; et al. Antitumoral Potential, Antioxidant Activity and Carotenoid Content of Two Southern Italy Tomato Cultivars Extracts: San Marzano and Corbarino. J. Cell. Physiol. 2018, 233, 1266–1277. [Google Scholar] [CrossRef]
- Fan, W.; Wang, F.; Jin, Z.; Zhu, L.; Zhang, J. Curcumin Synergizes with Cisplatin to Inhibit Colon Cancer through Targeting the MicroRNA-137-Glutaminase Axis. Curr. Med. Sci. 2022, 42, 108–117. [Google Scholar] [CrossRef]
- Arellano-Rodriguez, N.C.; Alvarez-Quezada, O.A.; Benavides, P.Z.; Vargas-Alanis, G.; Franco-Molina, M.; Zamora-Avila, D.; Arellano-Rodriguez, M.; Saavedra-Alonso, S.; Izaguirre-Álvarez, J.M.; Rodriguez-Padilla, C. Curcumin Sensitizes 4T1 Murine Breast Cancer Cells to Cisplatin through PAR4 Secretion. In Vivo 2022, 36, 2767–2773. [Google Scholar] [CrossRef]
- Sun, L.; Yao, X.; Liu, J.; Zhang, Y.; Hu, J. Curcumin Enhances the Efficacy of Docetaxel by Promoting Anti-Tumor Immune Response in Head and Neck Squamous Cell Carcinoma. Cancer Invest. 2023, 41, 524–533. [Google Scholar] [CrossRef]
- Deng, L.; Wu, X.; Zhu, X.; Yu, Z.; Liu, Z.; Wang, J.; Zheng, Y. Combination Effect of Curcumin with Docetaxel on the PI3K/AKT/MTOR Pathway to Induce Autophagy and Apoptosis in Esophageal Squamous Cell Carcinoma. Am. J. Transl. Res. 2021, 13, 57. [Google Scholar] [PubMed]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low Curcumin Concentration Enhances the Anticancer Effect of 5-Fluorouracil against Colorectal Cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, X.; Wang, R.; Guo, Y.; Xu, M. Effects of Curcumin on 5-Fluorouracil Resistance of Colon Cancer Cells through the PI3K/AKT/MTOR Pathway via MACC1. Eur. J. Integr. Med. 2022, 56, 102202. [Google Scholar] [CrossRef]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and Its Derivatives in Cancer Therapy: Potentiating Antitumor Activity of Cisplatin and Reducing Side Effects. Phyther. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef]
- Cheuk, I.W.; Chen, J.; Siu, M.; Ho, J.C.; Lam, S.S.; Shin, V.Y.; Kwong, A. Resveratrol Enhanced Chemosensitivity by Reversing Macrophage Polarization in Breast Cancer. Clin. Transl. Oncol. 2022, 1–10. [Google Scholar]
- Guo, W.; Wu, X.; Li, Y.; Gao, J.; Wang, F.; Jin, Y.; Chong, T.; Malhotra, A. Evaluation of Biophysical as Well as Biochemical Potential of Curcumin and Resveratrol during Prostate Cancer. J. Drug Target. 2020, 28, 41–45. [Google Scholar]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Gilardini Montani, M.S.; Cirone, M. New Insights into Curcumin-and Resveratrol-Mediated Anti-Cancer Effects. Pharmaceuticals 2021, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Altundaug, E.; Yilmaz, A.M.; Serdar, B.S.; Jannuzzi, A.T.; Koçtürk, S.; Yalçin, A.S. Synergistic Induction of Apoptosis by Quercetin and Curcumin in Chronic Myeloid Leukemia (K562) Cells: II. Signal Transduction Pathways Involved. Nutr. Cancer 2021, 73, 703–712. [Google Scholar] [CrossRef]
- Dian, C.; Qian, Z.; Ran, M.; Yan, X.; Dian, L. Co-Delivery of Docetaxel and Curcumin Functionalized Mixed Micelles for the Treatment of Drug-Resistant Breast Cancer by Oral Administration. Int. J. Nanomedicine 2024, 8603–8620. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Mohammadi, S.; Mahmoudi, R.; Fouani, M.H.; Ardakani, M.T.; Hadi, A.; Nikseresht, M.; Barmak, M.J.; Karimpour, F.; Bardania, H. Nanocodelivery of 5-Fluorouracil and Curcumin by RGD-Decorated Nanoliposomes Achieves Synergistic Chemotherapy for Breast Cancer. IET Nanobiotechnol. 2024, 2024, 4959295. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Shi, M.; Yu, Y.; Wang, H.; Ou, R.; Ge, R. CP41, a Novel Curcumin Analogue, Induces Apoptosis in Endometrial Cancer Cells by Activating the H3F3A/Proteasome-MAPK Signaling Pathway and Enhancing Oxidative Stress. Life Sci. 2024, 338, 122406. [Google Scholar]
- Rahim, N.F.C.; Hussin, Y.; Aziz, M.N.M.; Mohamad, N.E.; Yeap, S.K.; Masarudin, M.J.; Abdullah, R.; Akhtar, M.N.; Alitheen, N.B. Cytotoxicity and Apoptosis Effects of Curcumin Analogue (2E, 6E)-2, 6-Bis (2, 3-Dimethoxybenzylidine) Cyclohexanone (DMCH) on Human Colon Cancer Cells HT29 and SW620 in Vitro. Molecules 2021, 26, 1261. [Google Scholar] [CrossRef]
- Moordiani, M.; Novitasari, D.; Susidarti, R.A.; Kato, J.; Meiyanto, E. Curcumin Analogs PGV-1 and CCA-1.1 Induce Cell Cycle Arrest in Human Hepatocellular Carcinoma Cells with Overexpressed MYCN. Indones. Biomed. J. 2023, 15, 141–149. [Google Scholar] [CrossRef]
- Lima, F.T.; Seba, V.; Silva, G.; Torrezan, G.S.; Polaquini, C.R.; Pinhanelli, V.C.; Baek, S.J.; Fachin, A.L.; Regasini, L.O.; Marins, M. The Curcumin Analog CH-5 Exerts Anticancer Effects in Human Osteosarcoma Cells via Modulation of Transcription Factors P53/Sp1. Int. J. Mol. Sci. 2018, 19, 1909. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, L.; Chen, S.; Zheng, B.; Chen, H.; Zeng, T.; Sun, H.; Zhong, S.; Wu, W.; Lin, X.; et al. The Curcumin Analogue WZ35 Affects Glycolysis Inhibition of Gastric Cancer Cells through ROS-YAP-JNK Pathway. Food Chem. Toxicol. 2020, 137, 111131. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Baek, J.-H.; Sundaramoorthy, P.; Yoo, B.K. In Vitro Cytotoxicity and Bioavailability of Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin. AAPS PharmSciTech 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Baskaran, R.; Maeng, H.-J.; Yoo, B.K. Ginsenoside Improves Physicochemical Properties and Bioavailability of Curcumin-Loaded Nanostructured Lipid Carrier. Arch. Pharm. Res. 2017, 40, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.K.K.; Uzunhisarc\ikl\i, E.; Yerer, M.B.; Bishayee, A. The Golden Spice Curcumin in Cancer: A Perspective on Finalized Clinical Trials during the Last 10 Years. J. Cancer Res. Ther. 2022, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- de Man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Wu, Z.; Xu, Y.; Jiang, R. Curcumin for Treating Breast Cancer: A Review of Molecular Mechanisms, Combinations with Anticancer Drugs, and Nanosystems. Pharmaceutics 2024, 16, 79. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A. V Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; O’Neil, B.H.; McRee, A.J.; Sanoff, H.K.; Fallon, J.K.; Smith, P.C.; Ivanova, A.; Moore, D.T.; Dumond, J.; Asher, G.N. A Phase I Evaluation of the Effect of Curcumin on Dose-Limiting Toxicity and Pharmacokinetics of Irinotecan in Participants with Solid Tumors. Clin. Transl. Sci. 2022, 15, 1304–1315. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin Inhibits Cancer Stem Cell Phenotypes in Ex Vivo Models of Colorectal Liver Metastases, and Is Clinically Safe and Tolerable in Combination with FOLFOX Chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and Safety of Curcumin in Combination with Paclitaxel in Patients with Advanced, Metastatic Breast Cancer: A Comparative, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome Complex of Curcumin as Complementary Therapy of Advanced Pancreatic Cancer Improves Safety and Efficacy of Gemcitabine: Results of a Prospective Phase II Trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Joshi, P.; Bisht, A.; Paliwal, A.; Dwivedi, J.; Sharma, S. Recent Updates on Clinical Developments of Curcumin and Its Derivatives. Phyther. Res. 2023, 37, 5109–5158. [Google Scholar] [CrossRef]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, Distribution, Metabolism, and Excretion of Nanocarriers in Vivo and Their Influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef]
- Marin, E.; Briceño, M.I.; Torres, A.; Caballero-George, C. New Curcumin-Loaded Chitosan Nanocapsules: In Vivo Evaluation. Planta Med. 2017, 83, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kunati, S.R.; Yang, S.; William, B.M.; Xu, Y. An LC--MS/MS Method for Simultaneous Determination of Curcumin, Curcumin Glucuronide and Curcumin Sulfate in a Phase II Clinical Trial. J. Pharm. Biomed. Anal. 2018, 156, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P. V Enhancing Curcumin Oral Bioavailability through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Jiang, J.; Wei, P.; Sun, H. Triggered “On/off” Luminescent Polypeptide Bowl-Shaped Nanoparticles for Selective Lighting of Tumor Cells. Small 2025, 21, 2411432. [Google Scholar] [CrossRef]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Rebello, L.; Chepyala, S. Review on Nanoformulations of Curcumin (Curcuma longa Linn.): Special Emphasis on Nanocurcumin®. Int. J. Nat. Life Sci. 2019, 3, 1–12. [Google Scholar]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Curcumin: A Therapeutic Potential in Ageing-Related Disorders. PharmaNutrition 2020, 14, 100226. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Hosny, E.N.; Khadrawy, Y.A.; Magdy, M.; Attia, Y.S.; Sayed, O.A.; AbdElaal, M. Protective Effect of Curcumin Nanoparticles against Cardiotoxicity Induced by Doxorubicin in Rat. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 2020, 1866, 165665. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-Loaded Redox-Responsive Mesoporous Silica Nanoparticles for Targeted Breast Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 921–935. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Ebeling, M.C.; Khan, S.; Sundram, V.; Chauhan, N.; Gupta, B.K.; Puumala, S.E.; Jaggi, M.; Chauhan, S.C. Novel Curcumin-Loaded Magnetic Nanoparticles for Pancreatic Cancer Treatment. Mol. Cancer Ther. 2013, 12, 1471–1480. [Google Scholar] [CrossRef]
- Dourado, D.; Miranda, J.A.; de Oliveira, M.C.; Freire, D.T.; Xavier-Junior, F.H.; Paredes-Gamero, E.J.; Alencar, E. do N. Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for in Vivo Cancer Therapy. Pharmaceutics 2024, 16, 177. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive Efficacy and Pharmacokinetics of Curcumin in the Min/+ Mouse, a Model of Familial Adenomatous Polyposis. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 535–540. [Google Scholar]
- Park, J.; Conteas, C.N. Anti-Carcinogenic Properties of Curcumin on Colorectal Cancer. World J. Gastrointest. Oncol. 2010, 2, 169. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of Systemic Availability of Curcumin with That of Curcumin Formulated with Phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral Bioavailability of Curcumin in Rat and the Herbal Analysis from Curcuma longa by LC--MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Muller, R.H.; Gohla, S.; Keck, C.M. State of the Art of Nanocrystals-Special Features, Production, Nanotoxicology Aspects and Intracellular Delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Jahagirdar, P.S.; Gupta, P.K.; Kulkarni, S.P.; Devarajan, P. V Polymeric Curcumin Nanoparticles by a Facile in Situ Method for Macrophage Targeted Delivery. Bioeng. Transl. Med. 2019, 4, 141–151. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiang, Z.; Akakuru, O.U.; Li, J.; Wu, A. Nanoscale Covalent Organic Frameworks: From Controlled Synthesis to Cancer Therapy. Chem. Commun. 2021, 57, 12417–12435. [Google Scholar] [CrossRef]
- Nie, Y.; Li, D.; Peng, Y.; Wang, S.; Hu, S.; Liu, M.; Ding, J.; Zhou, W. Metal Organic Framework Coated MnO2 Nanosheets Delivering Doxorubicin and Self-Activated DNAzyme for Chemo-Gene Combinatorial Treatment of Cancer. Int. J. Pharm. 2020, 585, 119513. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Y.; Xu, J.; Zheng, Y.; Zhou, W.; Wang, Y.; Luo, C. Precisely Tailoring Molecular Structure of Doxorubicin Prodrugs to Enable Stable Nanoassembly, Rapid Activation, and Potent Antitumor Effect. Pharmaceutics 2024, 16, 1582. [Google Scholar] [CrossRef]
- Guo, L.; Fu, Z.; Li, H.; Wei, R.; Guo, J.; Wang, H.; Qi, J. Smart Hydrogel: A New Platform for Cancer Therapy. Adv. Colloid Interface Sci. 2025, 340, 103470. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.O.; Marques, M.B.D.F.; Kato, K.C.; Carneiro, G. Nanonization Techniques to Overcome Poor Water-Solubility with Drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Devel. Ther. 2024, 1469–1495. [Google Scholar] [CrossRef]
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent Developments in Formulation Design for Improving Oral Bioavailability of Curcumin: A Review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; de Cássia Ribeiro, T.; Carvalho, G.C.; Sábio, R.M.; Marchetti, J.M.; Chorilli, M. Folic Acid-Modified Curcumin-Loaded Liposomes for Breast Cancer Therapy. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 645, 128935. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Xu, M.; Luo, Y.; Wang, B.; Kuang, M.; Liu, X.; Sun, M.; Guo, Y.; Teng, L.; et al. Pulmonary Delivery of Liposomes Co-Loaded with SN38 Prodrug and Curcumin for the Treatment of Lung Cancer. Eur. J. Pharm. Biopharm. 2022, 179, 156–165. [Google Scholar] [CrossRef]
- Wu, J.; Qi, C.; Wang, H.; Wang, Q.; Sun, J.; Dong, J.; Yu, G.; Gao, Z.; Zhang, B.; Tian, G. Curcumin and Berberine Co-Loaded Liposomes for Anti-Hepatocellular Carcinoma Therapy by Blocking the Cross-Talk between Hepatic Stellate Cells and Tumor Cells. Front. Pharmacol. 2022, 13, 961788. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Sheashaa, R.F.; Moussa, E.A.; Mahfouz, M.E. Potential of Curcumin and Niacin-Loaded Targeted Chitosan Coated Liposomes to Activate Autophagy in Hepatocellular Carcinoma Cells: An in Vitro Evaluation in HePG2 Cell Line. Int. J. Biol. Macromol. 2023, 245, 125572. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Y.; Yan, F.; Zhang, M.; Wu, W. Novel Targeting Liposomes with Enhanced Endosomal Escape for Co-Delivery of Doxorubicin and Curcumin. Colloids Surfaces B Biointerfaces 2025, 245, 114267. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Du, S.; Guan, Y.; Qiu, J.; Chen, X.; Yuan, D.; Chen, T. Nanoparticles for Co-Delivery of Paclitaxel and Curcumin to Overcome Chemoresistance against Breast Cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 104050. [Google Scholar] [CrossRef]
- Ahmadi, F.; Akbari, J.; Saeedi, M.; Seyedabadi, M.; Ebrahimnejad, P.; Ghasemi, S.; Nokhodchi, A. Efficient Synergistic Combination Effect of Curcumin with Piperine by Polymeric Magnetic Nanoparticles for Breast Cancer Treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104624, Correction in J. Drug Deliv. Sci. Technol. 2025, 107416. [Google Scholar] [CrossRef]
- Sugumaran, A.; Sadhasivam, J.; Gawas, P.; Nutalapati, V.; Pandian, R.; Perumal, S.K. Curcumin Conjugated Dextran Coated Fe3O4 Nanoparticles: Cytotoxic Effect on Lung Cancer Cell Line A549. Mater. Sci. Eng. B 2022, 286, 116047. [Google Scholar] [CrossRef]
- Li, M.; Fang, G.; Zahid, F.; Saleem, R.; Ishrat, G.; Ali, Z.; Naeem, M.; ud Din, F. Co-Delivery of Paclitaxel and Curcumin Loaded Solid Lipid Nanoparticles for Improved Targeting of Lung Cancer: In Vitro and in Vivo Investigation. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Wei, Y.; Li, K.; Zhao, W.; He, Y.; Shen, H.; Yuan, J.; Pi, C.; Zhang, X.; Zeng, M.; Fu, S.; et al. The Effects of a Novel Curcumin Derivative Loaded Long-Circulating Solid Lipid Nanoparticle on the MHCC-97H Liver Cancer Cells and Pharmacokinetic Behavior. Int. J. Nanomed. 2022, 2225–2241. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ali, A.; Rahamathulla, M.; Salam, S.; Hani, U.; Wahab, S.; Warsi, M.H.; Yusuf, M.; Ali, A.; Mittal, V.; et al. Fabrication of Sustained Release Curcumin-Loaded Solid Lipid Nanoparticles (Cur-SLNs) as a Potential Drug Delivery System for the Treatment of Lung Cancer: Optimization of Formulation and in Vitro Biological Evaluation. Polymers 2023, 15, 542. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, W.; Zeng, M.; Yuan, J.; Shen, H.; Li, K.; Su, Z.; Liu, Z.; Wen, J.; Song, X.; et al. Anti-Lung Cancer Effect of Paclitaxel Solid Lipid Nanoparticles Delivery System with Curcumin as Co-Loading Partner in Vitro and in Vivo. Drug Deliv. 2022, 29, 1878–1891. [Google Scholar] [CrossRef]
- Nurjis, F.; Sarwar, U.; Ali, J.S.; Fayyaz, M. Doxorubicin and Curcumin-Loaded Nanomicelles Targeting Multidrug Resistance in Cancer. Bionanoscience 2024, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; He, K.; Cao, T.; Song, D.; Yang, H.; Li, L.; Lin, J. The Apoptosis of Liver Cancer Cells Promoted by Curcumin/TPP-CZL Nanomicelles with Mitochondrial Targeting Function. Front. Bioeng. Biotechnol. 2022, 10, 804513. [Google Scholar] [CrossRef]
- Mohebian, Z.; Babazadeh, M.; Zarghami, N. In Vitro Efficacy of Curcumin-Loaded Amine-Functionalized Mesoporous Silica Nanoparticles against MCF-7 Breast Cancer Cells. Adv. Pharm. Bull. 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, F.; Xing, Z.; Fan, L.; Li, Y.; Wang, S.; Ling, J.; Ouyang, X.-K. Efficient Delivery of Curcumin by Alginate Oligosaccharide Coated Aminated Mesoporous Silica Nanoparticles and in Vitro Anticancer Activity against Colon Cancer Cells. Pharmaceutics 2022, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhou, W.; Jiang, X.; Ma, J. Bovine Serum Albumin and Folic Acid-Modified Aurum Nanoparticles Loaded with Paclitaxel and Curcumin Enhance Radiotherapy Sensitization for Esophageal Cancer. Int. J. Radiat. Biol. 2024, 100, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Zeynalzadeh, S.; Dehghani, E.; Hassani, A.; Baradar Khoshfetrat, A.; Salami-Kalajahi, M. Effect of Curcumin-Loaded Poly (Amidoamine) Dendrimer on Cancer Cell Lines: A Comparison between Physical Loading and Chemical Conjugation of Drug. Polym. Bull. 2024, 81, 1439–1452. [Google Scholar] [CrossRef]
- Kang, N.; Choi, M.; Lee, M.; Choi, J.S. Dendrimeric Micelles Composed of Polyamidoamine Dendrimer-Peptide-Cholesterol Conjugates as Drug Carriers for the Treatment of Melanoma and Bacterial Infection. J. Ind. Eng. Chem. 2022, 114, 361–376. [Google Scholar]
- Roquito, T.; Colaço, M.; Costa, J.P.; Borges, O. Curcumin-Encapsulated Glucan Nanoparticles as an Oxidative Stress Modulator against Human Hepatic Cancer Cells. Colloids Surf. B Biointerfaces 2025, 245, 114326. [Google Scholar] [CrossRef] [PubMed]
- Kargar, B.; Fazeli, M.; Sobhani, Z.; Hosseinzadeh, S.; Solhjoo, A.; Akbarizadeh, A.R. Exploration of the Photothermal Role of Curcumin-Loaded Targeted Carbon Nanotubes as a Potential Therapy for Melanoma Cancer. Sci. Rep. 2024, 14, 10117. [Google Scholar] [CrossRef]
- Chattaraj, A.; Mishra, Y.; Aljabali, A.A.A.; Mishra, V. Development and Evaluation of Folic Acid Conjugated Curcumin-Loaded Functionalized Multiwalled Carbon Nanotubes for Enhanced Efficacy in Ovarian Cancer Treatment. Carbon Trends 2025, 100464. [Google Scholar] [CrossRef]
- Babaei, M.; Abrishami, A.; Iranpour, S.; Saljooghi, A.S.; Matin, M.M. Harnessing Curcumin in a Multifunctional Biodegradable Metal-Organic Framework (Bio-MOF) for Targeted Colorectal Cancer Theranostics. Drug Deliv. Transl. Res. 2024, 1–20. [Google Scholar] [CrossRef]
- Bazzazan, S.; Moeinabadi-Bidgoli, K.; Lalami, Z.A.; Bazzazan, S.; Mehrarya, M.; Yeganeh, F.E.; Hejabi, F.; Akbarzadeh, I.; Noorbazargan, H.; Jahanbakhshi, M.; et al. Engineered UIO-66 Metal-Organic Framework for Delivery of Curcumin against Breast Cancer Cells: An in Vitro Evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 104009. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the Oral Bioavailability of Curcumin Using Solid Lipid Nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Min, T. Curcumin, Curcumin Nanoparticles and Curcumin Nanospheres: A Review on Their Pharmacodynamics Based on Monogastric Farm Animal, Poultry and Fish Nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-Based Nanocarriers for Encapsulation and Delivery of Curcumin: A Review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. Aaps Pharmscitech 2019, 20, 69. [Google Scholar] [CrossRef]
- Kral, M.; Dendisova, M.; Matvejka, P.; Svoboda, J.; Pop-Georgievski, O. Infrared Imaging of Surface Confluent Polydopamine (PDA) Films at the Nanoscale. Colloids Surf. B Biointerfaces 2023, 221, 112954. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tang, J.; Xing, Y.; Wang, Z.; Ding, T.; Zhang, J.; Cai, K. Intervention of Polydopamine Assembly and Adhesion on Nanoscale Interfaces: State-of-the-Art Designs and Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Xu, Z.P. Understanding of Polydopamine Encapsulation of Hydrophobic Curcumin for Pleiotropic Drug Nanoformulation. Part. Part. Syst. Charact. 2023, 40, 2200132. [Google Scholar] [CrossRef]
- Azizi, N.; Eslami, R.; Goudarzi, S.; Cho, Y.H.; McPhee, J.B.; Zarrin, H. Bioinspired, Metal-Free Modification of Cotton Fabric Using Polydopamine-Coated Curcumin for Health-Protective Clothing. Cellulose 2024, 31, 3185–3204. [Google Scholar] [CrossRef]
- Yan, S.; Liao, X.; Xiao, Q.; Huang, Q.; Huang, X. Photostabilities and Anti-Tumor Effects of Curcumin and Curcumin-Loaded Polydopamine Nanoparticles. RSC Adv. 2024, 14, 13694–13702. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z. Polydopamine Nanoparticles-Coated Curcumin Upregulates the Expression of Nrf2 Through the Keap1/ARE Signaling Pathway to Improve Liver Injury in Mice with Liver Cancer. Pharmacogn. Mag. 2025, 09731296251313613. [Google Scholar] [CrossRef]
- Chen, X.; Lei, S.; Ning, Y.; Zhou, L.; Guo, Y.; Xu, R.; Wu, J. Injectable Polydopamine/Curcumin Dual-Modified Polylactic Acid/Polycaprolactone Coaxial Staple Fibers for Chronotropic Treatment of Oral Squamous Cell Carcinoma. Int. J. Biol. Macromol. 2025, 292, 139094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Nie, G. Biomembrane-Based Nanostructures for Cancer Targeting and Therapy: From Synthetic Liposomes to Natural Biomembranes and Membrane-Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113974. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.-X.; Chen, Z.-S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Zeng, M.; Guo, D.; Fernández-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Yang, H.; Liu, X.; Wang, Y.-C.; Zeng, Y.; et al. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharm. 2022, 20, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Aliakbari, F.; Volkening, K.; Strong, M.J.; Rohani, S. New Metal-Organic Framework Coated Sodium Alginate for the Delivery of Curcumin as a Sustainable Drug Delivery and Cancer Therapy System. Int. J. Biol. Macromol. 2024, 259, 128875. [Google Scholar] [CrossRef]
- Alavijeh, R.K.; Akhbari, K. Improvement of Curcumin Loading into a Nanoporous Functionalized Poor Hydrolytic Stable Metal-Organic Framework for High Anticancer Activity against Human Gastric Cancer AGS Cells. Colloids Surf. B Biointerfaces 2022, 212, 112340. [Google Scholar]
- Liu, J.; Movahedi, F.; Sun, B.; Sun, L.; Zhang, B.; Wang, J.; Li, L.; Xu, Z.P. Immunostimulatory Photochemotherapeutic Nanocapsule for Enhanced Colon Cancer Treatment. Nanophotonics 2021, 10, 3321–3337. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Lin, J.; Ge, G.; Fang, J.; Song, W.; Xiao, G.G.; Zhang, B.; Peng, X.; Duo, Y.; et al. Multifunctional Calcium-Manganese Nanomodulator Provides Antitumor Treatment and Improved Immunotherapy via Reprogramming of the Tumor Microenvironment. ACS Nano 2023, 17, 15449–15465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Huang, Y.; Xu, Y.; Zou, B. Nanotechnology and Curcumin: A Novel and Promising Approach in Digestive Cancer Therapy. Nanomedicine 2023, 18, 2081–2099. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Kuo, Y.-L.; Chen, C.-H.; Wu, H.-T.; Chen, H.-W.; Fang, W.-P. Improving the Stability and Bioactivity of Curcumin Using Chitosan-Coated Liposomes through a Combination Mode of High-Pressure Processing. LWT 2022, 168, 113946. [Google Scholar] [CrossRef]
- Hua, Y.; Qin, Z.; Gao, L.; Zhou, M.; Xue, Y.; Li, Y.; Xie, J. Protein Nanoparticles as Drug Delivery Systems for Cancer Theranostics. J. Control Release 2024, 371, 429–444. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, G.; Huang, J.; Wu, W.; Cui, Z.; Li, H.; Zhang, L.; Qi, H. Mussel-Inspired Protein-Based Nanoparticles for Curcumin Encapsulation and Promoting Antitumor Efficiency. Int. J. Biol. Macromol. 2024, 132965. [Google Scholar] [CrossRef]
- You, W.; Zhou, Z.; Li, Z.; Yan, J.; Wang, Y. From Foe to Friend: Rewiring Oncogenic Pathways through Artificial Selenoprotein to Combat Immune-Resistant Tumor. J. Pharm. Anal. 2025, 101322. [Google Scholar] [CrossRef]
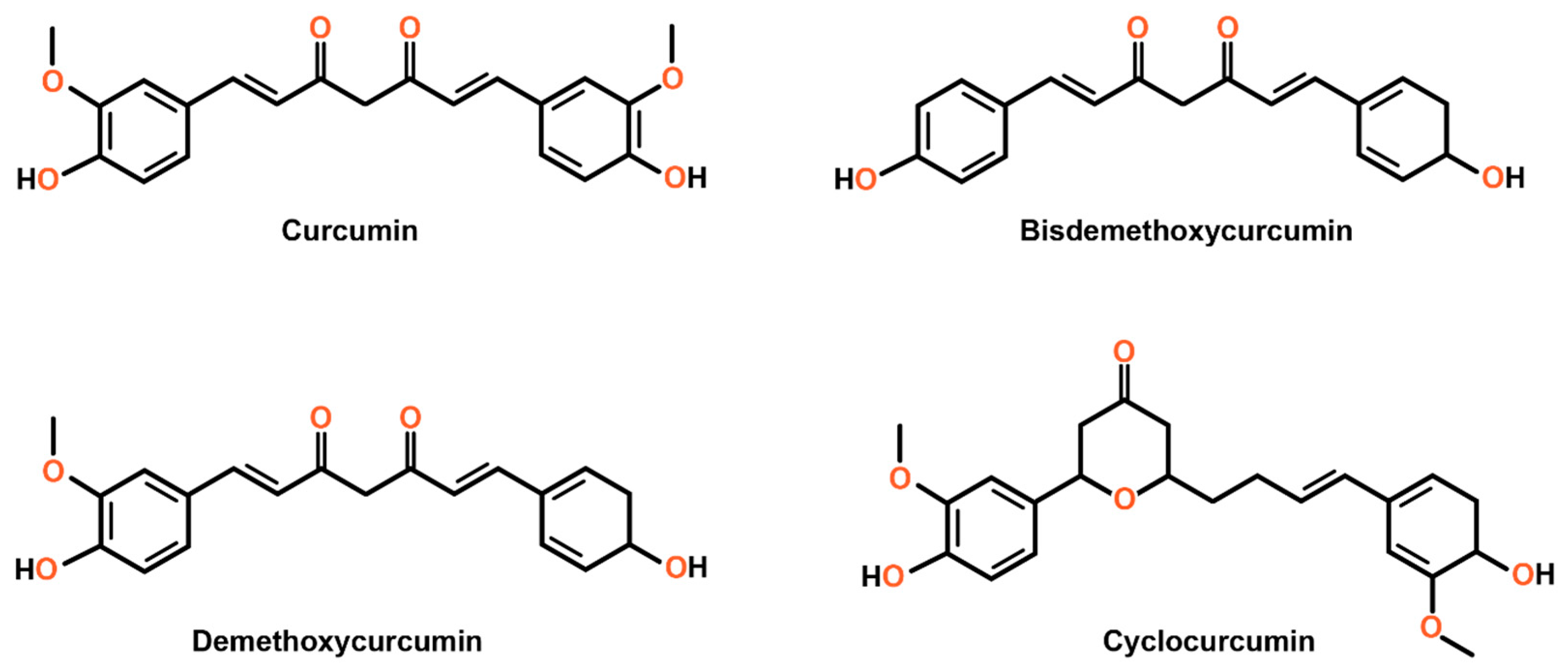
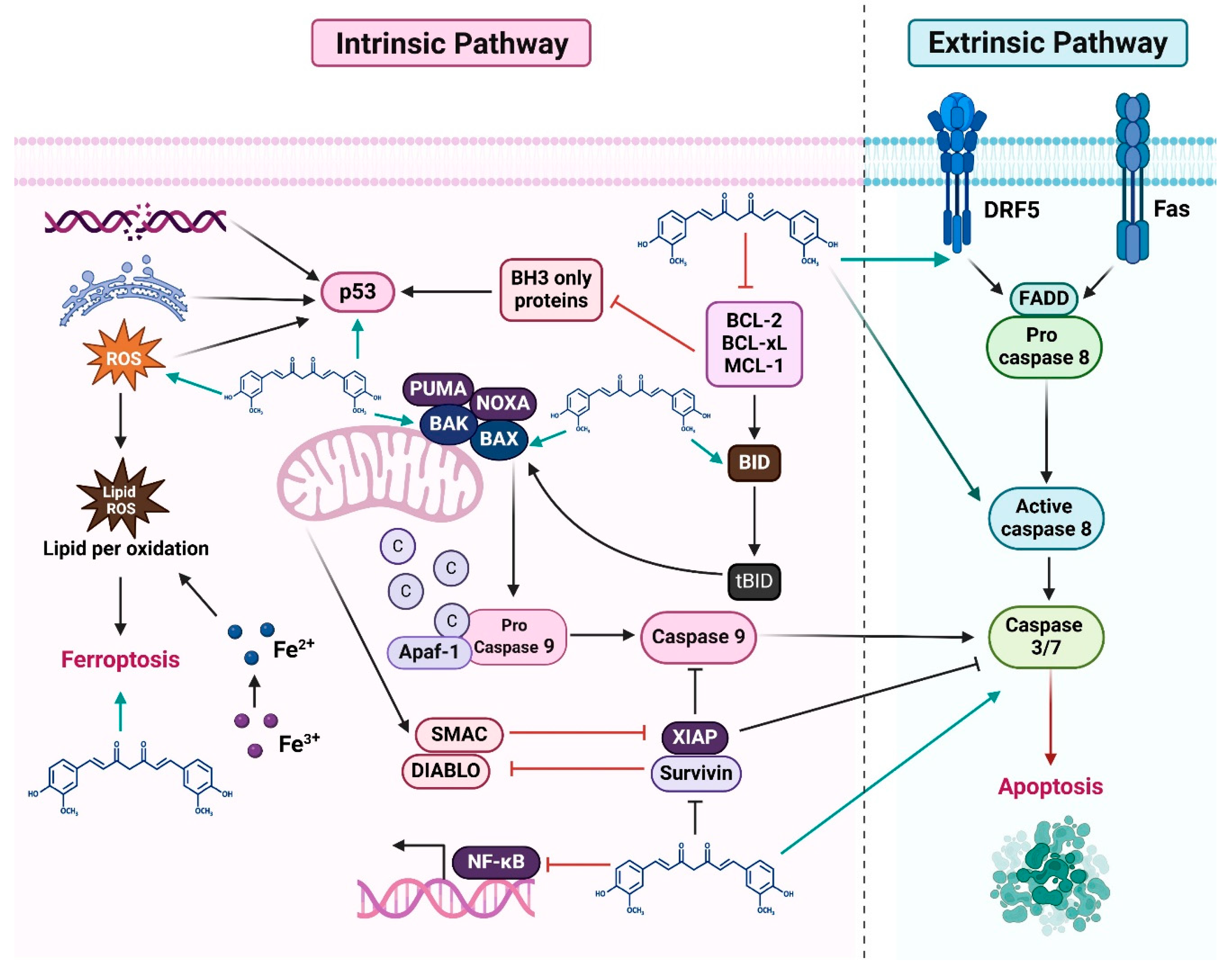
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, K.; Gul, K.; Mumtaz, S. Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations. Curr. Issues Mol. Biol. 2025, 47, 716. https://doi.org/10.3390/cimb47090716
Akter K, Gul K, Mumtaz S. Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations. Current Issues in Molecular Biology. 2025; 47(9):716. https://doi.org/10.3390/cimb47090716
Chicago/Turabian StyleAkter, Khadija, Kainat Gul, and Sohail Mumtaz. 2025. "Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations" Current Issues in Molecular Biology 47, no. 9: 716. https://doi.org/10.3390/cimb47090716
APA StyleAkter, K., Gul, K., & Mumtaz, S. (2025). Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations. Current Issues in Molecular Biology, 47(9), 716. https://doi.org/10.3390/cimb47090716







