The Extract of Periplaneta americana (L.) Promotes Hair Regrowth in Mice with Alopecia by Regulating the FOXO/PI3K/AKT Signaling Pathway and Skin Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Major Reagents and Instruments
2.2. Extraction Method of PA Extract PA-011
2.3. Network Pharmacology Analysis of PA-011 in Ap
2.3.1. Identification of PA-011 Components
2.3.2. Target Acquisition for PA-011
2.3.3. Acquisition of Ap Disease Targets
2.3.4. Construction of Protein–Protein Interaction (PPI) Network for Disease Targets
2.3.5. GO and KEGG Enrichment Analysis
2.3.6. Construction of Component-Target-Pathway Network
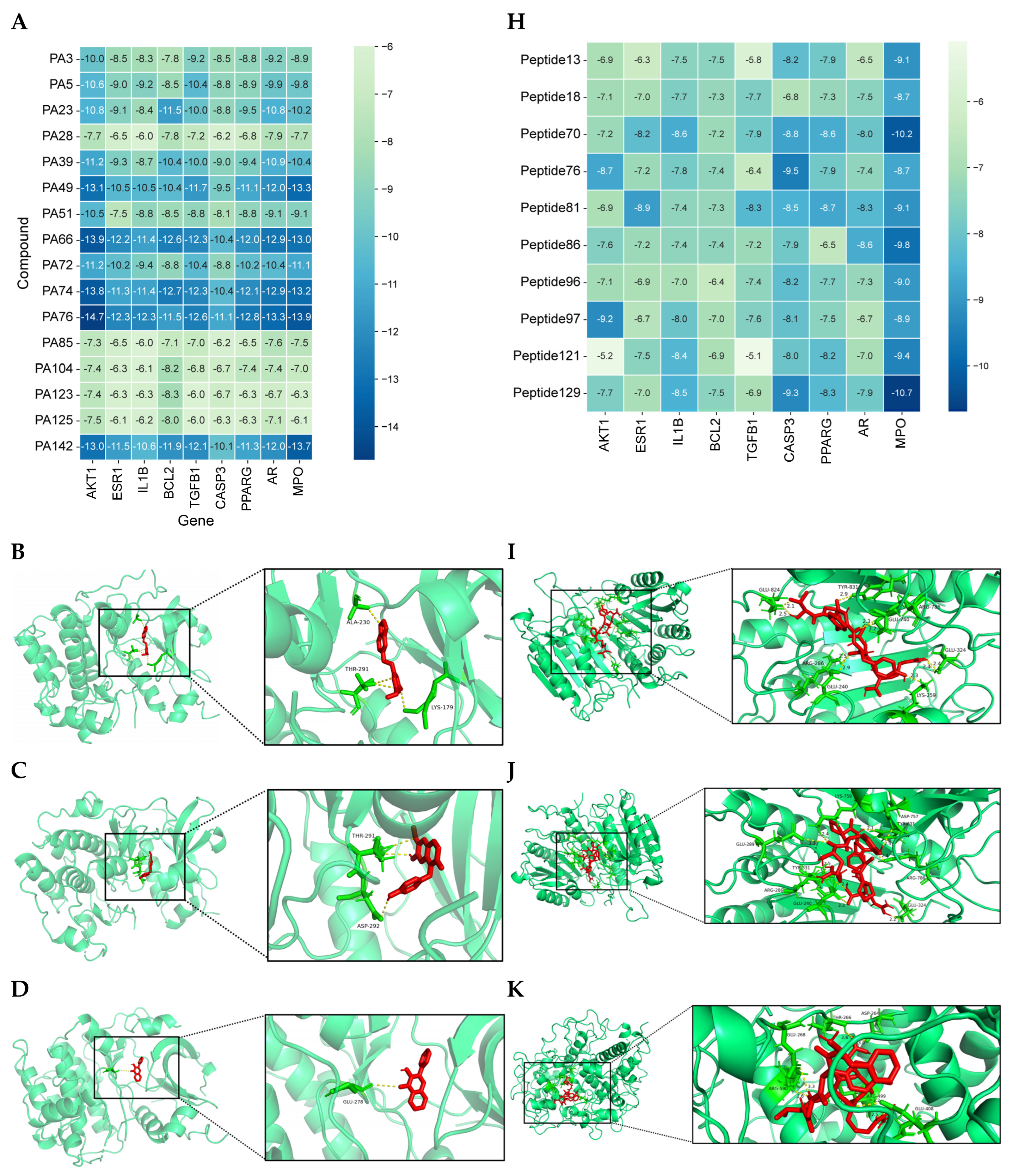
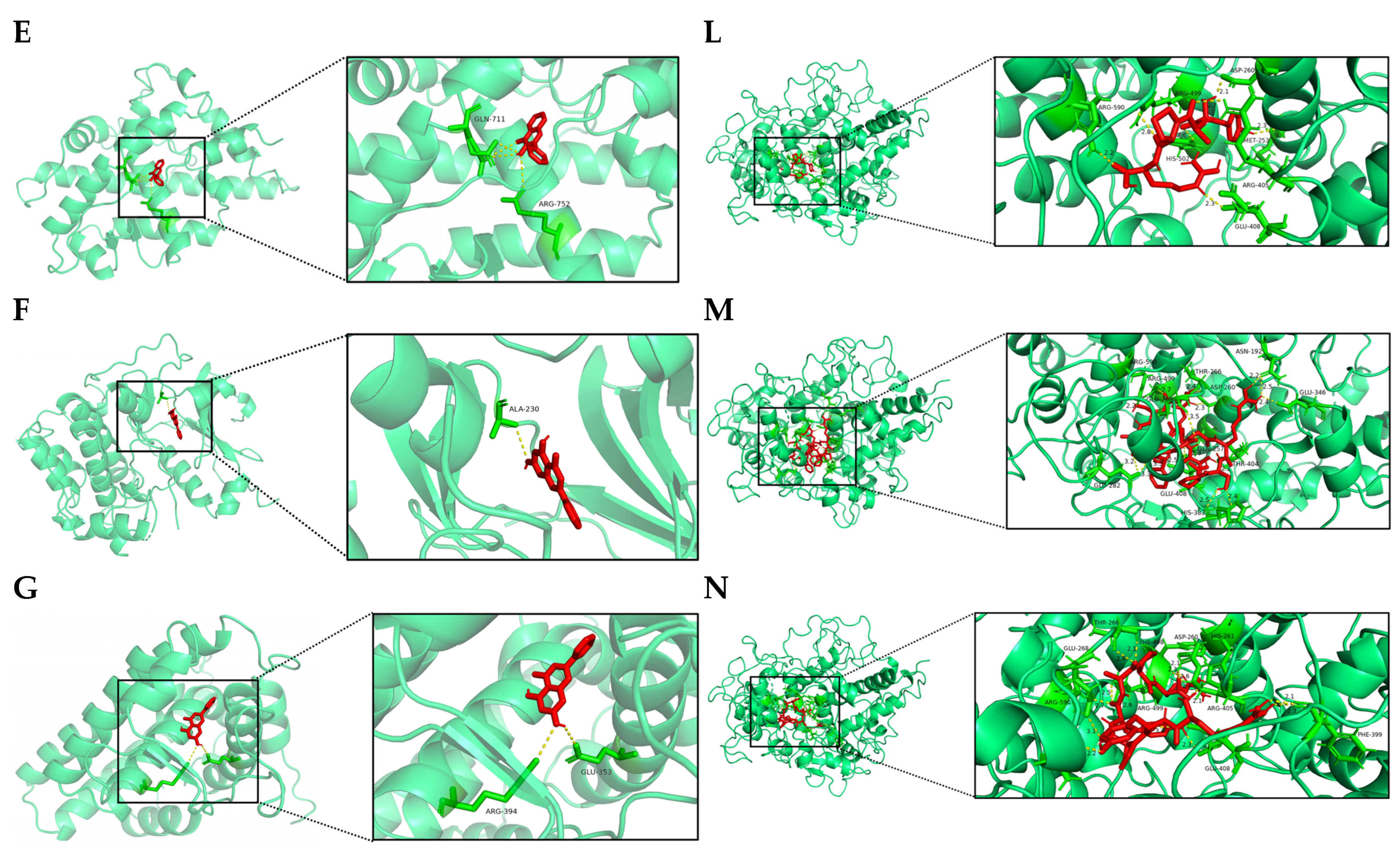
2.3.7. Molecular Docking
2.4. Experimental Animals
2.5. Establishment of Ap Mouse Model
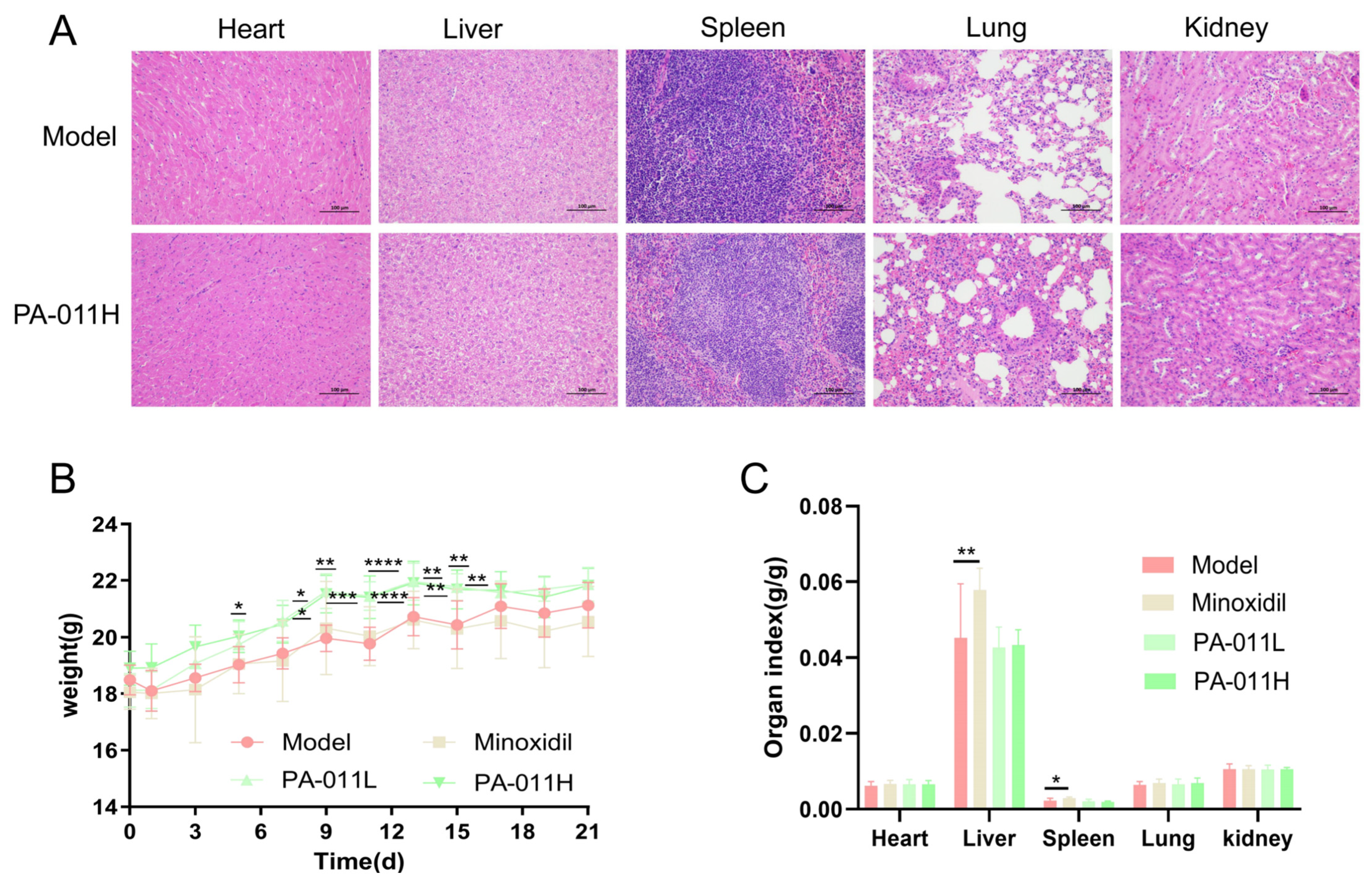
2.6. Safety Evaluation
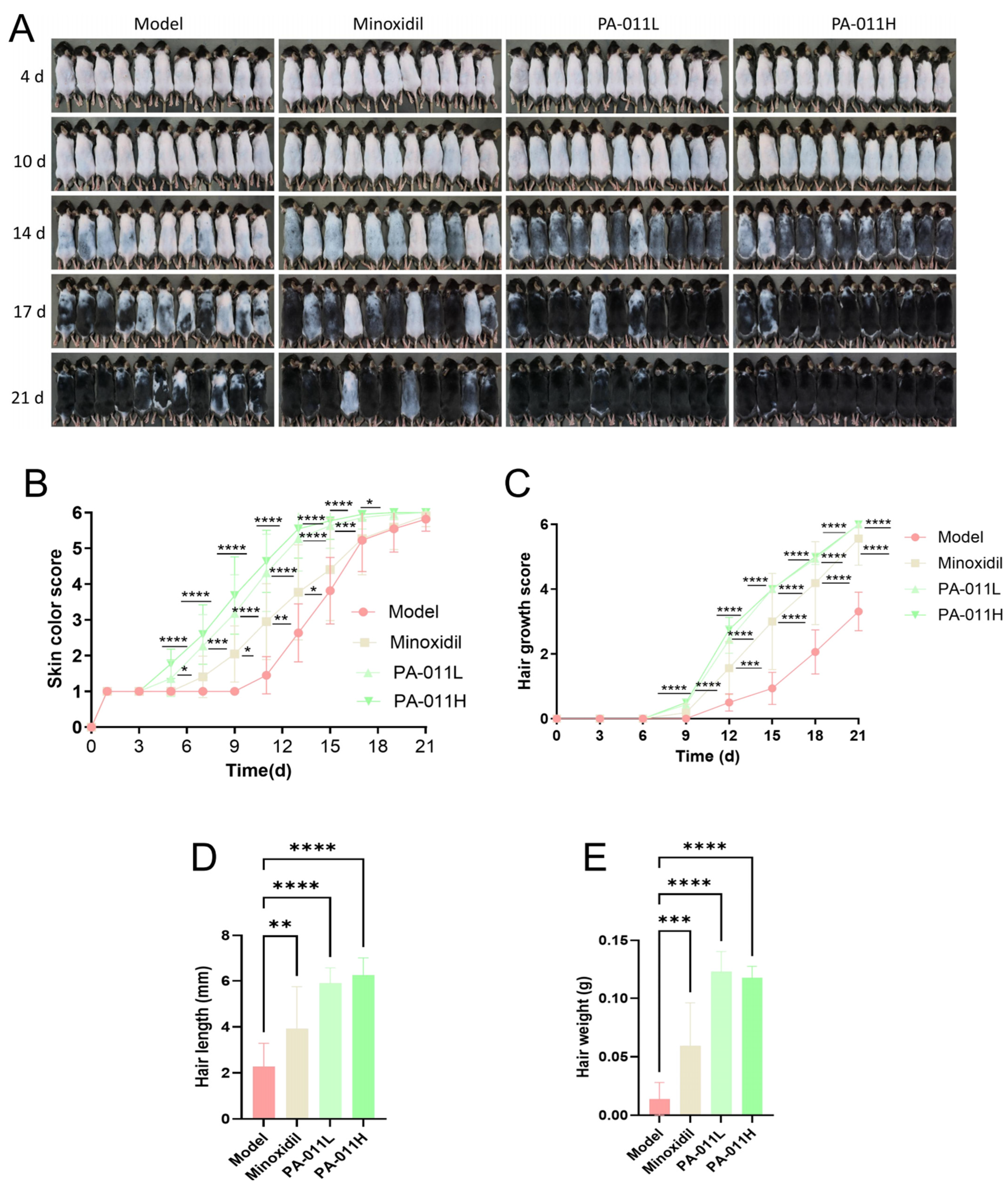
2.7. Observation of Hair Growth Status and Skin Color Scoring
2.8. Evaluation of Hair Growth in Mice
2.9. Detection of VEGF and SOD Levels in Skin Tissues
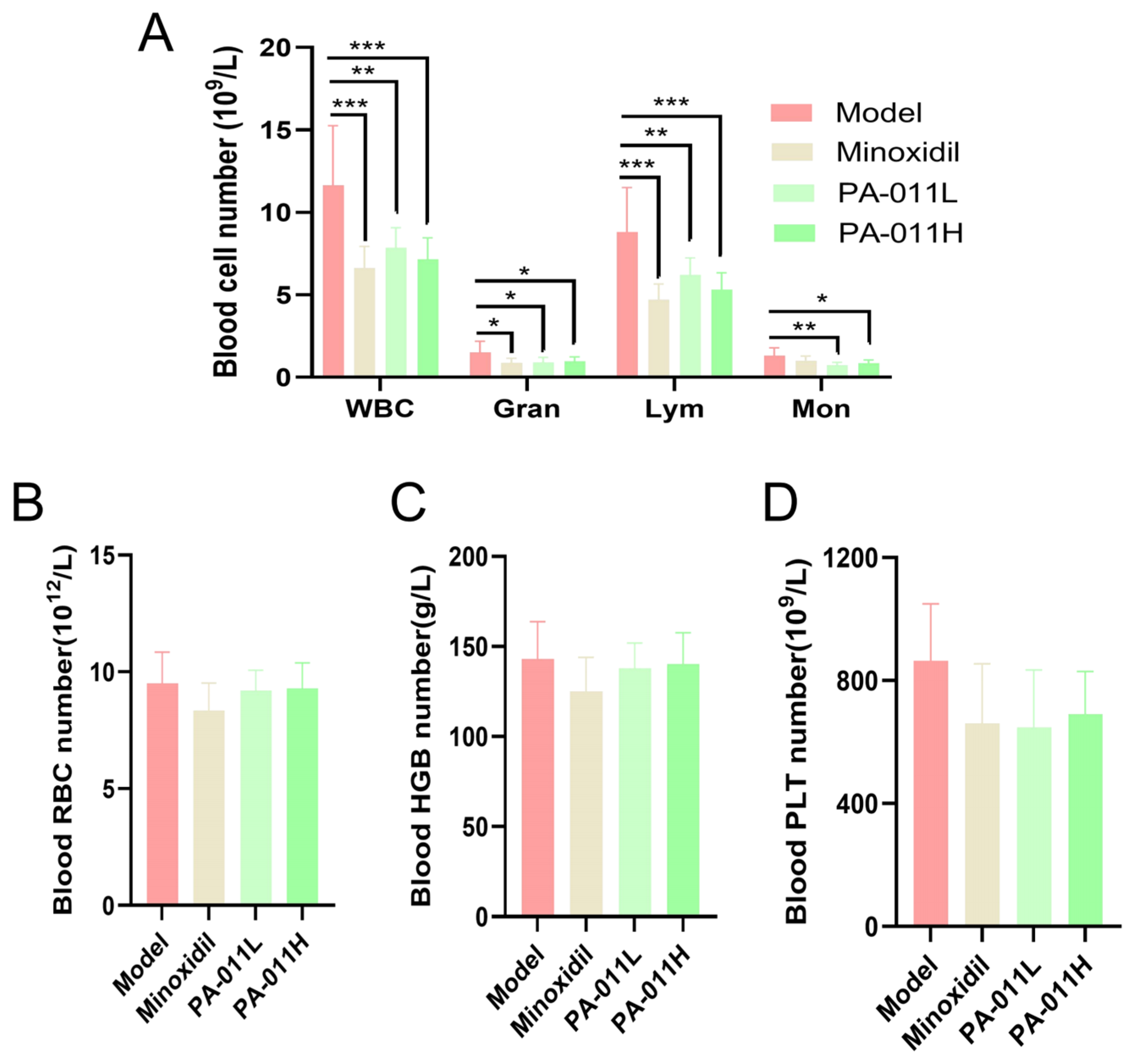
2.10. Blood Routine Analysis
2.11. Histological and Immunofluorescence Staining
2.12. Transcriptomic Analysis of Mouse Skin Tissues
2.13. Untargeted Metabolomic Analysis of Mouse Skin Tissues
2.14. Integrated Transcriptomic and Metabolomic Analysis of Mouse Skin Tissues
2.15. Real-Time Fluorescence Quantitative Reverse Transcription PCR (RT-qPCR)
2.16. 16 s rRNA Detection
2.17. Statistical Analysis
3. Results
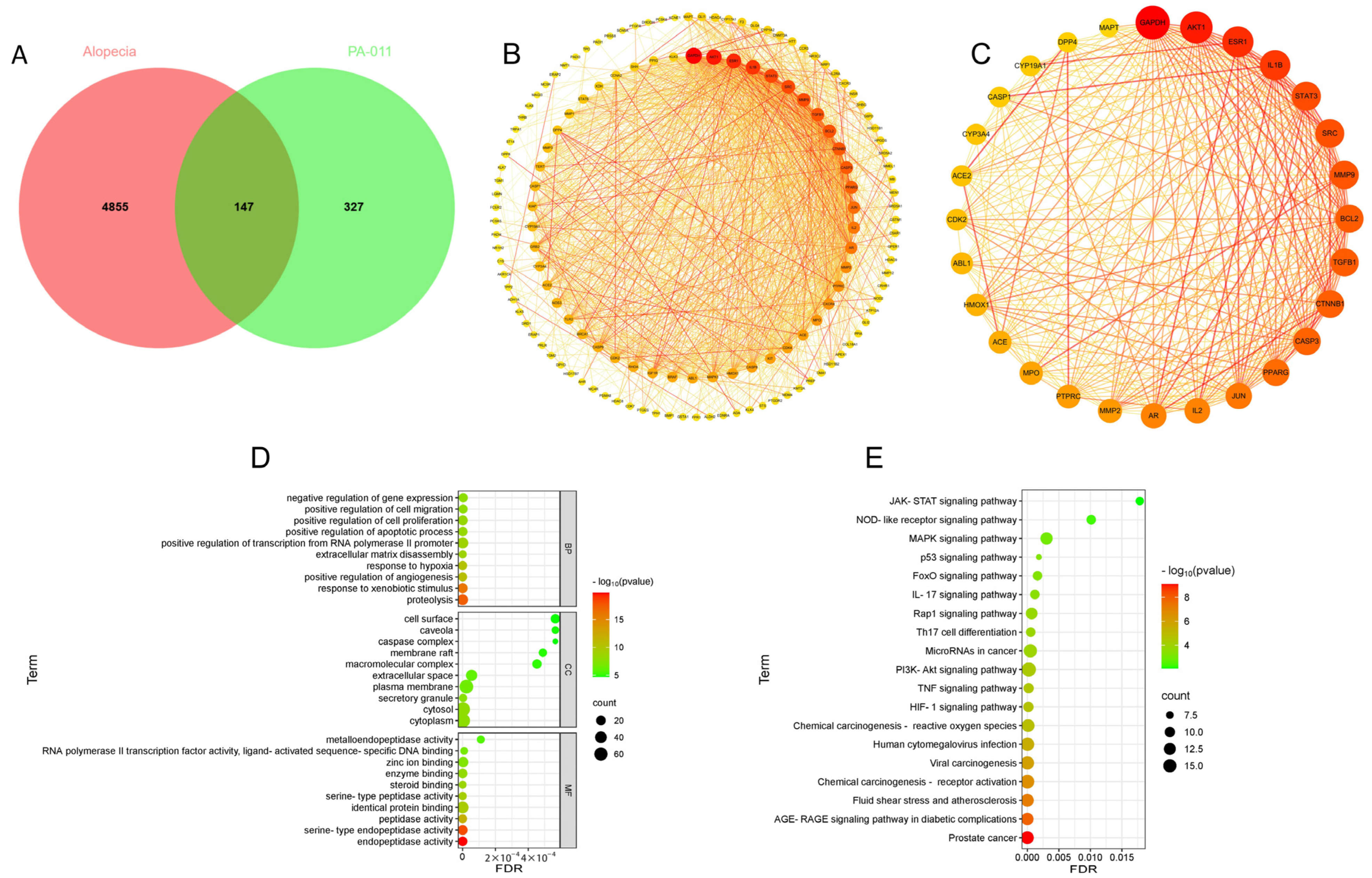
3.1. Network Pharmacology Results
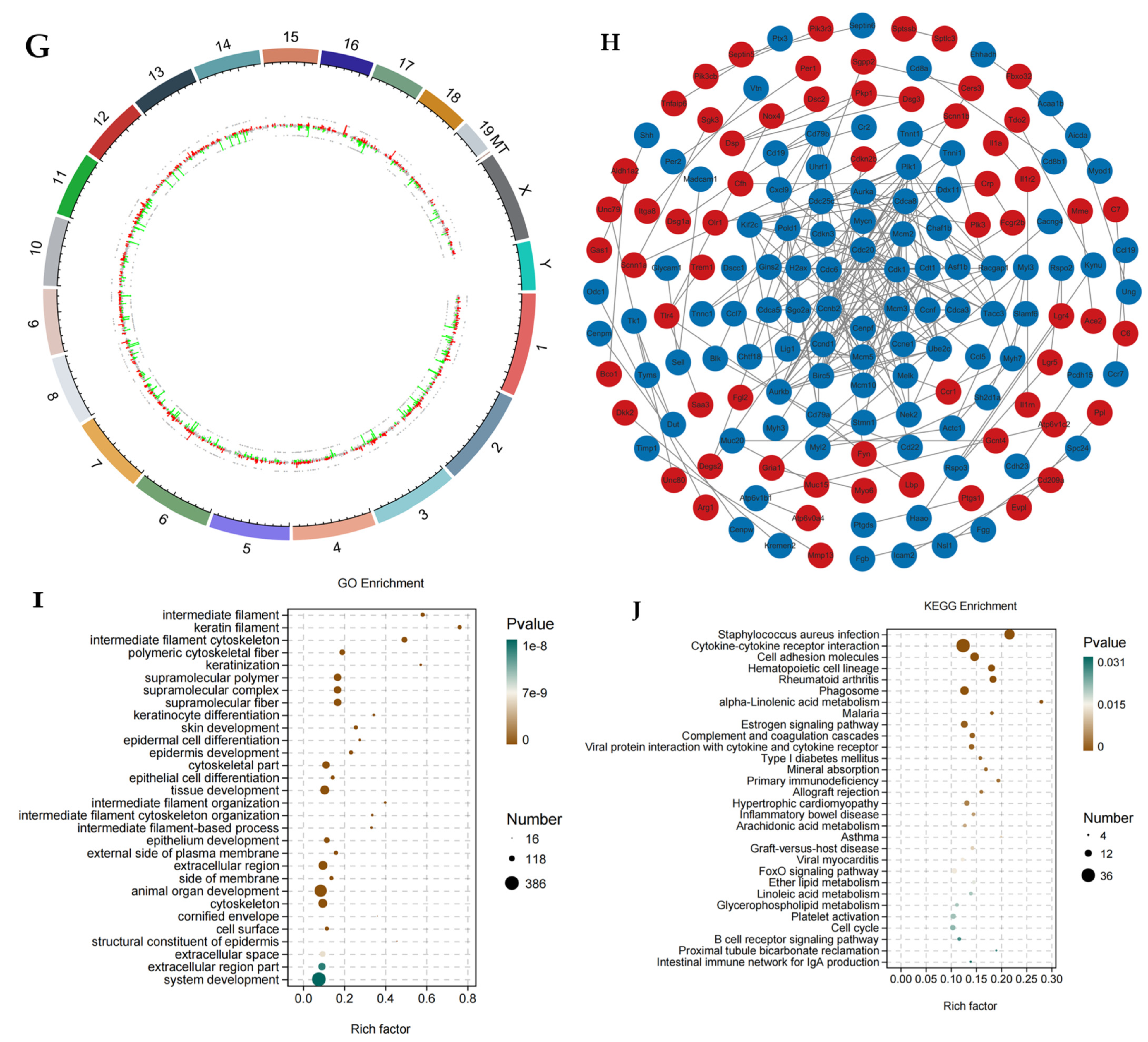
3.1.1. Identification of Core Targets
3.1.2. PPI Analysis of Core Targets
3.1.3. Enrichment Analysis
3.1.4. Drug-Target Pathway Correlation Network Analysis
3.1.5. Analysis of Molecular Docking Results
3.2. Safety Evaluation of PA-011
3.3. PA-011 Significantly Promotes Hair Regeneration in Depilated Mouse Skin
3.4. PA-011 Significantly Promotes HF Cell Proliferation
3.5. PA-011 Increases VEGF and SOD Expression Levels in Skin Tissues
3.6. Effects of PA-011 on Peripheral Blood Parameters in Mice
3.7. Transcriptomic Results of Skin Tissues
3.7.1. Sequencing Data Quality Control and Basic Analysis
3.7.2. Screening of Differentially Expressed Genes and Network Construction
3.7.3. Functional Enrichment and Pathway Analysis
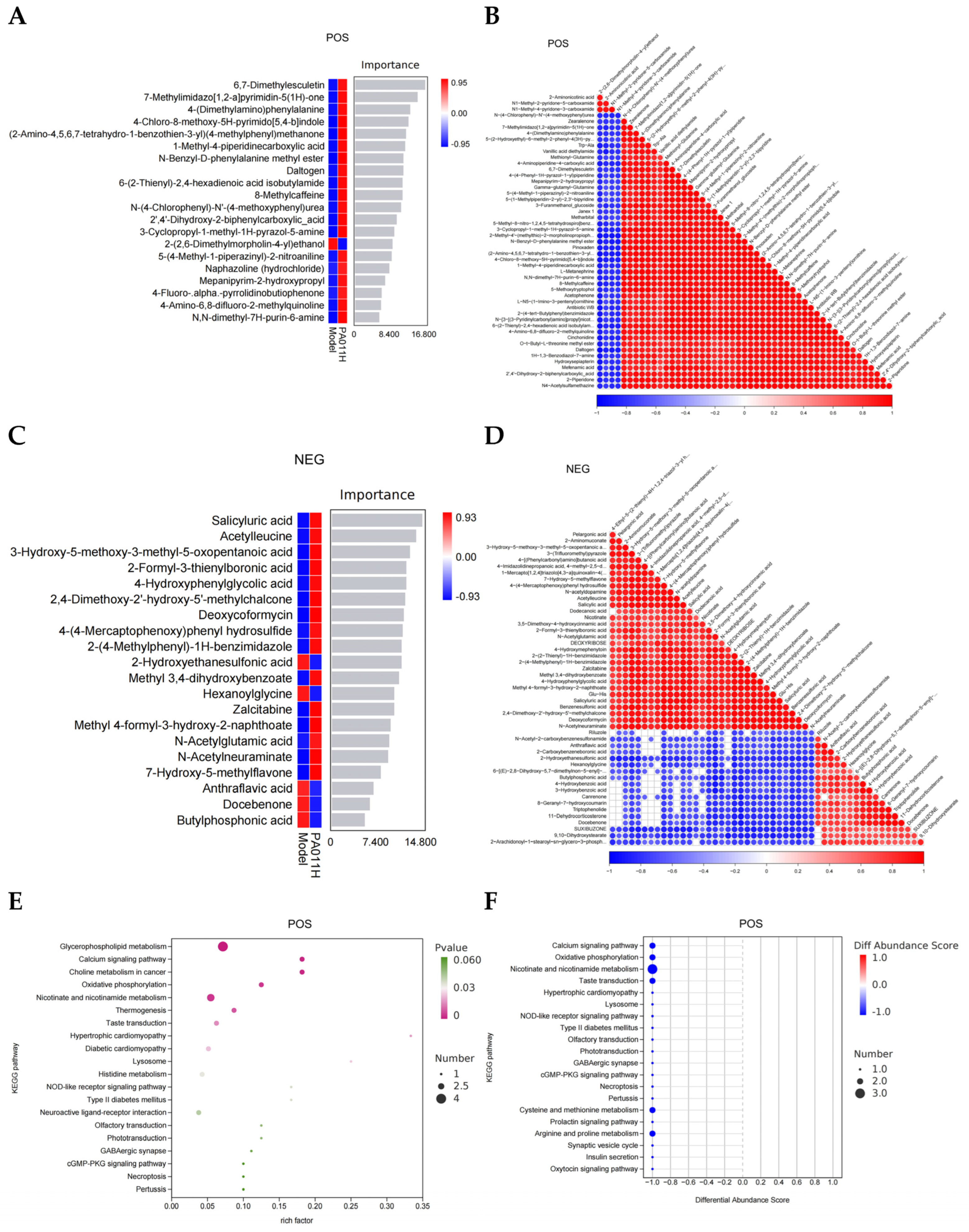
3.8. Metabolomic Analysis of Skin Tissues
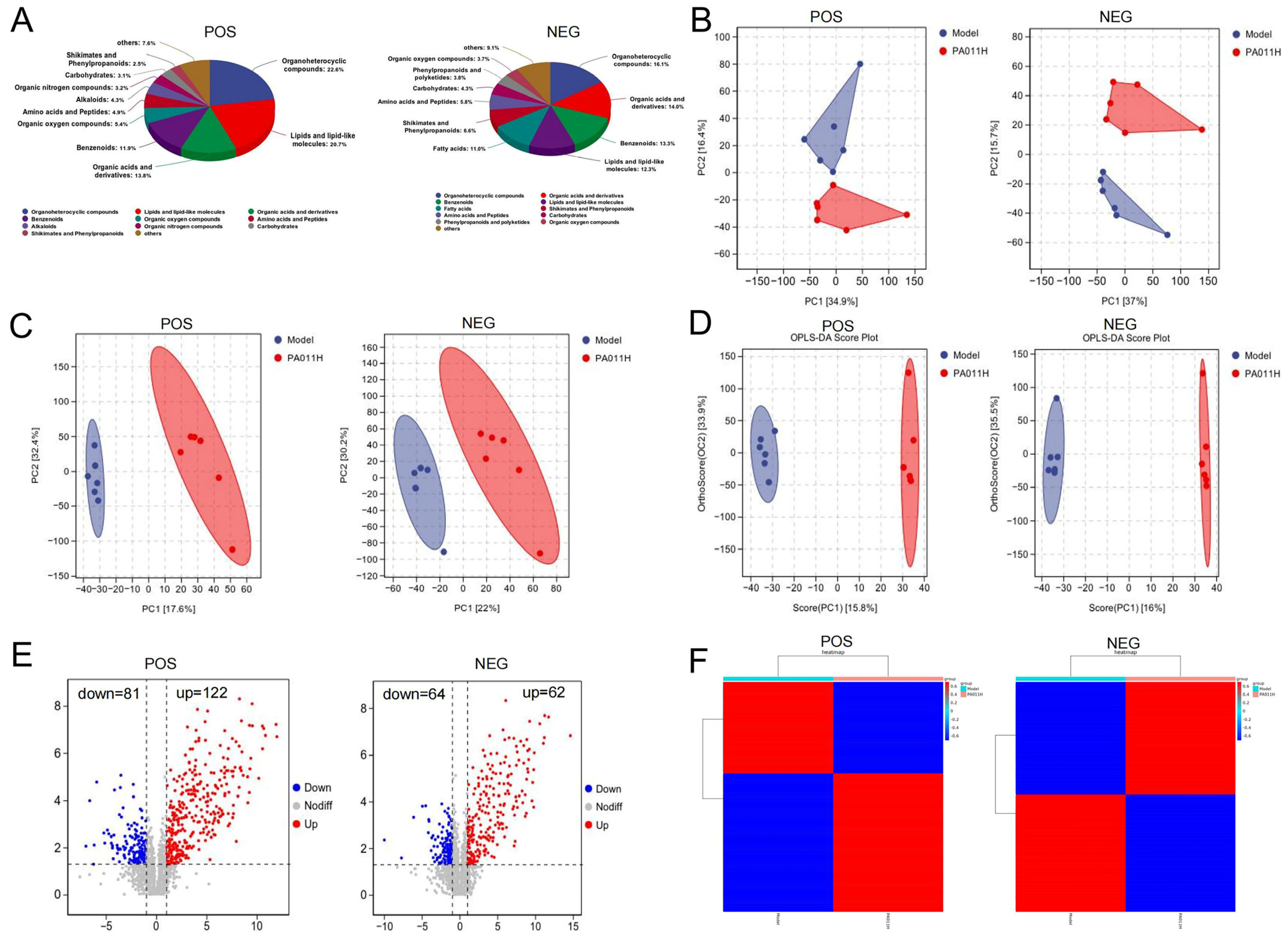
3.8.1. Metabolite Composition Analysis of Skin Tissue
3.8.2. Screening of Differential Metabolites and Functional Association
3.8.3. Correlation of Differential Metabolites
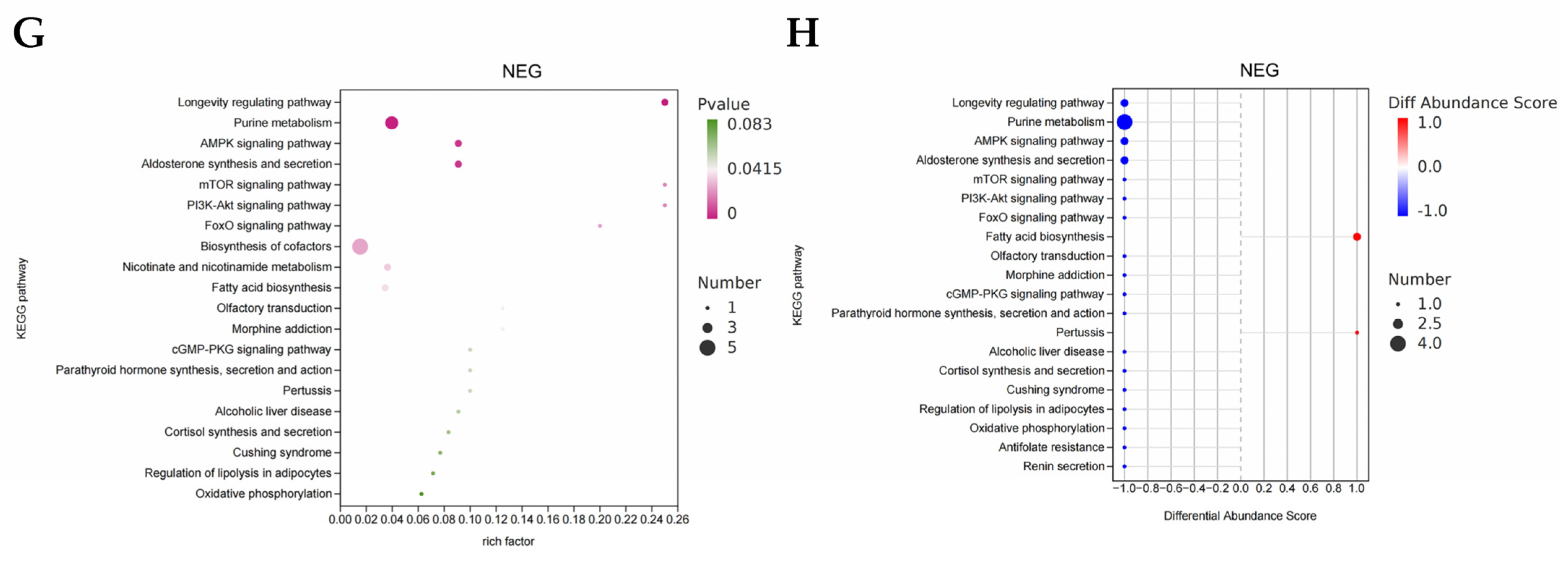
3.8.4. Functional Enrichment Analysis of Differential Metabolites
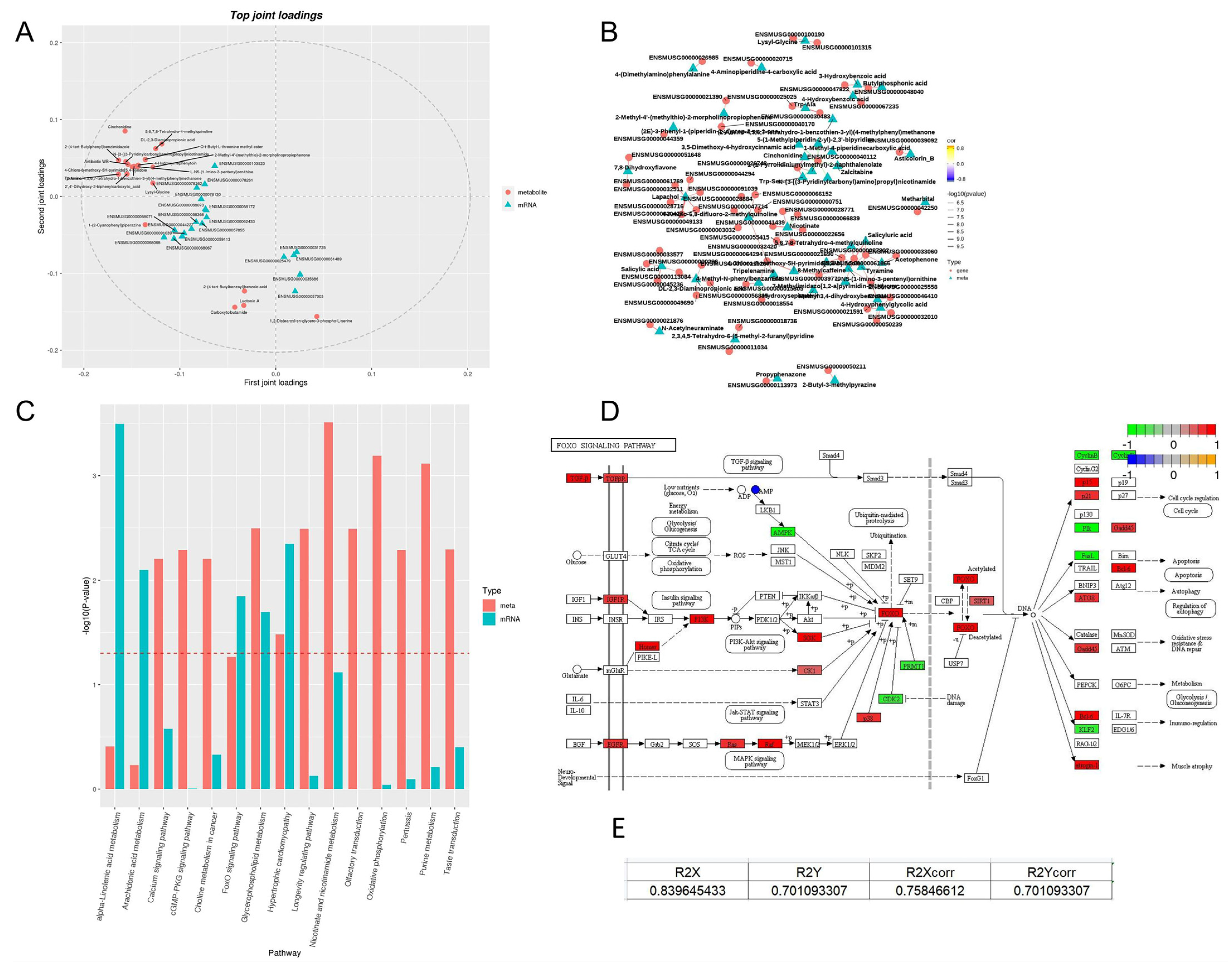
3.9. Multi-Omics Integration Reveals Core Action Pathways of PA-011
3.9.1. Construction of Cross-Omics Correlation Model
3.9.2. Analysis of Gene-Metabolite Interaction Networks
3.9.3. Validation of Pathway Cascade Regulation
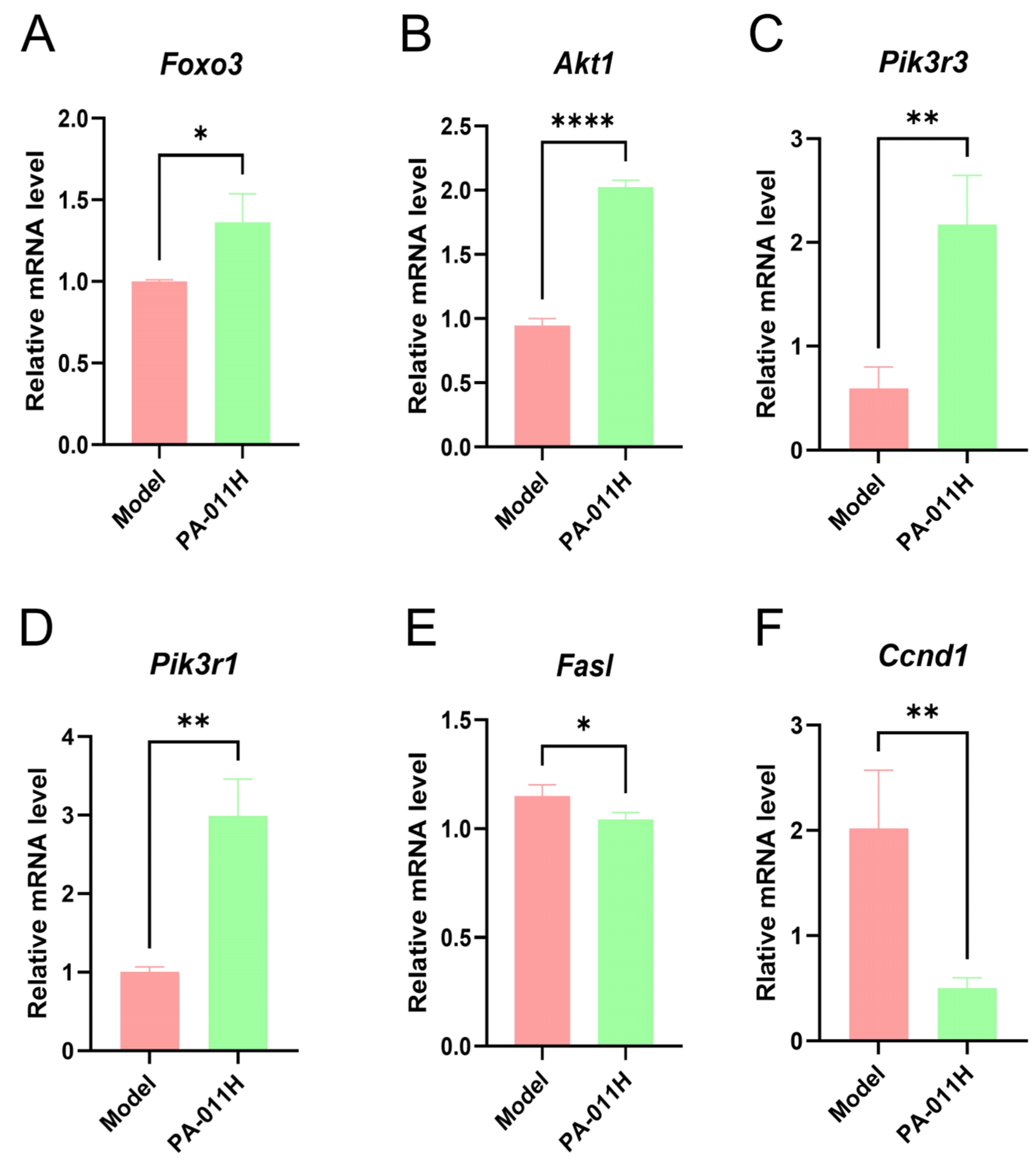
3.10. RT-qPCR Validation of Core Genes
3.11. Skin Microbiome Analysis
3.11.1. Alpha Diversity Analysis of Skin Microbiome
3.11.2. Beta Diversity Analysis of Skin Microbiome
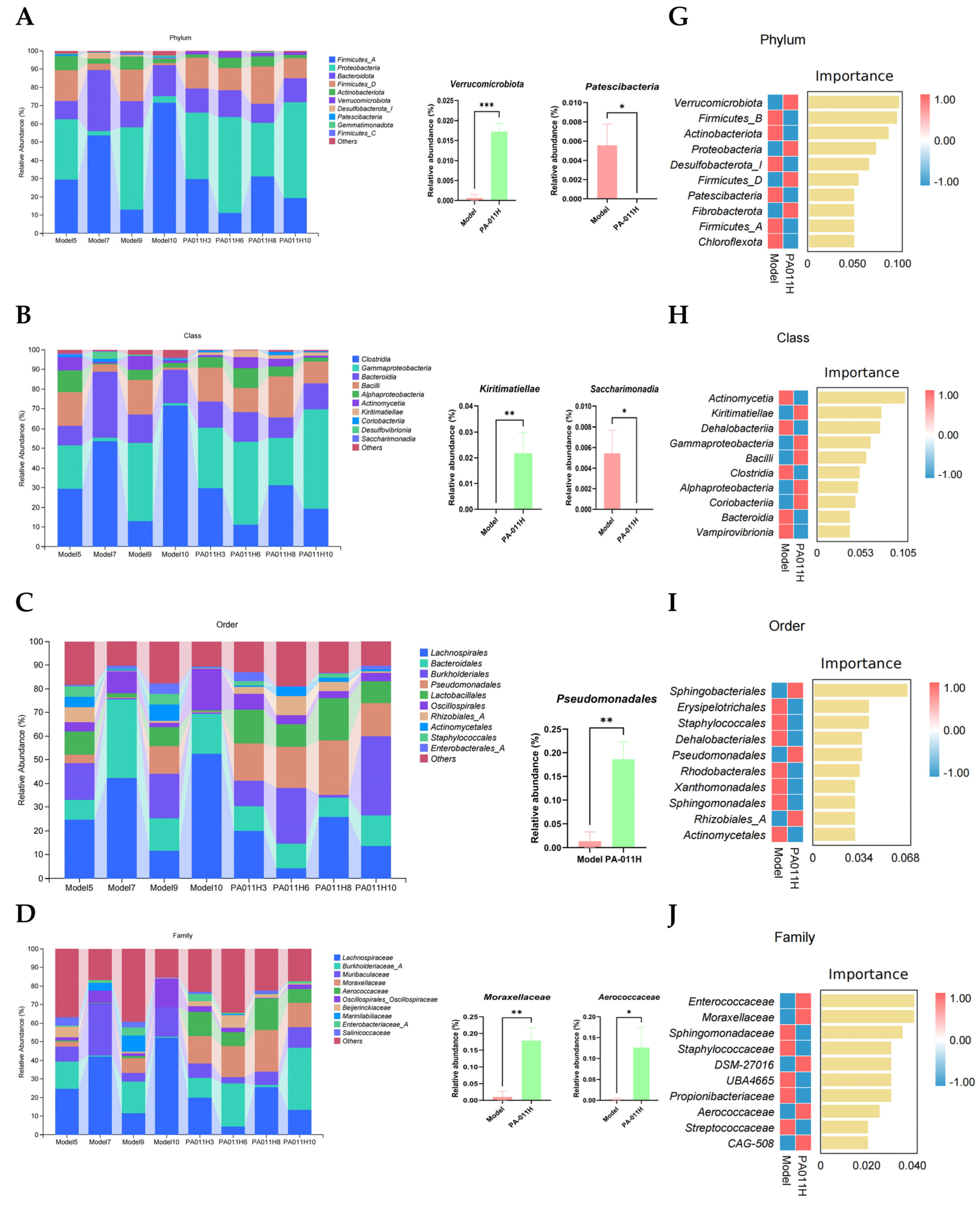
3.11.3. Skin Microbiome Species Analysis
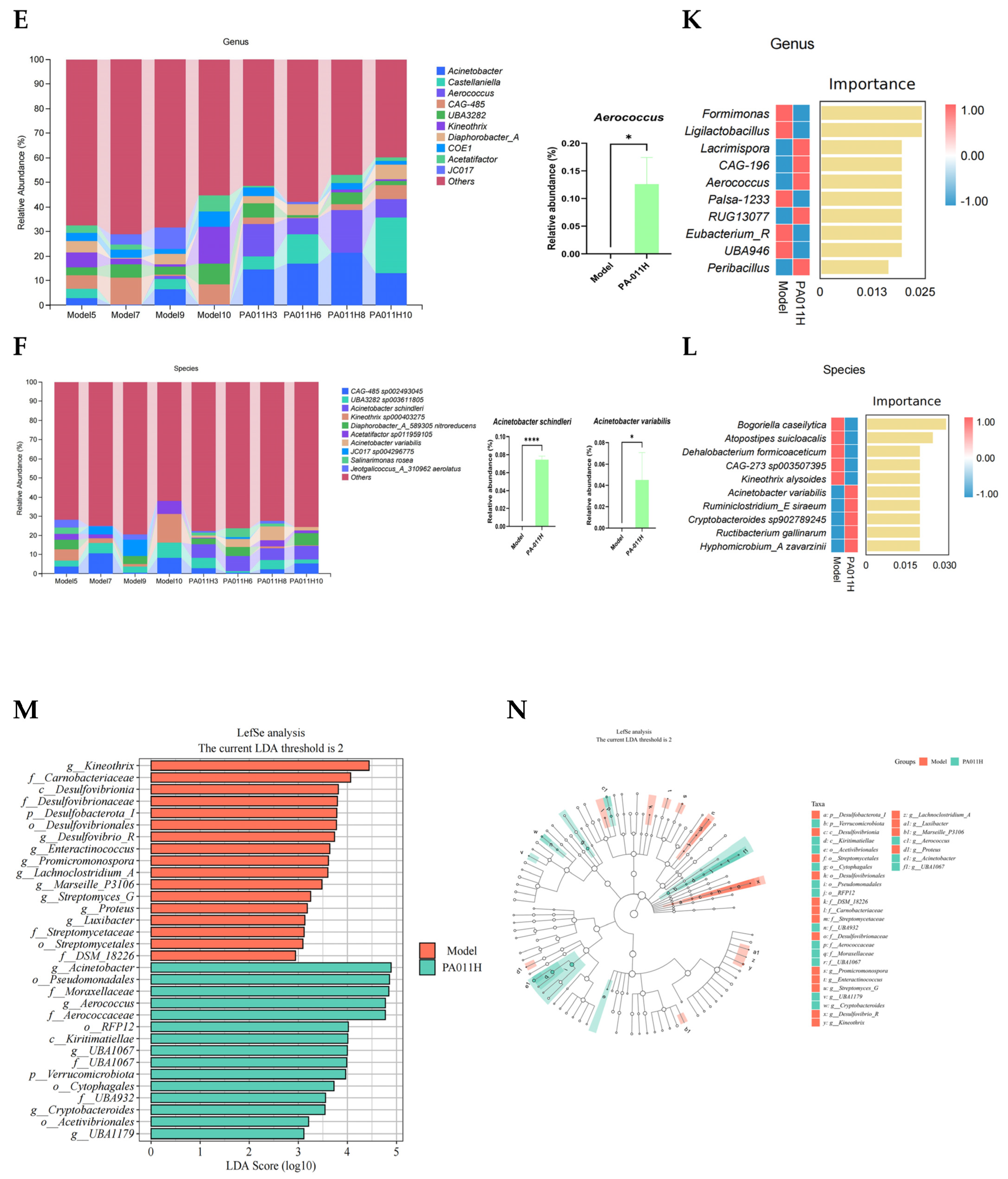
3.11.4. Biomarkers of Differential Skin Microbiome Between Groups
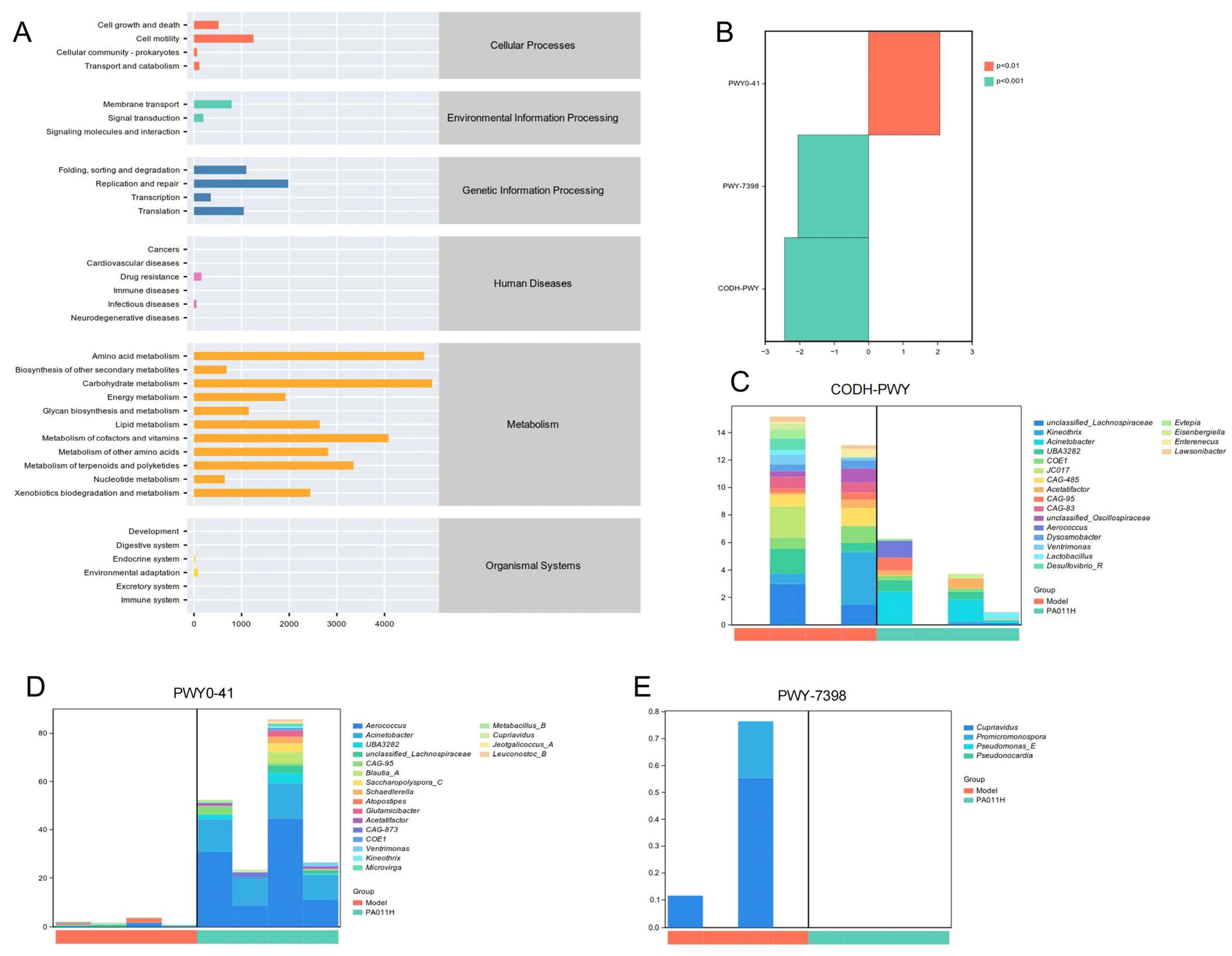
3.11.5. Predictive Analysis of Metabolic Pathways in Differential Bacterial Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ap | Alopecia |
| FDA | Food and Drug Administration |
| GO | Gene ontology |
| Gran | Granulocytes |
| HE | Hematoxylin & Eosin |
| HF | Hair follicle |
| HGB | Hemoglobin |
| IL-6 | Interleukin 6 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| Lym | Lymphocytes |
| Mon | Monocyte |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PA | Periplaneta americana |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares discriminant analysis |
| PLT | Platelet |
| PPI | Protein interaction |
| RBC | Red blood cell |
| SOD | Superoxide dismutase |
| TCM | Traditional Chinese medicine |
| VEGF | vascular endothelial growth factor |
| WBC | White blood cell |
References
- Zhao, Y.; Liang, W.; Liu, Z.; Chen, X.; Lin, C. Impact of SDF-1 and AMD3100 on Hair Follicle Dynamics in a Chronic Stress Model. Biomolecules 2024, 14, 1206. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Gong, Y.; Yu, Z.; Gan, Y.; Li, P.; Liu, W.; Wang, X. Curcumin-zinc framework encapsulated microneedle patch for promoting hair growth. Theranostics 2023, 13, 3675–3688. [Google Scholar] [CrossRef]
- Sintos, A.M.L.; Cabrera, H.S. Network Pharmacology Reveals Curcuma aeruginosa Roxb. Regulates MAPK and HIF-1 Pathways to Treat Androgenetic Alopecia. Biology 2024, 13, 497. [Google Scholar] [CrossRef]
- Wang, F.; He, G.; Liu, M.; Sun, Y.; Ma, S.; Sun, Z.; Wang, Y. Pilose antler extracts promotes hair growth in androgenetic alopecia mice by activating hair follicle stem cells via the AKT and Wnt pathways. Front. Pharmacol. 2024, 15, 1410810. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Y.; Chen, X.; Zhao, Y.; Ji, C.; Xu, R.; Jiang, R.; Liu, F.; Wang, M.; Sun, L. Deoxyshikonin from Arnebiae Radix promotes hair growth by targeting the Wnt/β-catenin signaling pathway. Phytomedicine 2025, 140, 156590. [Google Scholar] [CrossRef]
- Pillon, N.J.; Smith, J.A.B.; Alm, P.S.; Chibalin, A.V.; Alhusen, J.; Arner, E.; Carninci, P.; Fritz, T.; Otten, J.; Olsson, T.; et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv. 2022, 8, eabo3192. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.M.; Jang, Y.N.; Park, A.Y.; Kim, S.Y.; Kim, B.J.; Lee, J.O. Irisin promotes hair growth and hair cycle transition by activating the GSK-3β/β-catenin pathway. Exp. Dermatol. 2024, 33, e15155. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.F.; Shabaan, D.A. Effect of N-acetylcysteine on hair follicle changes in mouse model of cyclophosphamide-induced alopecia: Histological and biochemical study. Histochem. Cell Biol. 2024, 161, 477–491. [Google Scholar] [CrossRef]
- Huang, W.Y.; Hong, J.B.; Chang, M.; Wang, S.Y.; Lai, S.F.; Chien, H.F.; Lin, S.J. Lower proximal cup and outer root sheath cells regenerate hair bulbs during anagen hair follicle repair after chemotherapeutic injury. Exp. Dermatol. 2021, 30, 503–511. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Gong, Y.; Lu, W.; Sha, X.; Cao, C.; Li, Y.; Wang, J. Amygdalin ameliorates alopecia areata on C3H/HeJ mice by inhibiting inflammation through JAK2/STAT3 pathway. J. Ethnopharmacol. 2024, 331, 118317. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, Y.; Liu, J.; Xu, G.; Yu, W.; Cui, Q.; Lu, X.; Du, P.; An, L. Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides. Carbohydr. Polym. 2022, 278, 118811. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, J.U.; Lee, S.H.; Park, J.Y.; Lee, W.; Choi, K.M.; Kim, S.Y.; Park, M.H. Hair Growth Effect and the Mechanisms of Rosa rugosa Extract in DHT-Induced Alopecia Mice Model. Int. J. Mol. Sci. 2024, 25, 11362. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dai, J.; Ai, H.; Du, W.; Ji, H. β-Nicotinamide Mononucleotide Promotes Cell Proliferation and Hair Growth by Reducing Oxidative Stress. Molecules 2024, 29, 798. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Chen, L.; Jiang, H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J. Transl. Med. 2023, 21, 380. [Google Scholar] [CrossRef]
- Li, Y.; Dong, T.; Wan, S.; Xiong, R.; Jin, S.; Dai, Y.; Guan, C. Application of multi-omics techniques to androgenetic alopecia: Current status and perspectives. Comput. Struct. Biotechnol. J. 2024, 23, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Serrage, H.J.; O’Neill, C.A.; Uzunbajakava, N.E. Illuminating microflora: Shedding light on the potential of blue light to modulate the cutaneous microbiome. Front. Cell. Infect. Microbiol. 2024, 14, 1307374. [Google Scholar] [CrossRef]
- Jo, H.; Kim, S.Y.; Kang, B.H.; Baek, C.; Kwon, J.E.; Jeang, J.W.; Heo, Y.M.; Kim, H.-B.; Heo, C.Y.; Kang, S.M.; et al. Staphylococcus epidermidis Cicaria, a Novel Strain Derived from the Human Microbiome, and Its Efficacy as a Treatment for Hair Loss. Molecules 2022, 27, 5136. [Google Scholar] [CrossRef]
- Gómez-Arias, P.J.; Gay-Mimbrera, J.; Rivera-Ruiz, I.; Aguilar-Luque, M.; Juan-Cencerrado, M.; Mochón-Jiménez, C.; Gómez-García, F.; Sánchez-González, S.; Ortega-Hernández, A.; Gómez-Garre, D.; et al. Association Between Scalp Microbiota Imbalance, Disease Severity, and Systemic Inflammatory Markers in Alopecia Areata. Dermatol. Ther. 2024, 14, 2971–2986. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef]
- Grimshaw, S.G.; Smith, A.M.; Arnold, D.S.; Xu, E.; Hoptroff, M.; Murphy, B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS ONE 2019, 14, e0225796. [Google Scholar] [CrossRef]
- Lin, Q.; Panchamukhi, A.; Li, P.; Shan, W.; Zhou, H.; Hou, L.; Chen, W. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst. Eng. 2021, 44, 965–975. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Deng, Z.; Guo, R.Y.; Chai, J.W.; Li, R.; Zeng, Q.Y.; Lai, S.A.; Chen, X.; Xu, X.Q. Periplaneta americana Extract Ameliorates LPS-induced Acute Lung Injury Via Reducing Inflammation and Oxidative Stress. Curr. Med. Sci. 2023, 43, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lu, Q.; Tang, M.; Tao, L.; Zhao, H.; Zhang, C.; Yu, Y.; Wu, X.; Liu, H.; Cui, R. Periplaneta americana extract ameliorates dextran sulfate sodium-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacology 2022, 30, 907–918. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, C.; Wu, J.; Li, J.; Wang, L.; You, Y.; Peng, T.; Zhang, K.; Cao, M.; Hong, J. Periplaneta americana extract alleviates steatohepatitis in a mouse model by modulating HMGB1-mediated inflammatory response. Front. Pharmacol. 2022, 13, 995523. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Jin, J.; Chen, J.; Li, Z. Periplaneta americana (Insecta: Blattodea) and organ fibrosis: A mini review. Medicine 2022, 101, e32039. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, S.Q.; Su, T.H.; Tian, X.J.; Wen, W.J.; Pan, H.P.; Wang, X.F.; Zhang, W.; Zhong, J.L.; Dong, Z.S.; et al. Bioactive-Enriched Nanovesicles from American Cockroaches Enhance Wound Healing by Promoting Angiogenesis. ACS Appl. Mater. Interfaces 2025, 17, 13565–13576. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chu, J.; Xiang, Y.; He, M.; Ma, Q.; Duan, J.; Wang, Y.; Sun, S. Kangfuxin liquid reduces the ultraviolet B-induced photodamage of HaCaT cells by regulating autophagy. Biosci. Biotechnol. Biochem. 2023, 87, 1485–1494. [Google Scholar] [CrossRef]
- Li, L.J.; Xu, X.H.; Yuan, T.J.; Hou, J.; Yu, C.L.; Peng, L.H. Periplaneta americana L. as a novel therapeutics accelerates wound repair and regeneration. Biomed. Pharmacother. 2019, 114, 108858. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, Z.H.; Dai, D.M.; Fan, Z.-X.; Chen, J.; Wu, S.-L.; Liu, X.-M.; Miao, Y.; Hu, Z.-Q.; Qu, Q. Platelet lysate promotes hair growth: In vitro and in vivo mechanism and randomized, controlled trial. Biomed. Pharmacother. 2023, 161, 114517. [Google Scholar] [CrossRef]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, S.; Yuan, H.; Park, S. Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway. Pharmaceuticals 2021, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Qiang, W.; Jiang, J.; Hu, X.; Chen, Y.; Guo, Y.; Liu, H.; Sun, S.; Gao, H.; Zhang, Y.; et al. Safflower oil body nanoparticles deliver hFGF10 to hair follicles and reduce microinflammation to accelerate hair regeneration in androgenetic alopecia. Int. J. Pharm. 2022, 616, 121537. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Z.; Lv, C.; Geng, X.; Ren, Y.; Ren, J.; Wang, H.; Ai, F.; Zhang, B.; Yue, B.; et al. Medicinal and edible plant Allium macrostemon Bunge for the treatment of testosterone-induced androgenetic alopecia in mice. J. Ethnopharmacol. 2023, 315, 116657. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Mubki, T.; Rudnicka, L.; Olszewska, M.; Shapiro, J. Evaluation and diagnosis of the hair loss patient: Part I. History and clinical examination. J. Am. Acad. Dermatol. 2014, 71, 415.e1–415.e15. [Google Scholar] [CrossRef]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Zhang, W.; Xue, X.; Li, Y.; Lv, Y.; Chen, J.; Miao, X. Dissolving microneedles for alopecia treatment. Colloids Surf. B Biointerfaces 2023, 229, 113475. [Google Scholar] [CrossRef]
- Santana, F.F.V.; Lozi, A.A.; Gonçalves, R.V.; Da Silva, J.; Da Matta, S.L.P. Comparative effects of finasteride and minoxidil on the male reproductive organs: A systematic review of in vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2023, 478, 116710. [Google Scholar] [CrossRef]
- Fang, T.; Xu, R.; Sun, S.; He, Y.; Yan, Y.; Fu, H.; Luo, H.; Cao, Y.; Tao, M. Caizhixuan hair tonic regulates both apoptosis and the PI3K/Akt pathway to treat androgenetic alopecia. PLoS ONE 2023, 18, e0282427. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, G.; Wang, F.; Sun, Y.; Ma, S.; Hao, Y.; Wang, Y. Pilose antler extract promotes hair growth in androgenic alopecia mice by promoting the initial anagen phase. Biomed. Pharmacother. 2024, 174, 116503. [Google Scholar] [CrossRef]
- Kim, J.; An, J.; Lee, Y.K.; Ha, G.; Ban, H.; Kong, H.; Lee, H.; Song, Y.; Lee, C.K.; Kim, S.B.; et al. Hair Growth Promoting Effects of Solubilized Sturgeon Oil and Its Correlation with the Gut Microbiome. Pharmaceuticals 2024, 17, 1112. [Google Scholar] [CrossRef]
- Kim, J.; Joo, J.H.; Kim, J.; Rim, H.; Shin, J.Y.; Choi, Y.H.; Min, K.; Lee, S.Y.; Jun, S.H.; Kang, N.G. Platycladus orientalis Leaf Extract Promotes Hair Growth via Non-Receptor Tyrosine Kinase ACK1 Activation. Curr. Issues Mol. Biol. 2024, 46, 11207–11219. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.Y.; Lee, S.; Jang, S.; Hwang, S.T.; Kwon, Y.; Choi, J.; Kwon, O. Ganoderma lucidum extract attenuates corticotropin-releasing hormone-induced cellular senescence in human hair follicle cells. iScience 2024, 27, 109675. [Google Scholar] [CrossRef]
- Zeng, C.; Liao, Q.; Hu, Y.; Shen, Y.; Geng, F.; Chen, L. The Role of Periplaneta americana (Blattodea: Blattidae) in Modern Versus Traditional Chinese Medicine. J. Med. Entomol. 2019, 56, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, J.; Li, X.; Lin, R.; Wang, X.; Xiao, G.; Zeng, J.; Wang, Z. Periplaneta americana extract promotes infectious diabetic ulcers wound healing by downregulation of LINC01133/SLAMF9. Chin. J. Nat. Med. 2024, 22, 608–618. [Google Scholar] [CrossRef]
- Gao, Z.R.; Zhou, Y.H.; Zhao, Y.Q.; Zhao, J.; Ye, Q.; Zhang, S.H.; Feng, Y.; Tan, L.; Liu, Q.; Chen, Y.; et al. Kangfuxin Accelerates Extraction Socket Healing by Promoting Angiogenesis Via Upregulation of CCL2 in Stem Cells. J. Bone Miner. Res. 2023, 38, 1208–1221. [Google Scholar] [CrossRef]
- Xiao, S.; Deng, Y.; Mo, X.; Liu, Z.; Wang, D.; Deng, C.; Wei, Z. Promotion of hair growth by conditioned medium from extracellular matrix/stromal vascular fraction gel in C57BL/6 Mice. Stem Cells Int. 2020, 2020, 9054514. [Google Scholar] [CrossRef]
- Kumar, N.; Rungseevijitprapa, W.; Narkkhong, N.-A.; Suttajit, M.; Chaiyasut, C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. J. Ethnopharmacol. 2012, 139, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Su, C.H.; Chiang, C.Y.; Wu, C.N.; Kuan, Y.H. Observation of the Expression of Vascular Endothelial Growth Factor and the Potential Effect of Promoting Hair Growth Treated with Chinese Herbal BeauTop. Evid.-Based Complement. Altern. Med. 2021, 2021, 6667011. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Kim, D.; Lee, S.; Kim, W.; Yoo, J.W.; Kim, J.A.; Kwon, O.S.; Kim, D.D.; Min, D.S.; et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. Int. J. Mol. Sci. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Lee, M.K.; Hwang, T.; Choi, C.W.; Kim, M.S.; Kim, H.R.; Lee, B. The multi-functional roles of forkhead box protein O in skin aging and diseases. Redox Biol. 2021, 46, 102101. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Dhilip Kumar, S.S.; Houreld, N.N.; Abrahamse, H. Understanding the perspectives of forkhead transcription factors in delayed wound healing. J. Cell. Commun. Signal. 2019, 13, 151–162. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, F.; Yuan, Y.; Ma, H.; Qu, L. Paeonia suffruticosa Andrews root extract ameliorates photoaging via regulating IRS1/PI3K/FOXO pathway. Front. Pharmacol. 2025, 16, 1520392. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Ichraf, M.; Zhou, Y.; Song, Y.; Fu, X.; Liu, T.; Ma, J.; Zhuang, F.; Hu, X.; et al. Expression of FOXO3 in the skin follicles of goose embryos during embryonic development. Br. Poult. Sci. 2023, 64, 586–593. [Google Scholar] [CrossRef]
- Polak-Witka, K.; Rudnicka, L.; Blume-Peytavi, U.; Vogt, A. The role of the microbiome in scalp hair follicle biology and disease. Exp. Dermatol. 2020, 29, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.; Kanti, V.; Polak-Witka, K.; Blume-Peytavi, U.; Spyrou, G.M.; Vogt, A. The potential relevance of the microbiome to hair physiology and regeneration: The emerging role of metagenomics. Biomedicines 2021, 9, 236. [Google Scholar] [CrossRef]
- Jung, E.S.; Park, H.M.; Hyun, S.M.; Shon, J.C.; Singh, D.; Liu, K.H.; Whon, T.W.; Bae, J.W.; Hwang, J.S.; Lee, C.H. The green tea modulates large intestinal microbiome and exo/endogenous metabolome altered through chronic UVB-exposure. PLoS ONE 2017, 12, e0187154. [Google Scholar] [CrossRef] [PubMed]
- Dokoshi, T.; Chen, Y.; Cavagnero, K.J.; Rahman, G.; Hakim, D.; Brinton, S.; Schwarz, H.; Brown, E.A.; O’Neill, A.; Nakamura, Y.; et al. Dermal injury drives a skin to gut axis that disrupts the intestinal microbiome and intestinal immune homeostasis in mice. Nat. Commun. 2024, 15, 3009. [Google Scholar] [CrossRef] [PubMed]




| Name | Primer Direction | Primer Sequence (5′ to 3′) |
|---|---|---|
| Foxo3 | Forward | GTGTTTGGACCTTCGTCTCTGA |
| Reverse | GAGTGTCTGGTTGCCGTAGTGT | |
| Akt1 | Forward | GCTCTTCTTCCACCTGTCTCG |
| Reverse | CGCAGAATGTCTTCATAGTGGC | |
| Pik3r3 | Forward | ATTGACTTTGAGGAAGGGAGGA |
| Reverse | CTGTTGGAATCTGGATACTGGGT | |
| Pik3r1 | Forward | CACGGCGATTACACTCTTACACTA |
| Reverse | CACTGGGTAGAGCAACTTCACATC | |
| Fasl | Forward | CCTCTAAAGAAGAAGGACCACAACA |
| Reverse | ACGGAGTTCTGCCAGTTCCTT | |
| Ccnd1 | Forward | AGGCGGATGAGAACAAGCAG |
| Reverse | AAGAAAGTGCGTTGTGCGGTA | |
| Gapdh | Forward | CCTCGTCCCGTAGACAAAATG |
| Reverse | TGAGGTCAATGAAGGGGTCGT |
| Number | Name | ID | Molecular Formula | Structures |
|---|---|---|---|---|
| 1 | (R)-Methysticin | PA3 | C15H14O5 | 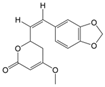 |
| 2 | (S)-N-Methylcoclaurine | PA5 | C18H21NO3 |  |
| 3 | 3-Hydroxyflavone | PA23 | C15H10O3 | 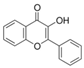 |
| 4 | 3-Methylindole | PA28 | C9H9N | 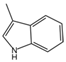 |
| 5 | 5,7-Dihydroxyflavone | PA39 | C15H10O4 |  |
| 6 | 17a-Estradiol | PA49 | C18H24O2 |  |
| 7 | all-trans-Retinoic acid | PA51 | C20H28O2 | 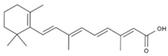 |
| 8 | Dehydroepiandrosterone | PA66 | C19H28O2 | 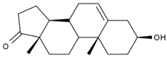 |
| 9 | Dodecanedioic acid | PA72 | C12H22O4 | 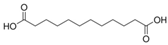 |
| 10 | Epiandrosterone | PA74 | C19H30O2 | 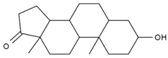 |
| 11 | Exemestane | PA76 | C20H24O2 |  |
| 12 | Hydroxyphenyllactic acid | PA85 | C9H10O4 | 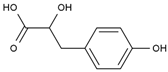 |
| 13 | Methyl jasmonate | PA104 | C13H20O3 | 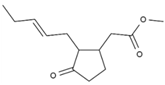 |
| 14 | Oleic acid | PA123 | C18H34O2 |  |
| 15 | Palmitoleic acid | PA125 | C16H30O2 | 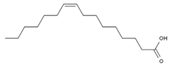 |
| 16 | Sotalol | PA142 | C12H20N2O3S | 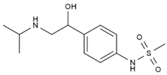 |
| Number | Peptide | ID | Charge |
|---|---|---|---|
| 1 | FQQRPQPQPQPQPQ | peptide13 | 1 |
| 2 | GGGAGGGAGGFGGGAGGGYR | peptide18 | 1 |
| 3 | FYGVVRAP | peptide70 | 1 |
| 4 | TPFYLR | Peptide76 | 1 |
| 5 | FGGANR | peptide81 | 1 |
| 6 | YAPR | Peptide86 | 1 |
| 7 | SSFGPR | Peptide96 | 1 |
| 8 | FGGGGAGGFGGGAGGR | Peptide97 | 1 |
| 9 | AGGGFGGGSGGFGGRSP | peptide121 | 1 |
| 10 | RYPYAPR | peptide129 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Yang, X.; Hong, C.; Zhang, Z.; Xiao, P.; Yang, Y.; Zhang, C.; He, Z. The Extract of Periplaneta americana (L.) Promotes Hair Regrowth in Mice with Alopecia by Regulating the FOXO/PI3K/AKT Signaling Pathway and Skin Microbiota. Curr. Issues Mol. Biol. 2025, 47, 619. https://doi.org/10.3390/cimb47080619
Guan T, Yang X, Hong C, Zhang Z, Xiao P, Yang Y, Zhang C, He Z. The Extract of Periplaneta americana (L.) Promotes Hair Regrowth in Mice with Alopecia by Regulating the FOXO/PI3K/AKT Signaling Pathway and Skin Microbiota. Current Issues in Molecular Biology. 2025; 47(8):619. https://doi.org/10.3390/cimb47080619
Chicago/Turabian StyleGuan, Tangfei, Xin Yang, Canhui Hong, Zehao Zhang, Peiyun Xiao, Yongshou Yang, Chenggui Zhang, and Zhengchun He. 2025. "The Extract of Periplaneta americana (L.) Promotes Hair Regrowth in Mice with Alopecia by Regulating the FOXO/PI3K/AKT Signaling Pathway and Skin Microbiota" Current Issues in Molecular Biology 47, no. 8: 619. https://doi.org/10.3390/cimb47080619
APA StyleGuan, T., Yang, X., Hong, C., Zhang, Z., Xiao, P., Yang, Y., Zhang, C., & He, Z. (2025). The Extract of Periplaneta americana (L.) Promotes Hair Regrowth in Mice with Alopecia by Regulating the FOXO/PI3K/AKT Signaling Pathway and Skin Microbiota. Current Issues in Molecular Biology, 47(8), 619. https://doi.org/10.3390/cimb47080619








