Xylitol Antioxidant Properties: A Potential Effect for Inflammation Reduction in Menopausal Women?—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nutrition Assessment
2.2.2. Determination of Antioxidant Capacity
2.2.3. DPPH Assay
2.2.4. ABTS Assay
2.3. Statistical Analysis
2.3.1. Study Design and Sample Size
2.3.2. Data Description and Assumptions
2.3.3. Selection of Statistical Tests
- Student’s t-test was used for normally distributed data.
- Mann–Whitney U test was applied for non-normally distributed data.
- Pearson’s correlation coefficient was used for normally distributed variables.
- Spearman’s rank correlation coefficient was applied for non-normally distributed variables.
2.3.4. Logistic Regression Models
2.3.5. Assessment of Antioxidant Potential: Delta TE Analysis
- (1)
- As a continuous variable, examining the influence of independent variables on the mean change in TE concentration;
- (2)
- As a binary variable, where, for the calculated delta values, cut-off points, median or mean values were used, depending on the distribution of the analyzed variable.
- For DPPH assay, delta values ≥ 50 µM TE/1 mL were coded as “effect,” and delta < 50 µM TE/1 mL as “too weak effect or no effect”.
- For ABTS assay, delta values ≥ 15 µM TE/1 mL were coded as “effect,” and delta < 15 µM TE/1 mL as “too weak effect or no effect”.
2.3.6. Effect Size Measures
2.3.7. Software
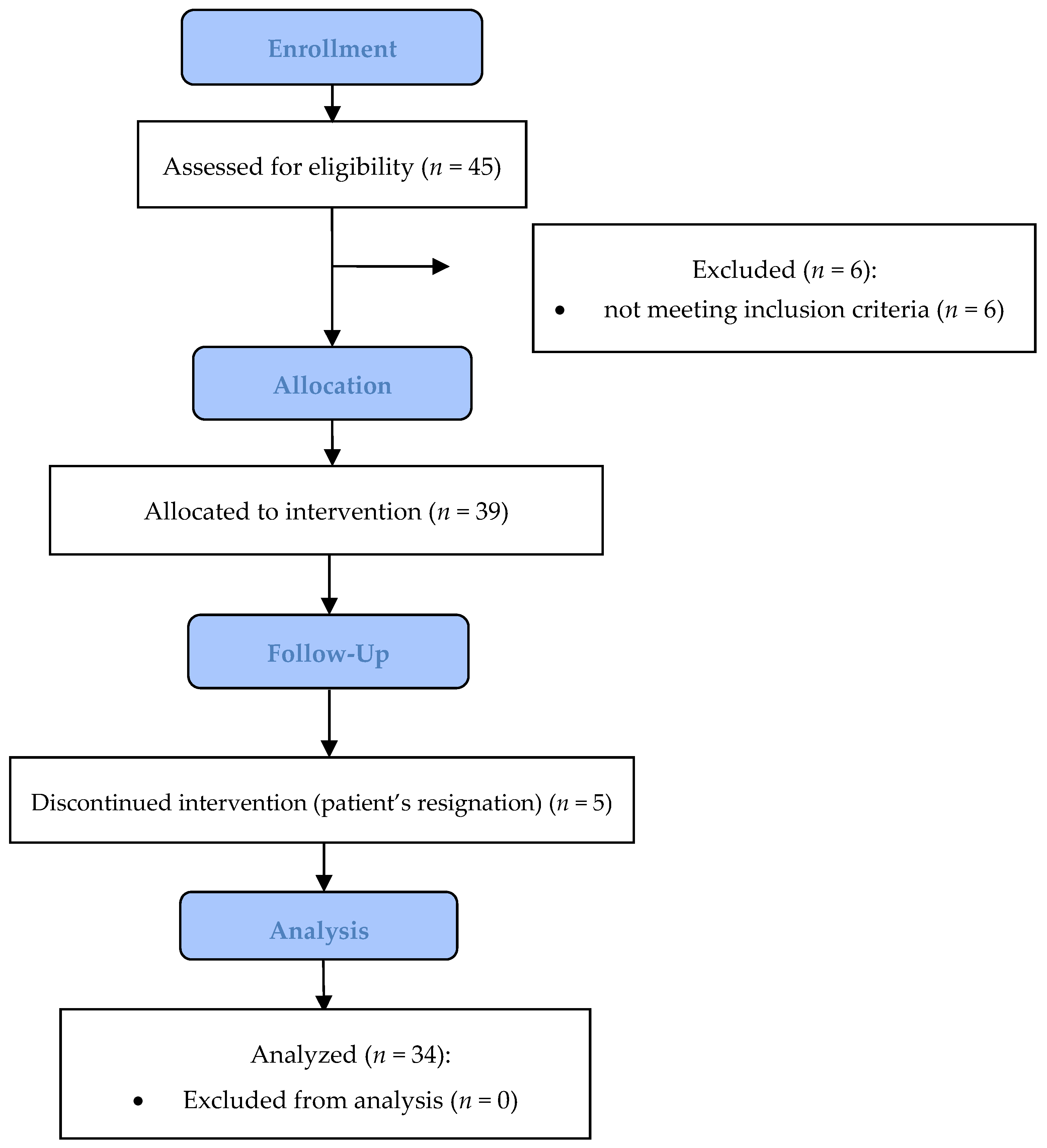
3. Results
Assessment of Effect Size
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPPH | 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| OS | oxidative stress |
| RSV | respiratory syncytial virus |
| TEAC | Trolox equivalent antioxidant capacity |
| TE | Trolox equivalent |
| %AA | % antioxidant activity |
| X | mean |
| SD | standard deviation |
| OR | odds ratios |
| Q | quartile |
| CI | confidence interval |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| ORAC | oxygen radical absorbance capacity |
| MnSOD | manganese superoxide dismutase |
| FODMAP | fermentable oligo-, di-, mono-saccharides, and polyols |
References
- Hyvönen, L.; Koivistoinen, P.; Voirol, F. Food Technological Evaluation of Xylitol. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Stewart, G.F., Eds.; Academic Press: Cambridge, MA, USA, 1982; Volume 28, pp. 373–403. [Google Scholar]
- Zacharis, C. Xylitol. In Sweeteners and Sugar Alternatives in Food Technology, 2nd ed.; O’Donnell, K., Kearsley, M.W., Eds.; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2012; pp. 347–377. ISBN 978-0-470-65968-7. [Google Scholar]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A Review on Bioproduction, Application, Health Benefits, and Related Safety Issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative Stress-Induced Diseases and Tea Polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Islam, S. Xylitol Improves Anti-Oxidative Defense System in Serum, Liver, Heart, Kidney and Pancreas of Normal and Type 2 Diabetes Model of Rats. Acta Pol. Pharm. 2017, 74, 817–826. [Google Scholar] [PubMed]
- Msomi, N.Z.; Erukainure, O.L.; Salau, V.F.; Olofinsan, K.A.; Islam, M.S. Xylitol Improves Antioxidant, Purinergic and Cholinergic Dysfunction, and Lipid Metabolic Homeostasis in Hepatic Injury in Type 2 Diabetic Rats. J. Food Biochem. 2022, 46, e14040. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Wi, G.R.; Kim, H.J.; Kim, H.-J. Ameliorating Effect of Dietary Xylitol on Human Respiratory Syncytial Virus (hRSV) Infection. Biol. Pharm. Bull. 2016, 39, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; La Mantia, I.; Brunese, F.P.; Varricchio, A.; Varricchio, A. Hypertonic Saline with Xylitol and Hyaluronate May Shorten the Viral Shedding Duration in Asymptomatic COVID-19 Positive Subjects: A Pilot Study. J. Biol. Regul. Homeost. Agents 2021, 35, 1151–1154. [Google Scholar] [CrossRef]
- Leanza, G.; Conte, C.; Cannata, F.; Isgrò, C.; Piccoli, A.; Strollo, R.; Quattrocchi, C.C.; Papalia, R.; Denaro, V.; Maccarrone, M.; et al. Oxidative Stress in Postmenopausal Women with or without Obesity. Cells 2023, 12, 1137. [Google Scholar] [CrossRef]
- Norma, P.M.; Virginia, N.-M.; Ral, R.-H.; Jos, C.E.; Cristbal, N.A. A Microassay for Quantification of 2,2-Diphenyl-1-Picrylhydracyl (DPPH) Free Radical Scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Guzik, P.; Więckowska, B. Data distribution analysis—A preliminary approach to quantitative data in biomedical research. J. Med. Sci. 2023, 92, e869. [Google Scholar] [CrossRef]
- Hameister, R.; Kaur, C.; Dheen, S.T.; Lohmann, C.H.; Singh, G. Reactive Oxygen/Nitrogen Species (ROS/RNS) and Oxidative Stress in Arthroplasty. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Al-Shahrani, M.M.; Zaman, G.S.; Amanullah, M. Measurement of Antioxidant Activity in Selected Food Products and Nutraceuticals. J. Nutr. Food Sci. 2013, 3, 205. [Google Scholar] [CrossRef]
- Kang, K.-W.; Kwak, S.-H.; Yun, S.-Y.; Kim, S.-K. Evaluation of Antioxidant Activity of Sugar Alcohols Using TOSC (Total Oxy-Radical Scavenging Capacity) Assay. Toxicol. Res. 2007, 23, 143–150. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Jo, S.-H.; Yoo, J.-I.; Cho, J.-B.; Kim, E.-J.; Apostolidis, E.; Kwon, Y.-I. Anti-Hyperglycemic Effect of Selected Sugar Alcohols. FASEB J. 2014, 28, 829–832. [Google Scholar] [CrossRef]
- Naknaen, P.; Itthisoponkul, T. Characteristics of Cantaloupe Jams as Affected by Substitution of Sucrose with Xylitol. Int. J. Fruit Sci. 2015, 15, 442–455. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Tai, N.D. Quality Characteristics and Antioxidant Activities of Aronia Jam Replacing Sucrose with Different Sugar Substances. Korean J. Food Nutr. 2014, 27, 888–896. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Lee, H.-J.; Cho, J.-S.; Choi, J.-Y.; Woo, J.-H.; Moon, K.-D. Effect of Sweeteners on the Quality Characteristics of Ginger (Zingiber Officinale Rosc) JungKwa. Korean J. Food Preserv. 2017, 24, 406–412. [Google Scholar] [CrossRef]
- Rutkowska, J.; Antoniewska, A.; Martinez-Pineda, M.; Nawirska-Olszańska, A.; Zbikowska, A.; Baranowski, D. Black Chokeberry Fruit Polyphenols: A Valuable Addition to Reduce Lipid Oxidation of Muffins Containing Xylitol. Antioxidants 2020, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, H.; Jiang, Y.; Ren, L.; Meng, Y.; Ma, R.; Wang, S.; Liu, Z.; Li, X.; Cui, F.; et al. Super Antioxidant and High Antibacterial Ability Bi-Functional Xylitol/2-Hydroxypropyl-β-Cyclodextrin Carbon Dots with Hydroxyl-Functionalized for Rainbow Trout Preservation. Food Res. Int. 2025, 203, 115792. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.D.S.; Guimarães, R.D.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.D.C.; Hiane, P.A.; De Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Tincu, R.C.; Cobilinschi, C.; Florea, I.A.; Cotae, A.-M.; Băetu, A.E.; Isac, S.; Ungureanu, R.; Droc, G.; Grintescu, I.M.; Mirea, L. Effects of Low-Level Organic Mercury Exposure on Oxidative Stress Profile. Processes 2022, 10, 2388. [Google Scholar] [CrossRef]
- Oku, T.; Nakamura, S. Threshold for Transitory Diarrhea Induced by Ingestion of Xylitol and Lactitol in Young Male and Female Adults. J. Nutr. Sci. Vitaminol. 2007, 53, 13–20. [Google Scholar] [CrossRef]
- Storey, D.; Lee, A.; Bornet, F.; Brouns, F. Gastrointestinal Tolerance of Erythritol and Xylitol Ingested in a Liquid. Eur. J. Clin. Nutr. 2007, 61, 349–354. [Google Scholar] [CrossRef]
- Storey, D.M.; Lee, A.; Zumbé, A. The Comparative Gastrointestinal Response of Young Children to the Ingestion of 25 g Sweets Containing Sucrose or Isomalt. Br. J. Nutr. 2002, 87, 291–297. [Google Scholar] [CrossRef][Green Version]
- de Roest, R.H.; Dobbs, B.R.; Chapman, B.A.; Batman, B.; O’Brien, L.A.; Leeper, J.A.; Hebblethwaite, C.R.; Gearry, R.B. The Low FODMAP Diet Improves Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome: A Prospective Study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. History of the Low FODMAP Diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K. Can the Pentitol-Hexitol Theory Explain the Clinical Observations Made with Xylitol? Med. Hypotheses 2000, 54, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, A.; Huang, R.; Shi, M.; Yang, R.; Wang, W.; Huang, Z.; Liu, Y.; Wu, J. Effect of xylitol on low-density lipoprotein-stimulated oxidative stress in THP-1 cells. Mol. Med. Rep. 2025, 32, 190. [Google Scholar] [CrossRef] [PubMed]
| Before Changing Sugar to Xylitol | After Changing Sugar to Xylitol | Total Change in Value | |||
|---|---|---|---|---|---|
| Analyzed parameter | %AA | µM TE/1 mL | %AA | µM TE/1 mL | Delta (µM TE/1 mL) |
| X ± SD | 20.2 ± 1.43 | 172 ± 19.6 | 24.4 ± 0.327 | 229 ± 4.50 | 56.9 ± 37.0 |
| Coefficient of variation (%) | 7.04 | 11.4 | 1.34 | 1.96 | 0.65 |
| Median | 20.0 | 168 | 25.6 | 246 | 49.5 |
| Minimum | 8.86 | 15.8 | 12.3 | 63.6 | −47.6 |
| Maximum | 27.0 | 265 | 32.9 | 346 | 148 |
| p-Value | p < 0.0001 | ||||
| Before Changing Sugar to Xylitol | After Changing Sugar to Xylitol | Total Change in Value | |||
|---|---|---|---|---|---|
| Analyzed parameter | %AA | µM TE/1 mL | %AA | µM TE/1 mL | Delta (µM TE/1 mL) |
| X ± SD | 87.1 ± 0.306 | 953 ± 4.05 | 88.3 ± 0.490 | 969 ± 6.49 | 15.6 ± 20.0 |
| Coefficient of variation (%) | 0.351 | 0.425 | 0.555 | 0.670 | 1.28 |
| Median | 88.7 | 974 | 90.5 | 997 | 17.0 |
| Minimum | 75.3 | 797 | 70.0 | 726 | −71.0 |
| Maximum | 93.2 | 1034 | 93.5 | 1038 | 60.0 |
| p-Value | p < 0.0001 | ||||
| Dependent Variable = Delta µM TE/1 mL Mean | |||
| Measures | |||
| Mean ± SD Median [Q1; Q3] | 56.9 ± 37 49.5 [43; 77.25] | ||
| Independent variables | Correlation coefficient | p-Value | |
| Zinc (mg) | 0.007 | 0.9707 | |
| Mean ± SD Median [Q1; Q3] | 8.73 ± 2.33 8.7 [7.2; 9.7] | ||
| Iron (mg) | 0.032 | 0.8578 | |
| Mean ± SD Median [Q1; Q3] | 11 ± 3.72 9.9 [8.68; 13.1] | ||
| Copper (mg) | −0.174 | 0.3256 | |
| Mean ± SD Median [Q1; Q3] | 1.17 ± 0.39 1.14 [0.92; 1.34] | ||
| Manganese (mg) | 0.036 | 0.8415 | |
| Mean ± SD Median [Q1; Q3] | 4.51 ± 1.35 4.11 [3.55; 5.25] | ||
| Selenium (µg) | −0.109 | 0.5407 | |
| Mean ± SD Median [Q1; Q3] | 48.54 ± 16.47 48.08 [37.54; 54.81] | ||
| Retinol (µg) | −0.015 | 0.9310 | |
| Mean ± SD Median [Q1; Q3] | 651.65 ± 971.47 348.11 [254.16; 459.67] | ||
| Vitamin A (µg) | −0.177 | 0.3154 | |
| Mean ± SD Median [Q1; Q3] | 1413.44 ± 1017.13 1071.55 [833.78; 1617.87] | ||
| β-carotene (µg) | −0.191 | 0.2784 | |
| Mean ± SD Median [Q1; Q3] | 9.75 ± 1.67 9.45 [8.55; 10.36] | ||
| Vitamin C (mg) | −0.199 | 0.2580 | |
| Mean ± SD Median [Q1; Q3] | 102.08 ± 53.53 96.6 [55.73; 142.93] | ||
| Vitamin E (mg) | −0.150 | 0.3973 | |
| Mean ± SD Median [Q1; Q3] | 7.32 ± 2.68 7.04 [5.37; 8.75] | ||
| Sucrose (g) | −0.153 | 0.3863 | |
| Mean ± SD Median [Q1; Q3] | 45.71 ± 20.04 40.86 [29.58; 59.75] | ||
| Glucose (g) | −0.086 | 0.6288 | |
| Mean ± SD Median [Q1; Q3] | 9.68 ± 6.27 6.96 [5.57; 12.31] | ||
| Galactose (g) | −0.147 | 0.4078 | |
| Mean ± SD Median [Q1; Q3] | 0.64 ± 0.71 0.42 [0.17; 0.93] | ||
| Lactose (g) | 0.074 | 0.6766 | |
| Mean ± SD Median [Q1; Q3] | 8.58 ± 4.78 7.82 [4.63; 11.57] | ||
| Maltose (g) | −0.156 | 0.3769 | |
| Mean ± SD Median [Q1; Q3] | 1.26 ± 0.33 1.16 [1.08; 1.42] | ||
| Fructose (g) | 0.024 | 0.8928 | |
| Mean ± SD Median [Q1; Q3] | 12 ± 8.36 8.95 [7.31; 13.1] | ||
| Lead (µg) | −0.050 | 0.7783 | |
| Mean ± SD Median [Q1; Q3] | 54.63 ± 18.17 50.94 [43.29; 60.64] | ||
| Cadmium (µg) | 0.041 | 0.8201 | |
| Mean ± SD Median [Q1; Q3] | 13.09 ± 4.98 12.03 [10.63; 13.14] | ||
| Mercury (µg) | −0.030 | 0.8644 | |
| Mean ± SD Median [Q1; Q3] | 738.21 ± 995.45 384.27 [347.13; 478.74] | ||
| Dependent Variable = Delta µM TE/1 mL Mean | |||
| Measures | |||
| Mean ± SD Median [Q1; Q3] | 15.65 ± 20.01 17 [6.5; 25.5] | ||
| Independent variables | Correlation coefficient | p-Value | |
| Zinc (mg) | 0.155 | 0.3814 | |
| Mean ± SD Median [Q1; Q3] | 8.73 ± 2.33 8.7 [7.2; 9.7] | ||
| Iron (mg) | 0.208 | 0.2376 | |
| Mean ± SD Median [Q1; Q3] | 11 ± 3.72 9.9 [8.68; 13.1] | ||
| Copper (mg) | 0.089 | 0.6176 | |
| Mean ± SD Median [Q1; Q3] | 1.17 ± 0.39 1.14 [0.92; 1.34] | ||
| Manganese (mg) | 0.196 | 0.2673 | |
| Mean ± SD Median [Q1; Q3] | 4.51 ± 1.35 4.11 [3.55; 5.25] | ||
| Selenium (µg) | −0.175 | 0.3229 | |
| Mean ± SD Median [Q1; Q3] | 48.54 ± 16.47 48.08 [37.54; 54.81] | ||
| Retinol (µg) | 0.273 | 0.1183 | |
| Mean ± SD Median [Q1; Q3] | 651.65 ± 971.47 348.11 [254.16; 459.67] | ||
| Vitamin A (µg) | 0.338 | 0.0507 | |
| Mean ± SD Median [Q1; Q3] | 1413.44 ± 1017.13 1071.55 [833.78; 1617.87] | ||
| β-carotene (µg) | 0.021 | 0.9042 | |
| Mean ± SD Median [Q1; Q3] | 9.75 ± 1.67 9.45 [8.55; 10.36] | ||
| Vitamin C (mg) | 0.147 | 0.4056 | |
| Mean ± SD Median [Q1; Q3] | 102.08 ± 53.53 96.6 [55.73; 142.93] | ||
| Vitamin E (mg) | 0.278 | 0.1113 | |
| Mean ± SD Median [Q1; Q3] | 7.32 ± 2.68 7.04 [5.37; 8.75] | ||
| Sucrose (g) | 0.108 | 0.5448 | |
| Mean ± SD Median [Q1; Q3] | 45.71 ± 20.04 40.86 [29.58; 59.75] | ||
| Glucose (g) | 0.009 | 0.9610 | |
| Mean ± SD Median [Q1; Q3] | 9.68 ± 6.27 6.96 [5.57; 12.31] | ||
| Galactose (g) | 0.201 | 0.2546 | |
| Mean ± SD Median [Q1; Q3] | 0.64 ± 0.71 0.42 [0.17; 0.93] | ||
| Lactose (g) | 0.198 | 0.2620 | |
| Mean ± SD Median [Q1; Q3] | 8.58 ± 4.78 7.82 [4.63; 11.57] | ||
| Maltose (g) | 0.081 | 0.6476 | |
| Mean ± SD Median [Q1; Q3] | 1.26 ± 0.33 1.16 [1.08; 1.42] | ||
| Fructose (g) | 0.038 | 0.8313 | |
| Mean ± SD Median [Q1; Q3] | 12 ± 8.36 8.95 [7.31; 13.1] | ||
| Lead (µg) | 0.001 | 0.9973 | |
| Mean ± SD Median [Q1; Q3] | 54.63 ± 18.17 50.94 [43.29; 60.64] | ||
| Cadmium (µg) | 0.038 | 0.8296 | |
| Mean ± SD Median [Q1; Q3] | 13.09 ± 4.98 12.03 [10.63; 13.14] | ||
| Mercury (µg) | −0.254 | 0.1479 | |
| Mean ± SD Median [Q1; Q3] | 738.21 ± 995.45 384.27 [347.13; 478.74] | ||
| Variable | N = 17 (>=50) [Effect] Mean ± SD Median [Q1; Q3] | N = 17 (<50) [Too Weak Effect or No Effect] Mean ± SD Median [Q1; Q3] | d-Cohen | p-Value | OR [95%CI] | p-Value |
|---|---|---|---|---|---|---|
| Zinc (mg) | 8.73 ± 1.52 8.68 [7.96; 9.55] | 8.73 ± 2.97 8.72 [6.81; 10.47] | 0.001 | 0.9971 | 1 [0.75–1.34] | 0.9970 |
| Iron (mg) | 11.31 ± 3.86 10.53 [9.09; 13.01] | 10.68 ± 3.67 9.65 [8.54; 13.13] | 0.167 | 0.7048 | 1 [0.87–1.26] | 0.6196 |
| Copper (mg) | 1.11 ± 0.27 1.11 [0.91; 1.26] | 1.23 ± 0.49 1.21 [0.97; 1.39] | 0.291 | 0.4047 | 0.4 [0.07–2.87] | 0.3967 |
| Manganese (mg) | 4.43 ± 1.14 4.45 [3.47; 5.17] | 4.59 ± 1.56 4.08 [3.68; 5.46] | 0.119 | 0.8497 | 0.9 [0.55–1.52] | 0.7210 |
| Selenium (µg) | 46.23 ± 18.96 43.52 [35.23; 51.36] | 50.85 ± 13.73 51.07 [40.19; 56.03] | 0.279 | 0.1792 | 1 [0.94–1.03] | 0.4163 |
| Retinol (µg) | 507.26 ± 620.89 349.64 [304.75; 432.17] | 796.03 ± 1231.54 323.88 [253.14; 581.11] | 0.296 | 0.9451 | 1 [0.999–1] | 0.4067 |
| Vitamin A (µg) | 1169.21 ± 602.56 1000.05 [903.4; 1437.52] | 1657.68 ± 1282.16 1414.3 [748.68; 1765.87] | 0.488 | 0.4282 | 0.999 [0.999–1] | 0.1876 |
| β-carotene (µg) | 9.59 ± 1.7 9.32 [8.08; 10.34] | 9.91 ± 1.68 9.59 [8.79; 10.88] | 0.186 | 0.5815 | 0.9 [0.59–1.35] | 0.5789 |
| Vitamin C (mg) | 100.98 ± 57.37 88.52 [54.61; 156.69] | 103.19 ± 51.15 97.48 [75.61; 119.48] | 0.041 | 0.9061 | 1 [0.99–1.01] | 0.9025 |
| Vitamin E (mg) | 6.66 ± 1.82 6.95 [4.91; 7.96] | 7.97 ± 3.26 7.12 [5.73; 10.56] | 0.494 | 0.1620 | 0.8 [0.62–1.09] | 0.1644 |
| Sucrose (g) | 40.06 ± 16.15 33.42 [28.74; 52.13] | 51.36 ± 22.36 45.81 [33.66; 64.26] | 0.579 | 0.1386 | 1 [0.93–1.01] | 0.1080 |
| Glucose (g) | 8.34 ± 4.47 6.26 [5.57; 10.78] | 11.02 ± 7.57 7.74 [6.02; 13.42] | 0.432 | 0.3014 | 0.9 [0.81–1.05] | 0.2307 |
| Galactose (g) | 0.48 ± 0.37 0.45 [0.25; 0.62] | 0.81 ± 0.91 0.36 [0.14; 1.14] | 0.480 | 0.7040 | 0.5 [0.14–1.47] | 0.1878 |
| Lactose (g) | 8.69 ± 5.55 9.81 [4.03; 11.88] | 8.47 ± 4.04 7.65 [5.66; 9.3] | 0.046 | 0.9451 | 1 [0.88–1.17] | 0.8905 |
| Maltose (g) | 1.16 ± 0.16 1.15 [1.07; 1.2] | 1.35 ± 0.43 1.19 [1.09; 1.59] | 0.587 | 0.3345 | 0.1 [0.01–1.83] | 0.1205 |
| Fructose (g) | 11.02 ± 7.68 8.43 [7.79; 10.82] | 12.98 ± 9.11 10.89 [6.91; 14.93] | 0.233 | 0.7565 | 1 [0.89–1.06] | 0.4942 |
| Lead (µg) | 52.63 ± 21.34 45.35 [40.33; 55.32] | 56.63 ± 14.72 51.8 [50.1; 61.96] | 0.219 | 0.1386 | 1 [0.95–1.03] | 0.5210 |
| Cadmium (µg) | 12.84 ± 5.17 12 [10.23; 13] | 13.34 ± 4.94 12.16 [10.85; 13.19] | 0.098 | 0.5815 | 1 [0.85–1.12] | 0.7697 |
| Mercury (µg) | 427.91 ± 180.4 374.78 [354.28; 445.19] | 1048.51 ± 1344.11 415.38 [345.68; 1024.79] | 0.647 | 0.3015 | 0.999 [0.996–1.001] | 0.1634 |
| Smoking | 0.6012 | 3.4 [0.32–36.83] | 0.3091 | |||
| yes | 3 (17.65%) | 1 (5.88%) | ||||
| no | 14 (82.35%) | 16 (94.12%) |
| Variable | N = 19 (>=15) [Effect] Mean ± SD Median [Q1; Q3] | N = 15 (<15) [Too Weak Effect or No Effect] Mean ± SD Median [Q1; Q3] | d-Cohen | p-Value | OR [95%CI] | p-Value |
|---|---|---|---|---|---|---|
| Zinc (mg) | 8.83 ± 1.91 8.6 [7.74; 9.46] | 8.61 ± 2.84 9.15 [6.79; 9.65] | 0.093 | 0.7885 | 1 [0.77–1.4] | 0.7809 |
| Iron (mg) | 10.94 ± 2.7 10.11 [8.99; 12.48] | 11.06 ± 4.83 9.49 [7.6; 13.23] | 0.032 | 0.9319 | 1 [0.82–1.19] | 0.9243 |
| Copper (mg) | 1.15 ± 0.36 1.11 [0.97; 1.25] | 1.2 ± 0.43 1.21 [0.88; 1.38] | 0.117 | 0.5908 | 0.7 [0.13–4.27] | 0.7279 |
| Manganese (mg) | 4.89 ± 1.38 4.63 [3.96; 5.33] | 4.03 ± 1.18 3.55 [3.22; 4.76] | 0.665 | 0.0461 | 1.8 [0.94–3.59] | 0.0759 |
| Selenium (µg) | 43.72 ± 11.09 43.52 [36.25; 51.22] | 54.64 ± 20.24 51.28 [41.86; 58.25] | 0.693 | 0.1270 | 1 [0.9–1.01] | 0.0829 |
| Retinol (µg) | 734.33 ± 1136.29 358.74 [307.21; 474.44] | 546.91 ± 737.2 273.89 [182.76; 412.45] | 0.191 | 0.1550 | 1 [0.999–1.001] | 0.5802 |
| Vitamin A (µg) | 1568.65 ± 1144.61 1414.3 [926.26; 1599.51] | 1216.84 ± 824.65 1066.39 [626.18; 1476.54] | 0.346 | 0.2981 | 1 [1–1.001] | 0.3293 |
| β-carotene (µg) | 9.73 ± 1.68 9.32 [8.69; 10.34] | 9.77 ± 1.72 9.61 [8.5; 10.61] | 0.022 | 0.9493 | 1 [0.65–1.49] | 0.9474 |
| Vitamin C (mg) | 98.26 ± 55.43 90.14 [51.42; 127.15] | 106.92 ± 52.53 98.69 [71.62; 151.16] | 0,160 | 0.6465 | 1 [0.98–1.01] | 0.6352 |
| Vitamin E (mg) | 7.85 ± 2.74 7.39 [5.94; 9.46] | 6.64 ± 2.54 5.77 [4.54; 8.18] | 0.458 | 0.1940 | 1.2 [0.91–1.61] | 0.1958 |
| Sucrose (g) | 47.39 ± 19.62 41.73 [31.46; 65.4] | 43.59 ± 21.05 33.66 [29.18; 56.19] | 0.188 | 0.5554 | 1 [0.98–1.05] | 0.5785 |
| Glucose (g) | 8.65 ± 4.03 6.67 [5.79; 11.36] | 10.98 ± 8.28 7.25 [5.51; 12.92] | 0.373 | 0.8082 | 0.9 [0.83–1.06] | 0.2948 |
| Galactose (g) | 0.68 ± 0.74 0.45 [0.34; 0.89] | 0.6 ± 0.69 0.3 [0.04; 0.99] | 0.107 | 0.5427 | 1.2 [0.43–3.17] | 0.7515 |
| Lactose (g) | 8.91 ± 5.31 9.3 [4.84; 11.54] | 8.16 ± 4.15 7.65 [5.23; 9.99] | 0.154 | 0.6596 | 1 [0.89–1.2] | 0.6487 |
| Maltose (g) | 1.22 ± 0.23 1.15 [1.1; 1.24] | 1.3 ± 0.44 1.18 [1.04; 1.5] | 0.218 | 0.9447 | 0.5 [0.06–4.21] | 0.5256 |
| Fructose (g) | 11.34 ± 6.53 9.55 [7.39; 12.99] | 12.85 ± 10.41 8.11 [7.35; 13.06] | 0.179 | 0.8896 | 1 [0.9–1.06] | 0.5983 |
| Lead (µg) | 49.96 ± 10.08 50.81 [42.5; 55.99] | 60.54 ± 24.09 51.8 [46.92; 66.67] | 0.600 | 0.3145 | 1 [0.91–1.01] | 0.1280 |
| Cadmium (µg) | 12.19 ± 2.94 12 [10.61; 13.85] | 14.22 ± 6.71 12.06 [10.71; 12.85] | 0.410 | 0.6900 | 0.9 [0.78–1.07] | 0.2599 |
| Mercury (µg) | 471.98 ± 443.54 374.78 [352.88; 404.18] | 1075.44 ± 1365.57 460.75 [338.87; 977.09] | 0.627 | 0.1270 | 0.999 [0.998–1] | 0.1566 |
| Smoking | 1 | 0.8 [0.09–6.17] | 0.8013 | |||
| yes | 2 (10.53%) | 2 (13.33%) | ||||
| no | 17 (89.47%) | 13 (86.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górna, I.; Kowalówka, M.; Więckowska, B.; Banaszak, M.; Kosewski, G.; Grządzielska, O.; Przysławski, J.; Drzymała-Czyż, S. Xylitol Antioxidant Properties: A Potential Effect for Inflammation Reduction in Menopausal Women?—A Pilot Study. Curr. Issues Mol. Biol. 2025, 47, 611. https://doi.org/10.3390/cimb47080611
Górna I, Kowalówka M, Więckowska B, Banaszak M, Kosewski G, Grządzielska O, Przysławski J, Drzymała-Czyż S. Xylitol Antioxidant Properties: A Potential Effect for Inflammation Reduction in Menopausal Women?—A Pilot Study. Current Issues in Molecular Biology. 2025; 47(8):611. https://doi.org/10.3390/cimb47080611
Chicago/Turabian StyleGórna, Ilona, Magdalena Kowalówka, Barbara Więckowska, Michalina Banaszak, Grzegorz Kosewski, Olivia Grządzielska, Juliusz Przysławski, and Sławomira Drzymała-Czyż. 2025. "Xylitol Antioxidant Properties: A Potential Effect for Inflammation Reduction in Menopausal Women?—A Pilot Study" Current Issues in Molecular Biology 47, no. 8: 611. https://doi.org/10.3390/cimb47080611
APA StyleGórna, I., Kowalówka, M., Więckowska, B., Banaszak, M., Kosewski, G., Grządzielska, O., Przysławski, J., & Drzymała-Czyż, S. (2025). Xylitol Antioxidant Properties: A Potential Effect for Inflammation Reduction in Menopausal Women?—A Pilot Study. Current Issues in Molecular Biology, 47(8), 611. https://doi.org/10.3390/cimb47080611







