1. Introduction
Thrombophilia, a predisposition to thrombosis due to inherited or acquired coagulation abnormalities, has been increasingly recognized as a contributor to adverse pregnancy outcomes [
1,
2,
3]. Inherited thrombophilic mutations such as Factor V Leiden and Prothrombin G20210A, along with other commonly investigated genetic polymorphisms of uncertain clinical significance (e.g., MTHFR variants, PAI-1 4G/4G, Factor XIII V34L), have been associated in the literature with placental vascular dysfunction, which may contribute to fetal growth restriction, preeclampsia, placental abruption, and stillbirth [
4,
5]. These mutations are known to affect maternal–fetal circulation by promoting microthrombi formation and altering vascular remodeling during placentation [
6,
7].
Although previous studies have explored associations between thrombophilia and clinical outcomes, fewer have systematically examined the histopathological characteristics of the placenta in genetically stratified cohorts [
2,
8]. Placental lesions such as infarctions, intervillous thrombosis, acute atherosis, and villous stasis reflect underlying hemodynamic disturbances and can serve as morphological markers of thrombophilic influence on pregnancy [
9,
10]. The umbilical cord is vital to fetal development, yet specific features like cord coiling remain underexplored. Coiling, quantified by the umbilical coiling index, is a physiological adaptation that helps protect the cord from external pressure [
11]. In parallel, the structure of the umbilical cord, including coiling pattern, insertion site, and hypertwisting, may also provide insight into fetal adaptive mechanisms in response to vascular stress [
11,
12,
13,
14].
This study aimed to evaluate the relationship between inherited thrombophilic mutations and histological features of the placenta and umbilical cord. By comparing placental pathology and morphometric parameters in women with and without thrombophilia, we sought to clarify the role of genetic prothrombotic risk in placental vascular compromise and fetal development. The findings may contribute to improved risk stratification, targeted screening, and early intervention strategies in thrombophilia-associated pregnancies.
It is important to acknowledge that certain polymorphisms included in this study—such as
MTHFR C677T,
PAI-1 4G/4G, and
Factor XIII V34L—have been the subject of substantial debate within the scientific community. While these variants have historically been associated with adverse pregnancy outcomes, recent expert consensus has questioned their clinical relevance in the context of thrombophilia screening. Notably, current guidelines explicitly discourage the routine testing of MTHFR polymorphisms, citing insufficient evidence of a causal link with thrombotic events or pregnancy complications. In light of these developments, the present study does not seek to endorse the clinical utility of these markers. Instead, their inclusion serves a purely exploratory purpose—to investigate potential associations between these common genetic variants and subclinical placental histopathological changes in a genetically stratified cohort [
15].
2. Materials and Methods
2.1. Study Design and Ethical Approval
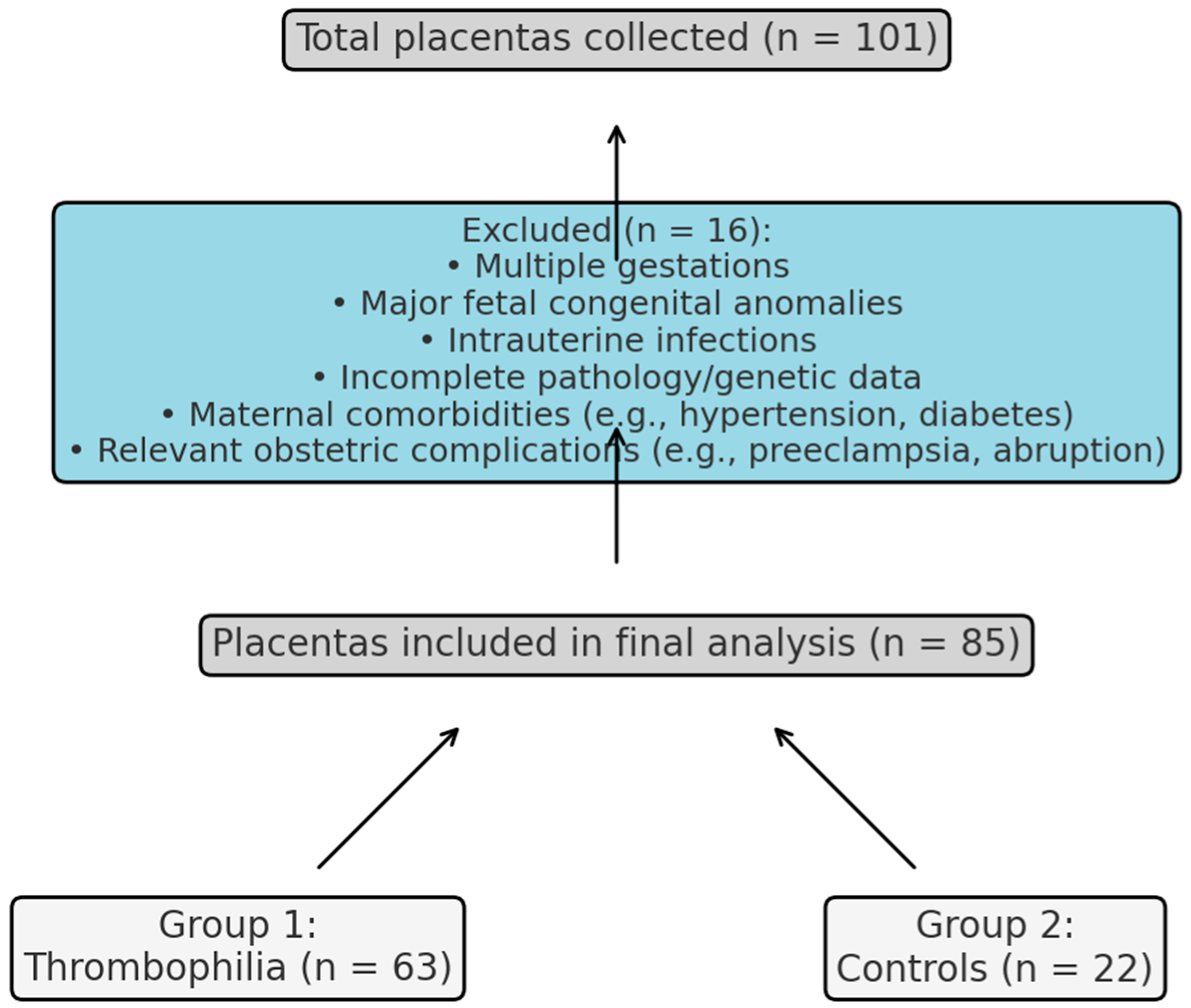
This retrospective observational study included 101 placental specimens collected at the Clinical County Emergency Hospital Bihor, Romania, between September 2020 and September 2024. After applying exclusion criteria, 85 placentas were eligible for analysis: 63 assigned to the thrombophilia group (Group 1), based on confirmed inherited prothrombotic mutations, and 22 to the control group (Group 2), from pregnancies without known maternal pathology. No formal a priori power calculation was performed; however, a post hoc estimation based on our final sample (
N = 85; 63 thrombophilia, 22 controls) suggests approximately 80% power to detect a moderate-to-large effect size (Cohen’s d ≈ 0.7) at α = 0.05. The selection process and final allocation of placentas are detailed in
Figure 1. The study protocol was approved by the local Institutional Review Board (IRB), and all procedures complied with the Declaration of Helsinki. Written informed consent was obtained from all participants before inclusion.
2.2. Study Population
Inclusion criteria for both groups encompassed the availability of complete placental inclusion criteria for both groups included the availability of complete placental assessment (macroscopic and histologic), validated genetic testing, and comprehensive clinical records with antenatal follow-up.
Exclusion criteria encompassed multiple gestations, major fetal congenital anomalies, intrauterine infections, incomplete pathology or genetic data, and known confounding maternal conditions that could influence placental morphology or vascular pathology. These included the following:
- -
Systemic comorbidities (e.g., chronic hypertension, diabetes mellitus, autoimmune diseases);
- -
Relevant obstetric complications (e.g., preeclampsia, placenta previa, placental abruption, preterm premature rupture of membranes).
Group 1 comprised placentas from women with confirmed inherited thrombophilic mutations, identified through standardized molecular testing. Group 2 included placentas from term or late preterm pregnancies without maternal pathology, genetic thrombophilia, or pregnancy complications.
2.3. Placental Collection and Macroscopic Examination
Placental samples were collected from all eligible term deliveries between January 2022 and December 2023, with 101 cases screened in total before applying exclusion criteria. Placentas were collected immediately after delivery and fixed in 10% neutral buffered formalin. A standardized protocol was applied for macroscopic assessment by a dedicated pathology team blinded to maternal thrombophilia status. Measured parameters included the following:
Placental weight (grams);
Maximum placental diameter (centimeters);
Placental thickness (centimeters);
Umbilical cord length (centimeters) and insertion type (central, eccentric, or marginal).
2.4. Histopathological Evaluation
Histological assessments were performed within the Department of Pathology at the Bihor County Emergency Clinical Hospital by pathologists experienced in placental evaluation. The observers were blinded to thrombophilia status, clinical outcomes, and study group allocation. Only maternal age and gestational age, routinely included in pathology forms, were available to guide lesion interpretation.
Sections representative of central and peripheral cotyledons, the umbilical cord, and membranes were processed and stained with hematoxylin and eosin. Histologic features were scored according to the Amsterdam Consensus (2016), classifying lesions under Maternal Vascular Malperfusion (MVM) or Fetal Vascular Malperfusion (FVM) where appropriate. Key lesions recorded included:
- •
Intervillous thrombi (excessive: MVM);
- •
Placental infarction (MVM);
- •
Decidual vasculopathy (acute atherosis; MVM);
- •
Villous stasis/delayed villous maturation (MVM);
- •
Stromal fibrosis (mild nonspecific; Other/MVM);
- •
Avascular or thrombosed fetal vessels (FVM).
A detailed overview of the placental lesions assessed, their classification under the Amsterdam Consensus criteria, and inclusion in the composite histological score is presented in
Table 1.
Morphometric analysis was performed on images acquired at 40× magnification using a standard light microscope. Vascular profiles within terminal villi were manually selected and measured by a single observer using ImageJ (Version 1.54p, NIH, USA), following a predefined protocol. Vessel inclusion criteria included regular shape, intact endothelial lining, and localization within terminal villi. To ensure consistency, measurements were repeated in a random subset of images, with acceptable intra-observer variability. A schematic of the analysis workflow and a representative example are provided in
Supplementary Figure S1.
2.5. Histologic Score Development
While established scoring systems for maternal vascular malperfusion—such as the Biron Shental index and the Khong MVM severity grading system—provide comprehensive assessment frameworks, they often rely on morphometric or semiquantitative parameters that are not consistently feasible in retrospective cohorts. To ensure applicability in routine histopathological evaluation, we developed a streamlined five-item composite score, aligned with the Amsterdam Consensus criteria.
The five lesions included—villous stasis, stromal fibrosis, intervillous thrombosis, placental infarction, and acute atherosis—were selected based on their frequent documentation in thrombophilia-associated placentas, as well as their reproducibility and pathophysiological relevance in maternal vascular malperfusion. These features have been repeatedly linked in the literature to altered uteroplacental circulation in thrombophilic pregnancies and were consistently observed in our cohort.
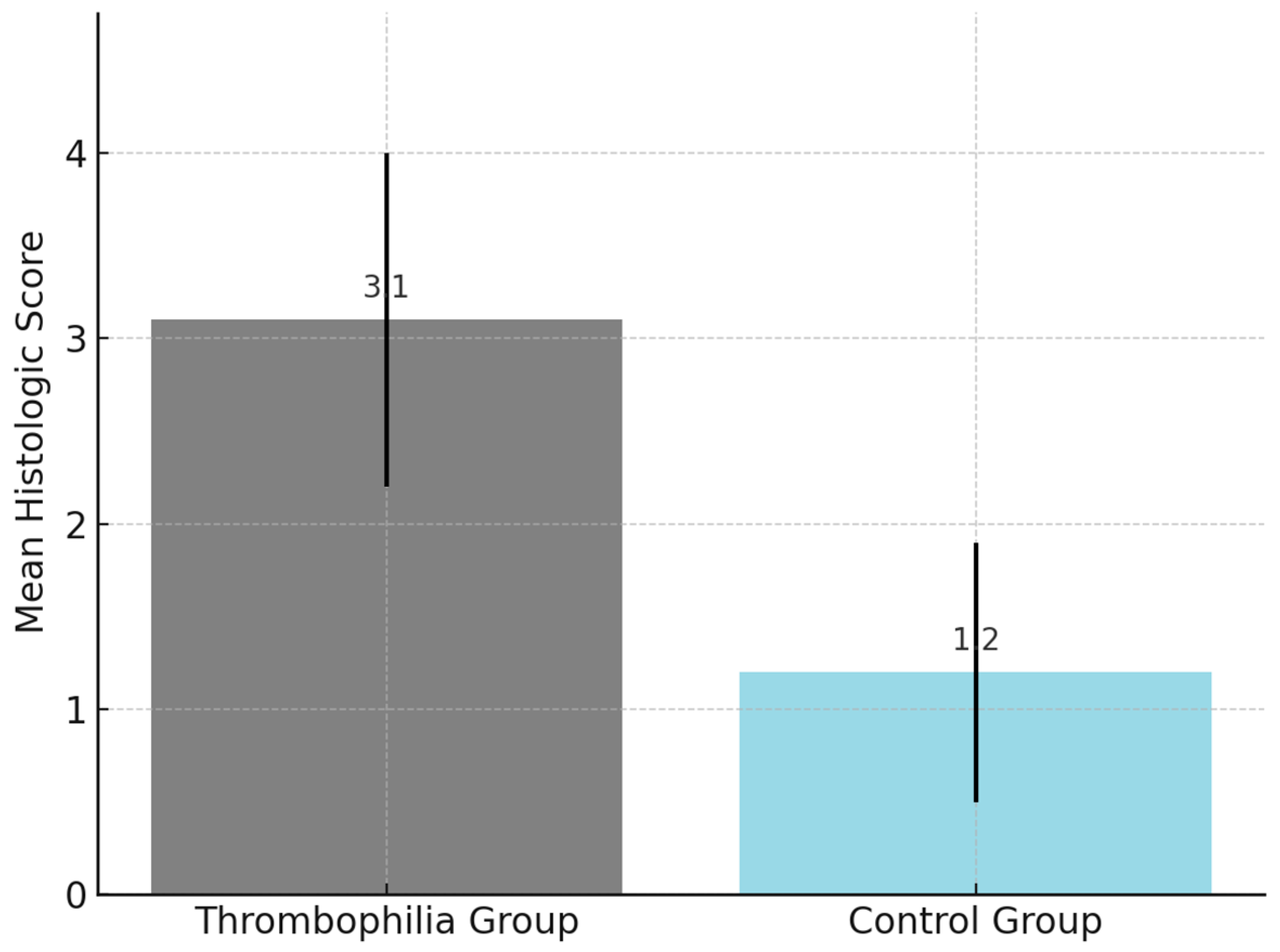
One point was assigned for the presence of each lesion, resulting in a total score ranging from 0 to 5. A cutoff value of ≥3 was pre-defined and explored for its discriminatory capacity between the thrombophilia and control groups. Internal validation showed a statistically significant association between scores ≥3 and known thrombophilic mutations. Although interobserver agreement was not formally tested, all assessments were performed within the Department of Pathology by experienced placental pathologists using standardized diagnostic criteria. The histopathologic findings are presented in
Figure 2.
2.6. Genetic Evaluation
Genetic testing had been performed before pregnancy in all women included in the thrombophilia group, based on clinical indications or family history. Genetic results were retrieved from patients’ medical records and confirmed to follow standardized diagnostic protocols. Peripheral blood samples had been collected at the time of initial evaluation, and DNA extraction was performed using silica-based column kits (QIAamp DNA Blood Mini Kit, Qiagen, Hilden, Germany; Cat. No. 51104) or automated extraction platforms. Genotyping was conducted by real-time polymerase chain reaction (PCR) using allele-specific primers and fluorescent probes (TaqMan chemistry, Coralville, IA, USA). Internal positive and negative controls were included in each run to ensure validity. The panel included the following: Factor V Leiden (G1691A), Prothrombin gene mutation (G20210A), MTHFR C677T, MTHFR A1298C, PAI-1 4G/5G polymorphism, Factor XIII V34L, and EPCR a1/a3 alleles. All analyses were conducted in an ISO-certified molecular diagnostics laboratory, and results were categorized as homozygous, heterozygous, or wild-type for each locus. Genetic data were then correlated with histopathological findings and clinical outcomes.
2.7. Statistical Analysis
Analyses were performed using SPSS (v20, Chicago, IL, USA) with SciPy and statsmodels libraries. Continuous variables were tested for normality using the Shapiro–Wilk test and compared by Student’s t-test or Mann–Whitney U test as appropriate. Categorical data were analyzed with a Chi-square test or Fisher’s exact test. Associations between the histologic score and continuous clinical or macroscopic parameters were evaluated with Pearson or Spearman correlation coefficients. A multivariate linear regression model identified independent predictors of the histologic score. Statistical significance was set at p < 0.05.
3. Results
3.1. Demographic and Birth Data—Summary
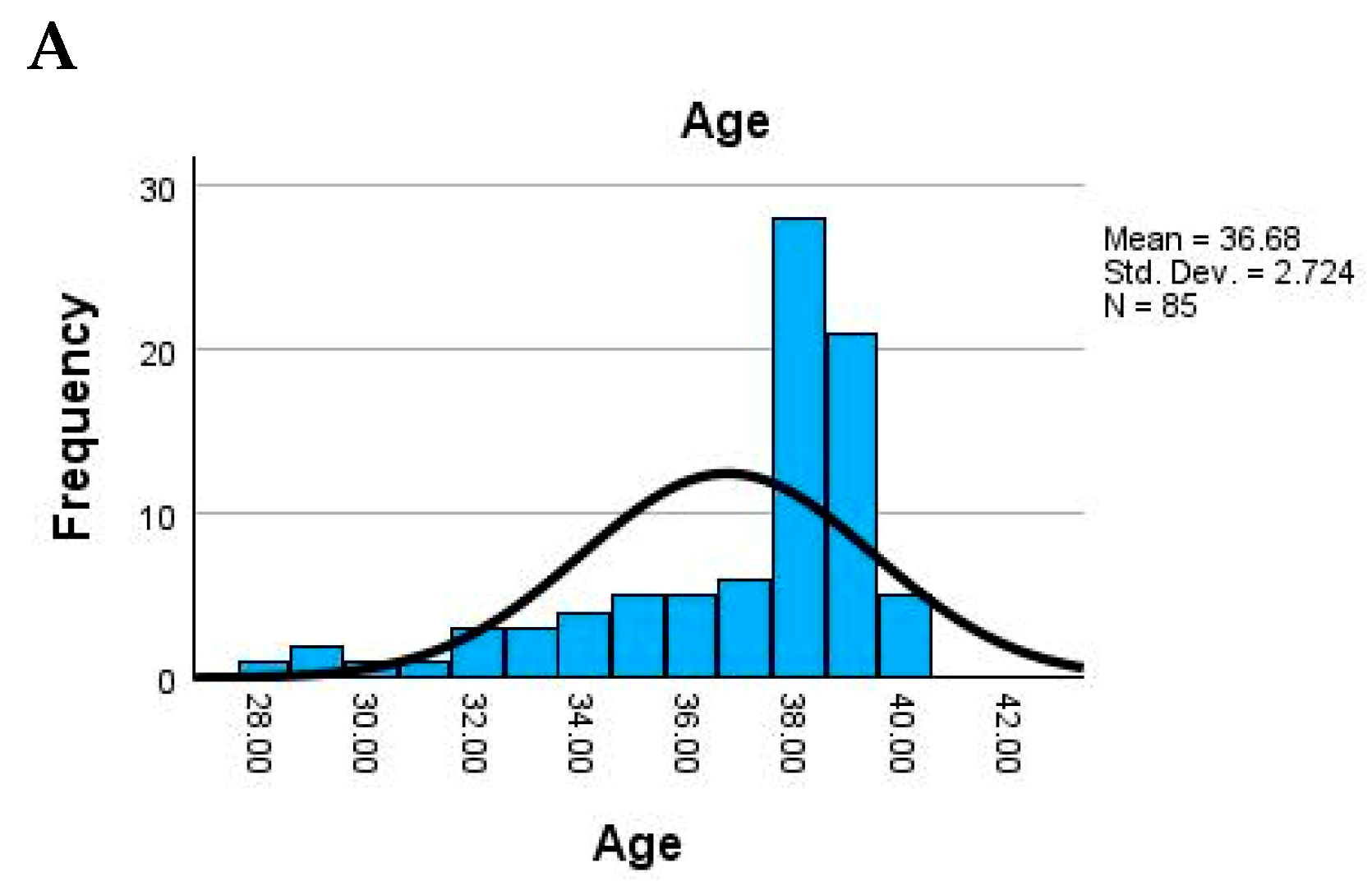
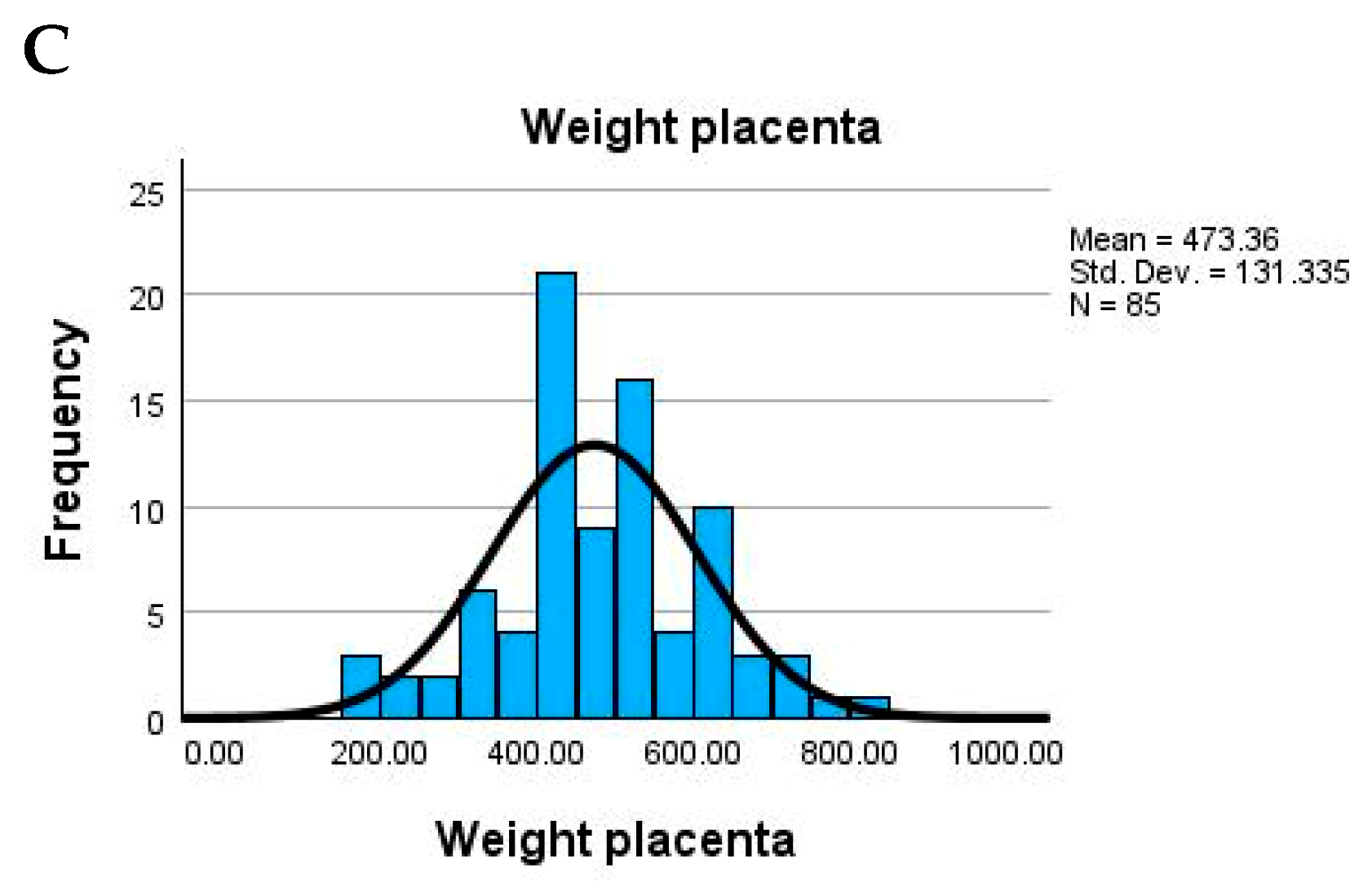
The study population consisted of adult women with a relatively narrow age range, the mean maternal age being 36.7 years (SD = 2.7), suggesting a homogeneous group. Newborn weight showed substantial variability, with an average of 2754.7 g and values ranging from very low birth weight (900 g) to normal (up to 4000 g). The placental weight also varied widely (mean = 473.4 g), possibly indicating underlying pathological changes in some cases (
Figure 3).
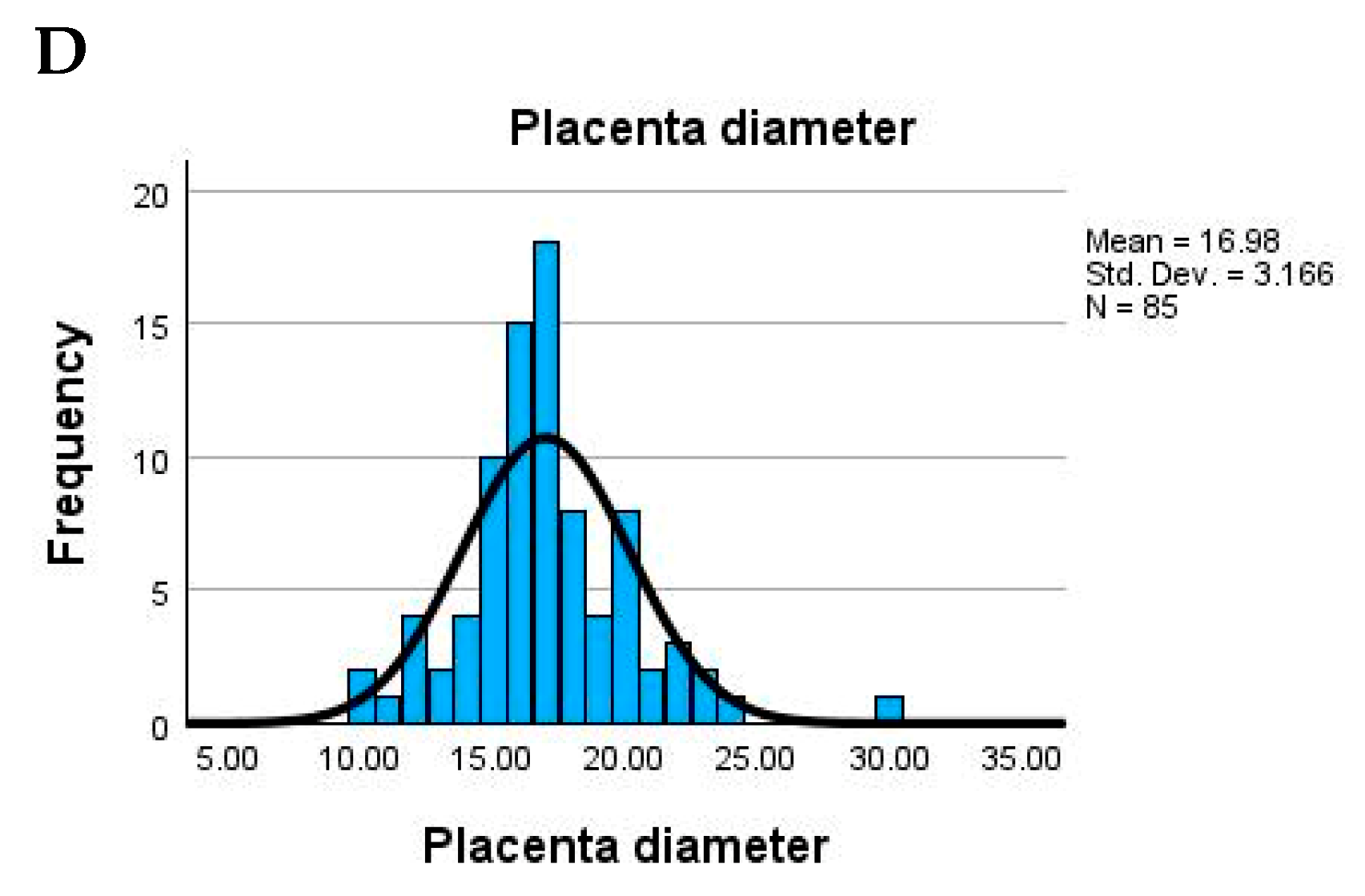
Placental size parameters were generally within expected limits. The average placental diameter was 17 cm (range: 10–30 cm), while thickness averaged 2.3 cm, with some extreme values (as low as 0 cm), potentially pointing to measurement issues or anomalies. The umbilical cord also exhibited significant variation: average length was 19.6 cm (ranging from 0 to 45 cm), and width averaged 1.13 cm. A minimum width or length of 0 could suggest missing data or abnormal development. The descriptive statistics of maternal and birth-related parameters in the study population, and a complete mapping of original descriptive terms to the Amsterdam classification is presented in
Table 1.
3.2. Baseline Characteristics
The baseline characteristics of the study participants are summarized in
Table 2. No statistically significant differences were observed between the thrombophilia and control groups regarding gestational age at delivery (36.5 ± 2.6 vs. 36.9 ± 3.1 weeks,
p = 0.2042), fetal birth weight (2754.7 ± 698.2 g vs. 2770.9 ± 630.4 g,
p = 0.8346), or placental macroscopic parameters. Specifically, mean placental weight, diameter, and thickness were comparable between groups, with
p-values > 0.4 in all comparisons. These findings suggest that gross placental morphology may not reliably differentiate pregnancies affected by thrombophilia from those without thrombotic risk, thereby highlighting the potential role of histopathological examination in detecting subclinical placental alterations.
Effect size analysis using Cohen’s d (
Table 2) revealed large differences between thrombophilia and control groups for several genetic mutations, including PAI-1 4G/4G (
d = 1.91), MTHFR C677T (
d = 1.85), EPCR (
d = 1.06), and Factor V Leiden (
d = 0.93), suggesting strong associations with thrombophilic status. Moderate to large effect sizes were also observed for placental lesions such as villous stasis (
d = 0.65), acute atherosis (
d = 0.60), and retroplacental hematoma (
d = 0.68), supporting the hypothesis that histopathological features reflect underlying thrombotic risk. Behavioral factors like smoking also showed a moderate effect (
d = 0.55), indicating potential lifestyle contributions to placental vascular dysfunction. These findings emphasize the potential utility of combining histological and genetic markers in evaluating thrombophilic risk.
The cohort was divided into two groups: individuals with at least one genetic mutation (Group 1) and those without mutations (Group 2). Overall, there were no statistically significant differences between the groups across the assessed parameters. Maternal age, newborn weight, placental weight and dimensions, as well as umbilical cord length and width, were all comparable between the two groups (
p > 0.05 for all). These findings suggest that the presence of genetic mutations did not significantly influence basic maternal or neonatal anthropometric characteristics in this sample. The comparison of maternal and placental morphometric parameters between thrombophilia and control groups is presented in
Table 3.
3.3. Genetic Polymorphisms (Heterozygote/Homozygote Values Range from 0 to 2)
The study assessed a panel of genetic variants, including both clinically validated thrombophilia markers (Factor V Leiden and Prothrombin G20210A) and additional polymorphisms frequently investigated in relation to pregnancy outcomes, such as MTHFR C677T, MTHFR A1298C, PAI-1 4G/4G, Factor XIII V34L, and EPCR variants. The most prevalent polymorphism in the cohort was PAI-1 4G/4G (mean = 0.98), followed by MTHFR C677T (mean = 0.76) and MTHFR A1298C (0.48). Factor V Leiden and Factor XIII V34L were present in approximately 26–48% of individuals, while Factor II G20210A and EPCR variants were less frequent (means ~0.26 and 0.34).
Placental pathology findings showed several common lesions. Intervillous thrombosis, villous agglutinations, and staza vilozitară (villous stasis) were each observed in around 38–50% of cases, suggesting widespread circulatory disturbances. Placental infarctions, acute atherosis, and marked villous edema were also common, each appearing in roughly a third of the sample. Inflammatory and ischemic findings such as chorioamnionitis, acute hypoxia/malperfusion, and retroplacental hematoma were present in 18–26% of placentas. Features like immature or fibrous villous appearance and hypertwisting of the cord were variably expressed, pointing to diverse placental responses to maternal and fetal stress.
A statistically significant difference was observed in the distribution of all analyzed genetic mutations between the two groups (
p < 0.05 for all variants). In Group 1 (with mutations), heterozygous and homozygous forms of Factor V Leiden, Factor II G20210A, MTHFR C677T, MTHFR A1298C, Factor XIII V34L, PAI-1 4G/4G, and EPCR variants were present in varying proportions. In contrast, Group 2 (without mutations) included only individuals with no detectable mutation for any of the investigated thrombophilia markers. These findings confirm the clear genetic separation between the two groups and validate the classification used in the study (
Table 4).
The distribution of thrombophilic genetic mutations is presented in
Figure 4.
3.4. Placental and Cord Pathology Features
Histopathological examination of the placentas revealed several frequent abnormalities. Staza vilozitară (villous stasis) and villous agglutinations were the most common, present in approximately 50% and 45% of cases, respectively, indicating significant circulatory stagnation and clustering of villi. Intervillous thrombosis and placental infarction were also prevalent (each around 38%), suggesting widespread disturbances in maternal-fetal blood flow. Marked villous edema (39%) and acute hypoxia or malperfusion (35%) pointed to signs of fetal vascular compromise.
Chronic lesions such as fibrous appearance (34%) and acute placental atherosis (34%) indicated long-term vascular stress or damage, possibly associated with maternal conditions like hypertension, which was noted in 22% of cases. Inflammatory changes were observed less frequently, with chorioamniotitis present in 18% of samples. Other findings included retroplacental hematoma (26%), boiled meat appearance (27%)—a nonspecific macroscopic marker of pathology—and immature villous structure (16%), which may suggest delayed placental development. Chorionic vasal thrombosis, while less common (13%), represents a severe vascular lesion when present (
Table 5).
Table 5.
Prevalence and interpretation of thrombophilia-related genetic mutations in the study cohort.
Table 5.
Prevalence and interpretation of thrombophilia-related genetic mutations in the study cohort.
| Variable | Mean | Interpretation |
|---|
| Factor V Leiden | 0.48 | Approx. 48% of the sample carries at least one mutated allele. |
| Factor II G20210A | 0.26 | About 26% have mutation, suggesting a moderate prevalence. |
| MTHFR C677T | 0.76 | Over 75% show some variant. |
| MTHFR A1298C | 0.48 | Roughly half have a mutation. |
| Factor XIII V34L | 0.26 | Mutation present in about 26%. |
| PAI-1 4G/4G | 0.98 | Very high frequency—nearly all subjects carry this variant. |
| EPCR (endothelial protein C receptor) | 0.34 | Detected in about 1/3 of the cohort. |
When comparing placental pathology between the two groups, several lesions were more frequent in individuals with genetic mutations (Group 1), although not all reached statistical significance. Notably, acute placental atherosis, retroplacental hematoma, signs of maternal hypertension, and villous stasis were significantly more common in Group 1 (
p < 0.05), suggesting a link between thrombophilic mutations and maternal vascular malperfusion. Other findings, such as boiled meat appearance, fibrous changes, placental infarction, and villous edema, were also more frequent in Group 1 but did not reach statistical significance. Inflammatory changes like chorioamnionitis and features such as chorionic vasal thrombosis and intervillous thrombosis showed no meaningful differences between groups. Overall, the presence of thrombophilia appears to be associated with a higher rate of placental vascular lesions (
Table 6).
3.5. Umbilical Cord Features
Assessment of umbilical cord characteristics revealed that non-central insertion was common, with a mean value of 0.72, indicating that most cords were marginal or eccentrically inserted. Approximately 39% of cases showed hypertwisting of the cord, a feature that may impact fetal blood flow or indicate intrauterine stress. Additionally, normal cord coiling was present in 72% of cases, although some variation was observed, suggesting mild structural abnormalities in a subset of the cohort (
Table 7).
When analyzing umbilical cord characteristics, insertion type (central, eccentric, or marginal) did not differ significantly between groups (
p = 0.281), with central insertion being most common overall (45%). However, significant differences were observed for both cord twisting and coiling. Hypertwisting was more frequent in the group without genetic mutations (Group 2), present in 17.6% compared to 21.2% in Group 1 (
p = 0.001), suggesting a possible compensatory or unrelated mechanical factor. Similarly, modified (abnormal) cord coiling was significantly more common in Group 1 (60%) versus 11.8% in Group 2 (
p = 0.001), indicating a potential association between thrombophilia and altered cord morphology (
Table 8).
The comparison of composite histologic scores between groups is presented in
Figure 5.
3.6. Correlations with General Aspects
Correlation analysis between genetic mutations and general parameters revealed no significant associations with maternal age. However, Factor II G20210A showed weak but statistically significant negative correlations with newborn weight (
r = −0.231,
p = 0.034) and placental weight (
r = −0.249,
p = 0.022), suggesting a possible impact on fetal growth. Similarly, Factor V and Factor II were both negatively correlated with placental diameter, and Factor V also with placental thickness, indicating that these mutations may be linked to subtle placental structural changes. Other correlations were weak and not statistically significant (
Table 9).
3.7. Correlations with Genetic Mutations
Several significant correlations were observed between the studied genetic mutations. Factor V was strongly correlated with Factor II G20210A (
r = 0.720,
p < 0.001), suggesting they frequently co-occur. Factor V also showed moderate associations with Factor XIII V34L and PAI-1 4G/4G. MTHFR C677T was significantly correlated with both PAI-1 4G/4G and EPCR, indicating a possible shared genetic predisposition. Similarly, MTHFR A1298C correlated with PAI-1 4G/4G and EPCR, while PAI-1 4G/4G itself showed multiple associations, including with Factor XIII V34L. These patterns suggest clusters of co-inherited thrombophilic mutations in the cohort (
Table 10).
3.8. Correlations with Placenta Appearance
Several genetic mutations were significantly associated with placental morphological changes. Factor V showed strong positive correlations with placental infarction, fibrous appearance, villous agglutinations, and intervillous thrombosis, suggesting a vascular impact. Factor II G20210A was also positively correlated with placental infarction, intervillous thrombosis, and chorionic vasal thrombosis. MTHFR C677T was weakly associated with villous edema, signs of maternal hypertension, and villous stasis, while MTHFR A1298C showed a mild link to boiled meat appearance. Factor XIII V34L had the strongest associations, significantly correlating with placental infarction, atherosis, and hypertension. PAI-1 4G/4G was linked to signs of hypertension, villous stasis, and villous edema, whereas EPCR showed an inverse correlation with placental infarction and intervillous thrombosis, but a positive one with villous edema and villous stasis. These findings suggest distinct vascular and inflammatory placental patterns associated with specific thrombophilic mutations (
Table 11).
3.9. Correlations with the Umbilical Cord
Most genetic mutations showed no significant correlation with umbilical cord length, width, or insertion site, indicating that these features are largely independent of thrombophilic status. However, he following notable associations emerged: MTHFR C677T was negatively correlated with hypertwisted cords (
r = −0.245,
p = 0.024), suggesting reduced twisting in carriers. PAI-1 4G/4G showed a strong negative correlation with hypertwisting (
r = −0.303,
p = 0.005), and a positive correlation with normal cord coiling (
r = 0.363,
p = 0.001), implying a protective or stabilizing effect. Additionally, MTHFR A1298C was positively correlated with normal coiling (
r = 0.278,
p = 0.010). These findings suggest certain genetic variants may subtly influence cord structure, particularly in relation to coiling and twisting (
Table 12).
The correlations between thrombophilia-associated genetic mutations and placental histopathological lesions are presented in
Table 13.
Table 13.
Correlations between thrombophilia-associated genetic mutations and placental histopathological lesions.
Table 13.
Correlations between thrombophilia-associated genetic mutations and placental histopathological lesions.
| Parameters | Factor_V | Factor II G20220A | MTHFR C677T | MTHFR A1298C | Factor XIII V34L | PAI 1 4G4G | EPCR |
|---|
| Boiled meat | r | 0.210 | 0.101 | 0.052 | 0.219 * | 0.094 | 0.046 | 0.176 |
| p | 0.054 | 0.356 | 0.634 | 0.044 | 0.392 | 0.678 | 0.107 |
| Immature appearance | r | 0.011 | 0.082 | −0.120 | 0.100 | −0.034 | 0.154 | −0.119 |
| p | 0.924 | 0.458 | 0.273 | 0.363 | 0.756 | 0.161 | 0.279 |
| Fibrous appearance | r | 0.267 * | 0.116 | 0.202 | 0.070 | 0.150 | 0.130 | −0.047 |
| p | 0.014 | 0.292 | 0.063 | 0.524 | 0.170 | 0.236 | 0.671 |
| Placental infarction | r | 0.442 ** | 0.350 ** | 0.120 | 0.087 | 0.535 ** | 0.211 | −0.354 ** |
| p | 0.000 | 0.001 | 0.274 | 0.427 | 0.000 | 0.053 | 0.001 |
| Acute placental atherosis | r | 0.200 | 0.209 | 0.168 | −0.104 | 0.279 ** | 0.213 | −0.099 |
| p | 0.066 | 0.055 | 0.125 | 0.344 | 0.010 | 0.050 | 0.367 |
| Marked villous edema | r | 0.067 | 0.111 | 0.229 * | 0.104 | 0.019 | 0.048 | 0.292 ** |
| p | 0.540 | 0.312 | 0.035 | 0.342 | 0.862 | 0.663 | 0.007 |
| Signs of HTN | r | 0.183 | 0.163 | 0.256 * | −0.086 | 0.298 ** | 0.267 * | −0.029 |
| p | 0.093 | 0.137 | 0.018 | 0.435 | 0.006 | 0.014 | 0.794 |
| Acute hypoxia malperfusion | r | −0.049 | −0.081 | 0.209 | 0.018 | 0.138 | 0.130 | −0.012 |
| p | 0.659 | 0.460 | 0.055 | 0.868 | 0.208 | 0.237 | 0.912 |
| Chorioamniotitis | r | 0.032 | 0.007 | −0.193 | −0.010 | 0.060 | −0.091 | 0.057 |
| p | 0.774 | 0.951 | 0.076 | 0.926 | 0.587 | 0.406 | 0.602 |
| Retroplacental hematoma | r | −0.094 | −0.035 | 0.195 | 0.015 | 0.014 | 0.106 | −0.029 |
| p | 0.392 | 0.751 | 0.074 | 0.894 | 0.897 | 0.336 | 0.795 |
| Intervillous thrombosis | r | 0.540 ** | 0.487 ** | −0.118 | 0.087 | 0.241 * | 0.129 | −0.303 ** |
| p | 0.000 | 0.000 | 0.282 | 0.427 | 0.027 | 0.239 | 0.005 |
| Chorionic vassal thrombosis | r | 0.362 ** | 0.272 * | −0.069 | −0.064 | 0.131 | −0.107 | −0.056 |
| p | 0.001 | 0.012 | 0.528 | 0.559 | 0.233 | 0.328 | 0.613 |
| Villous agglutinations | r | 0.275 * | 0.229 * | 0.164 | 0.055 | 0.130 | 0.077 | 0.102 |
| p | 0.011 | 0.035 | 0.134 | 0.614 | 0.237 | 0.486 | 0.355 |
| Villous stasis | r | 0.071 | 0.126 | 0.268 * | 0.207 | 0.199 | 0.263 * | 0.265 * |
| p | 0.517 | 0.249 | 0.013 | 0.058 | 0.068 | 0.015 | 0.014 |
| N | 85 | 85 | 85 | 85 | 85 | 85 | 85 |
The correlations between thrombophilic genetic mutations and umbilical cord morphological features are presented in
Table 14.
Table 14.
Correlations between thrombophilic genetic mutations and umbilical cord morphological features.
Table 14.
Correlations between thrombophilic genetic mutations and umbilical cord morphological features.
| Parameters | Factor V | Factor II G20220A | MTHFR C677T | MTHFR A1298C | FACTOR XIII V34L | PAI/1 4G4G | EPCR |
|---|
| Umbilical cord length | r | −0.055 | −0.019 | −0.052 | 0.031 | 0.105 | −0.143 | −0.014 |
| p | 0.619 | 0.861 | 0.634 | 0.779 | 0.340 | 0.190 | 0.899 |
| Umbilical cord width | r | −0.093 | −0.181 | 0.090 | −0.064 | 0.118 | 0.025 | −0.106 |
| p | 0.398 | 0.098 | 0.412 | 0.561 | 0.281 | 0.821 | 0.336 |
| Inset | r | −0.052 | −0.114 | −0.037 | 0.081 | −0.134 | −0.136 | −0.062 |
| p | 0.634 | 0.298 | 0.735 | 0.462 | 0.222 | 0.213 | 0.575 |
| Hypertwisted | r | −0.094 | −0.024 | −0.245 * | −0.166 | −0.106 | −0.303 ** | −0.013 |
| p | 0.390 | 0.824 | 0.024 | 0.128 | 0.333 | 0.005 | 0.905 |
| CO normal | r | 0.090 | 0.108 | 0.123 | 0.278 * | 0.010 | 0.363 ** | 0.066 |
| p | 0.411 | 0.325 | 0.263 | 0.010 | 0.931 | 0.001 | 0.551 |
| N | 85 | 85 | 85 | 85 | 85 | 85 | 85 |
3.10. Multiple Linear Regression
A multiple linear regression adjusting for smoking, BMI, gestational age, and parity showed that thrombophilia was independently associated with higher histologic scores (β = +2.3; 95% CI = 1.1–3.5;
p = 0.001). Gestational age was inversely associated with histologic score (β = –0.2; 95% CI = –0.4–0.0;
p = 0.045). Other covariates were not significant predictors (
Table 15).
These results confirm that thrombophilia status is independently associated with higher placental pathology scores, even after adjusting for smoking, BMI, gestational age, and parity.
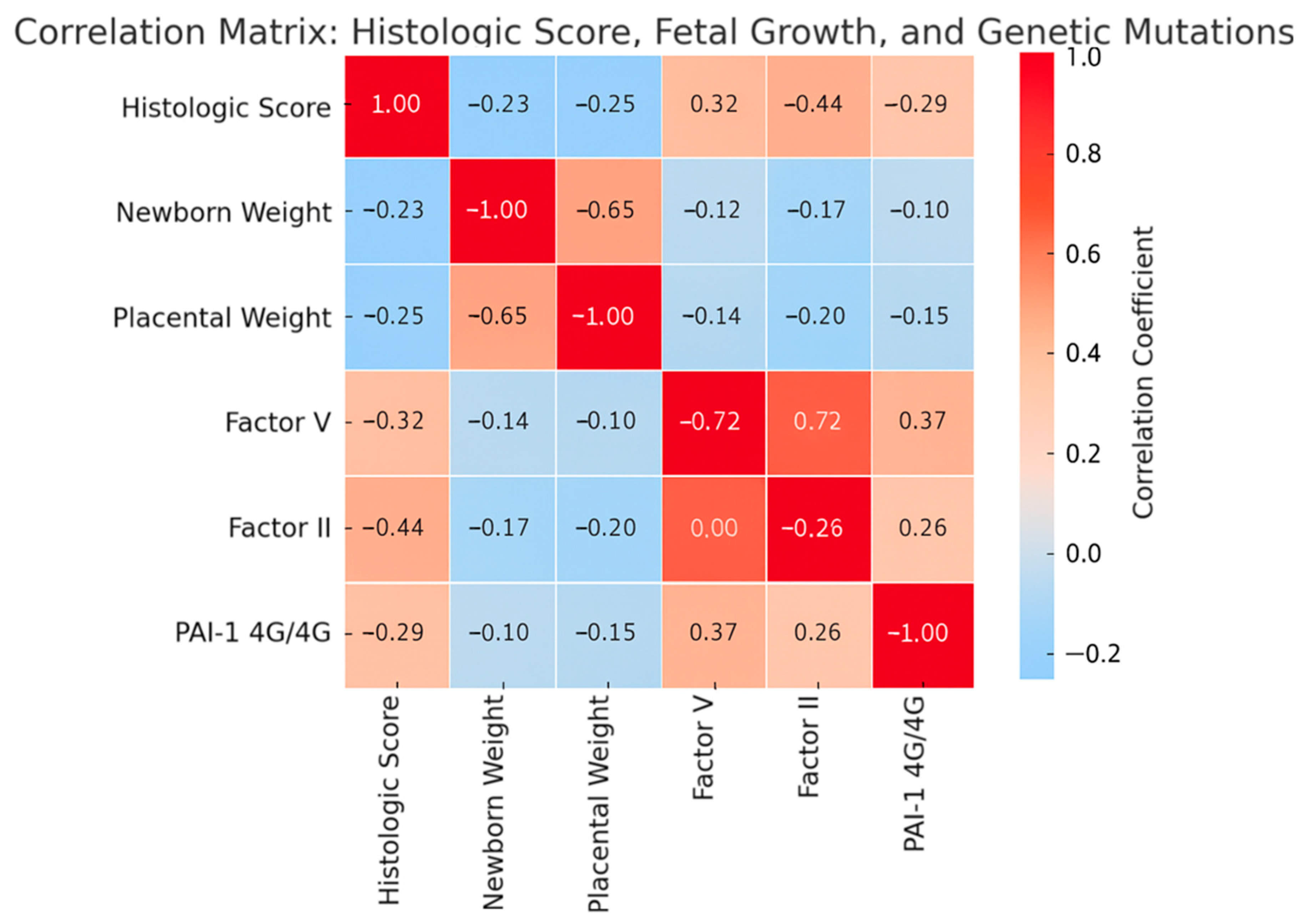
In addition to the regression analysis, the correlation matrix (
Figure 6) further supports these associations. Positive correlations were observed between the composite histologic score and thrombophilic genetic mutations (Factor V Leiden, Factor II G20210A, and PAI-1 4G/4G), suggesting a link between these variants and increased severity of placental vascular lesions. Conversely, negative correlations with newborn and placental weight highlight the impact of histological damage on fetal growth. These findings underscore the interplay between thrombophilia status, placental pathology, and fetal outcomes, complementing the adjusted regression results.
4. Discussion
This study investigated the relationship between inherited thrombophilic mutations and placental structural abnormalities, aiming to understand the histopathological footprint of thrombophilia in pregnancies without overt clinical complications. The findings demonstrate that placentas from women with hereditary thrombophilia exhibit a significantly higher frequency of vascular lesions, including infarctions, acute atherosis, intervillous thrombosis, stromal fibrosis, and villous stasis—hallmarks of maternal vascular malperfusion (MVM) [
16,
17,
18,
19,
20]. These results are consistent with and extend existing literature on thrombophilia-related placental dysfunction.
Previous studies have reported similar associations between Factor V Leiden or Prothrombin G20210A mutations and increased rates of placental infarction and decidual vasculopathy [
21,
22,
23,
24]. Our results confirm this link and further demonstrate that these mutations correlate with measurable changes in placental morphology, including reduced placental diameter and thickness, even in the absence of clinical complications such as preeclampsia or intrauterine growth restriction (IUGR). In line with the work by Gris et al. [
25], we observed that even asymptomatic thrombophilic women may exhibit subclinical placental damage, supporting the idea that genetic predisposition alone can impair placental perfusion [
26,
27,
28].
These findings underscore the need to explore the pathophysiological mechanisms by which inherited prothrombotic mutations affect placental vascular development. The proposed model suggests that mutations such as Factor V Leiden and Prothrombin G20210A induce a hypercoagulable maternal state, favoring the formation of microthrombi within the intervillous and fetal vascular compartments. These thrombotic events can compromise oxygen and nutrient exchange, leading to regional hypoxia, impaired spiral artery remodeling, and altered angiogenesis. As a result, the placenta may develop chronic vascular lesions such as infarctions, villous hypovascularity, and acute atherosis—even in clinically uncomplicated pregnancies. These histopathological features reflect subclinical maternal vascular malperfusion and may represent an early or compensated phase of placental insufficiency.
The high prevalence of PAI-1 4G/4G and MTHFR polymorphisms in our cohort and their association with villous edema, delayed maturation, and abnormal cord coiling patterns aligns with findings by Kupferminc et al. [
29] and Pasta et al. [
30], who noted similar morphological disturbances in placentas from thrombophilic pregnancies [
31,
32,
33,
34,
35]. Notably, our study is among the few to report significant correlations between these mutations and umbilical cord features—such as abnormal coiling and hypertwisting—which may represent subtle indicators of fetal adaptation to vascular stress. Nonetheless, these associations warrant cautious interpretation, as contemporary evidence does not substantiate the clinical utility of
MTHFR and
PAI-1 genotyping within standard thrombophilia screening protocols.
It is important to acknowledge that the high prevalence of certain mutations in our thrombophilia group—particularly PAI-1 4G/4G (98%) and MTHFR C677T (76%)—substantially exceeds reference frequencies reported in the general European population. This discrepancy likely reflects a selection bias, as participants in this group underwent genetic testing based on clinical suspicion or obstetric history rather than as part of routine population screening. Consequently, the reported frequencies should not be interpreted as representative of the Romanian population at large. Future studies incorporating matched population-level controls are warranted to determine the true distribution and clinical significance of these variants in our region.
Furthermore, the use of a composite histologic score allowed for the semi-quantitative assessment of thrombophilia-associated placental damage. A cutoff value of ≥3 effectively distinguished between the thrombophilia and control groups, suggesting its potential as a histological screening tool, particularly in cases lacking clinical suspicion.
The composite histologic score developed in this study, while designed to reflect reproducible and clinically relevant lesions, has not yet been validated in external populations. Future prospective studies are needed to assess its predictive performance and applicability across diverse clinical settings.
Our findings suggest that the proposed composite histological score, based on reproducible features of maternal vascular malperfusion, may aid in identifying placental patterns commonly associated with inherited thrombophilia. Incorporating such assessments into routine postnatal placental evaluation could improve risk stratification and support more personalized care in subsequent pregnancies.
Importantly, this score may also serve as a prospective trigger for targeted genetic testing. In cases where women experience serious obstetric complications—such as preeclampsia, fetal growth restriction, or placental abruption—without a known thrombophilia diagnosis, a high placental score may indicate an underlying prothrombotic condition. In such settings, histopathological findings could guide postnatal genetic screenings, ensuring earlier identification of thrombophilic mutations and enabling preventive strategies for future pregnancies or other hypercoagulable conditions.
While the primary focus of this study was on placental morphology in the context of maternal thrombophilia, available neonatal data were retrospectively reviewed. Most newborns had favorable short-term outcomes, and no consistent pattern of acute or chronic morbidity could be established based on genotype or histological findings. Due to limited long-term follow-up and the multifactorial nature of neonatal outcomes, a direct association between specific thrombophilic mutations or placental lesions and neonatal complications could not be reliably determined. Further prospective studies are required to assess the clinical impact of these findings on offspring health.
We acknowledge that smoking prevalence was significantly higher in the thrombophilia group (33% vs. 5%), representing a potential confounding factor for certain placental lesions. While our sample size limited formal multivariable adjustment, sensitivity analyses excluding smokers showed broadly consistent patterns of histopathologic differences, although residual confounding cannot be fully excluded. This limitation underscores the need for larger studies to disentangle the independent effects of thrombophilia and smoking on placental pathology.
Limitations
This study is subject to several limitations that warrant consideration. First, its retrospective design inherently limits the ability to establish causal relationships between inherited thrombophilic mutations and placental abnormalities. Second, the relatively modest sample size may affect both the generalizability of findings and the statistical power to detect more nuanced associations. Third, despite the blinding of pathologists to group allocation, the qualitative nature of certain histopathological assessments introduces the possibility of observer bias. Moreover, the study did not control for potential confounding variables such as maternal treatments—particularly anticoagulant therapy or folate supplementation—that may influence placental morphology.
An additional limitation lies in the scope of the genetic analysis, which was confined to a predefined panel of mutations, thereby excluding rarer or recently identified variants that may also play a role in placental pathology. Of particular note is the inclusion of certain polymorphisms, such as MTHFR C677T and PAI-1 4G/4G, which are no longer regarded as clinically relevant markers of inherited thrombophilia under current international guidelines. While these variants were historically investigated in the context of pregnancy complications, their thrombotic significance is not supported by contemporary evidence, and their use in clinical screening is presently discouraged. Their presence in the current analysis reflects both the retrospective character of the cohort and the historical prevalence of these tests in local diagnostic protocols. Importantly, the study does not endorse their clinical utility but rather incorporates them to provide a broader exploratory perspective on the potential relationships between commonly encountered genetic polymorphisms and placental histopathology.
5. Conclusions
This study provides evidence of a significant association between inherited prothrombotic genetic variants and histopathological markers of placental vascular compromise. Mutations such as Factor V Leiden and Prothrombin G20210A—recognized contributors to thrombotic risk—were found to correlate with a higher prevalence of placental infarctions, acute atherosis, intervillous thrombosis, and villous stasis, consistent with features of maternal vascular malperfusion. Notably, comparable clinical parameters between groups, including neonatal weight and placental dimensions, suggest that such histological alterations may precede or occur independently of overt fetal growth impairment.
Furthermore, exploratory associations between polymorphisms such as PAI-1 4G/4G and MTHFR A1298C and structural features of the umbilical cord—specifically coiling anomalies—may reflect subtle developmental responses to altered intrauterine hemodynamics. These findings, however, should be interpreted with caution. Several of the investigated variants, particularly MTHFR C677T and PAI-1 4G/4G, are no longer endorsed as clinically actionable thrombophilia markers in current consensus guidelines. Their inclusion in this study is intended to expand the understanding of potential morphopathological correlations rather than to imply diagnostic or therapeutic value.
In conclusion, while our results highlight histological patterns potentially linked to genetic predispositions, they do not support the clinical utility of non-validated thrombophilia testing. Future studies with prospective designs and expanded genetic panels are warranted to further delineate the biological relevance of these associations within the context of placental vascular pathology.