The Role of Plant-Derived Natural Products as a Regulator of the Tyrosine Kinase Pathway in the Management of Lung Cancer
Abstract
1. Introduction
2. Molecular Pathogenesis of Lung Cancer
2.1. Non-Small Cell Lung Cancer (NSCLC)
2.1.1. Adenocarcinoma
2.1.2. Squamous Cell Carcinoma (SCC)
2.1.3. Large Cell Carcinoma
2.2. Small Cell Lung Cancer (SCLC)
2.3. Other Rare Subtypes
3. Treatment Strategies for Lung Cancer
4. EGFR Signaling: Molecular Insights and Clinical Advances
5. Targeting Tyrosine Kinases (TKs) in Cancer Therapy: Molecular Mechanisms and Drug Development Strategies
6. TKs in Lung Cancer: Molecular Pathways and Clinical Implications
7. Advances in Targeted Therapy for Lung Cancer: Tyrosine Kinase Inhibitors (TKIs) and Resistance Pathways
8. Mechanisms of Action and Molecular Targets of TKIs
9. Therapeutic Role of TKIs Across Cancer Types Including Lung Cancer
10. Challenges for Implicating TKIs in Lung Cancer: Sensitivity and Resistance
11. Expanding Therapeutic Landscapes of Kinases: Complexities and Challenges in Designing Novel TKIs
12. Plant-Derived Natural Products in Cancer Therapy: Modulating Apoptosis, Cell Signaling, and Chemo-Resistance
13. Targeting TKI-Resistant Lung Cancer: Therapeutic Promise of Plant-Derived Natural Products
13.1. Alkaloids
13.1.1. Capsaicin
13.1.2. Oxymatrine
13.1.3. Tatrandrine
13.2. Flavonoids
13.2.1. Apigenin
13.2.2. Baicalein
13.2.3. Curcumin
13.2.4. Fisetin
13.2.5. Formononetin
13.2.6. Luteolin
13.2.7. Quercetin
13.3. Phenolic Molecules
13.3.1. Caffeic Acid
13.3.2. Epigallocatechin-3-Gallate (EGCG)
13.3.3. Ellagic Acid
13.3.4. Gossypol
13.3.5. Honokiol
13.3.6. Magnolol
13.4. Stilbene
Resveratrol
13.5. Saponins
13.5.1. Ginsenosides
13.5.2. Astragaloside IV
13.5.3. Polyphyllin
13.6. Triterpenes
13.6.1. Cucurbitacin
13.6.2. Betulinic Acid and Betulin
13.6.3. Leelamine
14. Translational Insights into Natural-Product-Enhanced Lung Cancer Combinational Therapy
15. Fourth-Generation TKIs
16. Caspase-Mediated Apoptosis Induced by Phytochemicals in Lung Cancer Models
17. Role of RTKs and Its Inhibitors for the Development and Treatment of BRAF Mutant Lung Cancers
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCLC | Small cell lung cancer |
| NSCLC | Non-small cell lung cancer |
| TKs | Tyrosine kinases |
| TKIs | Tyrosine kinase inhibitors |
| ATP | Adenosine triphosphate |
| EGFR | Epidermal growth factor receptor |
| COPD | Chronic obstructive pulmonary disease |
| EGFR | Epidermal growth factor receptor |
| ALK | Anaplastic lymphoma kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LCLC | Large cell carcinoma |
| SCC | Squamous cell carcinoma |
| EMT | Epithelial–mesenchymal Transition |
| TSGs | Tumor suppressor genes |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Serine-threonine kinase |
| TGF-β | Transforming growth factor β |
| GPNMB | Glycoprotein nonmetastatic melanoma protein B |
| HGF | Hepatocyte growth factor |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| EGFR-TKI | EGFR-tyrosine kinase inhibitor |
| MAPK | Mitogen-activated protein kinase |
| JAK-STAT | Janus kinase/Signal transducer and activator of transcription |
| DDRs | Discoidin domain receptor |
| Eph | Erythropoietin-producing human hepatocellular receptor |
| SRC | Proto-oncogene c-Src |
| SYK | Spleen tyrosine kinase |
| FLT3 | Fms-like tyrosine kinase 3 |
| TKIR | TKI-resistant |
| VEGFR | Vascular endothelial growth factor receptor |
| VEGF | Vascular endothelial growth factor |
| HER2 | Human epidermal growth factor receptor 2 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-2 | Matrix metalloproteinase-2 |
| Fak | Focal adhesion kinase |
| Bcl-2 | B-cell lymphoma/leukemia 2 |
| Bcl-xL | B-cell lymphoma ex-tra-large |
| NF-κB | Nuclear factor-kappa b |
| COX-2 | Cyclooxygenase-2 |
| STAT-3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor necrosis factor-alpha |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| PDK | Pyruvate dehydrogenase kinase |
| SAP | Signaling lymphocyte activation molecule-associated protein |
References
- Alrumaihi, F.; Raut, R.; Yahia, E.A.; Kumar, V.; Anwar, S. A Review on Risk Factors, Diagnostic Innovations, and Plant Based Therapies for the Management of Erectile Dysfunction. Uro 2024, 4, 60–88. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Anwar, S.; Verma, A.K.; Dev, K.; Rahmani, A.H. Cinnamon and its active compounds: A potential candidate in disease and tumour management through modulating various genes activity. Gene Rep. 2020, 21, 100966. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Raut, R.; Alhumaydhi, F.A. A comprehensive investigation on alleviating oxidative stress and inflammation in hyperglycaemic conditions through in vitro experiments and computational analysis. Saudi J. Biol. Sci. 2024, 31, 104003. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural products: Implication in cancer prevention and treatment through modulating various biological activities. Anti-Cancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.; Greten, F.R. Inflammation: The incubator of the tumor microenvironment. Trends Cancer 2022, 8, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Setton, J.; Lee, N.Y.; Riaz, N.; Powell, S.N. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat. Commun. 2018, 9, 3292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Holley, R. Control of growth of mammalian cells in cell culture. Nature 1975, 258, 487–490. [Google Scholar] [CrossRef]
- Raufi, A.G.; May, M.S.; Hadfield, M.J.; Seyhan, A.A.; El-Deiry, W.S. Advances in Liquid Biopsy Technology and Implications for Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 4238. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Zhuang, R.; Souza, V.G.P.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; Stewart, G.L.; Benard, K.; Marshall, E.A.; Reis, P.P.; et al. Mechanisms Contributing to the Comorbidity of COPD and Lung Cancer. Int. J. Mol. Sci. 2023, 24, 2859. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef]
- Park, W.; Han, J.H.; Wei, S.; Yang, E.-S.; Cheon, S.-Y.; Bae, S.-J.; Ryu, D.; Chung, H.-S.; Ha, K.-T. Natural Product-Based Glycolysis Inhibitors as a Therapeutic Strategy for Epidermal Growth Factor Receptor–Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 807. [Google Scholar] [CrossRef]
- Ibodeng, G.O.; Uche, I.N.; Mokua, R.; Galo, M.; Odigwe, B.; Galeas, J.N.; Dasgupta, S. A snapshot of lung cancer: Where are we now?—A narrative review. Ann. Transl. Med. 2023, 11, 261. [Google Scholar] [CrossRef]
- Clark, S.B.; Alsubait, S. Non–Small Cell Lung Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562307/ (accessed on 10 April 2025).
- Xie, S.; Wu, Z.; Qi, Y.; Wu, B.; Zhu, X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed. Pharmacother. 2021, 138, 111450. [Google Scholar] [CrossRef]
- Davidson, M.R.; Gazdar, A.F.; Clarke, B.E. The pivotal role of pathology in the management of lung cancer. J. Thorac. Dis. 2013, 5, S463–S478. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Gómez-López, S.; Whiteman, Z.E.; Janes, S.M. Mapping lung squamous cell carcinoma pathogenesis through in vitro and in vivo models. Commun. Biol. 2021, 4, 937. [Google Scholar] [CrossRef] [PubMed]
- Sabbula, B.R.; Gasalberti, D.P.; Mukkamalla, S.K.R.; Anjum, F. Squamous Cell Lung Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564510/ (accessed on 10 April 2025).
- Tai, Q.; Zhang, L.; Hu, X. Clinical characteristics and treatments of large cell lung carcinoma: A retrospective study using SEER data. Transl. Cancer Res. 2020, 9, 1455–1464. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fischer, B.; Marinov, M.; Arcaro, A. Targeting receptor tyrosine kinase signalling in small cell lung cancer (SCLC): What have we learned so far? Cancer Treat. Rev. 2007, 33, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cui, Y.; Zheng, X.; Zhao, Y.; Sun, G. Small-cell lung cancer brain metastasis: From molecular mechanisms to diagnosis and treatment. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166557. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Gazdar, A.F.; Minna, J.D. Molecular genetics of small cell lung carcinoma. Semin. Oncol. 2001, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hilal, T. Current understanding and approach to well differentiated lung neuroendocrine tumors: An update on classification and management. Ther. Adv. Med. Oncol. 2017, 9, 189–199. [Google Scholar] [CrossRef]
- Petty, W.J.; Paz-Ares, L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol. 2023, 9, 419–429. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, J.; Wang, W.; Lin, E.Y.; Wang, Y.; Pixley, F.; Stanley, E.R.; Graf, T.; Pollard, J.W.; Segall, J.; Condeelis, J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004, 64, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hagiwara, K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC). Target. Oncol. 2013, 8, 27–33. [Google Scholar] [CrossRef]
- Shin, S.H.; Koh, Y.G.; Lee, W.G.; Seok, J.; Park, K.Y. The use of epidermal growth factor in dermatological practice. Int. Wound J. 2023, 20, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef]
- Popat, S.; Ahn, M.J.; Ekman, S.; Leighl, N.B.; Ramalingam, S.S.; Reungwetwattana, T.; Siva, S.; Tsuboi, M.; Wu, Y.L.; Yang, J.C. Osimertinib for EGFR-Mutant Non-Small-Cell Lung Cancer Central Nervous System Metastases: Current Evidence and Future Perspectives on Therapeutic Strategies. Target. Oncol. 2023, 18, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and Cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Hunter, T.; Cooper, J.A. Protein-tyrosine kinases. Annu. Rev. Biochem. 1985, 54, 897–930. [Google Scholar] [CrossRef]
- Sengupta, P.; Das, R.; Majumder, P.; Mukhopadhyay, D. Connecting the ends: Signaling via receptor tyrosine kinases and cytoskeletal degradation in neurodegeneration. Explor. Neurosci. 2024, 3, 1–26. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signalling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Pawson, T.; Gish, G.D.; Nash, P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001, 11, 504–511. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Z.; Ou, Q.; Wu, X.; Wang, X.; Shao, Y.; Liu, H.; Yang, Y. Targeting FGFR in non-small cell lung cancer: Implications from the landscape of clinically actionable aberrations of FGFR kinases. Cancer Biol. Med. 2021, 18, 490–501. [Google Scholar] [CrossRef]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned so Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, H.; Wang, Y.; Yang, J.; Lin, C.; Shi, X.; Zou, Y.; Chen, J.; Jia, X.; Su, L. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer (Review). Oncol. Rep. 2024, 51, 16. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, L.; Niu, X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Laudadio, E.; Mangano, L.; Minnelli, C. Chemical Scaffolds for the Clinical Development of Mutant-Selective and Reversible Fourth-Generation EGFR-TKIs in NSCLC. ACS Chem. Biol. 2024, 19, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y. Receptor tyrosine kinases: Biological functions and anticancer targeted therapy. MedComm 2023, 4, e446. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Sig. Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Pophali, P.A.; Patnaik, M.M. The Role of New Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Cancer J. 2016, 22, 40–50. [Google Scholar] [CrossRef]
- Xu, L.; Xie, Y.; Gou, Q.; Cai, R.; Bao, R.; Huang, Y.; Tang, R. HER2-targeted therapies for HER2-positive early-stage breast cancer: Present and future. Front. Pharmacol. 2024, 15, 1446414. [Google Scholar] [CrossRef]
- Tomuleasa, C.; Tigu, A.B.; Munteanu, R.; Moldovan, C.-S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Sig. Transduct. Target. Ther. 2024, 9, 201. [Google Scholar] [CrossRef]
- Wang, X.S.; Bai, Y.F.; Verma, V.; Yu, R.L.; Tian, W.; Ao, R.; Deng, Y.; Zhu, X.Q.; Liu, H.; Pan, H.X.; et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2023, 115, 742–748. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, J.J.; Chen, Z.H.; Zhang, X.C.; Yan, H.H.; Xu, C.R.; Su, J.; Chen, H.J.; Tu, H.Y.; Zhong, W.Z.; et al. Serial cfDNA assessment of response and resistance to EGFR-TKI for patients with EGFR-L858R mutant lung cancer from a prospective clinical trial. J. Hematol. Oncol. 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- Yang, J.C.; Ahn, M.J.; Kim, D.W.; Ramalingam, S.S.; Sequist, L.V.; Su, W.C.; Kim, S.W.; Kim, J.H.; Planchard, D.; Felip, E.; et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J. Clin. Oncol. 2017, 35, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.; Bozorgmehr, F.; Brückner, L.; Chung, I.; Krisam, J.; Schneider, M.A.; Stenzinger, A.; Eickhoff, R.; Mueller, D.W.; Thomas, M. Brigatinib versus other second-generation ALK inhibitors as initial treatment of anaplastic lymphoma kinase positive non-small cell lung cancer with deep phenotyping: Study protocol of the ABP trial. BMC Cancer 2021, 21, 743. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.L.; Suri, Y.; Basu, A.; Koshkin, V.S.; Desai, A. Mechanisms of tyrosine kinase inhibitor resistance in renal cell carcinoma. Cancer Drug Resist. 2023, 6, 858–873. [Google Scholar] [CrossRef]
- Khaddour, K.; Jonna, S.; Deneka, A.; Patel, J.D.; Abazeed, M.E.; Golemis, E.; Borghaei, H.; Boumber, Y. Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies. Cancers 2021, 13, 3164. [Google Scholar] [CrossRef]
- Zhou, C. Multi-targeted tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: An era of individualized therapy. Transl. Lung Cancer Res. 2012, 1, 72–77. [Google Scholar] [CrossRef]
- Gazdar, A.F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009, 28 (Suppl. S1), S24–S31. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Wu, S.G.; Shih, J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef]
- Poudel, G.; Tolland, M.G.; Hughes, T.P.; Pagani, I.S. Mechanisms of Resistance and Implications for Treatment Strategies in Chronic Myeloid Leukaemia. Cancers 2022, 14, 3300. [Google Scholar] [CrossRef] [PubMed]
- Birnboim-Perach, R.; Benhar, I. Using Combination therapy to overcome diverse challenges of Immune Checkpoint Inhibitors treatment. Int. J. Biol. Sci. 2024, 20, 3911–3922. [Google Scholar] [CrossRef]
- Cui, J.J. A New Challenging and Promising Era of Tyrosine Kinase Inhibitors. ACS Med. Chem. Lett. 2014, 5, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Klaeger, S.; Heinzlmeir, S.; Wilhelm, M.; Polzer, H.; Vick, B.; Koenig, P.A.; Reinecke, M.; Ruprecht, B.; Petzoldt, S.; Meng, C.; et al. The target landscape of clinical kinase drugs. Science 2017, 358, eaan4368. [Google Scholar] [CrossRef]
- Codex, Y. Advancements in Targeted Kinase Inhibitors: Revolutionizing Personalized Medicine in Oncology. Yubetsu Codex Medicine & Physiology. Available online: https://codex.yubetsu.com/article/c020b487e78046d89a0ed3afaac73ec5 (accessed on 10 April 2025).
- Fukuda, S.; Suda, K.; Hamada, A.; Oiki, H.; Ohara, S.; Ito, M.; Soh, J.; Mitsudomi, T.; Tsutani, Y. Potential Utility of a 4th-Generation EGFR-TKI and Exploration of Resistance Mechanisms-An In Vitro Study. Biomedicines 2024, 12, 1412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products; Osbourn, A., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Anwar, S.; Kumar, V.; Kanwal, B.; Yahia, E.A. A review on utilization of ashoka plant in oxidative stress induced women reproductive health complications. IJCRT 2023, 11, f249–f258. [Google Scholar]
- Younus, H.; Anwar, S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. Int. J. Health Sci. 2016, 10, 261–277. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a major ingredient of ginger attenuates diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Babiker, A.Y.; Anwar, S. Hesperidin, a Bioflavonoid in Cancer Therapy: A Review for a Mechanism of Action through the Modulation of Cell Signaling Pathways. Molecules 2023, 28, 5152. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, G.; Peng, Q.; Li, X.; Zou, X.; Zhang, W.; Zhao, L.; Li, X.; Wu, P.; Luo, A.; et al. Natural products combating EGFR-TKIs resistance in cancer. Eur. J. Med. Chem. Rep. 2025, 13, 100251. [Google Scholar] [CrossRef]
- Paller, C.J.; Denmeade, S.R.; Carducci, M.A. Challenges of conducting clinical trials of natural products to combat cancer. Clin. Adv. Hematol. Oncol. 2016, 14, 447–455. [Google Scholar]
- Al-Yozbaki, M.; Wilkin, P.J.; Gupta, G.K.; Wilson, C.M. Therapeutic potential of natural compounds in lung cancer. Curr. Med. Chem. 2021, 28, 7988–8002. [Google Scholar] [CrossRef]
- Jeong, H.; Phan, A.N.H.; Choi, J.W. Anti-cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-resistant Non-small Cell Lung Cancer. Pharmacogn. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Thoennissen, N.H.; O’Kelly, J.; Lu, D.; Iwanski, G.B.; La, D.T.; Abbassi, S.; Leiter, A.; Karlan, B.; Mehta, R.; Koeffler, H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010, 29, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Yun, H.J.; Choi, J.H.; Han, E.H.; Kim, H.G.; Song, G.Y.; Kwon, K.I.; Jeong, T.C.; Jeong, H.G. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol. Nutr. Food Res. 2011, 55, 594–605. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, A.; Liu, R.; Zhang, Y. Simultaneous determination of fangchinoline and tetrandrine in Stephania tetrandra S. Moore by using 1-alkyl-3-methylimidazolium-based ionic liquids as the RP-HPLC mobile phase additives. Anal. Chim. Acta 2013, 767, 148–154. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, T.; Su, J.; Wang, K.; Li, X. Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 353–363. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Li, W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016, 7, 40800–40815. [Google Scholar] [CrossRef]
- Suh, Y.A.; Jo, S.Y.; Lee, H.Y.; Lee, C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int. J. Oncol. 2014, 46, 1405–1411. [Google Scholar] [CrossRef]
- Huang, S.; Yu, M.; Shi, N.; Zhou, Y.; Li, F.; Li, X.; Huang, X.; Jin, J. Apigenin and Abivertinib, a novel BTK inhibitor synergize to inhibit diffuse large B-cell lymphoma in vivo and vitro. J. Cancer 2020, 11, 2123–2132. [Google Scholar] [CrossRef]
- Leung, H.W.; Yang, W.H.; Lai, M.Y.; Lin, C.J.; Lee, H.Z. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem. Toxicol. 2007, 45, 403–411. [Google Scholar] [CrossRef]
- Hong, R.L.; Spohn, W.H.; Hung, M.C. Curcumin inhibits tyrosine kinase activity of p185 neu and also depletes p185 neu. Clin. Cancer Res. 1999, 5, 1884–1891. [Google Scholar] [PubMed]
- Kim, K.C.; Baek, S.H.; Lee, C. Curcumin-induced downregulation of Axl receptor tyrosine kinase inhibits cell proliferation and circumvents chemoresistance in non-small lung cancer cells. Int. J. Oncol. 2015, 47, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Cao, X.; Li, N.; Zhang, Y.; Lan, L.; Zhou, Y.; Pan, X.; Shen, L.; Yin, Z.; Luo, L. Luteolin is effective in the non-small cell lung cancer model with L858R/T790M EGF receptor mutation and erlotinib resistance. Br. J. Pharmacol. 2014, 171, 2842–2853. [Google Scholar] [CrossRef]
- Yu, X.; Gao, F.; Li, W.; Zhou, L.; Liu, W.; Li, M. Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2020, 39, 62. [Google Scholar] [CrossRef]
- Wang, L.; Chen, N.; Cheng, H. Fisetin inhibits vascular endothelial growth factor-induced angiogenesis in retinoblastoma cells. Oncol. Lett. 2020, 20, 1239–1244. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; de Takats, P.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Twum, Y.; Marshall, K.; Gao, W. Caffeic acid phenethyl ester surmounts acquired resistance of AZD9291 in non-small cell lung cancer cells. BioFactors 2023, 49, 1143–1157. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Govoni, M.; Spisni, E.; Papi, A. EGFR inhibition by (-)-epigallocatechin-3-gallate and IIF treatments reduces breast cancer cell invasion. Biosci. Rep. 2017, 37, BSR20170168. [Google Scholar] [CrossRef]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.; Wang, Q.; Zhang, P.; Wang, Y.; Zi, C.; Wang, X.; Sheng, J. (−)-Epigallocatechin-3-gallate derivatives combined with cisplatin exhibit synergistic inhibitory effects on non-small-cell lung cancer cells. Cancer Cell Int. 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, L.; Lamy, S.; Chapus, A.; Mihoubi, S.; Durocher, Y.; Cass, B.; Bojanowski, M.W.; Gingras, D.; Béliveau, R. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis 2005, 26, 821–826. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.; Xu, L.; Wu, D. Natural product gossypol and its derivatives in precision cancer medicine. Curr. Med. Chem. 2019, 26, 1849–1873. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, S.M.; Ye, Y.C.; Tashiro, S.i.; Onodera, S.; Ikejima, T. The tyrphostin AG1478 augments oridonin-induced A431 cell apoptosis by blockage of JNK MAPK and enhancement of oxidative stress. Free Radic. Res. 2012, 46, 1393–1405. [Google Scholar] [CrossRef]
- Pan, J.; Lee, Y.; Zhang, Q.; Xiong, D.; Wan, T.C.; Wang, Y.; You, M. Honokiol Decreases Lung Cancer Metastasis through Inhibition of the STAT3 Signaling Pathway. Cancer Prev. Res. 2017, 10, 133–141. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Fu, Y.; Ding, R.B.; Qi, X.; Zhou, X.; Sun, Z.; Bao, J. Magnolol as a Potential Anticancer Agent: A Proposed Mechanistic Insight. Molecules 2022, 27, 6441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Notas, G.; Nifli, A.P.; Kampa, M.; Vercauteren, J.; Kouroumalis, E.; Castanas, E. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim. Biophys. Acta 2006, 1760, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, T.; Jing, S.; Zuo, P.; Li, T.; Wang, Y.; Xing, S.; Zhang, J.; Wei, Z. 20 (S)-Ginsenoside Rg3 inhibits lung cancer cell proliferation by targeting EGFR-mediated Ras/Raf/MEK/ERK pathway. Am. J. Chin. Med. 2021, 49, 753–765. [Google Scholar] [CrossRef]
- Tan, Q.; Lin, S.; Zeng, Y.; Yao, M.; Liu, K.; Yuan, H.; Liu, C.; Jiang, G. Ginsenoside Rg3 attenuates the osimertinib resistance by reducing the stemness of non-small cell lung cancer cells. Environ. Toxicol. 2020, 35, 643–651. [Google Scholar] [CrossRef]
- Liu, T.; Zuo, L.; Guo, D.; Chai, X.; Xu, J.; Cui, Z.; Wang, Z.; Hou, C. Ginsenoside Rg3 regulates DNA damage in non-small cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed. Pharmacother. 2019, 120, 109483. [Google Scholar] [CrossRef]
- Xu, F.; Cui, W.Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.L.; Gong, W.-Y.; Dong, J.-C.; Liu, B.-J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 207. [Google Scholar] [CrossRef]
- Jiang, K.; Lu, Q.; Li, Q.; Ji, Y.; Chen, W.; Xue, X. Astragaloside IV inhibits breast cancer cell invasion by suppressing Vav3 mediated Rac1/MAPK signaling. Int. Immunopharmacol. 2017, 42, 195–202. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Zheng, F.; Tang, Q.; Wu, W.; Hann, S.S. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J. Exp. Clin. Cancer Res. 2016, 35, 112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, W.; Xu, Y.; Lv, X.; Zhang, M.; Jiang, H. Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol. Appl. Pharmacol. 2018, 356, 1–7. [Google Scholar] [CrossRef]
- Jing, S.-Y.; Wu, Z.-D.; Zhang, T.H.; Zhang, J.; Wei, Z.-Y. In vitro antitumor effect of cucurbitacin E on human lung cancer cell line and its molecular mechanism. Chin. J. Nat. Med. 2020, 18, 483–490. [Google Scholar]
- Xie, Y.L.; Tao, W.H.; Yang, T.X.; Qiao, J.G. Anticancer effect of cucurbitacin B on MKN-45 cells via inhibition of the JAK2/STAT3 signaling pathway. Exp. Ther. Med. 2016, 12, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Cao, D.; Ren, Q.N.; Zhang, S.S.; Zhou, N.N.; Mai, S.J.; Feng, B.; Wang, H.Y. Combination treatment with inhibitors of erk and autophagy enhances antitumor activity of betulinic acid in non–small-cell lung cancer in vivo and in vitro. Front. Pharmacol. 2021, 12, 684243. [Google Scholar] [CrossRef] [PubMed]
- Ahmadu, A.A.; Delehouzé, C.; Haruna, A.; Mustapha, L.; Lawal, B.A.; Udobre, A.; Baratte, B.; Triscornia, C.; Autret, A.; Robert, T.; et al. Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor. Molecules 2021, 26, 4599. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Potential Application of Leelamine as a Novel Regulator of Chemokine-Induced Epithelial-to-Mesenchymal Transition in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9848. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- DL McKay, J.B. Blumberg a review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Papay, Z.E.; Kosa, A.; Boddi, B.; Merchant, Z.; Saleem, I.Y.; Zariwala, M.G.; Klebovich, I.; Somavarapu, S.; Antal, I. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Huang, K.M. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 2013, 53, 376–383. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Gao, T.; Huang, Y.; Xue, J.; Xie, M.L. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lalpha in rats. Food Funct. 2016, 7, 1992–1998. [Google Scholar] [CrossRef]
- Ozcelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396. [Google Scholar] [CrossRef]
- Hu, W.J.; Liu, J.; Zhong, L.K.; Wang, J. Apigenin enhances the antitumor effects of cetuximab in nasopharyngeal carcinoma by inhibiting EGFR signaling. Biomed. Pharmacother. 2018, 102, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L.; et al. Apigenin Combined with Gefitinib Blocks Autophagy Flux and Induces Apoptotic Cell Death Through Inhibition of HIF-1α, c-Myc, p-EGFR, and Glucose Metabolism in EGFR L858R+T790M-Mutated H1975 Cells. Front. Pharmacol. 2019, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Maher, H.M.; Alzoman, N.Z.; Shehata, S.M.; Abahussain, A.O. Comparative pharmacokinetic profiles of selected irreversible tyrosine kinase inhibitors, neratinib and pelitinib, with apigenin in rat plasma by UPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 137, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Rathaur, P.; Vora, J.; Jha, P.C.; Johar, K.; Rawal, R.M.; Shrivastava, N. Reckoning apigenin and kaempferol as a potential multi-targeted inhibitor of EGFR/HER2-MEK pathway of metastatic colorectal cancer identified using rigorous computational workflow. Mol. Divers. 2022, 26, 3337–3356. [Google Scholar] [CrossRef]
- Kim, K.-C.; Choi, E.-H.; Lee, C. Axl receptor tyrosine kinase is a novel target of apigenin for the inhibition of cell proliferation. Int. J. Mol. Med. 2014, 34, 592–598. [Google Scholar] [CrossRef]
- Chang, J.H.; Cheng, C.W.; Yang, Y.C.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Chen, P.S.; Hsiao, M.; Lee, W.J.; Chien, M.H. Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses. J. Exp. Clin. Cancer Res. 2018, 37, 199. [Google Scholar] [CrossRef]
- Osada, M.; Imaoka, S.; Funae, Y. Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1α protein. FEBS Lett. 2004, 575, 59–63. [Google Scholar] [CrossRef]
- Ansó, E.; Zuazo, A.; Irigoyen, M.; Urdaci, M.C.; Rouzaut, A.; Martínez-Irujo, J.J. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem. Pharmacol. 2010, 79, 1600–1609. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Commun. Signal 2021, 19, 67. [Google Scholar] [CrossRef]
- Cathcart, M.C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 16, 707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, S.-H.; Choi, Y.-H.; Chi, G.-Y. The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3. Int. J. Mol. Sci. 2021, 22, 5181. [Google Scholar] [CrossRef]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the up regulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wei, C.; Xi, Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/β-catenin pathway. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 840–850. [Google Scholar] [CrossRef]
- Li, Y.; Chao, Y.; Fang, Y.; Wang, J.; Wang, M.; Zhang, H.; Ying, M.; Zhu, X.; Wang, H. MTA1 promotes the invasion and migration of non-small cell lung cancer cells by down regulating miR-125b. J. Exp. Clin. Cancer Res. 2013, 32, 33. [Google Scholar] [CrossRef]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Chang, G.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef]
- Chen, P.; Huang, H.; Wang, Y.; Jin, J.; Long, W.G.; Chen, K.; Zhao, X.H.; Chen, C.G.; Li, J. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019, 38, 254. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Izumi, Y.; Yamamoto, J.; Nomori, H. Coadministration of erlotinib and curcumin augmentatively reduces cell viability in lung cancer cells. Phytother. Res. 2014, 28, 728–735. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Zhu, F.; Fan, X.; Wu, X.; Zhao, H.; Jiang, L. Curcumin lowers erlotinib resistance in non-small cell lung carcinoma cells with mutated EGF receptor. Oncol. Res. 2014, 21, 137–144. [Google Scholar] [CrossRef]
- Ye, M.X.; Zhao, Y.L.; Li, Y.; Miao, Q.; Li, Z.K.; Ren, X.L.; Song, L.Q.; Yin, H.; Zhang, J. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine 2012, 19, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Chanvorachote, P.; Pongrakhananon, V.; Wannachaiyasit, S.; Luanpitpong, S.; Rojanasakul, Y.; Nimmannit, U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Investig. 2009, 27, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein [Internet]. Mol. Urol. 2000, 4, 1–6. [Google Scholar] [PubMed]
- Kim, J.H.; Xu, C.; Keum, Y.S.; Reddy, B.; Conney, A.; Kong, A.N.T. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with β-phenylethyl isothiocyanate and curcumin [Internet]. Carcinogenesis 2006, 27, 475–482. [Google Scholar] [CrossRef]
- A Open-label Prospective Cohort Trial of Curcumin Plus Tyrosine Kinase Inhibitors (TKI) for EGFR-Mutant Advanced NSCLC (CURCUMIN). Available online: https://clinicaltrials.gov/study/NCT02321293?term=Tyrosine%20Kinase%20Inhibitor&intr=Curcumin&rank=1 (accessed on 16 June 2025).
- Phase II Trial to Modulate Intermediate Endpoint Biomarkers in Former and Current Smokers. Available online: https://clinicaltrials.gov/study/NCT03598309?intr=Curcumin%20C3%20complex%C2%AE.%20Drug:%20Lovaza%C2%AE.&rank=1 (accessed on 16 June 2025).
- The Thoracic Peri-Operative Integrative Surgical Care Evaluation Trial—Stage III (POISE). Available online: https://clinicaltrials.gov/study/NCT04871412?intr=Curcumin&term=Thoracic%20Peri-Operative%20Integrative%20Surgical%20Care%20&rank=1 (accessed on 16 June 2025).
- Dev, S.S.; Farghadani, R.; Abidin, S.A.; Othman, I.; Naidu, R. Flavonoids as receptor tyrosine kinase inhibitors in lung cancer. J. Funct. Foods 2023, 110, 105845. [Google Scholar]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef]
- Tabasum, S.; Singh, R.P. Fisetin suppresses migration, invasion and stem-cell-like phenotype of human non-small cell lung carcinoma cells via attenuation of epithelial to mesenchymal transition. Chem. Biol. Interact. 2019, 303, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological effects and mechanisms of fisetin in cancer: A promising anti-cancer agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef]
- Sabarwal, A.; van Rooyen, J.C.; Caburet, J.; Avgenikos, M.; Dheeraj, A.; Ali, M.; Mishra, D.; de Meester, J.S.B.; Stander, S.; van Otterlo, W.A.L.; et al. A novel 4′-brominated derivative of fisetin induces cell cycle arrest and apoptosis and inhibits EGFR/ERK1/2/STAT3 pathways in non-small-cell lung cancer without any adverse effects in mice. FASEB J. 2022, 36, e22654. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Formononetin, a Type of Methoxylated Isoflavone, and Its Role in Cancer Therapy through the Modulation of Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9719. [Google Scholar] [CrossRef]
- Tay, K.C.; Tan, L.T.; Chan, C.K.; Hong, S.L.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, W.X.; Zheng, C.S.; Han, H.W.; Wang, X.; Zhang, Y.H.; Qiu, H.Y.; Tang, C.Y.; Qi, J.L.; Lu, G.H.; et al. Synthesis, characterization and biological evaluation of formononetin derivatives as novel EGFR inhibitors via inhibiting growth, migration and inducing apoptosis in breast cancer cell line. RSC Adv. 2017, 7, 48404–48419. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Hwang, J.J.; Lee, P.P.; Ke, F.C.; Huang, J.H.; Huang, C.J.; Kandaswami, C.; Middleton, E., Jr.; Lee, M.T. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 1999, 128, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Cai, X.; Zheng, X.; Zhu, X.; Feng, J.; Wang, X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell 2022, 75, 101740. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.O.; Divya, S.P.; Roy, R.V.; Hitron, J.A.; Wang, L.; Kim, D.; Dai, J.; Asha, P.; Zhang, Z.; et al. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol. Appl. Pharmacol. 2014, 281, 230–241. [Google Scholar] [CrossRef]
- Patel, N.; Patel, M.; Patel, A.; Patel, S.; Sakariya, D.; Parmar, A.; Sarkar, R.; Patel, M.; Rohit, S.; Patel, S.; et al. Investigating the role of natural flavonoids in VEGFR inhibition: Molecular modelling and biological activity in A549 lung cancer cells. J. Mol. Struct. 2025, 1322, 140392. [Google Scholar] [CrossRef]
- Huang, G.; Liu, X.; Jiang, T.; Cao, Y.; Sang, M.; Song, X.; Zhou, B.; Qu, H.; Cai, H.; Xing, D.; et al. Luteolin overcomes acquired resistance to osimertinib in non-small cell lung cancer cells by targeting the HGF-MET-Akt pathway. Am. J. Cancer Res. 2023, 13, 4145–4162. [Google Scholar]
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef]
- Sakurai, M.A.; Ozaki, Y.; Okuzaki, D.; Naito, Y.; Sasakura, T.; Okamoto, A.; Tabara, H.; Inoue, T.; Hagiyama, M.; Ito, A. Gefitinib and Luteolin Cause Growth Arrest of Human Prostate Cancer PC-3 Cells via Inhibition of Cyclin G-Associated Kinase and Induction of MiR-630. PLoS ONE 2014, 9, e100124. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Malik, M.Y.; Rashid, M.; Singh, S.; Tiwari, V.; Gupta, P.; Shukla, S.; Singh, S.; Wahajuddin, M. Mechanistic exploration of quercetin against metronidazole induced neurotoxicity in rats: Possible role of nitric oxide isoforms and inflammatory cytokines. Neurotoxicology 2020, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharm. Ther. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Elumalai, P.; Ezhilarasan, D.; Raghunandhakumar, S. Quercetin inhibits the epithelial to mesenchymal transition through suppressing Akt mediated nuclear translocation of β-catenin in lung cancer cell line. Nutr. Cancer 2022, 74, 1894–1906. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Interactions of quercetin with receptor tyrosine kinases associated with human lung carcinoma. Nat. Product Res. 2018, 32, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.H.; Bethapudi, P.; Majji Rambabu, F. Anti-angiogenesis property by Quercetin compound targeting VEGFR2 elucidated in a computational approach. Eur. J. Biotechnol. Biosci. 2014, 2, 30–46. [Google Scholar]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention on: Direct binding and molecular modeling. Antioxid. Redox Signal 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Navarro-Núñez, L.; Rivera, J.; Guerrero, J.A.; Martinez, C.; Vicente, V.; Lozano, M.L. Differential effects of quercetin, apigenin and genistein on signaling pathways of protease-activated receptors PAR1 and PAR4 in platelets. Br. J. Pharmacol. 2009, 158, 1548–1556. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and its role in biological functions: An updated review. EXCLI J. 2018, 17, 856–863. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Ren, X.; Xie, X.; Yan, P.; Wang, D.; Huang, X. A caffeic acid phenethyl ester analog inhibits the proliferation of nasopharyngeal carcinoma cells via targeting epidermal growth factor receptor. J. Biochem. Mol. Toxicol. 2020, 34, e22491. [Google Scholar] [CrossRef]
- Kuo, L.K.; Fu, Y.K.; Yeh, C.C.; Lee, C.Y.; Chung, C.J.; Shih, L.J.; Lu, H.Y.; Chuu, C.P. Combined Treatment of Caffeic Acid Phenethyl Ester with Docetaxel Inhibits Survival of Non-small-cell Lung Cancer Cells via Suppression of c-MYC. Anticancer Res. 2024, 44, 4915–4928. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Min, K.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar]
- Tsai, Y.J.; Chen, B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar]
- Chang, H.B.; Chen, B.H. Inhibition of lung cancer cells A549 and H460 by curcuminoid extracts and nanoemulsions prepared from Curcuma Longa Linnaeus. Int. J. Nanomed. 2015, 10, 5059–5080. [Google Scholar]
- Sadava, D.; Whitlock, E.; Kane, S.E. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem. Biophys. Res. Commun. 2007, 360, 233–237. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signaling pathway. Biomed. Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Tasaki, E.; Fujiki, H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int. J. Cancer 2006, 119, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Tighiouart, M.; Lee, J.E.; Shin, H.J.; Khuri, F.R.; Yang, C.S.; Chen, Z.; Shin, D.M. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int. J. Cancer 2008, 123, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Younus, H. Antiglycating potential of ellagic acid against glucose and methylglyoxal induced glycation of superoxide dismutase. J. Protein Proteom. 2017, 8, 1–2. [Google Scholar]
- Xie, C.; Kong, J.; Miao, F.; Wang, X.; Sheng, J. Combination effects of ellagic acid with erlotinib in a Ba/F3 cell line expressing EGFR H773_V774 insH mutation. Thorac. Cancer 2020, 11, 2101–2111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paunovic, D.; Rajkovic, J.; Novakovic, R.; Grujic-Milanovic, J.; Mekky, R.H.; Popa, D.; Calina, D.; Sharifi-Rad, J. The potential roles of gossypol as anticancer agent: Advances and future directions. Chin. Med. 2023, 18, 163. [Google Scholar] [CrossRef]
- Pal, D.; Sahu, P.; Sethi, G.; Wallace, C.E.; Bishayee, A. Gossypol and its natural derivatives: Multitargeted phytochemicals as potential drug candidates for oncologic diseases. Pharmaceutics 2022, 14, 2624. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, G.Y.; Cao, D.; Pan, H.; Li, Y.W. Gossypol overcomes EGFR-TKIs resistance in non-small cell lung cancer cells by targeting YAP/TAZ and EGFRL858R/T790M. Biomed. Pharmacother. 2019, 115, 108860. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.; Fan, X.; Luo, L.; Duan, F.; Jiang, Z.; Wang, Q.; Leung, E.L.; Liu, L.; Yao, X. Gossypol inhibits non-small cell lung cancer cells proliferation by targeting EGFRL858R/T790M. Front. Pharmacol. 2018, 9, 728. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M.A. Reviewing the role of resveratrol as a natural modulator of microglial activities. Curr. Pharm. Des. 2015, 21, 5277–5291. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, J.H.; Woodling, K.A.; Doerge, D.R.; Burns, S.T.; Hinson, J.A.; Mayeux, P.R. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem. Pharmacol. 2010, 80, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Calil, O.N.; Carvalho, G.S.G.; Franco, D.C.Z.; Silva, A.D.; Raposo, N.B.R. Antioxidant activity of Resveratrol Analogs. Lett. Drug Des. Discov. 2012, 9, 8–11. [Google Scholar] [CrossRef]
- Mendes, J.B.E.; Riekes, M.R.; Oliveira, V.M.; Michel, M.D.; Stulzer, H.K.; Zawadzki, S.F.; Mainardes, R.M.; Farago, P.V. PHBV/PCL Micro particles for Controlled Release of Resveratrol: Physicochemical characterization, antioxidant potential, and effect on hemolysis of human erythrocytes. Sci. World J. 2012, 2012, 542937. [Google Scholar] [CrossRef]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and Hydroxystilbene Compounds: Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef]
- Zhu, Y.; He, W.; Gao, X.; Li, B.; Mei, C.; Xu, R.; Chen, H. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci. Rep. 2015, 5, 17730. [Google Scholar] [CrossRef]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar] [PubMed]
- Varjas, T.; Nowrasteh, G.; Budan, F.; Nadasi, E.; Horvath, G.; Makai, S.; Gracza, T.; Cseh, J. Ember Chemo preventive effect of Panax ginseng. Phytother. Res. 2009, 23, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A potential neuroprotective agent. BioMed Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, W.; Sun, Q.; Tuohayi, J. Ginsenoside Rg3 promotes the antitumor activity of gefitinib in lung cancer cell lines. Exp. Ther. Med. 2018, 17, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Wang, X.; Wei, N.; Liu, H.; Zhang, X. CD146-mediated acquisition of stemness phenotype enhances tumour invasion metastasis after EGFR-TKI resistance in lung cancer. Clin. Respir. J. 2019, 13, 23–33. [Google Scholar] [CrossRef]
- Xu, T.; Jin, Z.; Yuan, Y.; Wei, H.; Xu, X.; He, S.; Chen, S.; Hou, W.; Guo, Q.; Hua, B. Ginsenoside Rg3 serves as an adjuvant chemotherapeutic agent and VEGF inhibitor in the treatment of non-small cell lung cancer: A meta-analysis and systematic review. Evid. Based Complement. Altern. Med. 2016, 2016, 7826753. [Google Scholar] [CrossRef]
- Chian, S.; Zhao, Y.; Xu, M.; Yu, X.; Ke, X.; Gao, R.; Yin, L. Ginsenoside Rd reverses cisplatin resistance in non-small-cell lung cancer A549 cells by downregulating the nuclear factor erythroid 2-related factor 2 pathway. Anticancer. Drugs 2019, 30, 838–845. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Y.; Yang, Y.; Zhang, Y.; Yue, Z.; Pan, Z.; Ren, X. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by down regulating PD-L1 and resuming immune. Biomed. Pharmacother. 2017, 96, 378–383. [Google Scholar] [CrossRef]
- Wang, Q.N.; Yang, X.F.; Song, Y.; Sun, X.W.; Li, W.T.; Zhang, L.; Hu, X.; Wang, H.; Zhao, N.; Zhuang, R.; et al. Astragaloside IV-targeting miRNA-1 attenuates lipopolysaccharide-induced cardiac dysfunction in rats through inhibition of apoptosis and autophagy. Life Sci. 2021, 275, 119414. [Google Scholar] [CrossRef]
- Liang, X.Y.; Hong, F.F.; Yang, S.L. Astragaloside IV alleviates liver inflammation, oxidative stress and apoptosis to protect against experimental non-alcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2021, 14, 1871–1883. [Google Scholar] [CrossRef]
- Xing, L.N.; Fang, J.; Zhu, B.B.; Wang, L.; Chen, J.L.; Wang, Y.M.; Huang, J.; Wang, H.; Yao, X. Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 2021, 269, 119068. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, G.; Chen, M.B.; Cai, R.Z. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Dev. Res. 2021, 83, 461–469. [Google Scholar] [CrossRef]
- Ye, Q.; Su, L.; Chen, D.G.; Zheng, W.Y.; Liu, Y. Astragaloside IV induced miR-134 expression reduces EMT and increases chemotherapeutic sensitivity by suppressing CREB1 signaling in colorectal cancer cell line SW-480. Cell. Physiol. Biochem. 2017, 43, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Wang, H.; Qi, H. Astragaloside IV inhibits the progression of liver cancer by modulating macrophage polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am. J. Transl. Res. 2022, 14, 1551–1566. [Google Scholar]
- Zheng, Y.F.; Dai, Y.; Liu, W.P.; Wang, N.; Cai, Y.L.; Wang, S.Q.; Zhang, F.; Liu, P.; Chen, Q.; Wang, Z. Astragaloside IV enhances taxol chemo sensitivity of breast cancer via caveolin-1-targeting oxidant damage. J. Cell. Physiol. 2018, 234, 4277–4290. [Google Scholar] [CrossRef]
- Dai, P.; Liu, D.; Zhang, L.; Ye, J.; Wang, Q.; Zhang, H.W.; Lin, X.H.; Lai, G.X. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumor. Biol. 2017; ahead of print. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, T.; Li, J. Research progress on the antitumor effects of astragaloside IV. Eur. J. Pharmacol. 2023, 938, 175449. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gong, G.Y.; Ma, L.L.; Wang, Z.Q.; Song, D.; Fang, M.Y. Anti-cancer effects of Polyphyllin I: An update in 5 years. Chem. Biol. Interact. 2020, 316, 108936. [Google Scholar] [CrossRef]
- Lou, W.; Chen, Y.; Zhu, K.Y.; Deng, H.; Wu, T.; Wang, J. Polyphyllin I Overcomes EMT-Associated Resistance to Erlotinib in Lung Cancer Cells via IL-6/STAT3 Pathway Inhibition. Biol. Pharm. Bull. 2017, 40, 1306–1313. [Google Scholar] [CrossRef]
- Zheng, R.; Jiang, H.; Li, J.; Liu, X.; Xu, H. Polyphyllin II Restores Sensitization of the Resistance of PC-9/ZD Cells to Gefitinib by a Negative Regulation of the PI3K/Akt/mTOR Signaling Pathway. Curr. Cancer Drug Targets 2017, 17, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Alghasham, A.A. Cucurbitacin—A promising target for cancer therapy. Int. J. Health Sci. 2013, 7, 77–89. [Google Scholar] [CrossRef]
- Clericuzio, M.; Mella, M.; Vita-Finzi, P.; Zema, M.; Vidari, G. Cucurbitane triterpenoids from Leucopaxillus gentianeus. J. Nat. Prod. 2004, 67, 1823–1828. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Wiart, C. The definition and significance of Cucurbitacin B a STAT3 inhibitors. Cancer Lett. 2013, 328, 188. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Tabasso, S.; Bianco, M.A.; Pratesi, G.; Beretta, G.; Tinelli, S.; Zunino, F.; Vidari, G. Cucurbitane triterpenes from the fruiting bodies and cultivated mycelia of Leucopaxillus gentianeus. J. Nat. Prod. 2006, 69, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, R.; Jraij, A.; Magdalou, J.; Ouaini, N.; Tome, D.; Greige-Gerges, H. Interaction of cucurbitacins with human serum albumin: Thermodynamic characteristics influence on the binding of site specific ligands. J. Photochem. Photobiol. B 2009, 95, 189–195. [Google Scholar] [CrossRef]
- Wakimoto, N.; Yin, D.; O’Kelly, J.; Haritunians, T.; Karlan, B.; Said, J.; Xing, H.; Koeffler, H.P. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008, 99, 1793–1797. [Google Scholar] [CrossRef]
- Kausar, H.; Munagala, R.; Bansal, S.S.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Cucurbitacin B potently suppresses non-small-cell lung cancer growth: Identification of intracellular thiols as critical targets. Cancer Lett. 2013, 332, 35–45. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Cai, S.; Yang, C.; Lin, Z.; Zhou, L.; Liu, L.; Cheng, X.; Zeng, W. Not all mutations of KRAS predict poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 957–967. [Google Scholar]
- Wei, L.; Qu, W.; Sun, J.; Wang, X.; Lv, L.; Xie, L.; Song, X. Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize NSCLC cells to cisplatin. Cancer Gene Ther. 2014, 21, 194–199. [Google Scholar] [CrossRef]
- Sablina, A.A.; Chen, W.; Arroyo, J.D.; Corral, L.; Hector, M.; Bulmer, S.E.; DeCaprio, J.A.; Hahn, W.C. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell 2007, 129, 969–982. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/Akt signaling axis in gefitinib-resistant non-small cell lung cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ku, J.M.; Lim, Y.S.; Lee, S.Y.; Kim, J.H.; Cheon, C.; Ko, S.G. Cucurbitacin D overcomes gefitinib resistance by blocking EGF binding to EGFR and inducing cell death in NSCLCs. Front. Oncol. 2020, 10, 62. [Google Scholar] [CrossRef]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulation. Ann. N. Y. Acad. Sci. 2018, 1421, 5. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic acid suppresses breast cancer metastasis by targeting GRP78-mediated glycolysis and ER stress apoptotic pathway Oxid. Med. Cell Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef]
- XLiu, I.; Jutooru, P.; Lei, K.; Kim, S.O.; Lee, L.K.; Brents, P.L.; Prather, S. Safe Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a: ZBTB10 in breast cancer Mol. Cancer Ther. 2012, 11, 1421–1431. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Gu, J.; Wang, S.; Wang, N.; Yang, B.; Zhang, F.; Wang, D.; Fu, W.; Wang, Z. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis. 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Silva, M.G.V.; Vieira, I.G.P.; Mendes, F.N.P.; Albuquerque, I.L.; Dos Santos, R.N.; Silva, F.O.; Morais, S.M. Variation of ursolic acid content in eight Ocimum species from northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, L.; Pfüller, I.; Scheffler, A. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleo gel-forming triterpene extract from the outer bark of birch (betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Ressmann, A.K.; Kremsmayr, T.; Gaertner, P.; Zirbs, R.; Bica, K. Toward a benign strategy for the manufacturing of betulinic acid. Green Chem. 2017, 19, 1014–1022. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Liu, J.; Xu, T.Y.; Fang, R.S.; Chen, Q.H.; He, G.Q. A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem. 2013, 136, 73–79. [Google Scholar] [CrossRef]
- Madsen, K.M.; Udatha, G.D.; Semba, S.; Otero, J.M.; Koetter, P.; Nielsen, J.; Ebizuka, Y.; Kushiro, T.; Panagiotou, G. Linking genotype and phenotype of Saccharomyces cerevisiae strains reveals metabolic engineering targets and leads to triterpene hyper-producers. PLoS ONE 2011, 6, 14763. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.Y.; Chen, Y. Betulinic acid and the pharmacological effects of tumour suppression. Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef]
- Lin, C.K.; Tseng, C.K.; Chen, K.H.; Wu, S.H.; Liaw, C.C.; Lee, J.C. Betulinic acid exerts anti-hepatitis C virus activity via the suppression of NF-κB and MAPK-ERK1/2-mediated COX-2 expression. Br. J. Pharm. 2015, 172, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product botuli Eur. J. Pharm. Sci. 2006, 29, 113. [Google Scholar] [CrossRef]
- Lingaraju, M.C.; Pathak, N.N.; Begum, J.; Balaganur, V.; Bhat, R.A.; Ramachandra, H.D.; Ayanur, A.; Ram, M.; Singh, V.; Kumar, D.; et al. Betulinic acid attenuates lung injury by modulation of inflammatory cytokine response in experimentally-induced polymicrobial sepsis in mice. Cytokine 2015, 71, 101–108. [Google Scholar] [CrossRef]
- Ko, J.L.; Lin, C.H.; Chen, H.C.; Hung, W.H.; Chien, P.J.; Chang, H.Y.; Wang, B.Y. Effects and mechanisms of betulinic acid on improving EGFR TKI-resistance of lung cancer cells. Environ. Toxicol. 2018, 33, 1153–1159. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Cornelio, G.; Yang, P.C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cao, J.; Chen, K.; Cheng, L.; Zhou, C.; Yan, B.; Qian, W.; Li, J.; Duan, W.; Ma, J.; et al. Betulinic acid inhibits stemness and EMT of pancreatic cancer cells via activation of AMPK signaling. Int. J. Oncol. 2019, 54, 98–110. [Google Scholar] [CrossRef]
- Yin, B.; Fang, D.M.; Zhou, X.L.; Gao, F. Natural products as important tyrosine kinase inhibitors. Eur. J. Med. Chem. 2019, 182, 111664. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Inamdar, G.S.; Kuzu, O.; Dinavahi, S.S.; Krzeminski, J.; Battu, M.B.; Voleti, S.R.; Amin, S.; Robertson, G.P. Identifying the structure-activity relationship of leelamine necessary for inhibiting intracellular cholesterol transport. Oncotarget 2017, 8, 28260–28277. [Google Scholar] [CrossRef]
- Jin, L.; Cho, M.; Kim, B.-S.; Han, J.H.; Park, S.; Lee, I.-K.; Ryu, D.; Kim, J.H.; Bae, S.-J.; Ha, K.-T. Drug evaluation based on phosphomimetic PDHA1 reveals the complexity of activity-related cell death in A549 non-small cell lung cancer cells. BMB Rep. 2021, 54, 563. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Yang, Z.; Tam, K.Y. Combination Strategies Using EGFR-TKi in NSCLC Therapy: Learning from the Gap between Pre-Clinical Results and Clinical Outcomes. Int. J. Biol. Sci. 2018, 14, 204–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foucquier, J.; Guedj, M. Analysis of Drug Combinations: Current Methodological Landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Zhong, W.; Qin, Y.; Chen, S.; Sun, T. Antitumor Effect of Natural Product Molecules against Lung Cancer. In A Global Scientific Vision-Prevention, Diagnosis, and Treatment of Lung Cancer; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castañeda, A.M.; Meléndez, C.M.; Uribe, D.; Pedroza-Díaz, J. Synergistic effects of natural compounds and conventional chemotherapeutic agents: Recent insights for the development of cancer treatment strategies. Heliyon 2022, 8, e09519. [Google Scholar] [CrossRef]
- Boța, M.; Vlaia, L.; Jîjie, A.-R.; Marcovici, I.; Crişan, F.; Oancea, C.; Dehelean, C.A.; Mateescu, T.; Moacă, E.-A. Exploring Synergistic Interactions between Natural Compounds and Conventional Chemotherapeutic Drugs in Preclinical Models of Lung Cancer. Pharmaceuticals 2024, 17, 598. [Google Scholar] [CrossRef] [PubMed]
- Gahtori, R.; Tripathi, A.H.; Kumari, A.; Negi, N.; Paliwal, A.; Tripathi, P.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Anticancer Plant-Derivatives: Deciphering Their Oncopreventive and Therapeutic Potential in Molecular Terms. Future J. Pharm. Sci. 2023, 9, 14. [Google Scholar] [CrossRef]
- Huang, T.H.; Wu, T.H.; Guo, Y.H.; Li, T.L.; Chan, Y.L.; Wu, C.J. The Concurrent Treatment of Scutellaria baicalensis Georgi Enhances the Therapeutic Efficacy of Cisplatin but Also Attenuates Chemotherapy-Induced Cachexia and Acute Kidney Injury. J. Ethnopharmacol. 2019, 243, 112075. [Google Scholar] [CrossRef]
- Han, S.Y.; Zhao, M.B.; Zhuang, G.B.; Li, P.P. Marsdenia Tenacissima Extract Restored Gefitinib Sensitivity in Resistant Non-Small Cell Lung Cancer Cells. Lung Cancer 2012, 75, 30–37. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Li, J.; Zhang, J.; Wang, X.; Wang, M. Sulforaphane reverses gefitinib tolerance in human lung cancer cells via modulation of sonic hedgehog signaling. Oncol. Lett. 2018, 15, 109–114. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Xu, Z.; Zhang, Y.; Cao, X.; Yu, J.; Li, J.; Xu, S. Identification of solamargine as a cisplatin sensitizer through phenotypical screening in cisplatin-resistant NSCLC organoids. Front. Pharmacol. 2022, 13, 802168. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Liu, Q.; Ho, I.H.; Wei, X.; Yin, T.; Zhan, Y.; Zhang, W.; Zhang, W.; Chen, B.; et al. Hederagenin potentiated cisplatin and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef]
- Shi, S.; Bai, X.; Ji, Q.; Wan, H.; An, H.; Kang, X.; Guo, S. Molecular mechanism of ion channel protein TMEM16A regulated by natural product of narirutin for lung cancer adjuvant treatment. Int. J. Biol. Macromol. 2022, 223, 1145–1157. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, H.; Chen, C.; Ren, K.; Xu, Y.; Liu, X.; He, L. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine 2018, 48, 51–61. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Chen, J.T.; Liu, Y. Targeted polysaccharide nanoparticle for adamplatin prodrug delivery. J. Med. Chem. 2013, 56, 9725–9736. [Google Scholar] [CrossRef]
- Pinmai, K.; Chunlaratthanabhorn, S.; Ngamkitidechakul, C.; Soonthornchareon, N.; Hahnvajanawong, C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: Doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J. Gastroenterol. 2008, 14, 1491–1497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, K.T.; Li, S.; Li, Y.R.; Cheng, S.-L.; Lin, S.-H.; Tung, Y.-T. Synergistic Anticancer Effect of a Combination of Paclitaxel and 5-Demethylnobiletin Against Lung Cancer Cell Line In Vitro and In Vivo. Appl. Biochem. Biotechnol. 2019, 187, 1328–1343. [Google Scholar] [CrossRef]
- Peng, M.; Zheng, Z.; Chen, S.; Fang, L.; Feng, R.; Zhang, L.; Tang, Q.; Liu, X. Sensitization of Non-Small Cell Lung Cancer Cells to Gefitinib and Reversal of Epithelial-Mesenchymal Transition by Aloe-Emodin Via PI3K/Akt/TWIS1 Signal Blockage. Front. Oncol. 2022, 12, 908031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiao, L.; Xu, J.; Sun, J.; Chen, Z.; Gong, Y.; Bi, L.; Lu, Y.; Yao, J.; Zhu, W.; Hou, A.; et al. Chinese Herbal Medicine Combined With EGFR-TKI in EGFR Mutation-Positive Advanced Pulmonary Adenocarcinoma (CATLA): A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2019, 10, 732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Sun, L.; Cui, J.; Wang, J.; Liu, X.; Aung, T.N.; Qu, Z.; Chen, Z.; Adelson, D.L.; Lin, L. Yiqi Chutan Tang Reduces Gefitinib-Induced Drug Resistance in Non-Small-Cell Lung Cancer by Targeting Apoptosis and Autophagy. Cytom. A 2020, 97, 70–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abd El-Hafeez, A.A.; Fujimura, T.; Kamei, R.; Hirakawa, N.; Baba, K.; Ono, K.; Kawamoto, S. Synergistic tumor suppression by a Perilla frutescens-derived methoxyflavanone and anti-cancer tyrosine kinase inhibitors in A549 human lung adenocarcinoma. Cytotechnology 2018, 70, 913–919. [Google Scholar] [CrossRef]
- Chen, Z.; Vallega, K.A.; Chen, H.; Zhou, J.; Ramalingam, S.S.; Sun, S.Y. The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 2022, 175, 105998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corvaja, C.; Passaro, A.; Attili, I.; Aliaga, P.T.; Spitaleri, G.; Signore, E.D.; de Marinis, F. Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging biological insights and therapeutic development. Cancer Treat. Rev. 2024, 130, 102824. [Google Scholar] [CrossRef] [PubMed]
- Eno, M.S.; Brubaker, J.D.; Campbell, J.E.; De Savi, C.; Guzi, T.J.; Williams, B.D.; Wilson, D.; Wilson, K.; Brooijmans, N.; Kim, J.; et al. Discovery of BLU-945, a Reversible, Potent, and Wild-Type-Sparing Next-Generation EGFR Mutant Inhibitor for Treatment-Resistant Non-Small-Cell Lung Cancer. J. Med. Chem. 2022, 65, 9662–9677. [Google Scholar] [CrossRef]
- Su, C.; Sun, S.Y. Fourth-generation epidermal growth factor receptor-tyrosine kinases inhibitors: Hope and challenges. Transl. Cancer Res. 2024, 13, 3929–3934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleem, M.; Kweon, M.H.; Yun, J.M.; Adhami, V.M.; Khan, N.; Syed, D.N.; Mukhtar, H. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005, 65, 11203–11213. [Google Scholar] [CrossRef]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekonomopoulou, M.T.; Babas, E.; Mioglou-Kalouptsi, E.; Malandri, M.; Iakovidou-Kritsi, Z. Changes in activities of caspase-8 and caspase-9 in human cervical malignancy. Int. J. Gynecol. Cancer 2011, 21, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002, 23, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011, 2, 348–356. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, H.Y.; Deng, J.S.; Chen, J.J.; Huang, S.S.; Lin, I.H.; Kuo, W.L.; Chao, W.; Huang, G.J. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am. J. Chin. Med. 2013, 41, 1439–1457. [Google Scholar] [CrossRef]
- Preljević, K.; Pašić, I.; Vlaović, M.; Matić, I.Z.; Krivokapić, S.; Petrović, N.; Stanojković, T.; Živković, V.; Perović, S. Comparative analysis of chemical profiles, antioxidant, antibacterial, and anticancer effects of essential oils of two Thymus species from Montenegro. Fitoterapia 2024, 174, 105871. [Google Scholar] [CrossRef]
- Su, C.W.; Kao, S.H.; Chen, Y.T.; Hsieh, Y.H.; Yang, W.E.; Tsai, M.Y.; Lin, C.W.; Yang, S.F. Curcumin analog L48H37 induces apoptosis in human Oral cancer cells by activating caspase cascades and downregulating the inhibitor of apoptosis proteins through JNK/p38 signaling. Am. J. Chin. Med. 2024, 52, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Auwal, A.; Al Banna, M.H.; Pronoy, T.U.; Hossain, M.M.; Rashel, K.M.; Kabir, S.R.; Ansary, M.R.; Islam, F. In vitro and In vivo Growth Inhibition and apoptosis of cancer cells by ethyl 4-[(4-methylbenzyl) oxy] benzoate complex. Anti-Cancer Agents Med. Chem. 2025; ahead of print. [Google Scholar]
- Huang, S.T.; Huang, C.C.; Huang, W.L.; Lin, T.K.; Liao, P.L.; Wang, P.W.; Liou, C.W.; Chuang, J.H. Tanshinone IIA induces intrinsic apoptosis in osteosarcoma cells both in vivo and in vitro associated with mitochondrial dysfunction. Sci. Rep. 2017, 7, 40382. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Z.; Torres, N.M.; Tao, A.; Gao, Y.J.; Luo, L.S.; Li, Ł.Q.; de Stanchina, E.; Abdel-Wahab, O.; Solit, D.B.; Poulikakos, P.I.; et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms That Determine Their Sensitivity to Pharmacologic Inhibition. Cancer Cell 2015, 28, 370–383. [Google Scholar] [CrossRef]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with Class 3 BRAF Mutants are Sensitive to the Inhibition of Activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF Alterations in Cancer: New Rational Therapeutic Strategies for Actionable Mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Paik, P.K.; Arcila, M.E.; Fara, M.; Sima, C.S.; Miller, V.A.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Clinical Characteristics of Patients with Lung Adenocarcinomas Harboring BRAF Mutations. J. Clin. Oncol. 2011, 29, 2046–2051. [Google Scholar] [CrossRef]
- Litvak, A.M.; Paik, P.K.; Woo, K.M.; Sima, C.S.; Hellmann, M.D.; Arcila, M.E.; Ladanyi, M.; Rudin, C.M.; Kris, M.G.; Riely, G.J. Clinical Characteristics and Course of 63 Patients with BRAF Mutant Lung Cancers. J. Thorac. Oncol. 2014, 9, 1669–1674. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Biersack, B.; Tahtamouni, L.; Höpfner, M. Role and Function of Receptor Tyrosine Kinases in BRAF Mutant Cancers. Receptors 2024, 3, 58–106. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Ebi, H.; Turke, A.B.; Coffee, E.M.; Nishino, M.; Cogdill, A.P.; Brown, R.D.; Della Pelle, P.; Dias-Santagata, D.; Hung, K.E.; et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012, 2, 227–235. [Google Scholar] [CrossRef]
- Held, M.A.; Langdon, C.G.; Platt, J.T.; Graham-Steed, T.; Liu, Z.; Chakraborty, A.; Bacchiocchi, A.; Koo, A.; Haskins, J.W.; Bosenberg, M.W.; et al. Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer Discov. 2013, 3, 52–67. [Google Scholar] [CrossRef]
- Baur, F.; Nietzer, S.L.; Kunz, M.; Saal, F.; Jeromin, J.; Matschos, S.; Linnebacher, M.; Walles, H.; Dandekar, T.; Dandekar, G. Connecting cancer pathways to tumor engines: A stratification tool for colorectal cancer combining human in vitro tissue models with Boolean in silico models. Cancers 2020, 12, 28. [Google Scholar] [CrossRef]
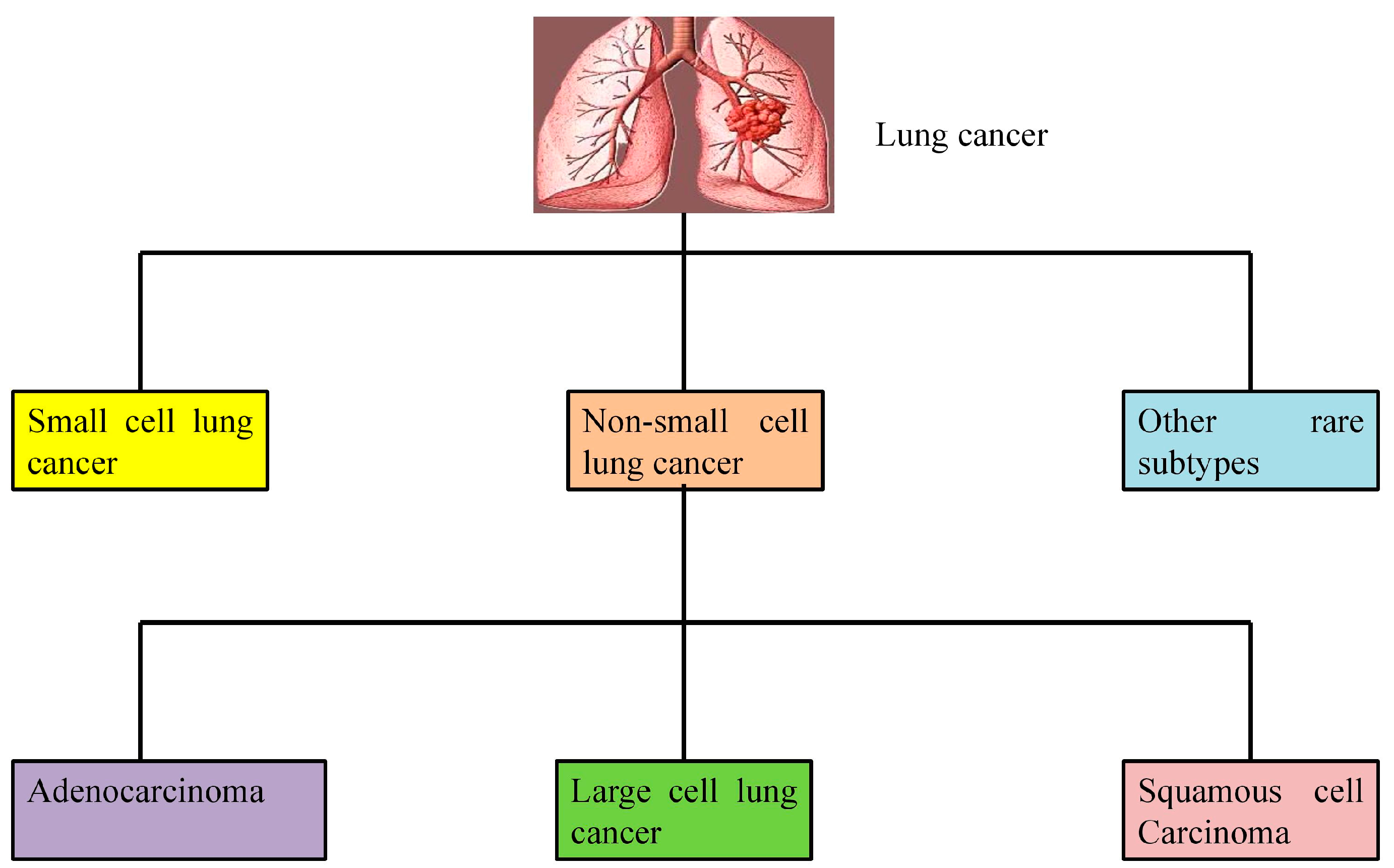
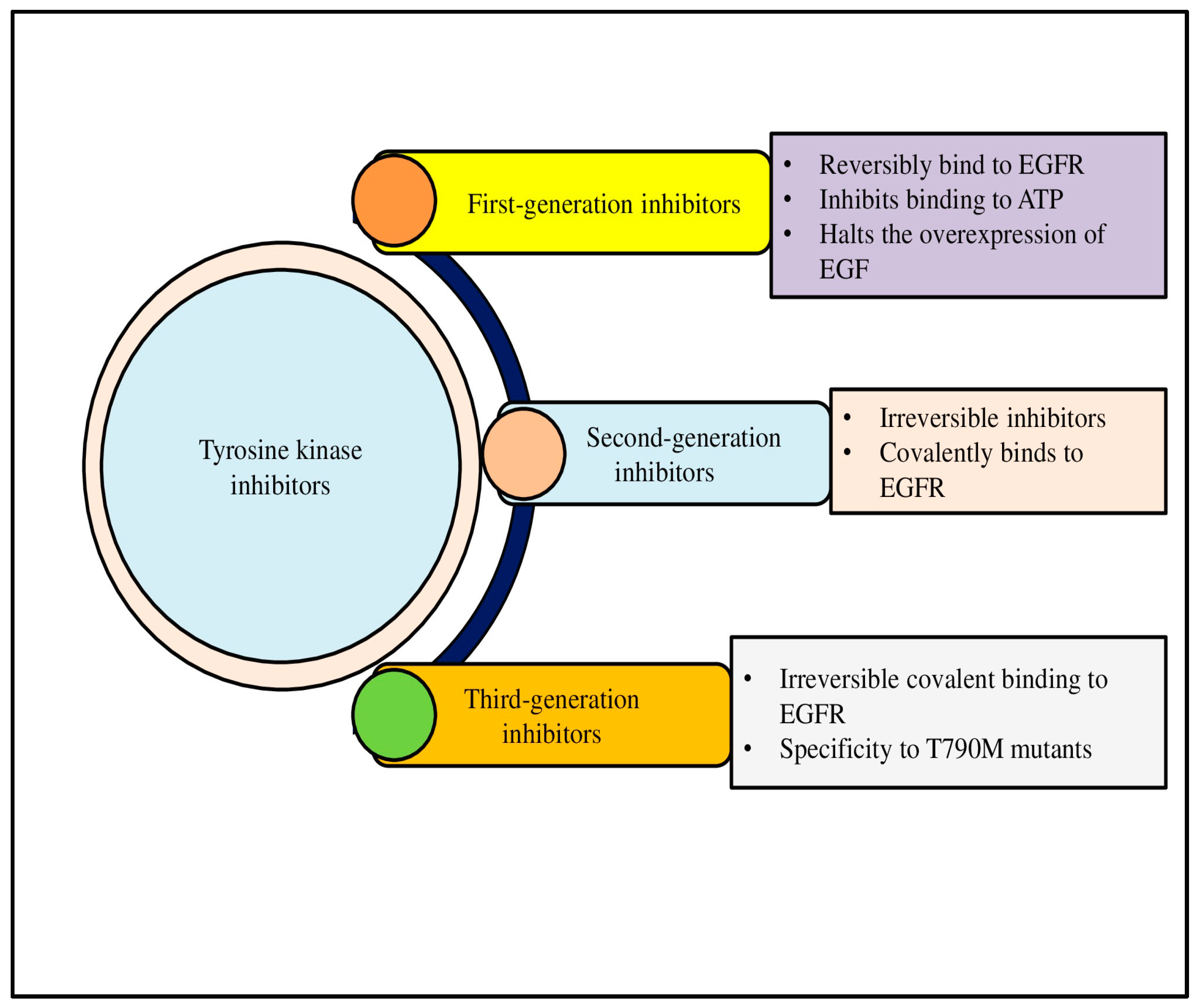
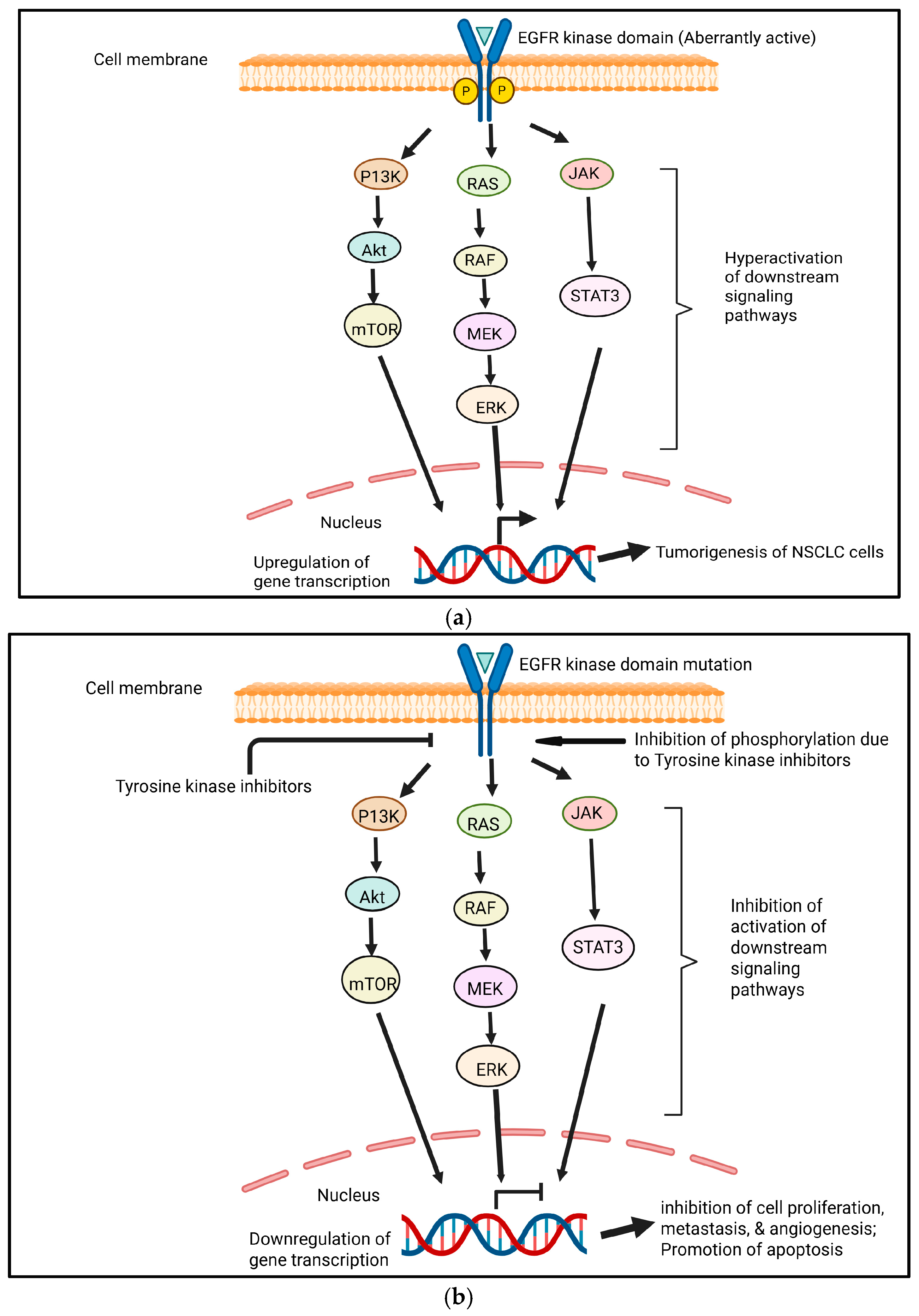
| EGFR TKIs | Clinical Trial Number | Outcomes/Goals | References |
|---|---|---|---|
| Gefitinib, erlotinib, or icotinib | NCT02893332 | For EGFR-mutated NSCLC, the addition of upfront local treatment utilizing RT statistically significantly improved PFS and OS as compared to a first-line TKI alone. | [62] |
| Erlotinib with gefitinib | NCT01024413 | At the moment of peak response to EGFR-TKI, the amount of L858R dropped and hit its lowest level. | [63] |
| AZD9291 | NCT01802632 | Patients with EGFR T790M-mutant lung cancer who had seen disease progression after previous EGFR tyrosine kinase inhibitor therapy showed high levels of AZD9291 activity. | [64] |
| Osimertinib | NCT01802632 | After EGFR-TKI treatment, osimertinib produced a high objective response rate, favorable progression-free survival, and persistent response in patients with EGFRm T790M advanced non-small cell lung cancer. | [65] |
| Brigatinib | NCT04318938 | The primary goals of the ABP trial are to enhance knowledge of the underlying biology and enable the creation of a framework for the customized treatment of ALK+ NSCLC based on molecular characteristics, in addition to assisting enrolled patients in making treatment decisions. | [66] |
| Natural Product | Type | Source | Cancer Type | Conclusion | Reference |
|---|---|---|---|---|---|
| Capsaicin | Alkaloids | Capsicum annuum L. | Human fibrosarcoma (HT-1080 cells) | Inhibits EGF-induced activation of MMP-9 and MMP-2, as well as tumor cell invasion and migration. | [90] |
| Human fibrosarcoma cells | Capsaicin suppressed EGF-induced invasion and migration of human fibrosarcoma cells via EGFR-dependent FAK/Akt, PKC/Raf/ERK, p38 mitogen-activated protein kinase (MAPK), and AP-1 signaling, resulting in decreased matrix metalloproteinase 9 (MMP-9) expression. | [91] | |||
| Oxymatrine | Alkaloids | Sophora flavescens Aiton | Human malignant glioma (U251MG cells) | Reduced cell growth, halted the cell cycle at the G0/G1 phase, and reduced production of cell cycle regulatory proteins. | [92] |
| Gastric cancer cells | Oxymatrine decreased the proliferation and invasion of gastric cells by inhibiting the EGFR/Cyclin D1/CDK4/6, EGFR/Akt, and MEK-1/ERK1/2/MMP2 pathways by inhibiting EGFRp-Tyr845. | [93] | |||
| Tetrandrine | Alkaloid | Stephania tetrandra S. Moore | Human colorectal adenocarcinoma (HT29 cells) | Inhibited the phosphorylation of EGFR and its downstream signaling pathways. | [94] |
| Apigenin | Flavone | Many fruits, vegetables, and seasonings | SKOV3 and SKOV3/TR cells | Apigenin’s cytotoxic action is explained by the considerable reduction in the mRNA and protein expression of Tyro3 and Axl receptor tyrosine kinases (RTKs). | [95] |
| DLBCL cells | To promote G2/M phase arrest, apigenin inhibits cell cycle proteins such as CDK2/CDK4/CDK6/CDC2/p-RB. Our data mechanically show that apigenin inhibits the survival of DLBCL cells by significantly reducing the expression of the pro-proliferative pathway PI3K/mTOR. | [96] | |||
| Baicalein | Flavonoid | Scutellaria baicalensis, Thymus vulgaris, Oroxylum indicum | NSCLC | Induced apoptosis and changes in the regulation of cell cycle and altered expression of apoptotic regulatory proteins. | [97] |
| Curcumin | Flavonoid | Curcuma longa Lin | Breast cancer cell lines | By interfering with the p185neu protein’s binding to the chaperone GRP94, curcumin reduced the amount of p185neu in vivo and inhibited it in vitro. | [98] |
| Non-small cell lung cancer (NSCLC) A549 and H460 cells | Curcumin has an anti-proliferative effect on both parental and chemo-resistant NSCLC cells via its new target, Axl RTK. | [99] | |||
| Luteolin | Flavone | Celery, carrots, peppers, thyme, oregano, etc. | NSCLC | Inhibits EGFR autophosphorylation. | [100] |
| Formononetin | Isoflavone | Astragalus membranaceus | NSCLC | Promoted the efficacy of EGFR-TKI by modulating the EGFR-AKT-Mcl-1 axis in a ubiquitination-dependent manner. | [101] |
| Fisetin | Flavonol | Apples, strawberries, cucumbers, persimmons, acacia plants and shrubs | Retinoblastoma angiogenesis | Blocked the VEGF/VEGFR signaling pathway. | [102] |
| Quercein | Flavonol | Fruits, vegetables, and beverages | Cisplatin-resistant ovarian cancer | An antiproliferative effect and the inhibition of lymphocyte TK activity were found. | [103] |
| Caffeic acid | Phenolic molecule | Coffee, wine, and tea, etc. | Breast cancer cells | Inhibits the phosphorylation of EGFR. | [104] |
| (-)-Epigallocatechin-3-gallate (EGCG) | Phenolics | Camellia sinensis | Hepatocellular carcinoma and colorectal cancer | Prevented the EGFR-TKs from activating. | [105] |
| Breast cancer cell invasion | In MCF-7, MCF-7TAM, and MDA-MB-231 cells, EGCG and IIF treatments decreased the migratory tendency and changed the molecular network based on the interdependence of EGFR, CD44, and EMMPRIN expression. | [106] | |||
| Colorectal adenomas | GTCs work as chemopreventive and anticancer agents by preventing the activation of certain RTKs, particularly EGFR, IGF-1R, and VEGFR2. | [107] | |||
| NSCLC | Reduced the growth of erlotinib-sensitive and -resistant cell lines, including those with overexpressed c-Met and with developed erlotinib resistance. | [108] | |||
| NSCLC | Treatment with EGCG decreases cell migration and alters the expression of vinculin and meta-vinculin. | [109] | |||
| NSCLC | Combining EGCG derivatives with cisplatin inhibits the EGFR signaling pathway and reduces p-EGFR, p-AKT, and p-ERK expression both in vitro and in vivo. | [110] | |||
| Ellagic acid | Polyphenol | Fruits and nuts | Not specified | Dual inhibitor of VEGF and PDGF receptors indicated that it has significant antiangiogenic qualities. | [111] |
| Gossypol | Polyphenol | Gossypium sp. | Different cancer types in vitro and in vivo | Cell apoptosis but also autophagy, cell cycle arrest, and other abnormal cellular phenomena were found. | [112] |
| Honokiol | Phenolics | Magnolia | NSCLC | Reduced Akt phosphorylation and upregulated PTEN expression to downregulate the PI3K/Akt/mTOR pathway. | [113] |
| Lung cancer | Regardless of the epidermal growth factor receptor (EGFR) mutation status, HNK specifically suppresses STAT3 phosphorylation, and STAT3 knockdown eliminated HNK’s anti-proliferative and anti-metastatic activities. | [114] | |||
| Magnolol | Hydroxylated biphenyl | Magnolia sps. | NSCLC | Multiple signaling pathways relayed by TKs were targeted. | [115] |
| Resveratrol | Stilbene | Grapes, peanuts, apples, blueberries, raspberries, etc. | HepG2 hepatocellular carcinoma cells | Effectively stops proliferation of cells, lowers reactive oxygen species generation, and triggers apoptosis, stopping the cell cycle in the G1 and G2/M phases. Additionally, it alters the NO/NOS system. | [116] |
| 20(S)-ginsenoside Rg3 | Triterpenoid saponins | Panax ginseng | Lung cancer | 20(S)-Rg3 may inhibit CDK2, Cyclin A2, and Cyclin E1 by blocking the cell cycle at the G0/G1 phase. | [117] |
| Ginsenosides | Saponins | Panax ginseng | NSCLC | Decreased stemness marker expression and reduced spheroid formation were found. | [118] |
| NSCLC | Inhibited the growth of NSCLC cells by activating the tumor suppressor p53-binding protein-1 and upregulating vaccinia-related kinase 1. | [119] | |||
| Astragaloside IV | Saponine | Astragali Radix | Lung cancer | AS-IV inhibited the M2 polarization of macrophages partially via the AMPK signaling pathway, which decreased the proliferation, invasion, migration, and angiogenesis of lung cancer. | [120] |
| Breast cancer MDA-MB-231 cells | Orthotopic breast tumor development and lung metastases were inhibited by astragaloside IV. | [121] | |||
| Polyphyllin | Steroidal saponin | Paris polyphylla | NSCLC | Activation of SAPK/JNK pathway, suppression of p65 and DNMT1 expression. | [122] |
| NSCLC | Downregulation of MALAT1 and inhibition of suppression of STAT3 phosphorylation. | [123] | |||
| Cucurbitacin E | Tetracyclic triterpenes | Cucurbitaceo-us plants | Non-small cell lung cancer (NSCLC) cell line A549 | Cucurbitacins E exhibited anti-proliferative effect against A549 cells by targeting the EGFR/MAPK signaling pathway. | [124] |
| Cucurbitacin B | MKN-45 gastric carcinoma cells | The JAK2/STAT3 signaling pathway may be inhibited by cucurbitacin B, which decreases MKN-45 cell proliferation and promotes apoptosis. | [125] | ||
| Betulinic Acid | Triterpenoid | Many plants and herbs | Human NSCLC cell lines | In NSCLC cells, betulinic acid (BA) causes apoptosis and protective autophagy while suppressing cell division. BA’s ability to destroy cancer cells is increased by inhibiting autophagy. | [126] |
| Betulin | Triterpenoid | Many plants and herbs | Human chronic myelogenous leukemia cell line | Betulin modulates the mitogen-activated protein (MAP) kinase pathway with activity comparable to that of the well-known ABL1 kinase inhibitor imatinib mesylate. | [127] |
| Leelamine | Diterpene | Pine bark | Myelogenous leukemia cells | Modulation of the STAT5 pathway, autophagy, and death. | [128] |
| ClinicalTrials.gov ID & Reference | Title of Study | Objective | Intervention/Treatment |
|---|---|---|---|
| NCT02321293 [159] | An Open-Label Prospective Cohort Trial of Curcumin Plus Tyrosine Kinase Inhibitors (TKI) for EGFR-Mutant Advanced NSCLC (CURCUMIN) | To evaluate the safety and tolerability of curcumin in combination with EGFR-TKIs in patients with advanced, non-resectable, and EGFR-mutant NSCLC | Dietary Supplement: CurcuVIVA™. Drug: Tyrosine Kinase Inhibitor Gefitinib (Iressa). Drug: Tyrosine Kinase Inhibitor Erlotinib (Tarceva). |
| NCT03598309 [160] | Phase II Trial to Modulate Intermediate Endpoint Biomarkers in Former and Current Smokers | To find out if an investigational combination drug called Lovaza (made with fish oils)+Curcumin C3 Complex (made from a root called curcumin) can help reduce the size of lung nodules | Drug: Curcumin C3 complex®. Drug: Lovaza®. |
| NCT04871412 [161] | The Thoracic Peri-Operative Integrative Surgical Care Evaluation Trial—Stage III (POISE) | Thoracic POISE project aims to improve outcomes by integrating complementary and individualized care approaches to enhance recovery, reduce adverse events, and extend survival in real-world clinical settings | Vit D, Provitalix Pure Whey Protein, Theracurmin 2X, Green Tea Extract, Trident SAP 66:33 Lemon, Probiotic Pro12. |
| Natural Product | Combined Drug (TKI/Chemotherapy) | Target | Observed Anticancer Effects | Reference |
|---|---|---|---|---|
| Emodin | Sorafenib, afatinib, cisplatin, paclitaxel, gemcitabine, and endoxifen | Lung adenocarcinoma and non-small cell lung cancer | Enhanced anticancer activity | [281] |
| Scutellaria baicalensis | Cisplatin | Lewis lung carcinoma cells, or LLC and in vivo C57BL/6J-tumor-inoculated mouse model | Showed synergistic effects, prevented tumor growth in vitro | [282] |
| Marsdenia tenacissima | Gefitinib | H460, H1975, and H292 cell lines | Caused apoptosis in resistant cells, decreased EGFR downstream signaling pathways | [283] |
| Sulforaphane | Gefitinib | Gefitinib-resistant lung cancer cells | Decreased the growth of gefitinib-resistant lung cancer cells, reversed gefitinib resistance, and inhibited the expression of SHH, SMO, and GLI1 | [284] |
| Solamargine | Cisplatin | Lung cancer cell lines NCI-H1299 and NCI-H460 | Increased apoptosis and anti-proliferative effects | [285] |
| Hederagenin | Paclitaxel, cisplatin | Lung cancer cells | Enhanced cytotoxicity via autophagy suppression | [286] |
| Narirutin | Cisplatin | Lung cancer cells | Dose-dependently inhibited TMEM16A, synergistic cytotoxicity | [287] |
| Curcumin | Cisplatin | NSCLC | Improved cisplatin efficacy | [288] |
| Curcumin | Cisplatin | Lung cancer cell lines A549, H460, and H1299 | Improved cisplatin efficacy | [289] |
| Phyllanthus emblica and Termanalia bellerica | Doxorubicin, cisplatin | Lung and liver cells | Synergistic growth inhibition | [290] |
| 5-Demethylnobiletin | Paclitaxel | Lung cancer cell lines | Synergistically inhibited proliferation | [291] |
| Aloe-emodin | Gefitinib | NSCLC | Increased sensitivity to gefitinib and reversed EMT | [292] |
| Chinese herbal medicine | Erlotinib, gefitinib, or icotinib | Advanced NSCLC | Increased PFS in patients with advanced NSCLC with an EGFR mutation | [293] |
| Yiqi Chutan Tang (YQCT) | Gefitinib | EFGR-TKI-resistant lung cancer cells | Reduced drug resistance and improved anticancer effects when associated with gefitinib, enhancement of apoptosis and autophagy | [294] |
| Methoxyflavanone derivative | Nilotinib, bosutinib, dasatinib, and ponatinib | A549 cells | Inhibited the proliferation of A549 cells in combination | [295] |
| Berberine | Osimertinib | MET-amplified osimertinib-resistant lung cancer | Reduced the survival of multiple MET-amplified EGFR-mutant osimertinib-resistant NSCLC cell lines and had increased apoptosis induction | [296] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrumaihi, F.; Rahmani, A.H.; Prabhu, S.V.; Kumar, V.; Anwar, S. The Role of Plant-Derived Natural Products as a Regulator of the Tyrosine Kinase Pathway in the Management of Lung Cancer. Curr. Issues Mol. Biol. 2025, 47, 498. https://doi.org/10.3390/cimb47070498
Alrumaihi F, Rahmani AH, Prabhu SV, Kumar V, Anwar S. The Role of Plant-Derived Natural Products as a Regulator of the Tyrosine Kinase Pathway in the Management of Lung Cancer. Current Issues in Molecular Biology. 2025; 47(7):498. https://doi.org/10.3390/cimb47070498
Chicago/Turabian StyleAlrumaihi, Faris, Arshad Husain Rahmani, Sitrarasu Vijaya Prabhu, Vikalp Kumar, and Shehwaz Anwar. 2025. "The Role of Plant-Derived Natural Products as a Regulator of the Tyrosine Kinase Pathway in the Management of Lung Cancer" Current Issues in Molecular Biology 47, no. 7: 498. https://doi.org/10.3390/cimb47070498
APA StyleAlrumaihi, F., Rahmani, A. H., Prabhu, S. V., Kumar, V., & Anwar, S. (2025). The Role of Plant-Derived Natural Products as a Regulator of the Tyrosine Kinase Pathway in the Management of Lung Cancer. Current Issues in Molecular Biology, 47(7), 498. https://doi.org/10.3390/cimb47070498









