TP53 Is a Potential Target of Juglone Against Colorectal Cancer: Based on a Combination of Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Pharmacological Analysis of Juglone
2.1.1. Juglone and CRC Gene Collection and Target Prediction
2.1.2. Construction of a Protein-Protein Interaction (PPI) Network for Common Targets Between Juglone and Colorectal Cancer (CRC)
2.1.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
2.1.4. Molecular Docking
3. Experimental Verification
3.1. Experimental Material
Reagents and Instruments
3.2. Cell Cultivation
3.3. Cell Viability Assay
3.4. Cell Apoptosis Assay
3.5. Detection of ROS
3.6. Western Blot
3.7. Statistical Analysis
4. Results
4.1. Network Pharmacology Analysis
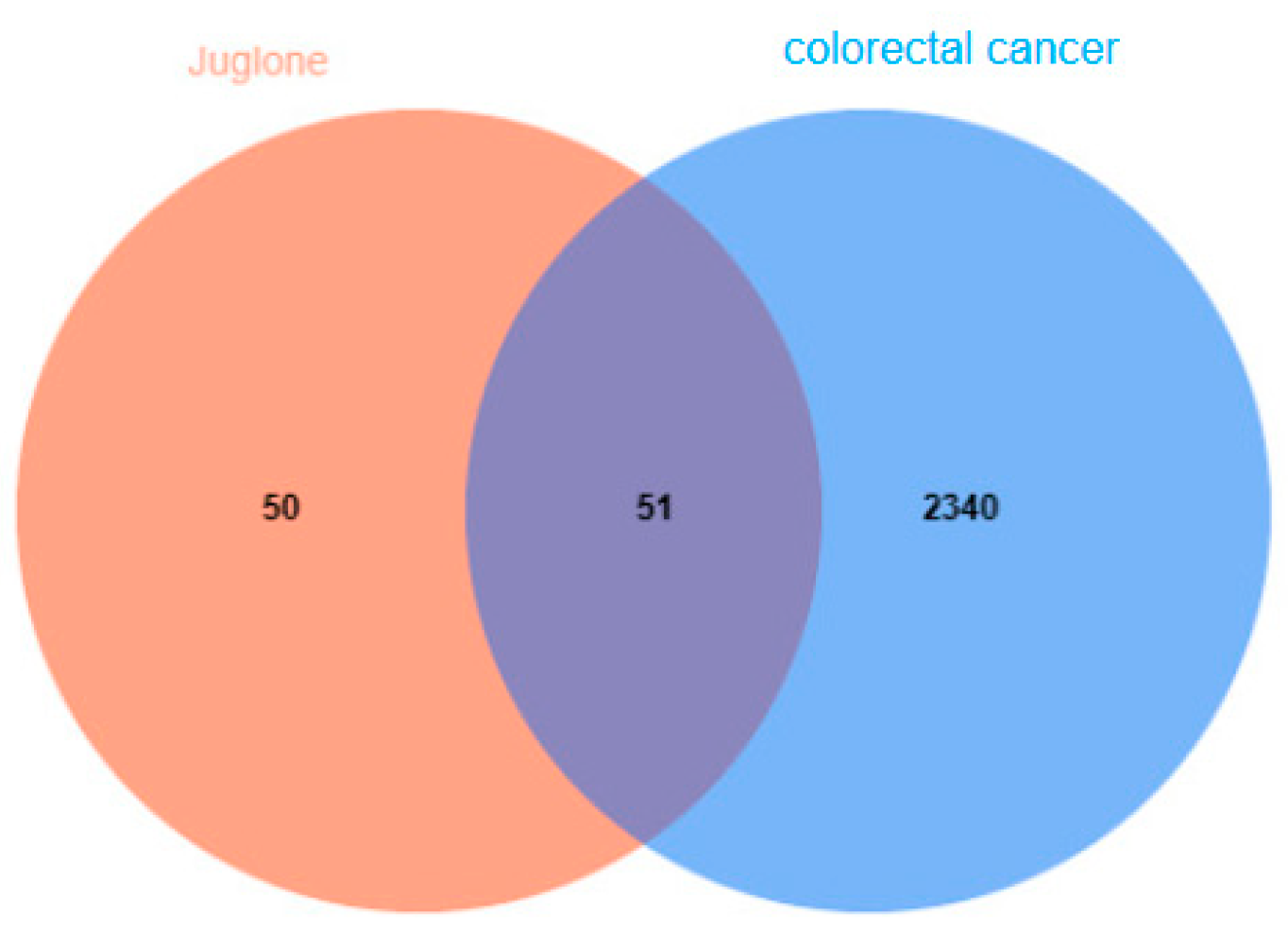
4.1.1. CRC Gene Collection
4.1.2. Network Diagram of Juglone Targets
4.1.3. PPI Network Construction for Active Ingredients and Disease Common Targets
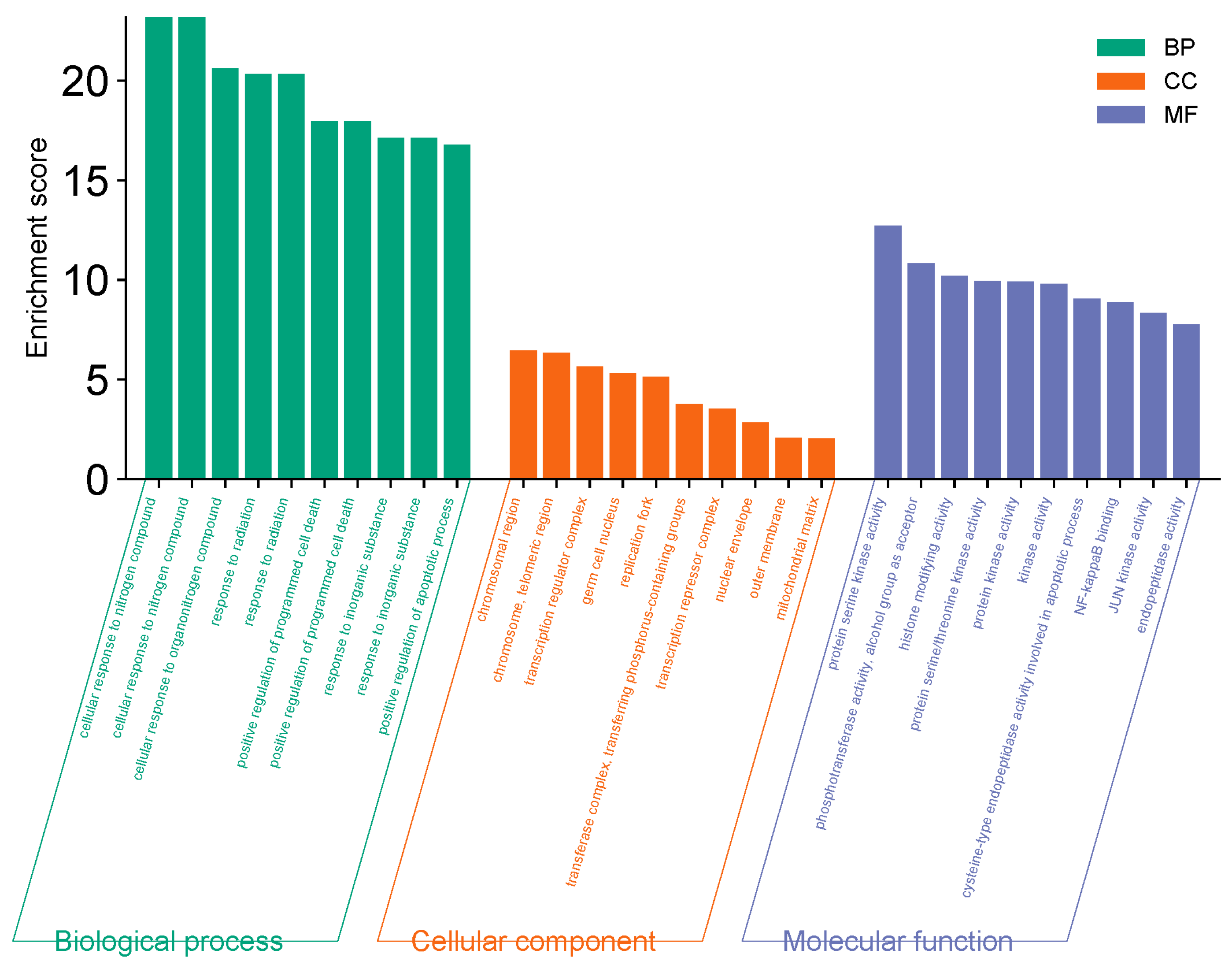
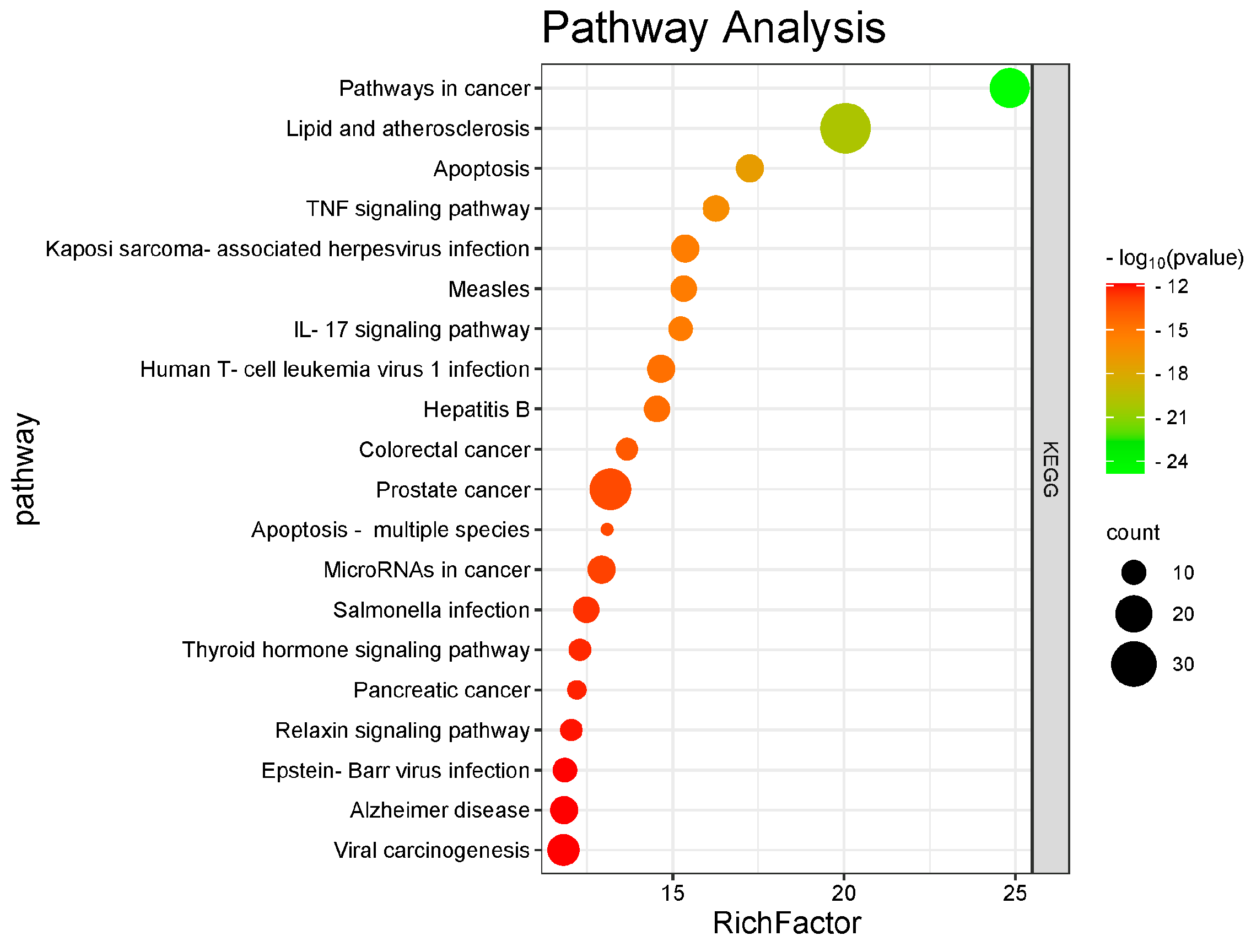
4.1.4. GO and KEGG Enrichment Analysis
4.1.5. Analysis of Molecular Docking Results
4.1.6. Analysis of Molecular Dynamics Simulation Results
4.2. Analysis of Cell Cytotoxicity and Proliferation Assay Results
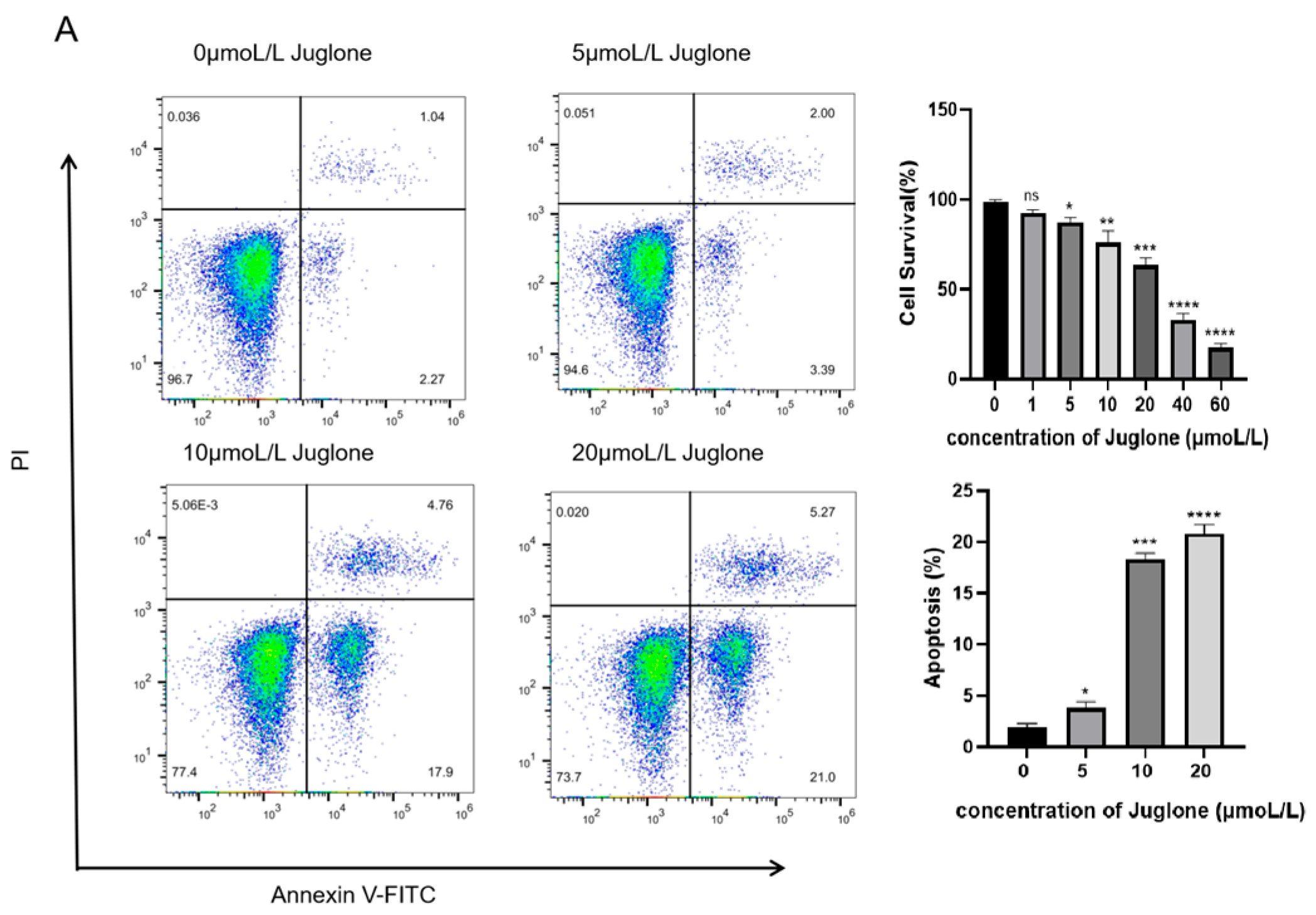
4.2.1. The Anti-Proliferative and Pro-Apoptotic Effects of Juglone on CT26 Cells
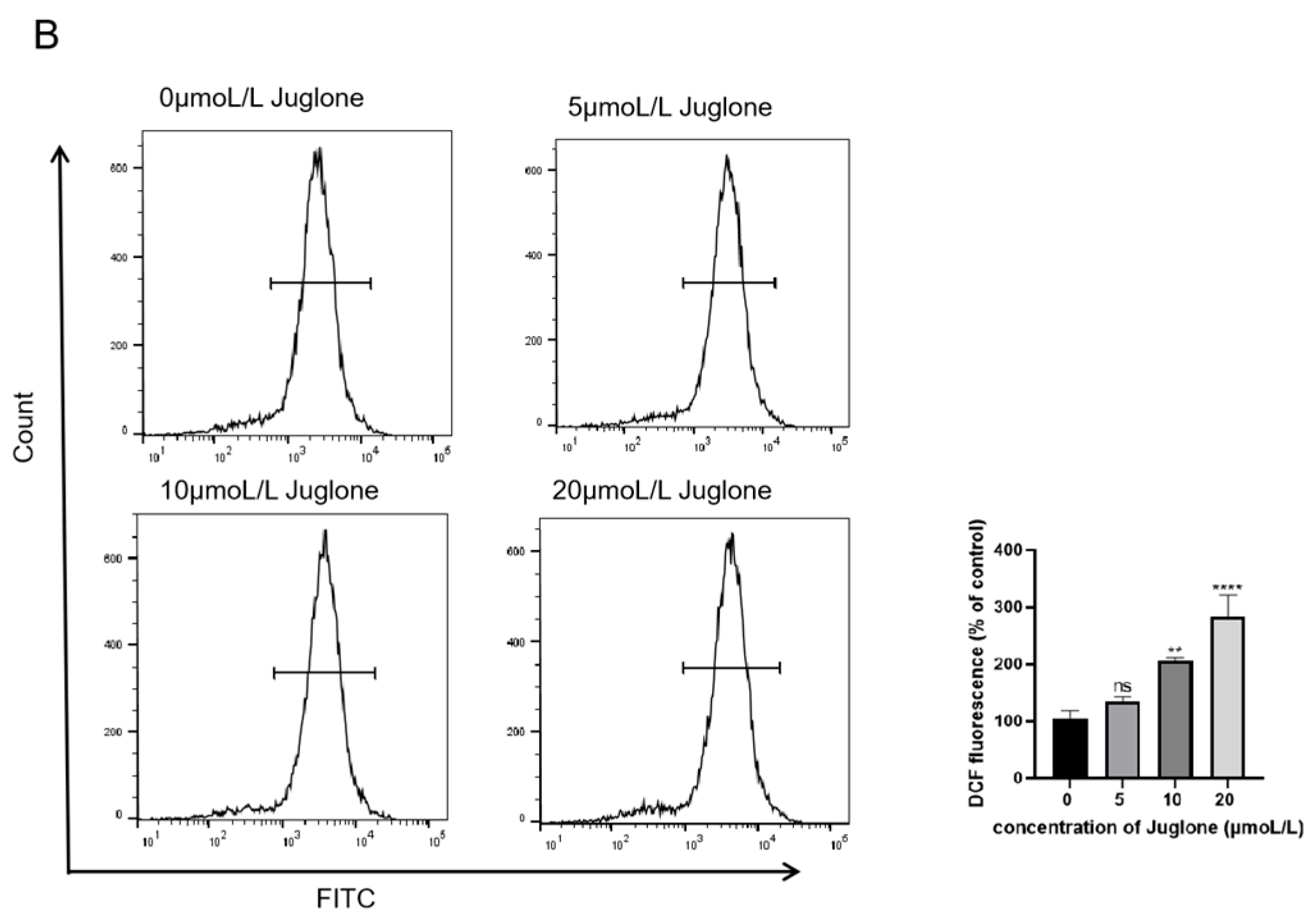
4.2.2. ROS Production Induces Cell Apoptosis in CT26 Cells
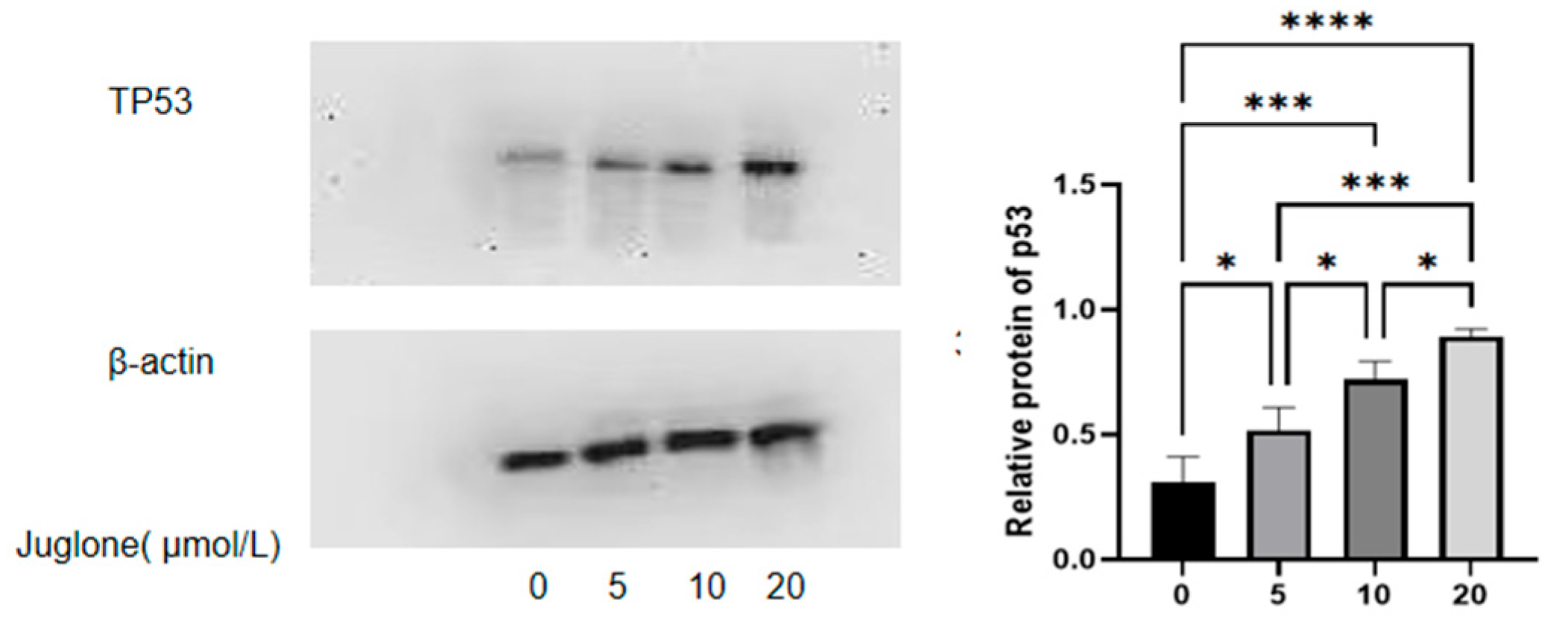
4.2.3. CT26 Cells Increase p53 Protein Expression to Promote Cell Apoptosis
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Lui, R.N.; Tsoi, K.K.F.; Ho, J.M.W.; Lo, C.M.; Chan, F.C.H.; Kyaw, M.H.; Sung, J.J.Y. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1275–1282. [Google Scholar] [CrossRef]
- Spaander, M.C.W.; Zauber, A.G.; Syngal, S.; Blaser, M.J.; Sung, J.J.; You, Y.N.; Kuipers, E.J. Young-onset colorectal cancer. Nat. Rev. Dis. Primers 2023, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., 3rd; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Housini, M.; Dariya, B.; Ahmed, N.; Stevens, A.; Fiadjoe, H.; Nagaraju, G.P.; Basha, R. Colorectal cancer: Genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene 2024, 892, 147857. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.K.; Mangrulkar, S.V.; Kushwaha, S.; Ved, A.; Kale, M.B.; Wankhede, N.L.; Taksande, B.G.; Upaganlawar, A.B.; Umekar, M.J.; Koppula, S.; et al. Recent Perspectives on Cardiovascular Toxicity Associated with Colorectal Cancer Drug Therapy. Pharmaceuticals 2023, 16, 1441. [Google Scholar] [CrossRef]
- Bozali, K.; Metin Guler, E.; Kocyigit, A. A Study on Thymoquinone: Antioxidant Capacity and Anticancer Activities in LoVo Colorectal Cancer Cells. Chem. Biodivers. 2024, 21, e202301886. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, X.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma. J. Nanobiotechnology 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Erkoc-Kaya, D.; Arikoglu, H.; Guclu, E.; Dursunoglu, D.; Menevse, E. Juglone-ascorbate treatment enhances reactive oxygen species mediated mitochondrial apoptosis in pancreatic cancer. Mol. Biol. Rep. 2024, 51, 340. [Google Scholar] [CrossRef]
- Guo, F.; Ling, G.; Qiu, J.; Li, J.; Gan, Y.; Yu, Y.; Tang, J.; Mo, L.; Piao, H. Juglone induces ferroptosis in glioblastoma cells by inhibiting the Nrf2-GPX4 axis through the phosphorylation of p38MAPK. Chin. Med. 2024, 19, 52. [Google Scholar] [CrossRef]
- Shi, J.Y.; Huang, Z.R.; Gao, H.Y.; Xu, X.L. Anticancer effects of juglone in OVCAR-3 human ovarian carcinoma are facilitated through programmed cell death, endogenous ROS production, inhibition of cell migration and invasion and cell cycle arrest. J. BUON 2021, 26, 1188. [Google Scholar]
- Shang, L.; Wang, Y.; Li, J.; Zhou, F.; Xiao, K.; Liu, Y.; Zhang, M.; Wang, S.; Yang, S. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 302 Pt A, 115876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Jandova, Z.; Vargiu, A.V.; Bonvin, A. Native or Non-Native Protein-Protein Docking Models? Molecular Dynamics to the Rescue. J. Chem. Theory Comput. 2021, 17, 5944–5954. [Google Scholar] [CrossRef]
- Jin, Z.; Sato, Y.; Kawashima, M.; Kanehisa, M. KEGG tools for classification and analysis of viral proteins. Protein Sci. 2023, 32, e4820. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Hua, H.; Jiang, Q.; Sun, P.; Xu, X. Risk factors for early-onset colorectal cancer: Systematic review and meta-analysis. Front. Oncol. 2023, 13, 1132306. [Google Scholar] [CrossRef]
- Wu, J.; Liu, N.; Chen, J.; Tao, Q.; Lu, C.; Li, Q.; Chen, X.; Peng, C. Clofarabine induces tumor cell apoptosis, GSDME-related pyroptosis, and CD8+ T-cell antitumor activity via the non-canonical P53/STING pathway. J. Immunother. Cancer 2025, 13, e010252. [Google Scholar] [CrossRef]
- Fang, F.; Qin, Y.; Qi, L.; Fang, Q.; Zhao, L.; Chen, S.; Li, Q.; Zhang, D.; Wang, L. Juglone exerts antitumor effect in ovarian cancer cells. Iran. J. Basic. Med. Sci. 2015, 18, 544–548. [Google Scholar] [PubMed]
- Gu, Y.; Zhang, J.; Zheng, H.; Qin, Y.; Zheng, M.; Hu, Y.; Xin, J. Therapeutic Effect of Shikimic Acid on Heat Stress-Induced Myocardial Damage: Assessment via Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Pharmaceuticals 2024, 17, 1485. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Sun, R.; Kim, J. Juglone and KPT6566 Suppress the Tumorigenic Potential of CD44+CD133+ Tumor-Initiating Caco-2 Cells In Vitro and In Vivo. Front. Cell Dev. Biol. 2022, 10, 861045. [Google Scholar] [CrossRef]
- Ramezani, M.; Shamsabadi, F.T.; Shahbazi, M. Harnessing the TP53INP1/TP53I3 axis for inhibition of colorectal cancer cell proliferation through MEG3 and Linc-ROR Co-expression. Heliyon 2024, 10, e34075. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Wang, M.; Lai, L.; Zheng, L.; Lu, H. Ursolic acid alleviates cancer cachexia by inhibiting STAT3 signaling pathways in C2C12 myotube and CT26 tumor-bearing mouse model. Eur. J. Pharmacol. 2024, 969, 176429. [Google Scholar] [CrossRef] [PubMed]
- van der Haar Àvila, I.; Zhang, T.; Lorrain, V.; de Bruin, F.; Spreij, T.; Nakayama, H.; Iwabuchi, K.; García-Vallejo, J.J.; Wuhrer, M.; van Kooyk, Y.; et al. Limited impact of cancer-derived gangliosides on anti-tumor immunity in colorectal cancer. Glycobiology 2024, 34, cwae036. [Google Scholar] [CrossRef]
- Juang, Y.P.; Tsai, J.Y.; Gu, W.L.; Hsu, H.C.; Lin, C.L.; Wu, C.C.; Liang, P.H. Discovery of 5-Hydroxy-1,4-naphthoquinone (Juglone) Derivatives as Dual Effective Agents Targeting Platelet-Cancer Interplay through Protein Disulfide Isomerase Inhibition. J. Med. Chem. 2024, 67, 3626–3642. [Google Scholar] [CrossRef]
- Williams, D.S.; Mouradov, D.; Browne, C.; Palmieri, M.; Elliott, M.J.; Nightingale, R.; Fang, C.G.; Li, R.; Mariadason, J.M.; Faragher, I.; et al. Overexpression of TP53 protein is associated with the lack of adjuvant chemotherapy benefit in patients with stage III colorectal cancer. Mod. Pathol. 2020, 33, 483–495. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, J.; Sung, C.O.; Hong, Y.S.; Kim, S.Y.; Lee, H.; Kim, T.W.; Kim, J.I. High prevalence of TP53 loss and whole-genome doubling in early-onset colorectal cancer. Exp. Mol. Med. 2021, 53, 446–456. [Google Scholar] [CrossRef]
- Fang, D.; Hu, H.; Zhao, K.; Xu, A.; Yu, C.; Zhu, Y.; Yu, N.; Yao, B.; Tang, S.; Wu, X.; et al. MLF2 Negatively Regulates P53 and Promotes Colorectal Carcinogenesis. Adv. Sci. 2023, 10, e2303336. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Huang, J.; Wong, C.C.; Zhai, J.; Li, C.; Wei, G.; Zhao, L.; Wang, G.; Wei, H.; et al. TRIM67 Activates p53 to Suppress Colorectal Cancer Initiation and Progression. Cancer Res. 2019, 79, 4086–4098. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Zheng, P. USP7 regulates growth and maintains the stemness of p53-mutant colorectal cancer cells via stabilizing of mutant p53. Front. Oncol. 2024, 14, 1427663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wei, L.; Lin, G.; Tong, Y.; Zhang, J.; Jiang, X.; He, Q.; Lu, X.; Zhu, D.D.; Chen, Y.Q. Metabolic Alterations Induced by Kudingcha Lead to Cancer Cell Apoptosis and Metastasis Inhibition. Nutr. Cancer 2020, 72, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Poyya, J.; Kumar, D.J.; Nagendra, H.G.; Dinesh, B.; Aditya Rao, S.J.; Joshi, C.G. Receptor based virtual screening of potential novel inhibitors of tigar TP53 (tumour protein 53)-induced glycolysis and apoptosis regulator. Med. Hypotheses 2021, 156, 110683. [Google Scholar] [CrossRef]
- Eichhoff, O.M.; Stoffel, C.I.; Käsler, J.; Briker, L.; Turko, P.; Karsai, G.; Zila, N.; Paulitschke, V.; Cheng, P.F.; Leitner, A.; et al. ROS Induction Targets Persister Cancer Cells with Low Metabolic Activity in NRAS-Mutated Melanoma. Cancer Res. 2023, 83, 1128–1146. [Google Scholar] [CrossRef]
- Li, H.; Fan, T.J.; Zou, P.; Xu, B. Diclofenac Sodium Triggers p53-Dependent Apoptosis in Human Corneal Epithelial Cells via ROS-Mediated Crosstalk. Chem. Res. Toxicol. 2021, 34, 70–79. [Google Scholar] [CrossRef]
- Monge, C.; Waldrup, B.; Manjarrez, S.; Carranza, F.G.; Velazquez-Villarreal, E. Detecting PI3K and TP53 Pathway Disruptions in Early-Onset Colorectal Cancer Among Hispanic/Latino Patients. Cancer Med. 2025, 14, e70791. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Zhang, Z.; He, Y.; Teng, Y.; Li, J.; Yuan, S.; Pan, Y.; Liang, H.; Yang, H.; et al. Pancreatic cancer cell apoptosis is induced by a proteoglycan extracted from Ganoderma lucidum. Oncol. Lett. 2021, 21, 34. [Google Scholar] [CrossRef]

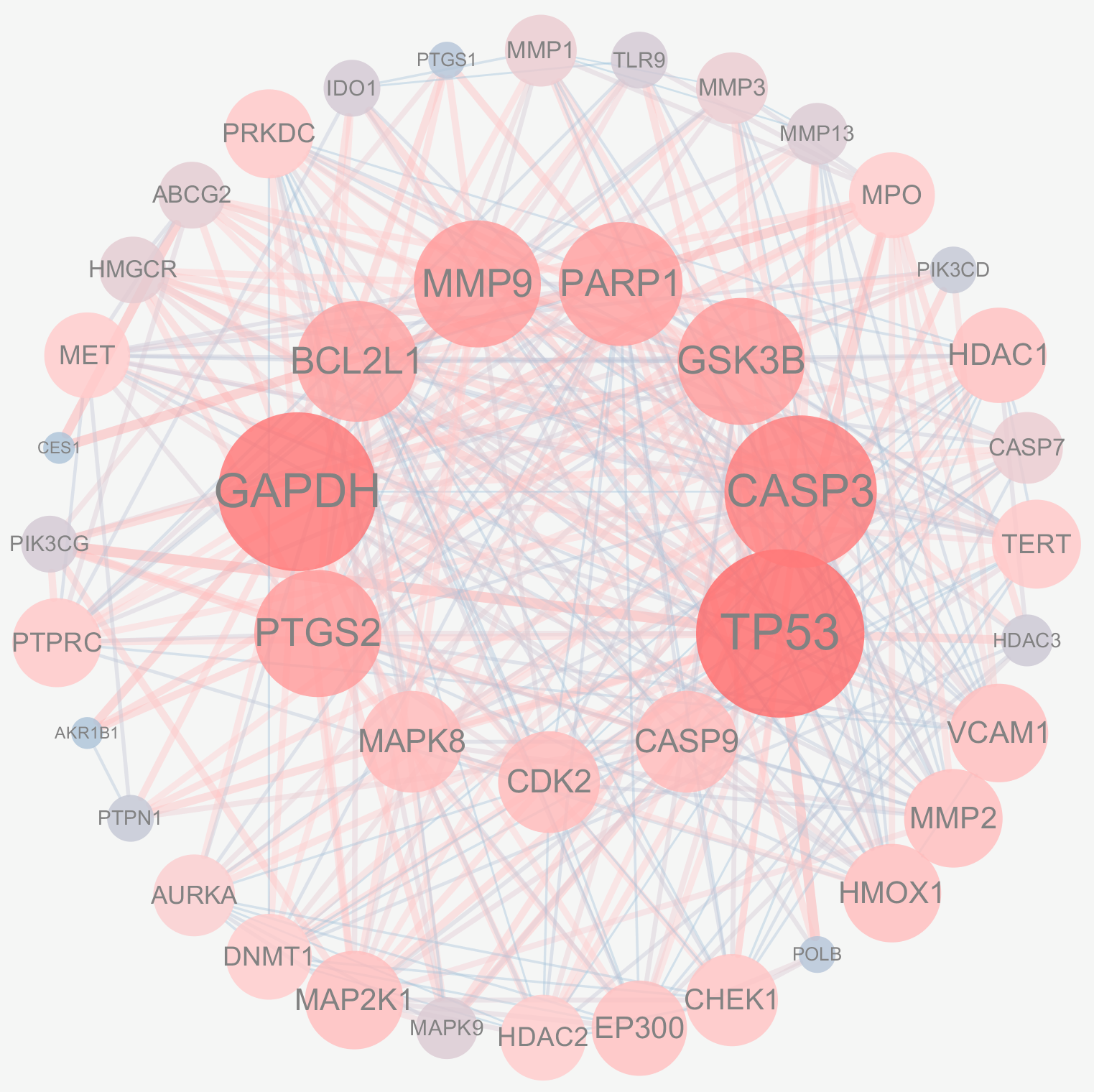

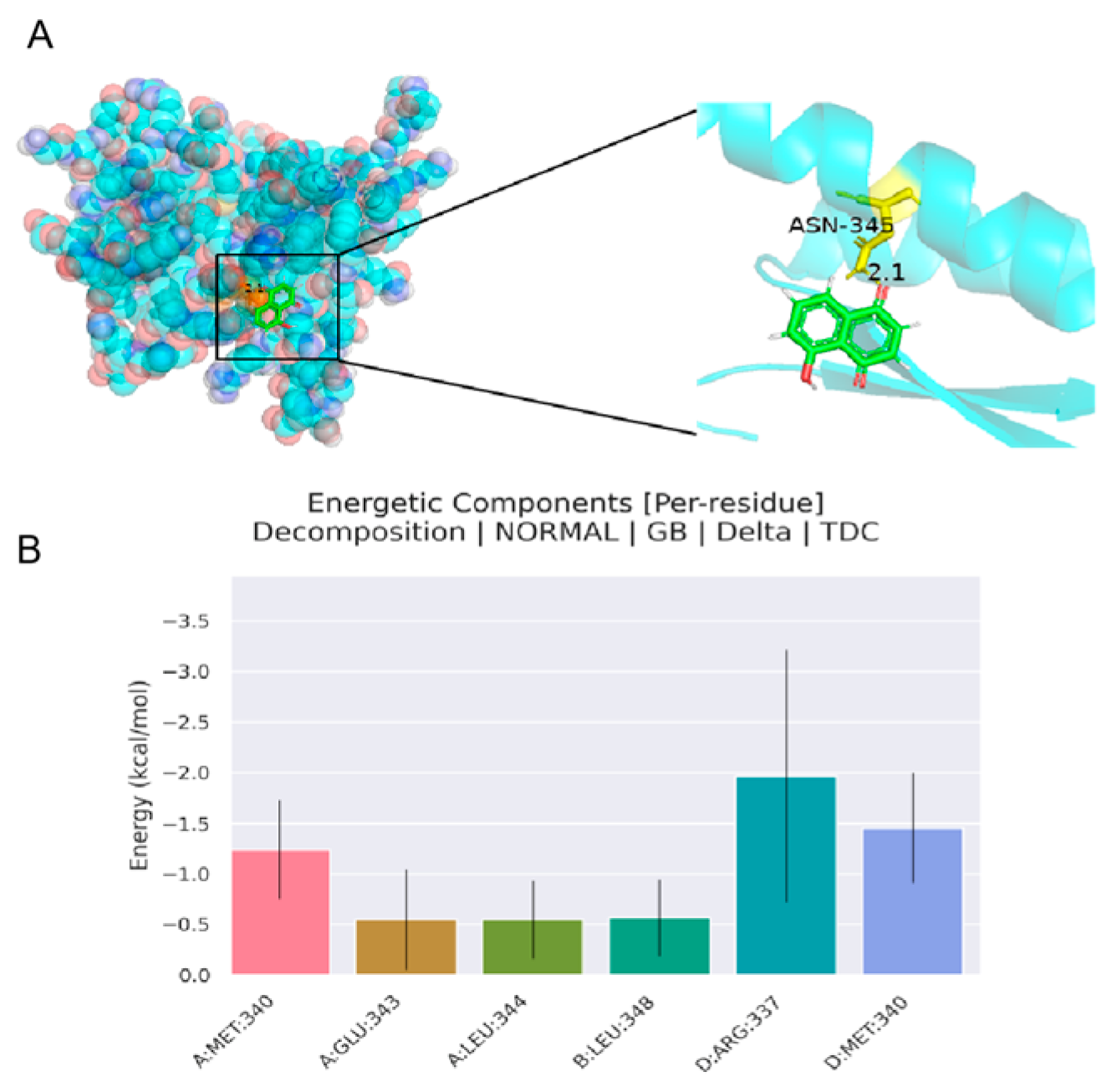
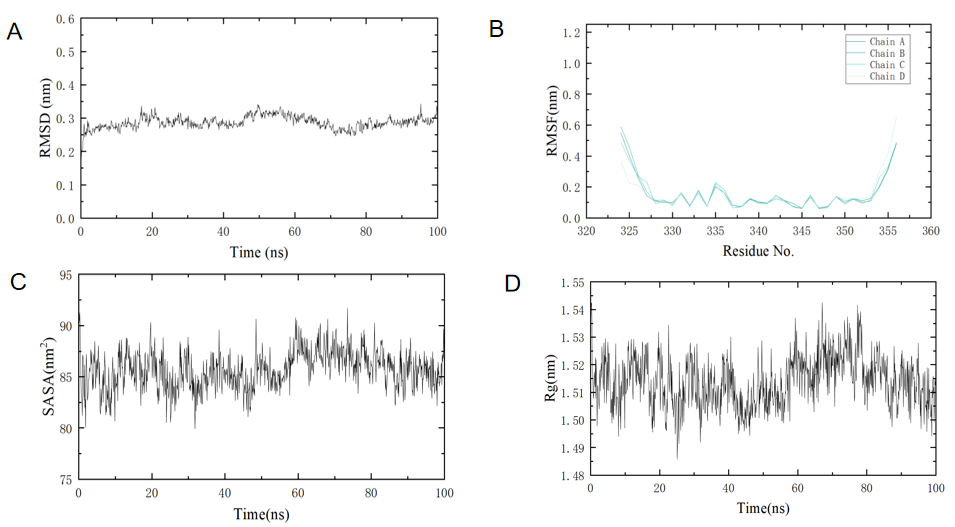
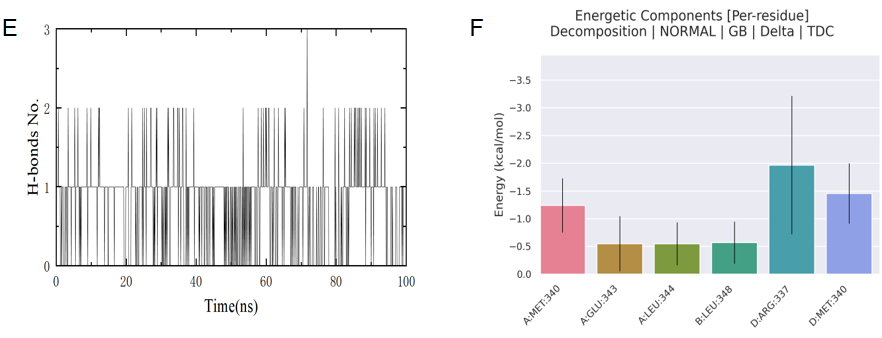
| Gene Name | Betweenness Centrality | Closeness Centrality | Neighborhood Connectivity | Radiality | Degree |
|---|---|---|---|---|---|
| TP53 | 0.17660225 | 0.85964912 | 16.14634146 | 0.95918367 | 41 |
| GAPDH | 0.09942267 | 0.80327869 | 16.94736842 | 0.93877551 | 38 |
| CASP3 | 0.07554407 | 0.77777778 | 17.58333333 | 0.92857143 | 36 |
| GSK3B | 0.04488729 | 0.68055556 | 19.03571429 | 0.88265306 | 28 |
| MMP9 | 0.03629503 | 0.69014085 | 18.85714286 | 0.8877551 | 28 |
| PTGS2 | 0.05012007 | 0.7 | 18.67857143 | 0.89285714 | 28 |
| PARP1 | 0.02609519 | 0.66216216 | 19.59259259 | 0.87244898 | 27 |
| BCL2L1 | 0.02295318 | 0.65333333 | 20.11538462 | 0.86734694 | 26 |
| MAPK8 | 0.03274029 | 0.59756098 | 21.3 | 0.83163265 | 20 |
| CDK2 | 0.00417775 | 0.59036145 | 22.6 | 0.82653061 | 20 |
| Target | Protein Affinity kcal/mol Compound |
|---|---|
| p53 | −5.39 |
| Caspase-3 | −4.67 |
| GAPDH | −5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhang, Y.; Chen, X.; Wang, W.; Huo, J. TP53 Is a Potential Target of Juglone Against Colorectal Cancer: Based on a Combination of Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Curr. Issues Mol. Biol. 2025, 47, 439. https://doi.org/10.3390/cimb47060439
Deng Y, Zhang Y, Chen X, Wang W, Huo J. TP53 Is a Potential Target of Juglone Against Colorectal Cancer: Based on a Combination of Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Current Issues in Molecular Biology. 2025; 47(6):439. https://doi.org/10.3390/cimb47060439
Chicago/Turabian StyleDeng, Yunting, Yanan Zhang, Xinghai Chen, Weiming Wang, and Jinhai Huo. 2025. "TP53 Is a Potential Target of Juglone Against Colorectal Cancer: Based on a Combination of Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments" Current Issues in Molecular Biology 47, no. 6: 439. https://doi.org/10.3390/cimb47060439
APA StyleDeng, Y., Zhang, Y., Chen, X., Wang, W., & Huo, J. (2025). TP53 Is a Potential Target of Juglone Against Colorectal Cancer: Based on a Combination of Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Current Issues in Molecular Biology, 47(6), 439. https://doi.org/10.3390/cimb47060439







