Biomarker Discovery and Molecular Docking Reveal Marsdenia tenacissima Fermentation Product’s Anti-Lung Cancer Components
Abstract
1. Introduction
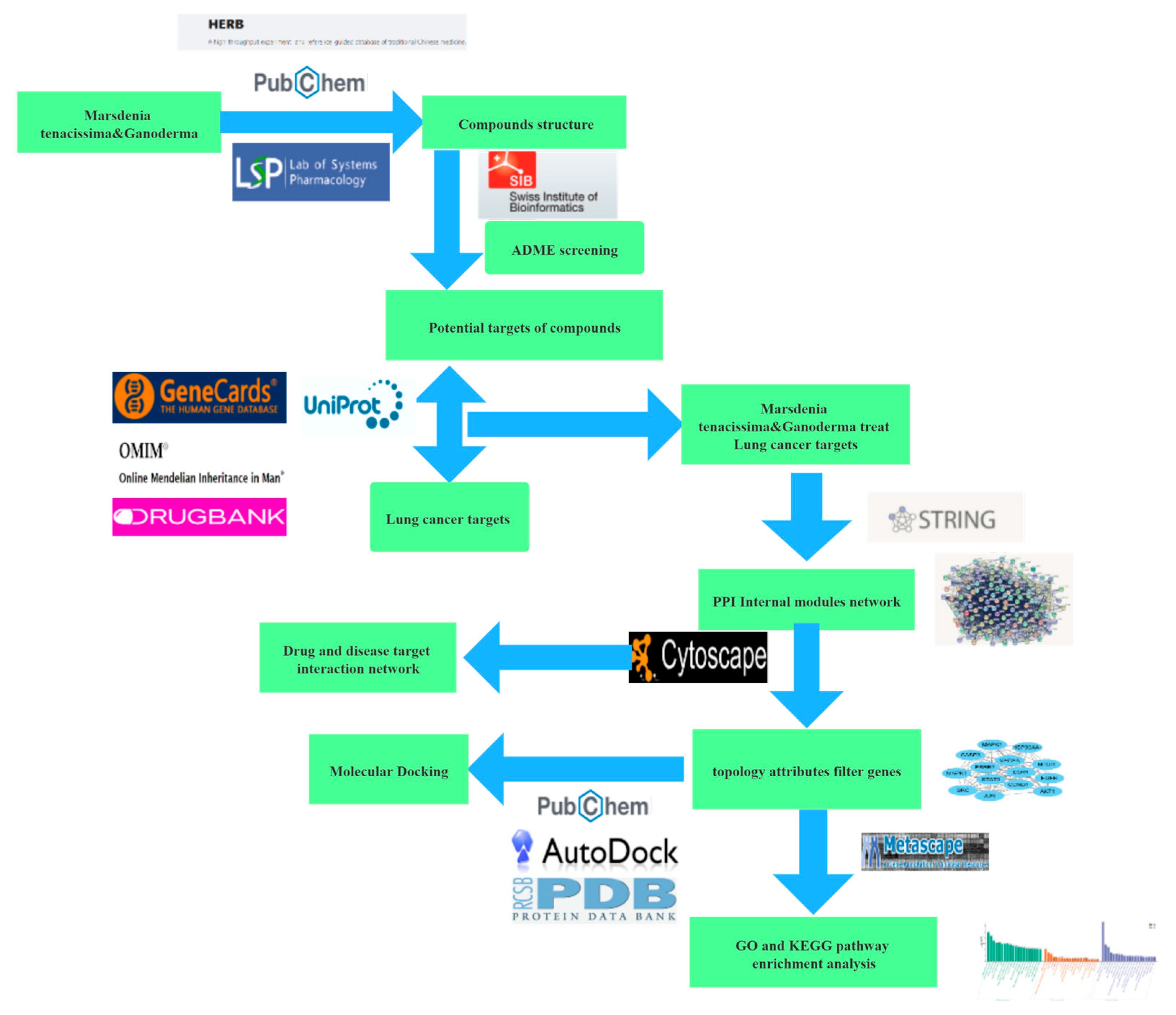
2. Materials and Methods
2.1. Preparation of MGF Methanol Extract and UPLC-Q-TOF/MS Analysis
2.2. Data Collection
2.3. Core Gene Analysis
2.4. Construction and Screening of Drug–Target Interaction Network
2.5. Functional Enrichment Analysis of the Drug–Target Network
2.6. Molecular Docking Studies
2.7. MD Simulation
3. Results
3.1. MS Data Processing
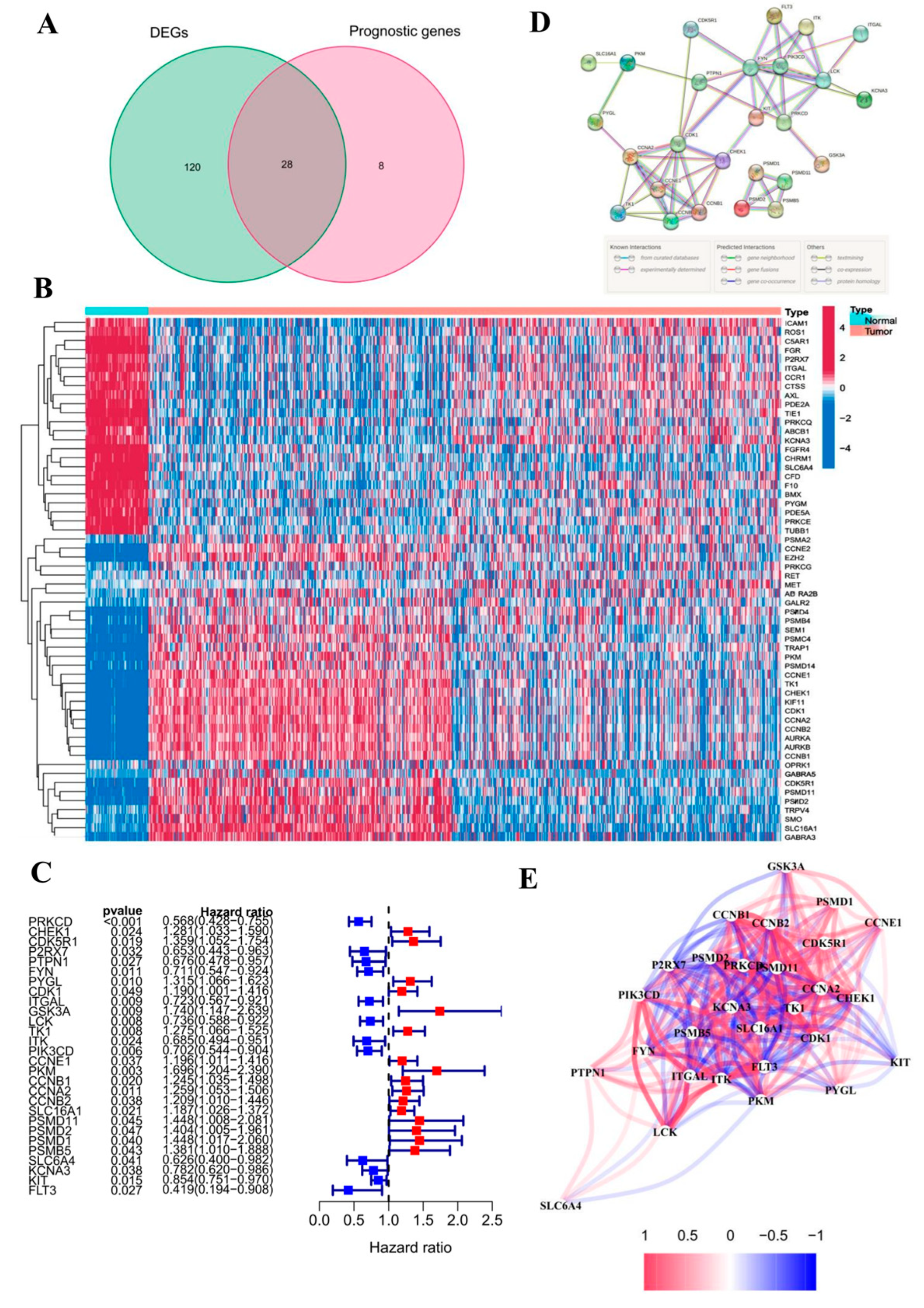
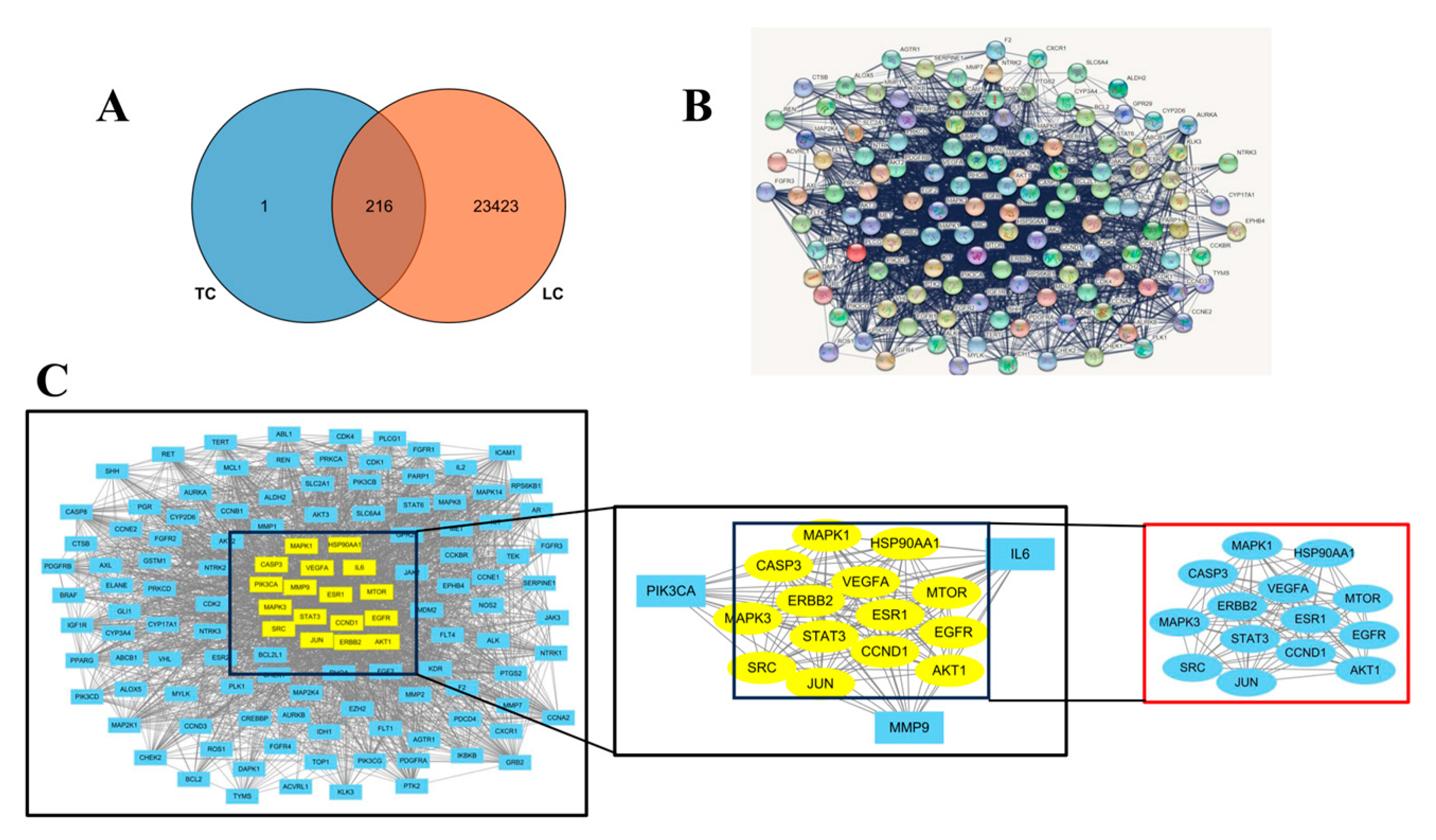
3.2. Identification and Analysis of DEGs
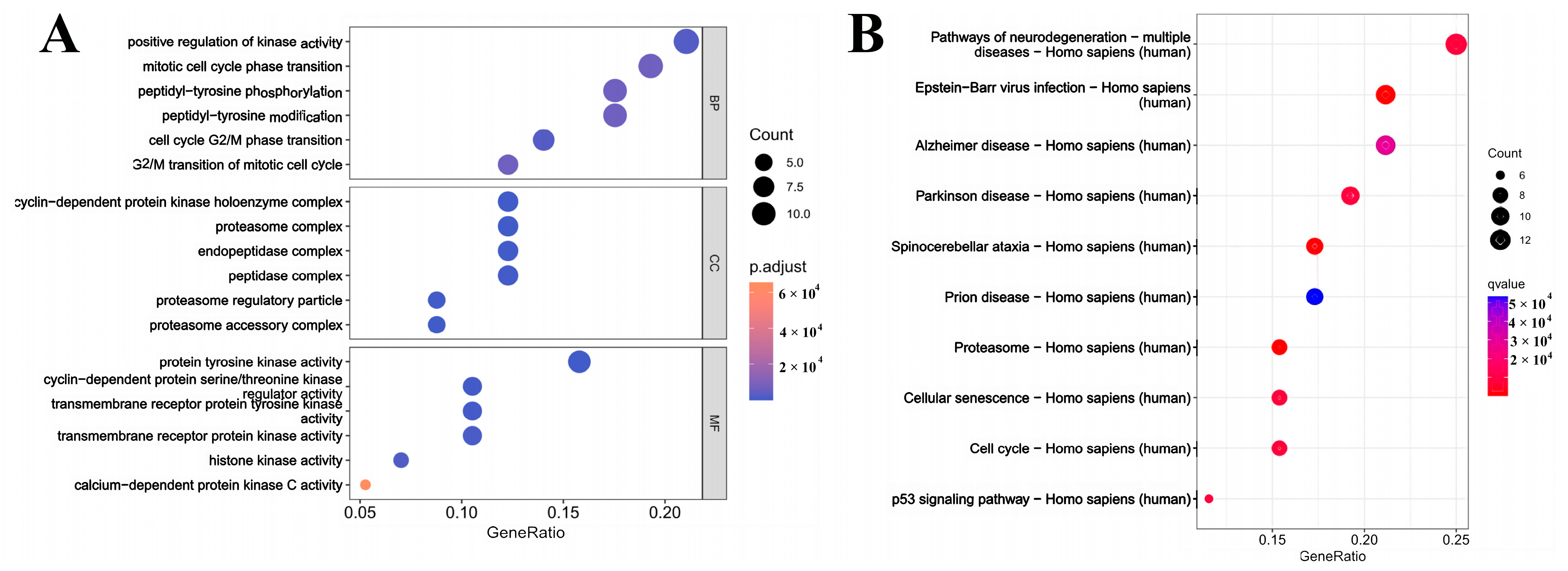
3.3. Analysis of the GO and KEGG
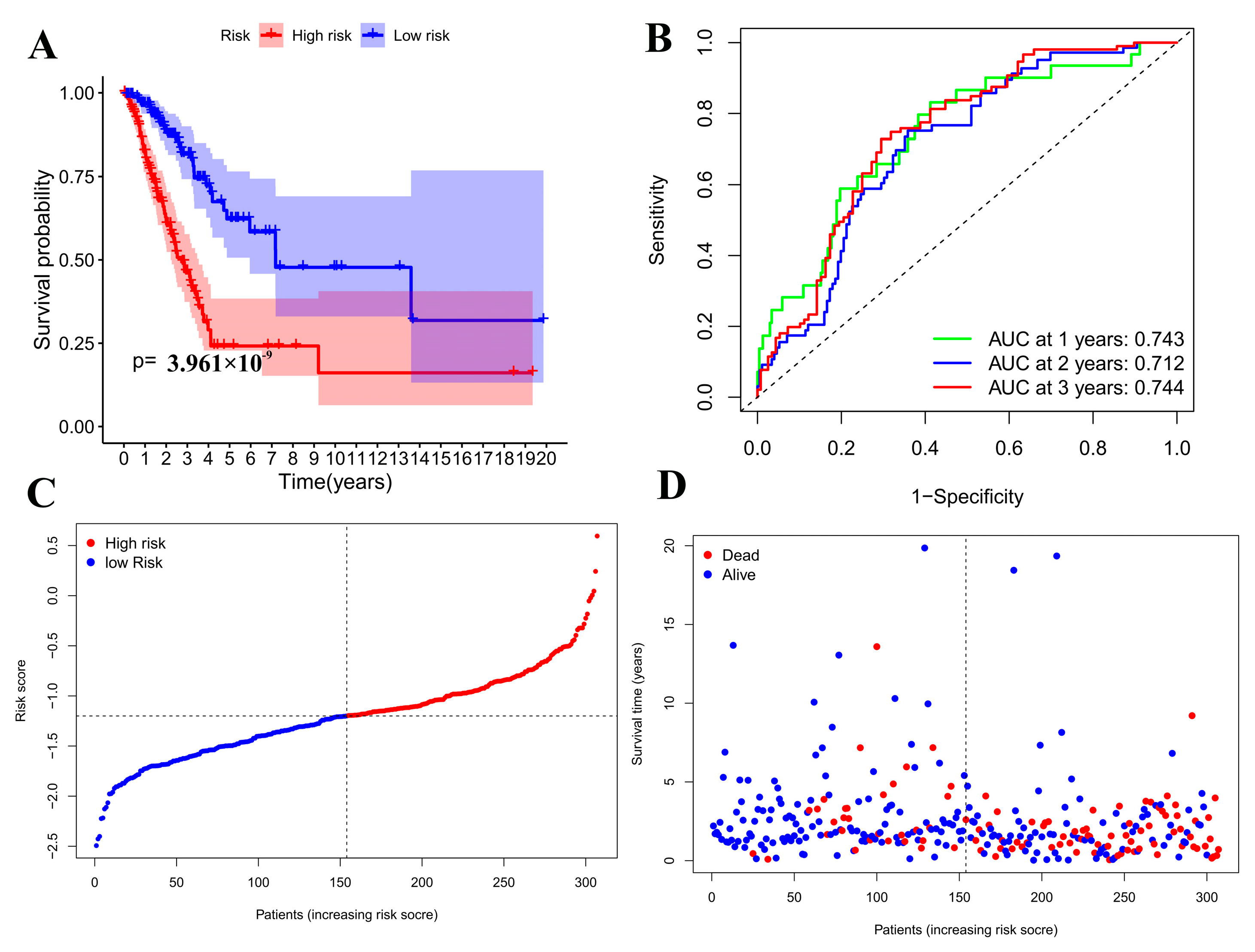
3.4. Prognostic Model Validation Analysis
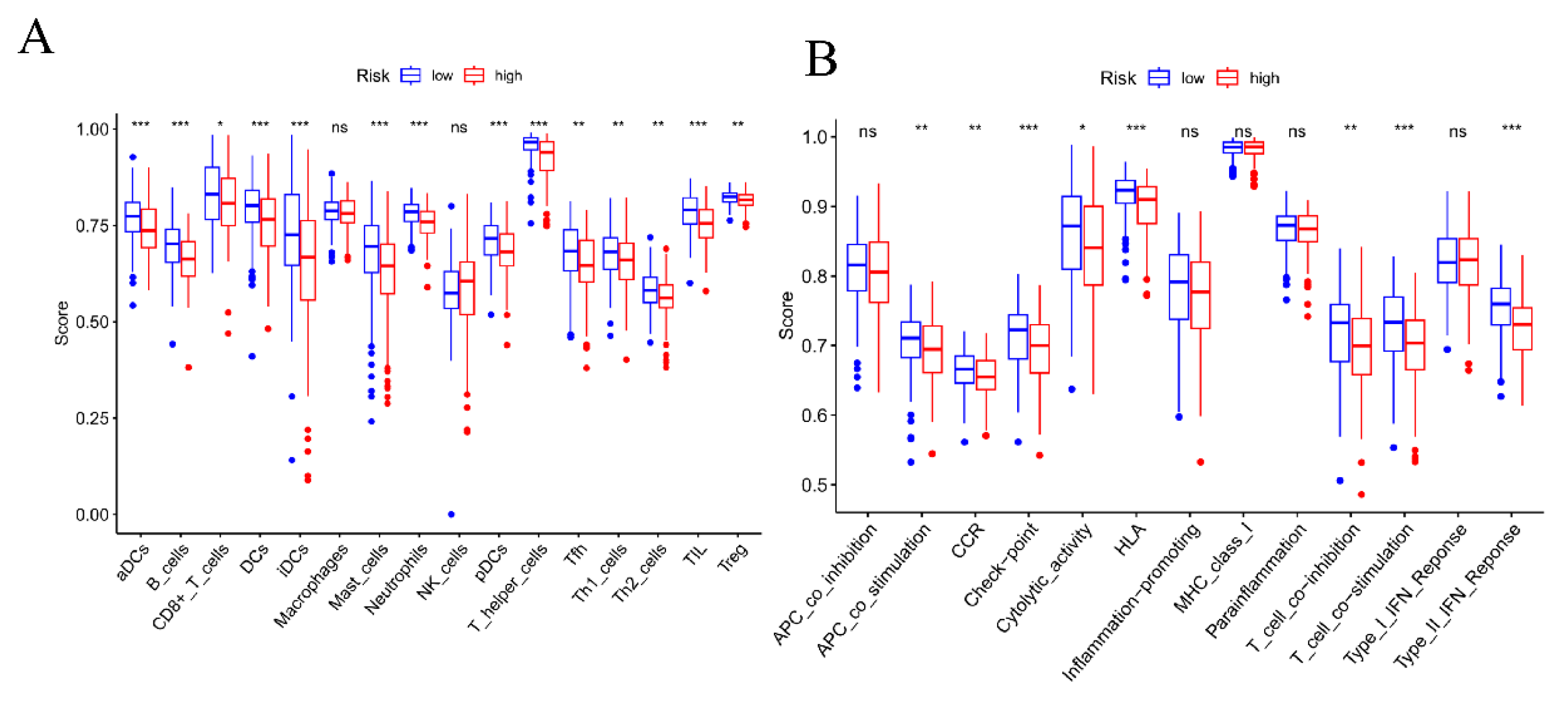
3.5. Analysis and Evaluation of Tumor Immune Cell Infiltration and Immune Environment
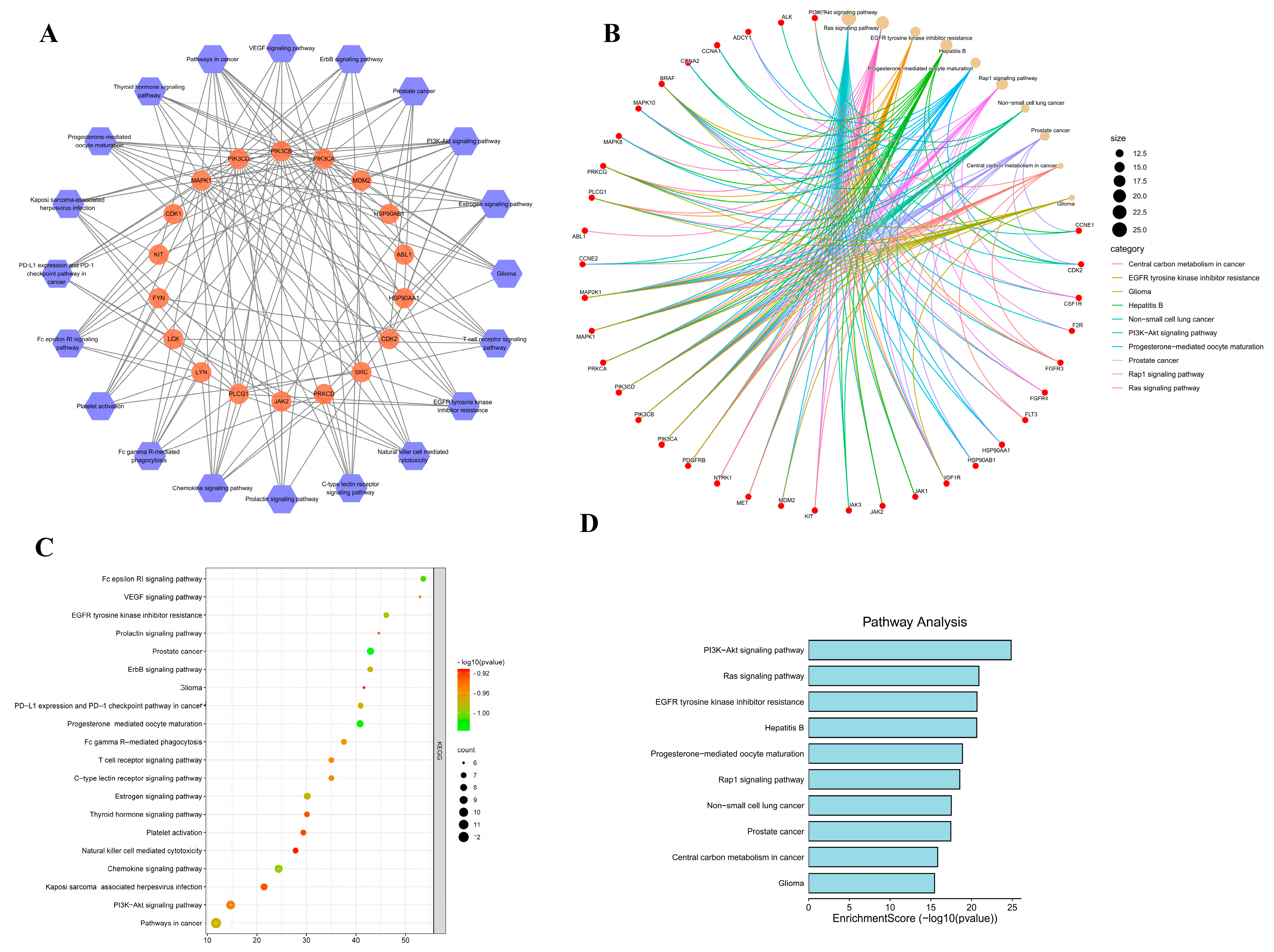
3.6. Network Pharmacology Analysis with “Ingredient-Target-Disease”
3.7. GO Enrichment Analysis and KEGG Pathway Analysis
3.8. Molecular Docking Simulation Verification
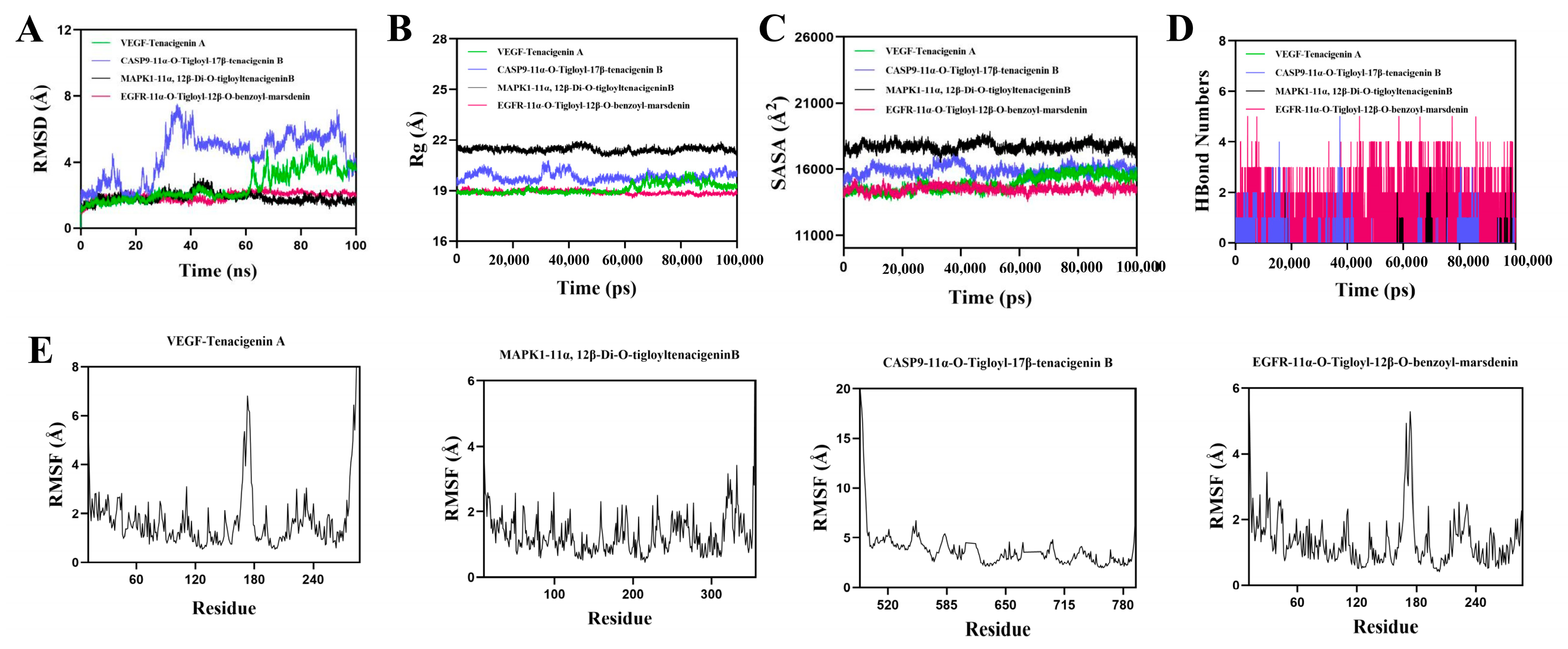
3.9. Molecular Dynamics Simulation of Protein–Ligand Complexes
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MGF | Ganoderma lucidum and Marsdenia tenacissima |
| TCGA | The Cancer Genome Atlas Program |
| LUAD | lung adenocarcinoma |
| AUC | Area Under the Curve |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology |
| PPI | Protein–Protein Interaction |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| PI3K | Phosphatidylinositol 3-kinase |
| Rap | Ras-related protein |
| PCA | Principal Component Analysis |
| mTOR | mammalian target of rapamycin |
| Ras | rat sarcoma |
| Casp3 | Caspase-3 |
| Casp9 | Caspase-9 |
| EGFR | epidermal growth factor receptor |
| VEGF | vascular endothelial growth factor |
| MAPK1 | mitogen-activated protein kinase 1 |
| BP | biological process |
| CC | Cellular Component |
| MF | molecular function |
| ROC | Receiver Operating Characteristic |
References
- Luo, G.; Zhang, Y.; Rumgay, H.; Morgan, E.; Langselius, O.; Vignat, J.; Colombet, M.; Bray, F. Estimated worldwide variation and trends in incidence of lung cancer by histological subtype in 2022 and over time: A population-based study. Lancet. Respir. Med. 2025, 13, 348–363. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F.J.G. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Pezeshki, Z.; Khosravi, A.; Nekuei, M.; Khoshnood, S.; Zandi, E.; Eslamian, M.; Talebi, A.; Emami, N.E.D.; Nematbakhsh, M.J.J.N. Time course of cisplatin-induced nephrotoxicity and hepatotoxicity. J. Nephropathol. 2017, 6, 163–167. [Google Scholar] [CrossRef]
- Li, Z.; Feiyue, Z.; Gaofeng, L.J.B. Pharmacotherapy, Traditional Chinese medicine and lung cancer—From theory to practice. Biomed. Pharmacother. 2021, 137, 111381. [Google Scholar] [CrossRef]
- Xing, W.; Chen, B.; Mi, H.; Wu, Y. Textual research of wuguteng. J. Chin. Med. Mater. 2003, 26, 524–526. [Google Scholar]
- Wang, P.; Yang, J.; Zhu, Z.; Zhang, X. Marsdenia tenacissima: A Review of Traditional Uses, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2018, 46, 1449–1480. [Google Scholar] [CrossRef]
- Feng, F.; Huang, J.; Wang, Z.; Zhang, J.; Han, D.; Wu, Q.; He, H.; Zhou, X. Xiao-ai-ping injection adjunct with platinum-based chemotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 3. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, B.; Jike, W.; Li, R.; Su, X.; Yu, J.; Liu, T. The Protective Effect of Marsdenia tenacissima against Cisplatin-Induced Nephrotoxicity Mediated by Inhibiting Oxidative Stress, Inflammation, and Apoptosis. Molecules 2023, 28, 7582. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, P.; Kang, L.; Li, J.; Li, R.; Liu, T. An Interdisciplinary Journal Devoted to Bioscientific Research on Indigenous Drugs, Mechanism of Marsdenia tenacissima extract promoting apoptosis of lung cancer by regulating Ca2+/CaM/CaMK signaling. J. Ethnopharmacol. 2020, 251, 112535. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Su, X.; Yu, J.; Lu, L.; Liu, T. Nontargeted metabolomics study and pharmacodynamic evaluation of bidirectional fermentation for Ganoderma lucidum with Marsdenia tenacissima. Front. Pharmacol. 2022, 13, 1012063. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Wang, S.; Yu, J.; Su, X.; Lu, L.; Liu, T. Co-Fermentation of Marsdenia tenacissima with Ganoderma lucidum and Anti-Lung Cancer Effect of the Fermentation Products. J. Vis. Exp. JoVE 2022, 190, e64687. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Zhang, B.; Jiang, Y.; Wang, X.; Guo, Y.; Liu, H.; Li, S.; Tong, X. A network pharmacology approach to determine active compounds and action mechanisms of ge-gen-qin-lian decoction for treatment of type 2 diabetes. Evid.-Based Complement. Altern. Med. eCAM 2014, 2014, 495840. [Google Scholar] [CrossRef]
- Li, S. Network Systems Underlying Traditional Chinese Medicine Syndrome and Herb Formula. Curr. Bioinform. 2009, 4, 188–196. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Sierra, J.R.; Chang, A.; Moffat, J.; Neel, B.G.; Tsao, M.S. Abstract 1232: Overcoming resistance to EGFR-tyrosine kinase inhibitor therapy in non-small cell lung cancer. Cancer Res. 2012, 72 (Suppl. S8), 1232. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Wilson, W.; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S. Control of PD-L1 Expression by Oncogenic Activation of the AKT–mTOR Pathway in Non–Small Cell Lung Cancer. Cancer Res. 2015, 76, 227–238. [Google Scholar] [CrossRef]
- Falestiny, M.N.; Cardona, J.J.; Zhou, J.; Zhukov, T.; Lee, H. Transforming Growth Factor B type II receptor expression in non-small cell lung cancer, viral transformed bronchial cells and normal bronchial epithelial cells: A comparitive study. BMC Cancer 1998, 25, 484. [Google Scholar]
- Wu, G.; Sakai, R.; Xu, K.; Hangauer, D.; Roth, J.; Li, J. Abstract #2823: The Src-PI3K-Akt and apoptosis signaling pathway-targeted therapy with novel pro-apoptotic FUS1-nanoparticles and Src kinase inhibitors for lung cancer. Cancer Res. 2009, 69, 2823. [Google Scholar]
- Hatapakki, B.C.; Hukkeri, V.I. Antipyretic Activity of Root of Marsdenia tenacissima in Rats. J. Nat. Remedies 2011, 11, 98–102. [Google Scholar]
- Li, Y.; Wang, Y.; Yu, X.; Yu, T.; Zheng, X.; Chu, Q. Radix Tetrastigma Inhibits the Non-Small Cell Lung Cancer via Bax/Bcl-2/Caspase-9/Caspase-3 Pathway. Nutr. Cancer 2021, 74, 320–332. [Google Scholar] [CrossRef]
- Malapelle, U.; Passiglia, F.; Cremolini, C.; Reale, M.L.; Pepe, F.; Pisapia, P.; Avallone, A.; Cortinovis, D.; De Stefano, A.; Fassan, M.; et al. RAS as a positive predictive biomarker: Focus on lung and colorectal cancer patients. Eur. J. Cancer 2021, 146, 74–83. [Google Scholar] [CrossRef]
- Xie, B.; Lu, Y.Y.; Luo, Z.-H.; Qu, Z.; Zheng, C.-G.; Huang, X.-A.; Zhou, H.-Y.; Hu, Y.-J.; Shen, X.-L. Tenacigenin B ester derivatives from Marsdenia tenacissima actively inhibited CYP3A4 and enhanced in vivo antitumor activity of paclitaxel. J. Ethnopharmacol. 2019, 235, 309–319. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wang, X.-Y.; Huang, B.-X.; Zhu, F.; Zhang, R.-G.; Wu, G. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: Relation to prognosis. Med. Oncol. 2011, 28, 673–678. [Google Scholar] [CrossRef]
- Pilotto, S.; Grizzi, G.; Sperduti, I.; Simbolo, M.; Vicentini, C.; Caliò, A.; Caccese, M.; Mafficini, A.; Carbognin, L.; Corbo, V.; et al. P2.04-12 A Genomic Signature [JAK2, JAK3, PIAS4, PTPN2, STAT3, IFNAR2] Predicts Baseline Resistance to Nivolumab in Advanced NSCLC. J. Thorac. Oncol. 2018, 13, S734–S735. [Google Scholar] [CrossRef]
- So, T.; Takenoyama, M.; Mizukami, M.; Ichiki, Y.; Sugaya, M.; Hanagiri, T.; Sugio, K.; Yasumoto, K. Haplotype Loss of HLA Class I Antigen as an Escape Mechanism from Immune Attack in Lung Cancer. Cancer Res. 2005, 65, 5945. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Xu, Q.; Gu, C.; Hu, D. Tenacissoside G synergistically potentiates inhibitory effects of 5-fluorouracil to human colorectal cancer. Phytomedicine 2021, 86, 153553. [Google Scholar] [CrossRef]
- Jeong, C.H.; Park, H.B.; Jang, W.J.; Jung, S.H.; Seo, Y.H.; Letters, M.C. Discovery of hybrid Hsp90 inhibitors and their anti-neoplastic effects against gefitinib-resistant non-small cell lung cancer (NSCLC). Bioorg. Med. Chem. Lett. 2013, 24, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.Z.; Lu, A.X.; Zhang, K.F.; Li, B.J. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol. Lett. 2014, 7, 1159–1164. [Google Scholar] [CrossRef] [PubMed]

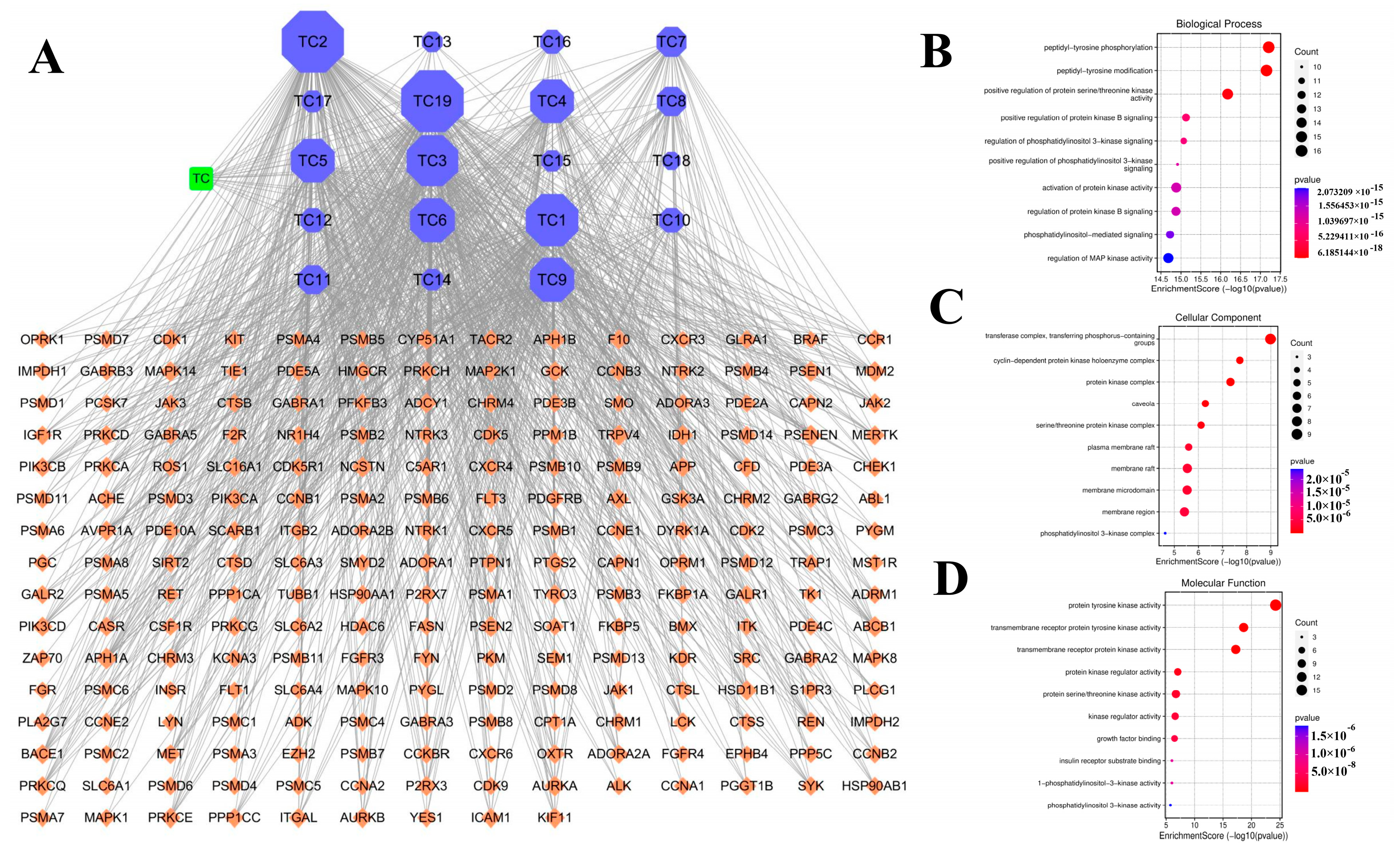

| NO | EM | Tentative Identification | Adducts | Formula | Mass Error (ppm) |
|---|---|---|---|---|---|
| TC1 | 553.3134 m/z | 11α-O-2-Methylbutyryl-12β-O-2-benzoyltenacigeninB | [M + H]+ | C33H44O7 | −4.69 |
| TC2 | 531.3321 m/z | 11α-O-2-Methylbutyryl-12β-O-2-tigloyltenacigeninB | [M + H]+ | C31H44O7 | −3.01 |
| TC3 | 529.3150 m/z | 11α, 12β-Di-O-tigloyltenacigeninB | [M + H]+ | C31H44O7 | −2.45 |
| TC4 | 551.2981 m/z | 11α-O-Tigloyl-12β-O-benzoyl-marsdenin | [M + H]+ | C33H42O7 | −3.99 |
| TC5 | 529.3147 m/z | 11α,12β-O,O-Ditigloyl-17β-tenacigenin B | [M + H]+ | C31H44O7 | −2.45 |
| TC6 | 511.2696 m/z | 11α-O-Benzoyl-12β-O-acetyltenacigenin B | [M + H]+ | C30H38O7 | −3.71 |
| TC7 | 489.2840 m/z | 11α-O-Tigloyl-12β-O-acetyltenacigenin B | [M + H]+ | C28H40O7 | −1.43 |
| TC8 | 447.2730 m/z | 11α-O-Tigloyl-17β-tenacigenin B | [M + H]+ | C26H38O6 | −2.45 |
| TC9 | 383.2418 m/z | Tenacigenin C | [M + H]+ | C26H38O7 | −2.34 |
| TC10 | 363.2174 m/z | Tenacigenin A | [M − H]− | C21H32O5 | −0.55 |
| TC11 | 363.2166 m/z | Tenacigenin B | [M − H]− | C21H32O6 | −2.75 |
| TC12 | 837.4424 m/z | Tenacissoside I | [M + Na]+ | C44H62O14 | −0.95 |
| TC13 | 815.4134 m/z | Tenacissoside G | [M + Na]+ | C42H64O14 | −1.34 |
| TC14 | 829.4216 m/z | Tenacissimoside H | [M − H]− | C47H76O22 | −0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, L.; Li, R.; Wu, H.; Dou, G. Biomarker Discovery and Molecular Docking Reveal Marsdenia tenacissima Fermentation Product’s Anti-Lung Cancer Components. Curr. Issues Mol. Biol. 2025, 47, 427. https://doi.org/10.3390/cimb47060427
Li R, Li L, Li R, Wu H, Dou G. Biomarker Discovery and Molecular Docking Reveal Marsdenia tenacissima Fermentation Product’s Anti-Lung Cancer Components. Current Issues in Molecular Biology. 2025; 47(6):427. https://doi.org/10.3390/cimb47060427
Chicago/Turabian StyleLi, Runtian, Lintao Li, Runzhi Li, Haiyang Wu, and Guifang Dou. 2025. "Biomarker Discovery and Molecular Docking Reveal Marsdenia tenacissima Fermentation Product’s Anti-Lung Cancer Components" Current Issues in Molecular Biology 47, no. 6: 427. https://doi.org/10.3390/cimb47060427
APA StyleLi, R., Li, L., Li, R., Wu, H., & Dou, G. (2025). Biomarker Discovery and Molecular Docking Reveal Marsdenia tenacissima Fermentation Product’s Anti-Lung Cancer Components. Current Issues in Molecular Biology, 47(6), 427. https://doi.org/10.3390/cimb47060427








