The current cross-sectional study was conducted at the Internal Clinic, Rheumatology Department and Psychiatry Clinic of the University Clinical Center of the Republic of Srpska (UCC RS) from November 2021 to January 2023. It included patients aged 18 to 81 years with a confirmed diagnosis of RA who were treated at the Rheumatology Department. The diagnosis of RA was made according to the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria and the ACR 1991 revised criteria [
29,
30]. The sample size for the study group was calculated to achieve statistical significance using G*Power software, version 3.1.9.4. We had two groups of patients: the first one was the RA patient with depression group (
group I,
n = 116), and the second was the control group (group II), which included patients with primary depression, matched in age and gender, who were treated at the Psychiatry Clinic (
n = 45).
2.1. Inclusion and Exclusion Criteria
The inclusion criteria for patients with RA were as follows: (1) informed consent; (2) a diagnosis of RA confirmed by data from medical records according to criteria from the 2010 ACR/EULAR and the ACR 1991 revised criteria for functional global status in RA; [
29,
30] (3) moderate disease activity in patients taking a stable dose of disease-modifying antirheumatic drugs (methotrexate, leflunomide, sulfasalazine, or antimalarial) for the preceding six months (moderate disease activity is expressed by the DAS 28 index (Disease Activity Score 28) for a value of 3.2–5.1) [
31]; and (4) patients who had been taking a stable dose of nonsteroidal anti-inflammatory drugs for the preceding six months. The exclusion criteria were as follows: (1) patients diagnosed with another autoimmune disease (Hashimoto thyroiditis, systemic lupus erythematosus, scleroderma, vasculitis syndromes, mixed connective tissue disease, or multiple sclerosis); (2) patients with depression diagnosed before RA; (3) patients with psychiatric disorders (schizophrenia, alcoholism, or drug addiction); (4) patients with an acute infectious disease; (5) pregnant women; (6) patients with polytrauma; and (7) patients treated with biological drugs (anti-TNF therapy or IL-6 receptor blockers).
The inclusion criteria for patients with depression were as follows: (1) informed consent; (2) the diagnosis of depression confirmed by data from medical records and mild and moderate disease forms (according to the Beck scale of depression, values of 14–19 for mild and 20–28 for moderate) [
32]; and (3) patients taking a stable dose of antidepressant medication for the preceding six months. The exclusion criteria for patients with depression were as follows: (1) patients diagnosed with an autoimmune disease (RA, Hashimoto thyroiditis, systemic lupus erythematosus, scleroderma, vasculitis syndromes, mixed connective tissue disease, or multiple sclerosis); (2) patients with other psychiatric disorders (schizophrenia, alcoholism, or drug addiction); (3) patients with an acute infectious disease; (4) pregnant women; and (5) patients with polytrauma.
2.2. Group I Tests (RA Patients with Depressive Syndrome)
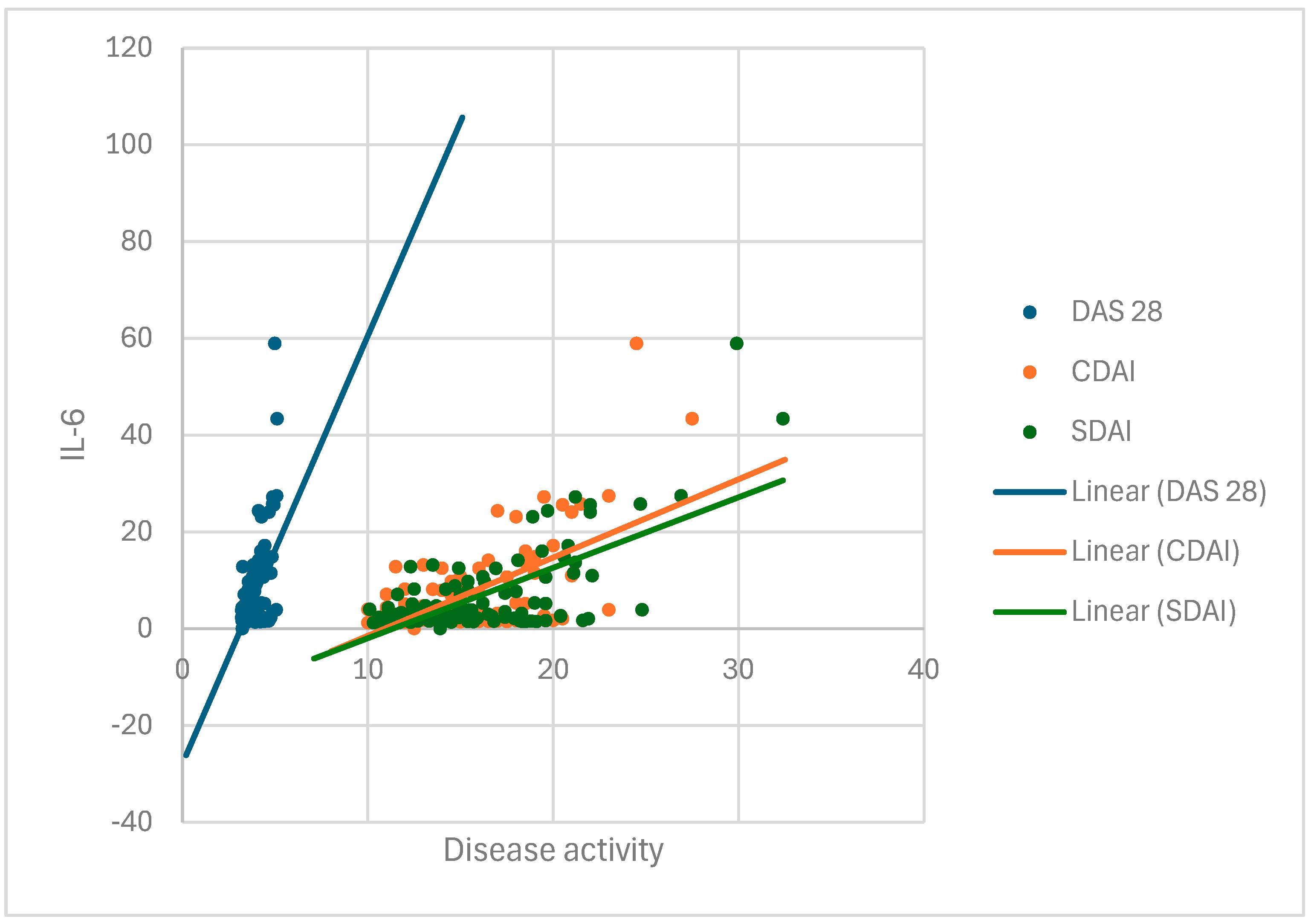
In group I, we tested the degree of disease activity based on the Disease Activity Score 28 (DAS28), clinical disease activity index (CDAI), and Simplified Disease Activity Index (SDAI), which involve a global assessment of the condition by the patient on a visual analog scale of 1 to 10 (VAS), a global assessment of the patient’s condition by a physician on a visual analog scale of 1 to 10 (VAS), and establishing the number of joints with active arthritis based on a rheumatologic examination [
31,
33,
34]. Independently, the patients completed the Health Assessment Questionnaire (HAQ), the Rheumatoid Arthritis Quality of Life (RaQoL) questionnaire, and the 36-item Short Form Survey (SF-36) [
35,
36,
37]. There are no defined psychiatric questionnaires to diagnose depressive syndrome in RA, so we used two scales to safely and more precisely detect depression: the Beck and Hamilton depression scales [
32,
38]. The Beck and Hamilton depression scales were completed by the patients with the assistance of a psychiatrist employed at the UCC RS Psychiatry Clinic.
The patients were sampled for biomarkers of inflammation—the erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) concentration—on the same day that disease activity was assessed.
DAS 28 is an index used for assessing disease activity in patients with RA, recommended by the European Alliance of Associations of Rheumatology (EULAR) [
31]. DAS 28 is calculated based on the number of tender joints (TJs) and swollen joints (SJs) out of 28 recommended joints, the patient’s assessment of their health on a visual analog scale (VAS), and the erythrocyte sedimentation rate (ESR). Recommended joints for examination are the shoulder, elbow, wrist, metacarpophalangeal, proximal interphalangeal, and knee joints. The formula for calculating the index is DAS28 = 0.56 × TJ + 0.28 × SJ + 0.7 × ln (ESR) + 0.014 × VAS, but in practice, the DAS28 calculator is used. The index result is in the range of 0.49–9.07 and interpreted as follows: disease remission (for a value of less than 2.6), low disease activity (2.6–3.2), moderate activity (3.2–5.1), and high disease activity (over 5.1) [
33].
The CDAI is a clinical index of disease activity and represents a simple sum of the numbers of tender and swollen joints out of a total of 28 joints using a scale for disease assessment completed by the patient and the physician on a VAS (0–10 cm) [
33]. The index value is a numeric value ranging from 0 to 76 and is interpreted as follows: disease remission (for a CDAI value less than 2.8), low disease activity (2.8–10), moderate disease activity (10–22), and high disease activity (over 22) [
33].
The SDAI is a simplified index used to quickly assess disease activity in patients with RA [
34]. It is calculated based on the sum of the numbers of tender and swollen joints out of a total of 28 joints, the completion of the disease assessment scale by the patient and the physician on the VAS (0–10 cm), and the CRP value. The index is a numeric value ranging from 0 to 86 and is interpreted as disease remission (for an SDAI value less than 3.3), low disease activity (3.3–11), moderate disease activity (11–26), and high disease activity (26–86) [
34].
The patients’ functional abilities were examined using the Health Assessment Questionnaire (HAQ), which contains 20 questions divided into eight functional categories related to grasping, reaching for objects, dressing, personal care, getting up, feeding, usual daily activities, walking, and personal hygiene [
35]. The patients filled out the questionnaire independently, answering questions about the possibility of performing certain functions as follows: 0—no difficulty; 1—with minor difficulties; 2—with major difficulties; and 3—the inability to perform the function. The total value is then divided by 20, and the HAQ index, a number with three decimal places, is calculated. The HAQ index has a three-tiered grading system: 0–1, mild impairment of function in daily life; 1–2, moderate-to-severe impairment in all segments; and 2–3, severe impairment and total disability with the need for someone else’s help. The time required to fill out the questionnaire is 10 min [
35].
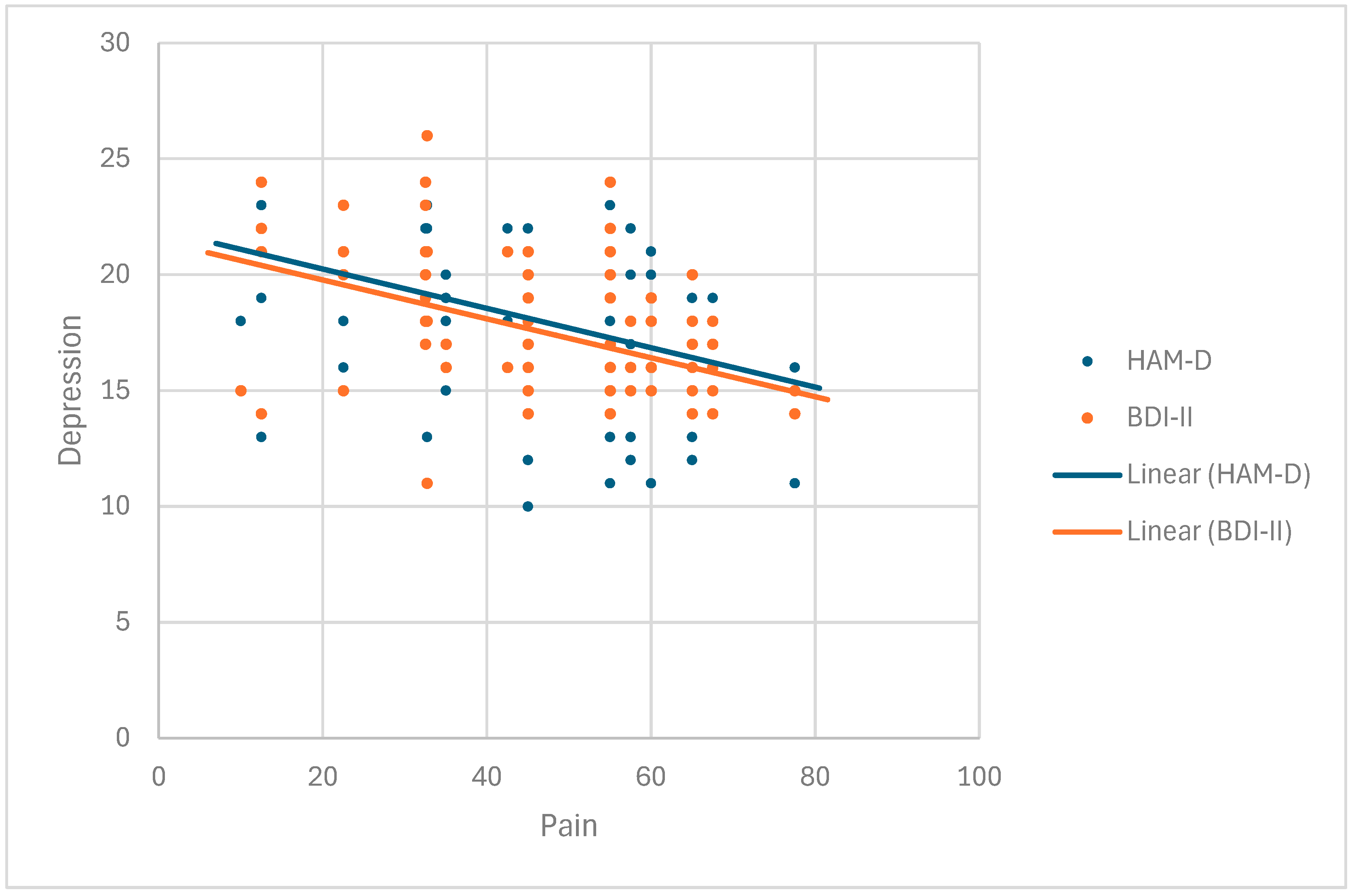
The Beck Depression Inventory—second edition (BDI-II) is a self-assessment measure of depressive symptoms [
32]. It contains 21 items that are evaluated on a four-point scale (0–3), with higher values indicating a greater intensity of symptoms. The assessment is made concerning the preceding two weeks. The maximum number of points is 63. Results between 0 and 13 indicate minimal depression, results ranging from 14 to 19 indicate mild depression, those ranging from 20 to 28 indicate moderate depression, and results of 29 and above indicate severe depression. The psychiatrist fills out the questionnaire during the interview with the patient, with the questionnaire taking 10 min [
32].
The Hamilton Depression Rating Scale (HAM-D) is one of the earliest-developed and most widely used scales that assesses the severity of depressive symptoms or the severity of a depressive episode [
38]. The HAM-D has become a standard in clinical trials of depression. It is performed by a psychiatrist who interviews a patient. This scale consists of 17 items rated from 0 to 4 (0—non-existent; 4—significantly expressed). A sum of less than 7 points means that the patient is not depressed, a range of 8–13 represents mild depression, a sum of points between 14 and 18 indicates moderately severe depression, sums larger than 19 and 22 represent a severe depressive episode, and more than 23 points is a sign of very severe depression. The time required to fill out the questionnaire is 20 min [
38].
The RAQoL questionnaire contains 30 questions related to the physical and psychological components of the quality of life of patients with RA [
36]. Each question is scored with 1 point for a total of 30. A lower score represents a better quality of life. The patient fills out the questionnaire independently, which takes 10 min [
36].
The SF 36 questionnaire (36-item Short Form Survey) is a short health status questionnaire consisting of 36 questions related to mental and physical health and social functioning, and it is frequently used for assessing quality of life [
37]. The questions are divided into eight areas of examination. The advantage of this questionnaire is that it can be widely used since it does not refer to a specific age, disease, or population. The result of the test is expressed as a value in the range of 0–100, with low results showing less functionality and a worse health assessment, while high results indicate good health, without pain or functional limitations. The patient fills out the questionnaire independently; the time required to fill out the questionnaire is 20–30 min [
37].
2.6. Statistical Analysis
A comprehensive statistical analysis was conducted to examine clinical, demographic, and laboratory differences between two cohorts: rheumatoid arthritis (RA) patients with depression (group I) and patients with primary depression (group II). Based on normality tests (Kolmogorov–Smirnov and Shapiro–Wilk), the majority of variables in group I displayed significant deviations from normality (p < 0.05), necessitating the use of non-parametric methods for comparison and correlation analysis. Exceptions included the SF-36 variable, which followed a normal distribution. Descriptive statistical methods were employed in the study, along with the non-parametric tests (Mann–Whitney U test, Spearman’s rank correlation coefficient) and linear regression analysis.
These methods provided robust insights into the relationships between disease activity, inflammatory markers, and depressive symptoms in RA patients with depression versus those with primary depression.