Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Malignant Melanoma Cell Culture
2.2. Tested Drugs
2.3. Cell Viability Assessment—MTT Test
2.4. Cell Cytotoxicity—LDH Test
2.5. Statistical Analysis
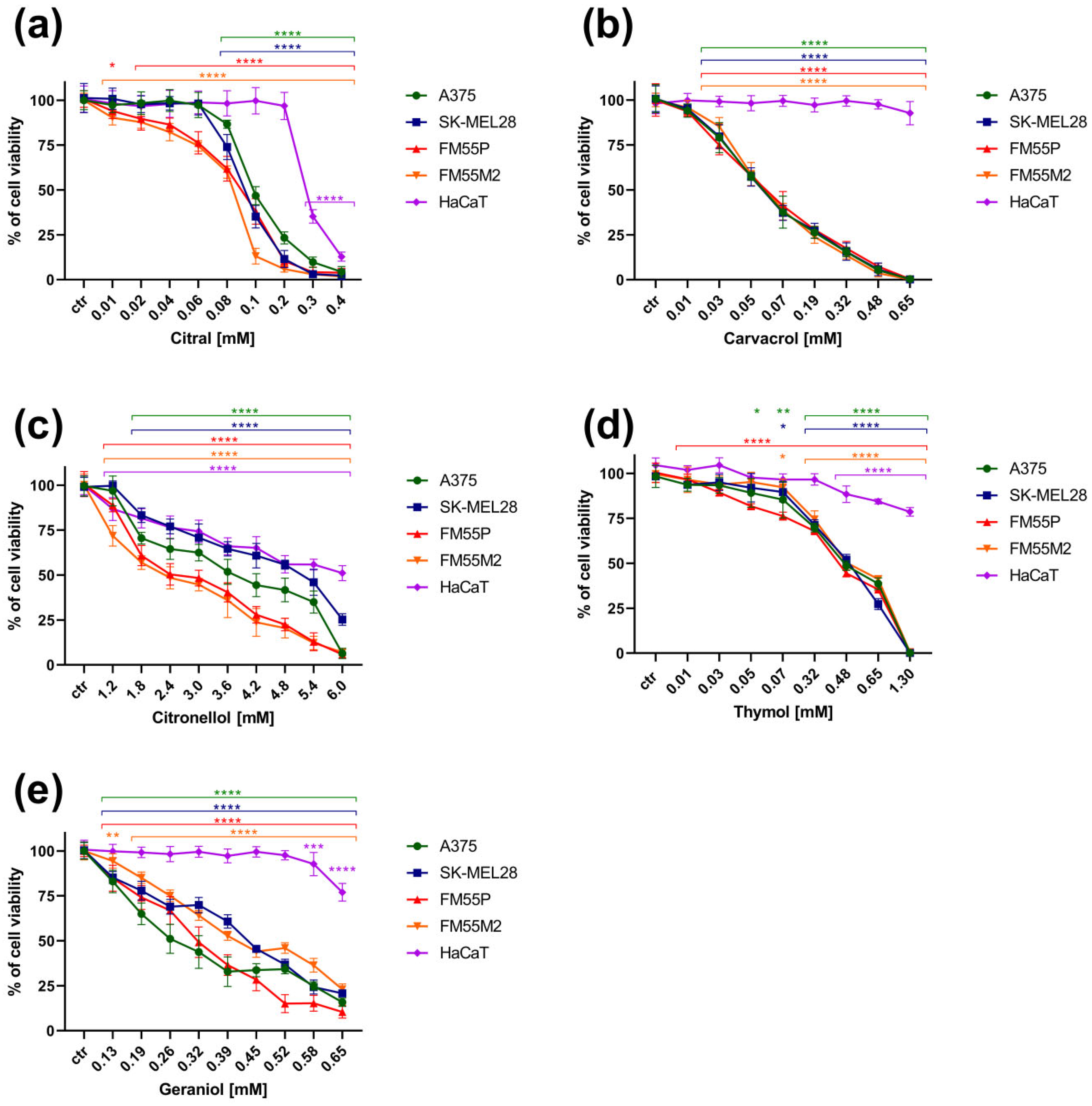
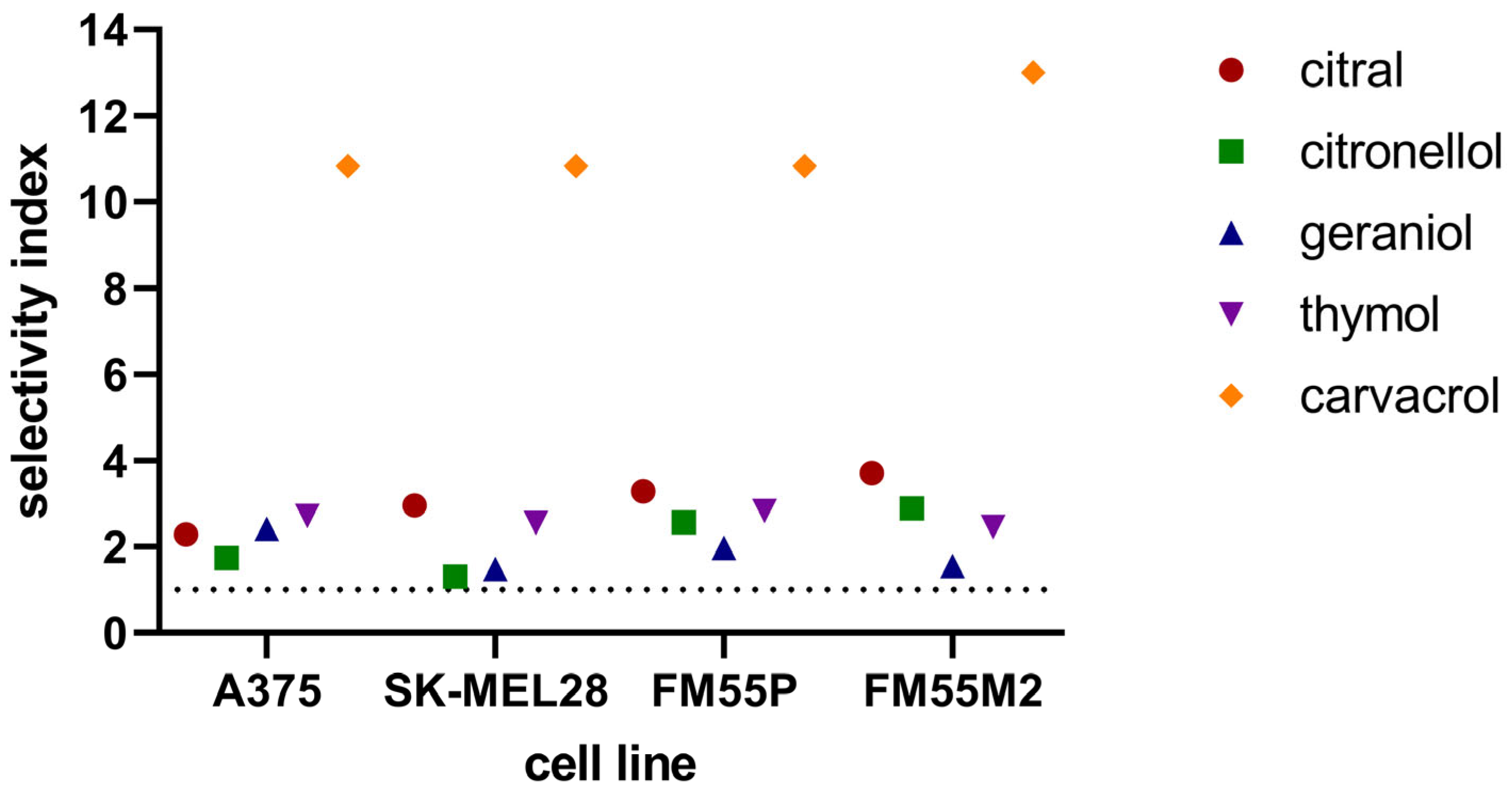
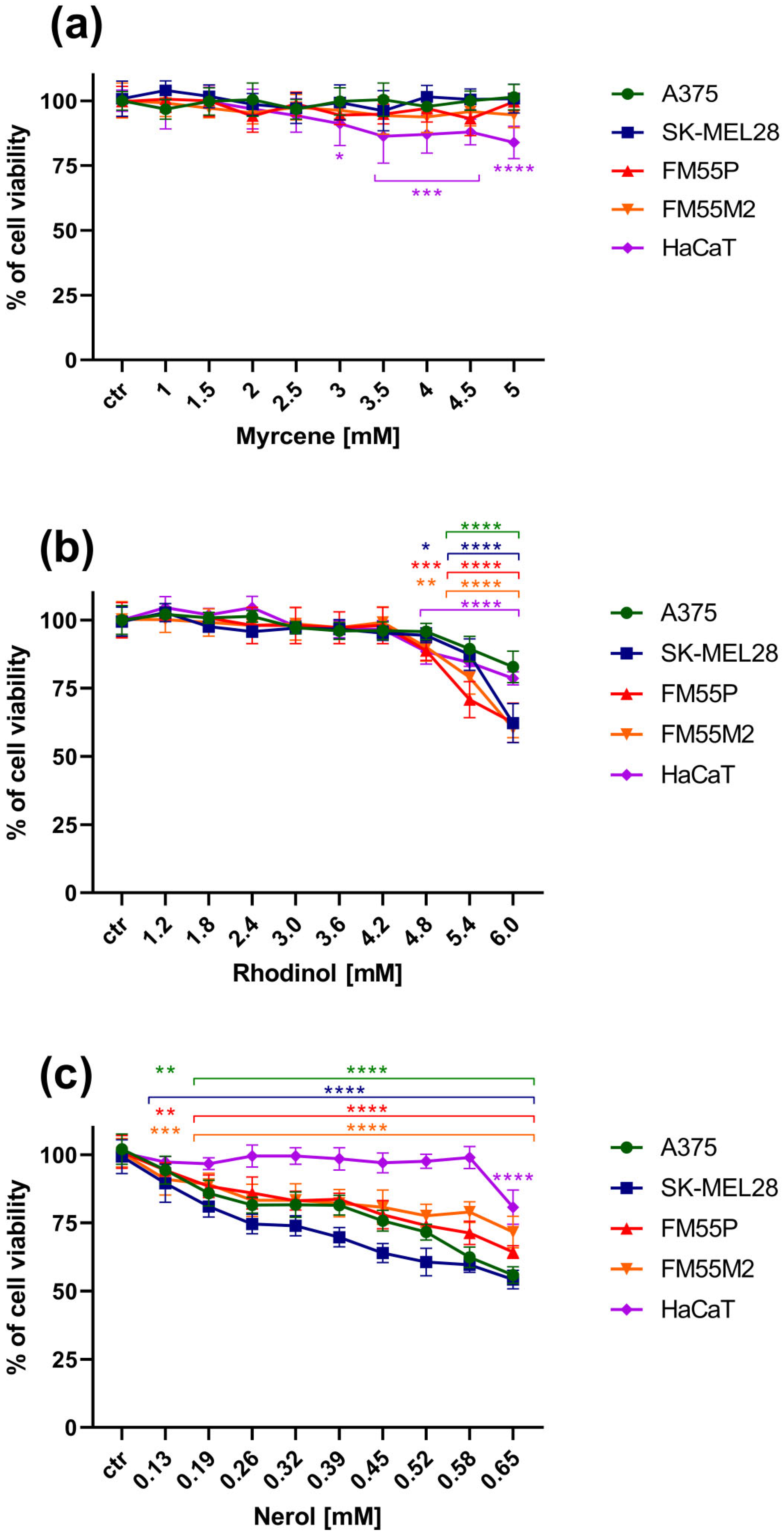
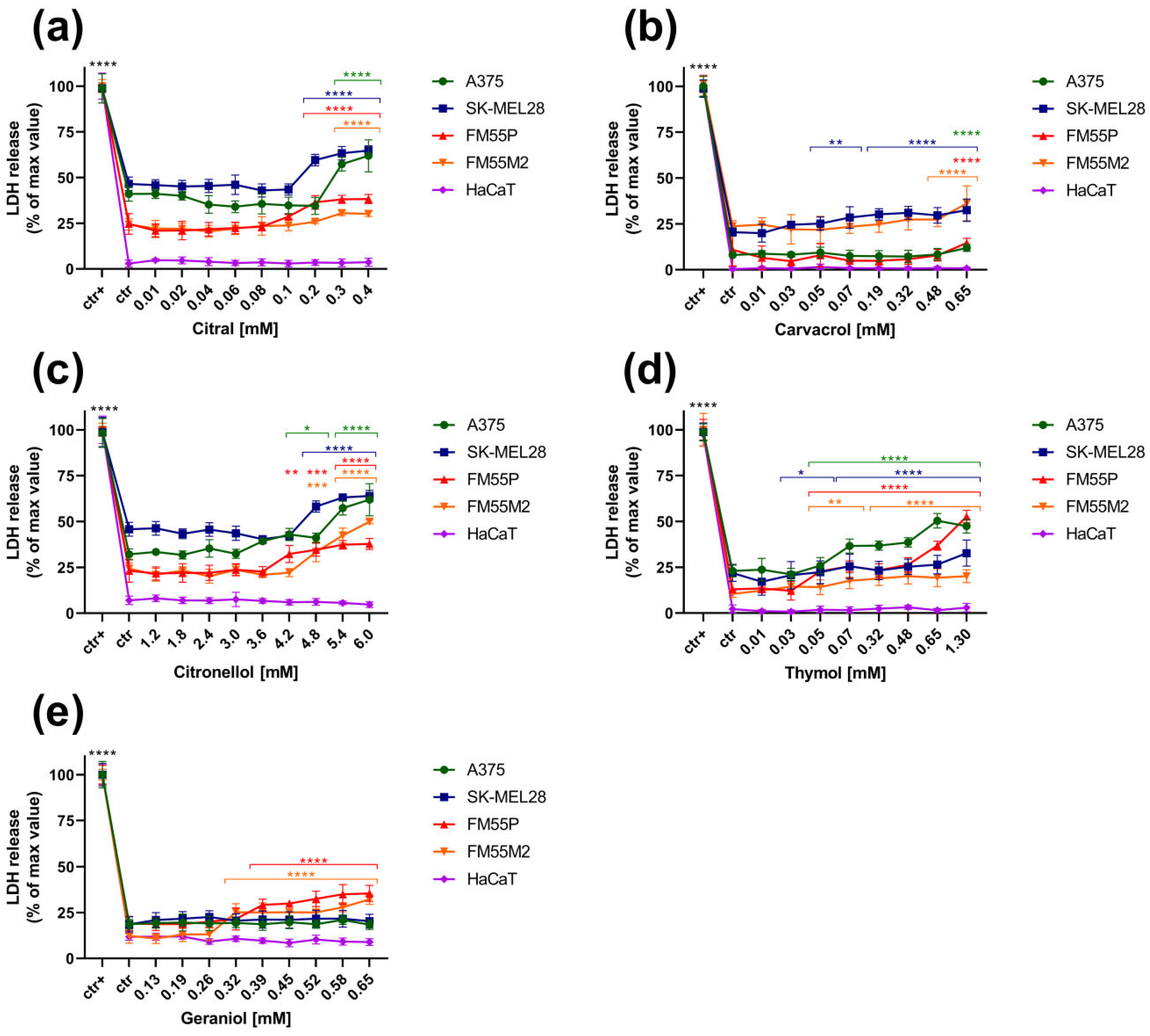
3. Results
3.1. The MTT Assay
3.2. The LDH Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, 93–102. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bargieł, J.; Łuszczki, J.J. Anticancer effect of terpenes: Focus on malignant melanoma. Pharmacol. Rep. 2023, 75, 1115–1125. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Hwang, E.; Ngo, H.T.T.; Park, B.; Seo, S.A.; Yang, J.E.; Yi, T.H. Myrcene, an Aromatic Volatile Compound, Ameliorates Human Skin Extrinsic Aging via Regulation of MMPs Production. Am. J. Chin. Med. 2017, 45, 1113–1124. [Google Scholar] [CrossRef]
- Luis, S.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; da Silva, T.B.; Machado, W.J.; Prata, A.P.; Costa, E.V.; Moraes, V.R.; Nogueira, P.C.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef]
- Rajendran, J.; Pachaiappan, P.; Thangarasu, R. Citronellol, an Acyclic Monoterpene Induces Mitochondrial-Mediated Apoptosis through Activation of Proapoptotic Factors in MCF-7 and MDA-MB-231 Human Mammary Tumor Cells. Nutr. Cancer 2021, 73, 1448–1458. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Jayaganesh, R.; Pugalendhi, P.; Murali, R. Effect of citronellol on NF-kB inflammatory signaling molecules in chemical carcinogen-induced mammary cancer in the rat model. J. Biochem. Mol. Toxicol. 2020, 34, e22441. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.N.; Lai, Y.J.; Ma, J.W.; Ho, C.T.; Hung, S.W.; Chen, Y.H.; Chen, C.T.; Kao, J.Y.; Way, T.D. Citronellol Induces Necroptosis of Human Lung Cancer Cells via TNF-α Pathway and Reactive Oxygen Species Accumulation. In Vivo 2019, 33, 1193–1201. [Google Scholar] [CrossRef]
- Zhuang, S.R.; Chen, S.L.; Tsai, J.H.; Huang, C.C.; Wu, T.C.; Liu, W.S.; Tseng, H.C.; Lee, H.S.; Huang, M.C.; Shane, G.T.; et al. Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother. Res. 2009, 23, 785–790. [Google Scholar] [CrossRef]
- Silva, G.D.S.E.; Marques, J.N.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar]
- Shen, X.; Cui, X.; Cui, H.; Jin, Y.; Jin, W.; Sun, H. Geraniol and lupeol inhibit growth and promote apoptosis in human hepatocarcinoma cells through the MAPK signaling pathway. J. Cell. Biochem. 2019, 120, 5033–5041. [Google Scholar] [CrossRef]
- Kuzu, B.; Cüce, G.; Ayan, İ.Ç.; Gültekin, B.; Canbaz, H.T.; Dursun, H.G.; Şahin, Z.; Keskin, İ.; Kalkan, S.S. Evaluation of Apoptosis Pathway of Geraniol on Ishikawa Cells. Nutr. Cancer 2021, 73, 2532–2537. [Google Scholar] [CrossRef]
- Duncan, R.E.; Lau, D.; El-Sohemy, A.; Archer, M.C. Geraniol and beta-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem. Pharmacol. 2004, 68, 1739–1747. [Google Scholar] [CrossRef]
- Kim, S.H.; Bae, H.C.; Park, E.J.; Lee, C.R.; Kim, B.J.; Lee, S.; Park, H.H.; Kim, S.J.; So, I.; Kim, T.W.; et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134. [Google Scholar] [CrossRef]
- Dolghi, A.; Buzatu, R.; Dobrescu, A.; Olaru, F.; Popescu, G.A.; Marcovici, I.; Pinzaru, I.; Navolan, D.; Cretu, O.M.; Popescu, I.; et al. Phytochemical Analysis and In Vitro Cytotoxic Activity against Colorectal Adenocarcinoma Cells of Hippophae rhamnodies L.; Cymbopogon citratus (D.C.) Stapf, and Ocimum basilicum L. Essential Oils. Plants 2021, 10, 2752. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2021, 17, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Nguyen Quang, T.; Dat, N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef]
- Balan, D.J.; Rajavel, T.; Das, M.; Sathya, S.; Jeyakumar, M.; Devi, K.P. Thymol induces mitochondrial pathway-mediated apoptosis via ROS generation, macromolecular damage and SOD diminution in A549 cells. Pharmacol. Rep. 2021, 73, 240–254. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L.; and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Satooka, H.; Kubo, I. Effects of thymol on B16-F10 melanoma cells. J. Agric. Food Chem. 2012, 60, 2746–2752. [Google Scholar] [CrossRef]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The Essential Oil of Cymbopogon citratus Stapt and Carvacrol: An Approach of the Antitumor Effect on 7,12-Dimethylbenz-[α]-anthracene (DMBA)-Induced Breast Cancer in Female Rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef]
- Jung, C.Y.; Kim, S.Y.; Lee, C. Carvacrol Targets AXL to Inhibit Cell Proliferation and Migration in Non-small Cell Lung Cancer Cells. Anticancer Res. 2018, 38, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ansari, I.A. Carvacrol Exhibits Chemopreventive Potential against Cervical Cancer Cells via Caspase-Dependent Apoptosis and Abrogation of Cell Cycle Progression. Anticancer Agents Med. Chem. 2021, 21, 2224–2235. [Google Scholar] [CrossRef]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol Induces Reactive Oxygen Species (ROS)-mediated Apoptosis Along with Cell Cycle Arrest at G0/G1 in Human Prostate Cancer Cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Singh, V.K.; Saeed, M.; Kausar, M.A.; Ansari, I.A. Carvacrol Induced Program Cell Death and Cell Cycle Arrest in Androgen-Independent Human Prostate Cancer Cells via Inhibition of Notch Signaling. Anticancer Agents Med. Chem. 2019, 19, 1588–1608. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Veerappan, K.; Huq, M.A.; Rajeshkumar, S.; Lakshmi, T.; Kim, Y.J. Citral Induced Apoptosis through Modulation of Key Genes Involved in Fatty Acid Biosynthesis in Human Prostate Cancer Cells: In Silico and In Vitro Study. BioMed Res. Int. 2020, 2020, 6040727. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Ramani, S.; Natarajan, S.; Kim, Y.J.; Perumalsamy, H. Integrated transcriptome and in vitro analysis revealed anti-proliferative effect of citral in human stomach cancer through apoptosis. Sci. Rep. 2019, 9, 4883. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Grabarska, A.; Florek-Łuszczki, M.; Plewa, Z.; Łuszczki, J.J. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 537. [Google Scholar] [CrossRef]
- Romashin, D.D.; Rusanov, A.L.; Kozhin, P.M.; Karagyaur, M.N.; Tikhonova, O.V.; Zgoda, V.G.; Luzgina, N.G. Impact of p53 knockdown on protein dataset of HaCaT cells. Data Brief 2022, 42, 108274. [Google Scholar] [CrossRef]
- Luszczki, J.J. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: A practical application. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef]
- Krzywik, J.; Mozga, W.; Aminpour, M.; Janczak, J.; Maj, E.; Wietrzyk, J.; Tuszyński, J.A.; Huczyński, A. Synthesis, antiproliferative activity and molecular docking studies of novel doubly modified colchicine amides and sulfonamides as anticancer agents. Molecules 2020, 25, 1789. [Google Scholar] [CrossRef] [PubMed]
- Russin, W.A.; Hoesly, J.D.; Elson, C.E.; Tanner, M.A.; Gould, M.N. Inhibition of rat mammary carcinogenesis by monoterpenoids. Carcinogenesis 1989, 10, 2161–2164. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mo, H.; Hadisusilo, S.; Qureshi, A.A.; Elson, C.E. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J. Nutr. 1997, 127, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Selvan, M.V. Chemopreventive potential of geraniol in 7,12-dimethylbenz(a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. J. Environ. Biol. 2012, 33, 255–260. [Google Scholar]
- Yu, S.G.; Hildebrandt, L.A.; Elson, C.E. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J. Nutr. 1995, 125, 2763–2767. [Google Scholar]
- Fatima, K.; Wani, Z.A.; Meena, A.; Luqman, S. Geraniol exerts its antiproliferative action by modulating molecular targets in lung and skin carcinoma cells. Phytother. Res. 2021, 35, 3861–3874. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef]
- Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer activity of thymol: A literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef]
- De La Chapa, J.J.; Singha, P.K.; Lee, D.R.; Gonzales, C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018, 47, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Calò, R.; Visone, C.M.; Marabini, L. Thymol and Thymus Vulgaris, L. activity against UVA- and UVB-induced damage in NCTC 2544 cell line. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 791, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cornaghi, L.; Arnaboldi, F.; Calò, R.; Landoni, F.; Baruffaldi Preis, W.F.; Marabini, L.; Donetti, E. Effects of UV Rays and Thymol/Thymus vulgaris L. Extract in an ex vivo Human Skin Model: Morphological and Genotoxicological Assessment. Cells Tissues Organs 2016, 201, 180–192. [Google Scholar] [CrossRef]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol Promotes Cell Cycle Arrest and Apoptosis through PI3K/AKT Signaling Pathway in MCF-7 Breast Cancer Cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Khan, I.; Bhardwaj, M.; Shukla, S.; Lee, H.; Oh, M.H.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Carvacrol encapsulated nanocarrier/nanoemulsion abrogates angiogenesis by downregulating COX-2, VEGF and CD31 in vitro and in vivo in a lung adenocarcinoma model. Colloids Surf. B Biointerfaces 2019, 181, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Arnold, N.A.; Conforti, F.; Menichini, F.; Formisano, C.; Piozzi, F.; Senatore, F. Characterisation of the essential oil of Nepeta glomerata Montbret et Aucher ex Bentham from Lebanon and its biological activities. Nat. Prod. Res. 2011, 25, 614–626. [Google Scholar] [CrossRef]
- Ellithy, M.M.A.; Abdrabo, R.A.M. Plant Based Extract Oil-Based Nano emulsions: Impact on Human Melanoma Cell Line. Asian Pac. J. Cancer Prev. 2024, 25, 1663–1671. [Google Scholar] [CrossRef]

| Cell Line/ Terpenes [mM] | A375 | SK-MEL28 | FM55P | FM55M2 | HaCaT |
|---|---|---|---|---|---|
| Citral | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.00 | 0.29 ± 0.01 |
| Citronellol | 3.63 ± 0.56 | 4.81 ± 0.29 | 2.45 ± 0.38 | 2.17 ± 0.30 | 6.27 ± 1.15 |
| Geraniol | 0.27 ± 0.03 | 0.44 ± 0.03 | 0.33 ± 0.02 | 0.42 ± 0.02 | >0.65 |
| Thymol | 0.48 ± 0.04 | 0.51 ± 0.03 | 0.46 ± 0.05 | 0.53 ± 0.05 | >1.3 |
| Carvacrol | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.00 | >0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska-Łuczka, P.; Kulenty, L.; Załuska-Ogryzek, K.; Góralczyk, A.; Łuszczki, J.J. Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines. Curr. Issues Mol. Biol. 2025, 47, 97. https://doi.org/10.3390/cimb47020097
Wróblewska-Łuczka P, Kulenty L, Załuska-Ogryzek K, Góralczyk A, Łuszczki JJ. Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines. Current Issues in Molecular Biology. 2025; 47(2):97. https://doi.org/10.3390/cimb47020097
Chicago/Turabian StyleWróblewska-Łuczka, Paula, Laura Kulenty, Katarzyna Załuska-Ogryzek, Agnieszka Góralczyk, and Jarogniew J. Łuszczki. 2025. "Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines" Current Issues in Molecular Biology 47, no. 2: 97. https://doi.org/10.3390/cimb47020097
APA StyleWróblewska-Łuczka, P., Kulenty, L., Załuska-Ogryzek, K., Góralczyk, A., & Łuszczki, J. J. (2025). Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines. Current Issues in Molecular Biology, 47(2), 97. https://doi.org/10.3390/cimb47020097






