Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Preparation
2.2. Hs68 Cell Transfection with siRNA for RNAi Experiments
2.3. RNA Extraction and RT-qPCR
2.4. UV Irradiation
2.5. Flow Cytometry Analysis of Mitochondrial Membrane Potential (ΔΨm)
2.6. ATP Assay
2.7. Procollagen Type I C-Peptide Assay
2.8. Reconstructed Three-Dimensional (3D) Human Skin
2.9. Human Clinical Test
2.10. Statistical Analysis
3. Results and Discussion
3.1. Functional Study of Wrinkle-Related Gene, PPARGC1B, in Hs68 Cells
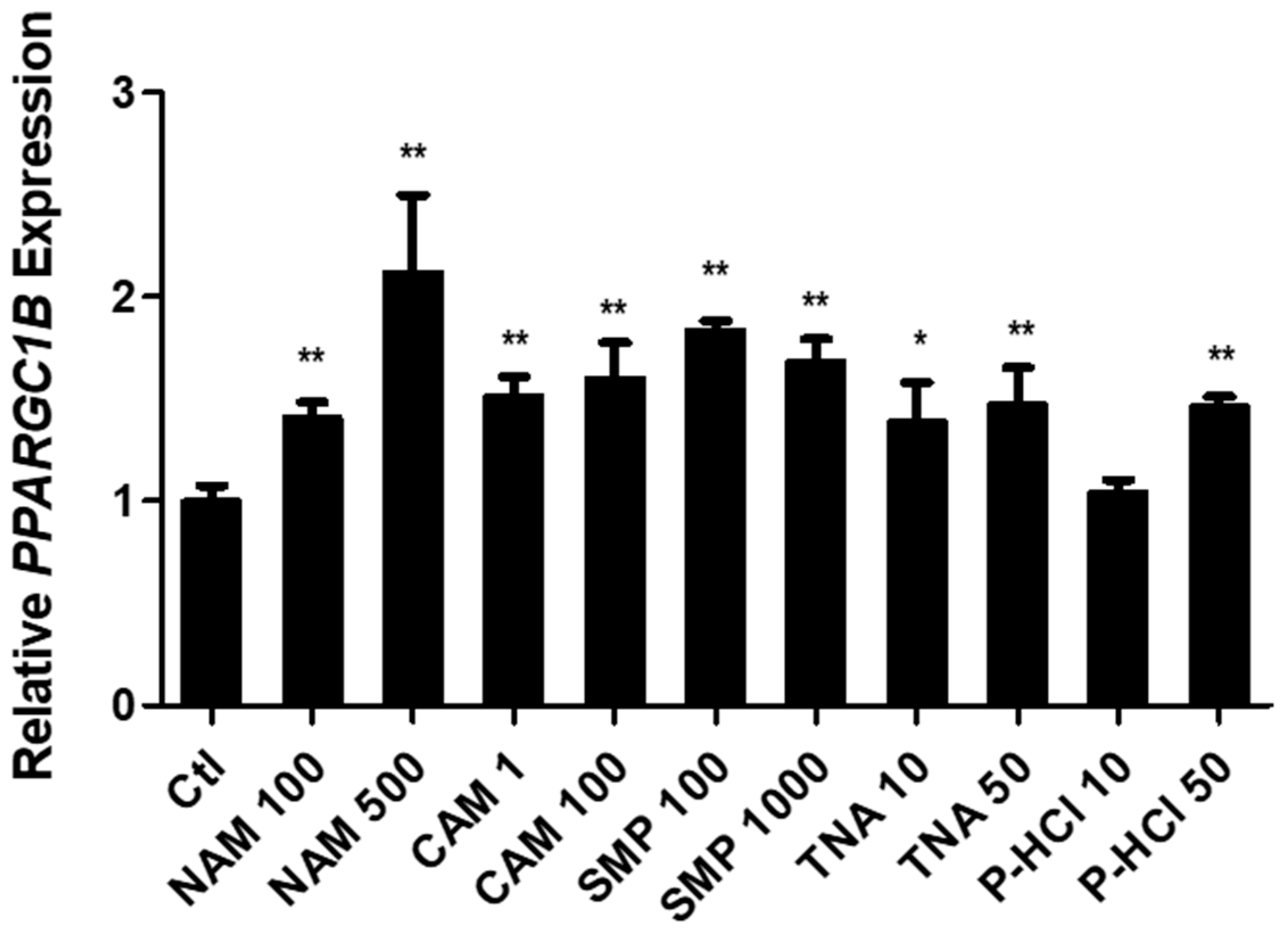
3.2. Screening Active Materials That Regulate PPARGC1B Expression
3.3. Antiaging Effect of PPARGC1B Expression-Regulating Materials In Vitro
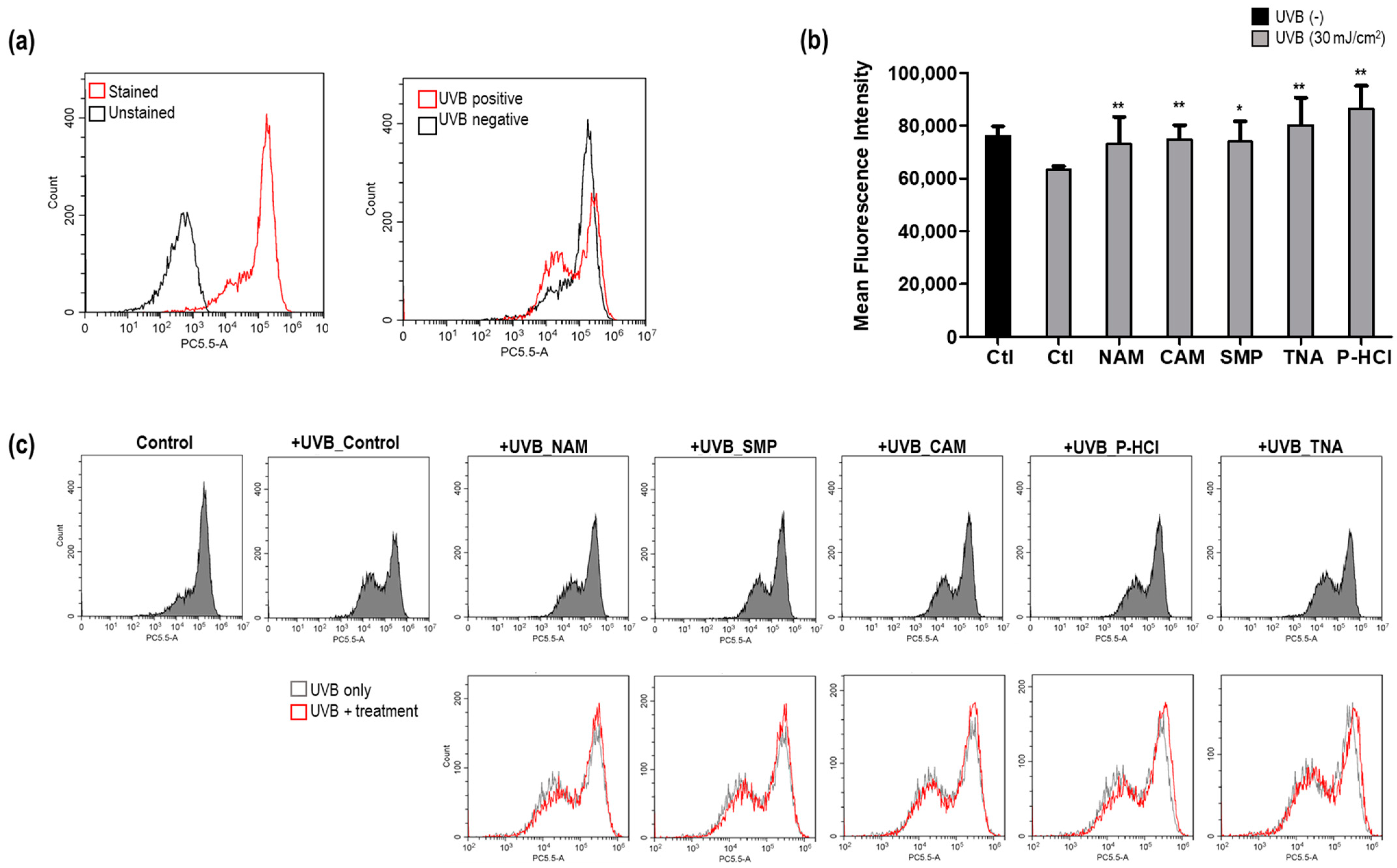
3.3.1. Recovery of UVB-Induced Decrease in ΔΨm
3.3.2. Recovery of UVB-Induced Decrease in ATP Synthesis and Promotion of ATP Synthesis
3.3.3. Effect of Enhanced Type I Procollagen Synthesis
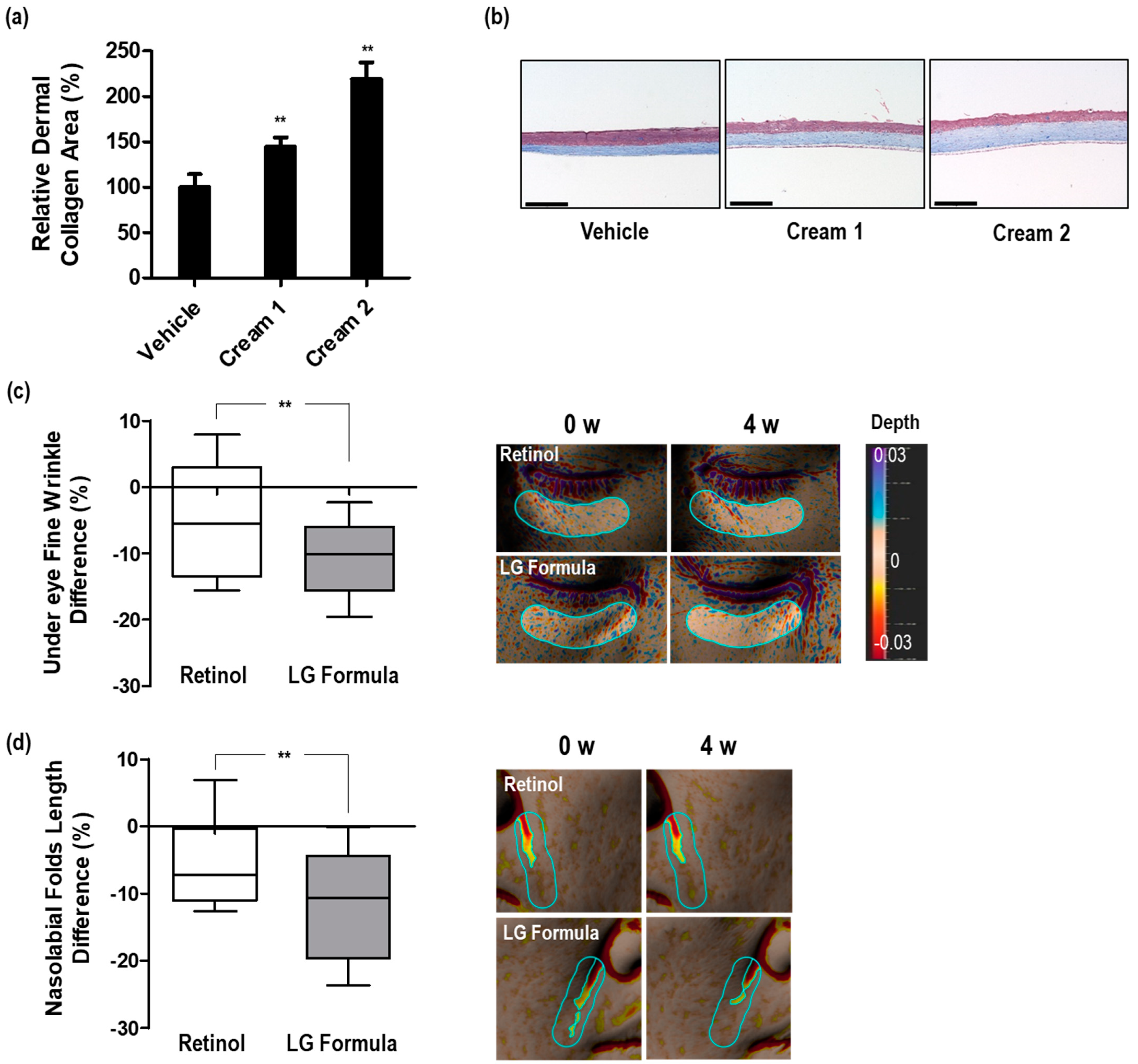
3.4. Dermal Collagen Increases Efficacy of the Selected Materials in 3D Skin Equivalents
3.5. Improvement in Skin Wrinkles by LG Formula-Containing Materials That Increased PPARGC1B Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Casanova, J.; Torro-Alves, N.; Fukusima, S. How much older do you get when a wrinkle appears on your face? Modifying age estimates by number of wrinkles. Aging Neuropsychol. Cogn. 2010, 17, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Lourith, N.; Kanlayavattanakul, M. Biopolymeric agents for skin wrinkle treatment. J. Cosmet. Laser Ther. 2016, 18, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.K.; Struble, S.L.; Quatela, V.C. Evaluating the effectiveness and safety of radiofrequency for face and neck rejuvenation: A systematic review. Lasers Surg. Med. 2022, 54, 27–45. [Google Scholar] [CrossRef]
- Keller, E.C. Home-use devices in aesthetic dermatology. Semin. Cutan. Med. Surg. 2014, 33, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Hanna, R.; Birch-Machin, M.A. What is the role of mitochondrial dysfunction in skin photoaging? Exp. Dermatol. 2018, 27, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.; Birch-Machin, M. Mitochondria’s role in skin ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological signaling functions of reactive oxygen species in stem cells: From flies to man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Barrientos, A.; Casademont, J.; Cardellach, F.; Estivill, X.; Urbano-Marquez, A.; Nunes, V. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res. Mol. Brain Res. 1997, 52, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Turkaly, P.J.; Minkler, P.E.; Hoppel, C.L. Aging skeletal muscle mitochondria in the rat: Decreased uncoupling protein-3 content. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1054–E1062. [Google Scholar] [CrossRef]
- Corsetti, G.; Pasini, E.; D’Antona, G.; Nisoli, E.; Flati, V.; Assanelli, D.; Dioguardi, F.S.; Bianchi, R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am. J. Cardiol. 2008, 101, 26E–34E. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): A complex relationship regulated by the cAMP/PKA signaling pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Shao, D.; Liu, Y.; Liu, X.; Zhu, L.; Cui, Y.; Cui, A.; Qiao, A.; Kong, X.; Liu, Y.; Chen, Q.; et al. PGC-1 beta-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR alpha. Mitochondrion 2010, 10, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calvo, R.; Jové, M.; Coll, T.; Camins, A.; Sánchez, R.M.; Alegret, M.; Merlos, M.; Pallàs, M.; Laguna, J.C.; Vázquez-Carrera, M. PGC-1β down-regulation is associated with reduced ERRα activity and MCAD expression in skeletal muscle of senescence-accelerated mice. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 773–780. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Lin, J.; Krauss, S.; Tarr, P.T.; Yang, R.; Newgard, C.B.; Spiegelman, B.M. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J. Biol. Chem. 2003, 278, 26597–26603. [Google Scholar] [CrossRef] [PubMed]
- Eltania, F.; Lesmana, R.; Sudigdoadi, S.; Sudigdoadi, S.; Khairani, A.F.; Goenawan, H.; Citrawan, A.; Yuniarti, R.A.; Wahyudianingsih, R.; Gunadi, J.W.; et al. Tranexamic acid cream protects ultraviolet B-induced photoaging in Balb/c mice skin by increasing mitochondrial markers: Changes lead to improvement of histological appearance. Photochem. Photobiol. 2020, 96, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Shin, J.-G.; Kim, Y.; Leem, S.; Park, S.-G.; Won, H.-H.; Kang, N.G. Identification of Genetic Loci Associated with Facial Wrinkles in a Large Korean Population. J. Investig. Dermatol. 2022, 142, 2824–2827. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T. (Ed.) New Cosmetic Science; 5—Raw materials of cosmetics; Elsevier: Amsterdam, The Netherlands, 1997; pp. 121–147. [Google Scholar] [CrossRef]
- Lee, S.; Ye, S.; Kim, M.; Lee, H.; Jun, S.H.; Kang, N.G. Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression. Biomolecules 2024, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Nguyen, T.M.; Shim, D.S.; Kim, J.Y.; Lee, J.; Kim, J. Development of Multifunctional Cosmetic Cream Using Bioactive Materials from Streptomyces sp. T65 with Synthesized Mesoporous Silica Particles SBA-15. Antioxidants 2020, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, H.; Jun, S.-H.; Park, S.-G.; Kang, N.G. Enhancement of efficacy of retinoids through enhancing retinoid-induced RAR activity and inhibiting hydroxylation of retinoic acid, and its clinical efficacy on photo-aging. Pharmaceutics 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef]
- Schreiber, S.N.; Emter, R.; Benjamin Hock, M.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Lee, H.C.; Wei, Y.H. Mitochondria and aging. Adv. Exp. Med. Biol. 2012, 942, 311–327. [Google Scholar] [CrossRef]
- Aghaei, S.; Nilforoushzadeh, M.A.; Aghaei, M. The role of peroxisome proliferator-activated receptor-coactivator-1 gene in skin aging. J. Res. Med. Sci. 2016, 21, 36. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, B.; Cui, Y.; Shi, M.; Zhao, Y.; Quan, T.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell Commun. Signal. 2023, 17, 523–529. [Google Scholar] [CrossRef]
- Guak, H.; Sheldon, R.D.; Beddows, I.; Ark, A.V.; Weiland, M.J.; Shen, H.; Jones, R.G.; St-Pierre, J.; Ma, E.H.; Krawczyk, C.M. PGC-1β maintains mitochondrial metabolism and restrains inflammatory gene expression. Sci. Rep. 2022, 12, 16028. [Google Scholar] [CrossRef]
- Khan, M.; Couturier, A.; Kubens, J.F.; Most, E.; Mooren, F.-C.; Krüger, K.; Ringseis, R.; Eder, K. Niacin supplementation induces type II to type I muscle fiber transition in skeletal muscle of sheep. Acta Vet. Scand. 2013, 55, 85. [Google Scholar] [CrossRef]
- Le, S.B.; Holmuhamedov, E.L.; Narayanan, V.L.; Sausville, E.A.; Kaufmann, S.H. Adaphostin and other anticancer drugs quench the fluorescence of mitochondrial potential probes. Cell Death Differ. 2006, 13, 151–159. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Jogdand, P.S.; Singh, S.K.; Christiansen, M.; Dziegiel, M.H.; Singh, S.; Theisen, M. Flow cytometric readout based on Mitotracker Red CMXRos staining of live asexual blood stage malarial parasites reliably assesses antibody dependent cellular inhibition. Malar. J. 2012, 11, 235. [Google Scholar] [CrossRef]
- de Brito Monteiro, L.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieira, P.M.M. Using flow cytometry for mitochondrial assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef]
- Al-Atif, H. Collagen supplements for aging and wrinkles: A paradigm shift in the fields of dermatology and cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A comprehensive review on collagen type I development of biomaterials for tissue engineering: From biosynthesis to bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Smith, L.T.; Holbrook, K.A.; Madri, J.A. Collagen types I, III, and V in human embryonic and fetal skin. Am. J. Anat. 1986, 175, 507–521. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef]
- Messaraa, C.; Metois, A.; Walsh, M.; Hurley, S.; Doyle, L.; Mansfield, A.; O’Connor, C.; Mavon, A. Wrinkle and roughness measurement by the Antera 3D and its application for evaluation of cosmetic products. Skin Res. Technol. 2018, 24, 359–366. [Google Scholar] [CrossRef]
- Messaraa, C.; Doyle, L.; Mansfield, A.; O’Connor, C.; Mavon, A. Ageing profiles of Caucasian and Chinese cohorts—Focus on hands skin. Int. J. Cosmet. Sci. 2019, 41, 79–88. [Google Scholar] [CrossRef]
- Tanaka, O.M.; Cavassin, L.D.; Gasparello, G.C.; Meira, T.M.; Miyoshi, C.S.; Hartmann, G.C. The esthetics of the nasolabial fold and age in the elderly via eye-tracking. Contemp. Clin. Dent. 2023, 14, 18–24. [Google Scholar] [CrossRef]
- Yi, K.-H.; Lee, J.-J.; Hur, H.-W.; Bae, H.; Kim, H.J. Hyaluronic acid filler injection for deep nasolabial folds: A novel intraoral approach. Clin. Anat. 2022, 35, 820–823. [Google Scholar] [CrossRef]
- Fang, R.-Y.; Zhang, H.-C.; Liu, Y.-G.; Sun, Q.N. Quantitative evaluation of rejuvenation treatment of nasolabial fold wrinkles by regression model and 3D photography. J. Cosmet. Dermatol. 2021, 20, 338–345. [Google Scholar] [CrossRef]
- Tucker-Samaras, S.; Zedayko, T.; Cole, C.; Miller, D.; Wallo, W.; Leyden, J.J. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study. J. Drugs Dermatol. 2009, 8, 932–936. [Google Scholar]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Ye, S.; Kim, J.; Jun, S.-H.; Kang, N.-G. Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function. Curr. Issues Mol. Biol. 2024, 46, 5037-5051. https://doi.org/10.3390/cimb46060302
Lee H, Ye S, Kim J, Jun S-H, Kang N-G. Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function. Current Issues in Molecular Biology. 2024; 46(6):5037-5051. https://doi.org/10.3390/cimb46060302
Chicago/Turabian StyleLee, Hyejin, Sanghyun Ye, Juhyun Kim, Seung-Hyun Jun, and Nae-Gyu Kang. 2024. "Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function" Current Issues in Molecular Biology 46, no. 6: 5037-5051. https://doi.org/10.3390/cimb46060302
APA StyleLee, H., Ye, S., Kim, J., Jun, S.-H., & Kang, N.-G. (2024). Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function. Current Issues in Molecular Biology, 46(6), 5037-5051. https://doi.org/10.3390/cimb46060302





