Serine/Arginine-Rich Splicing Factor 7 Knockdown Inhibits Aerobic Glycolysis and Growth in HepG2 Cells by Regulating PKM2 Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Pancancer Analysis and Survival Analysis
2.2. Culture and Cell Lines
2.3. Transfection of SiRNA and Plasmids
2.4. Real-Time Quantitative PCR (qRT-PCR)
2.5. Western Blot Analysis
2.6. Cell Proliferation Assay
2.7. Colony Formation Assay
2.8. Transwell Invasion Assay
2.9. Glucose Assay
2.10. Lactate Assay
2.11. Mitochondrial Membrane Potential Assay
2.12. PKM Splicing Assays
2.13. Statistical Analysis
3. Results
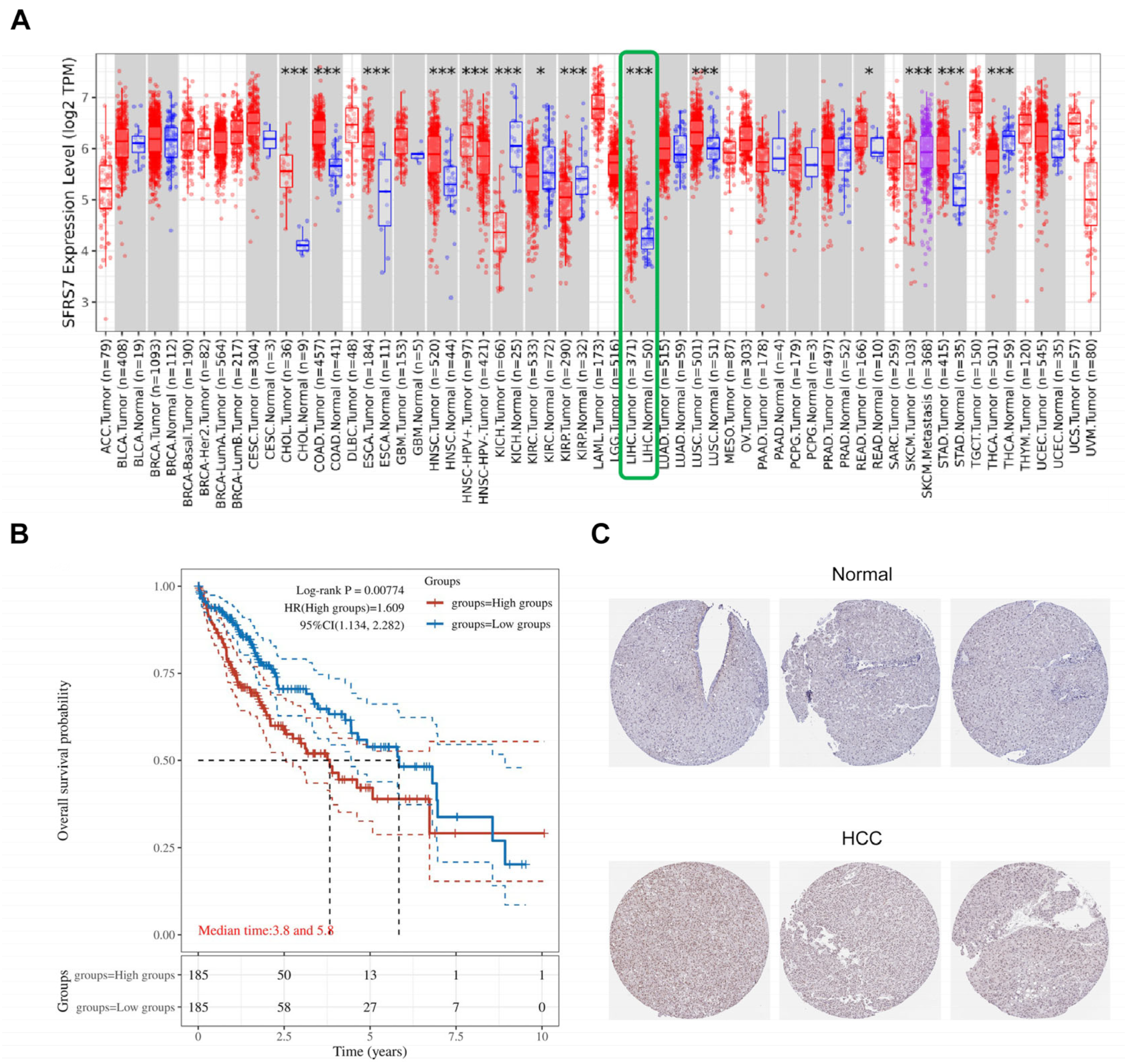
3.1. SRSF7 Was Highly Expressed in Liver Cancer Patients and Was Associated with a Poor Prognosis
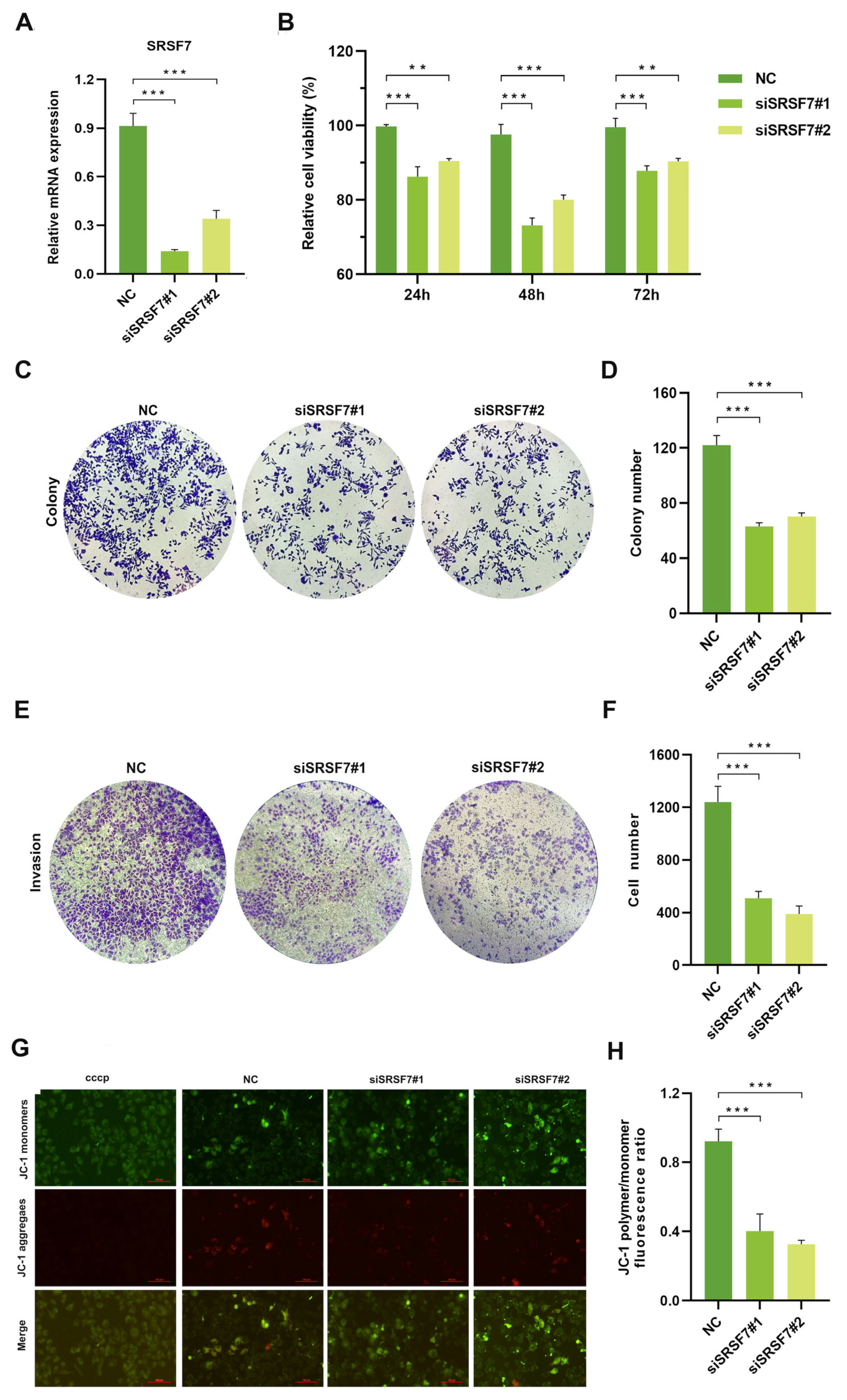
3.2. SRSF7 Knockdown Reduces Proliferation and Invasion while Enhancing Apoptosis in HepG2 Cells In Vitro
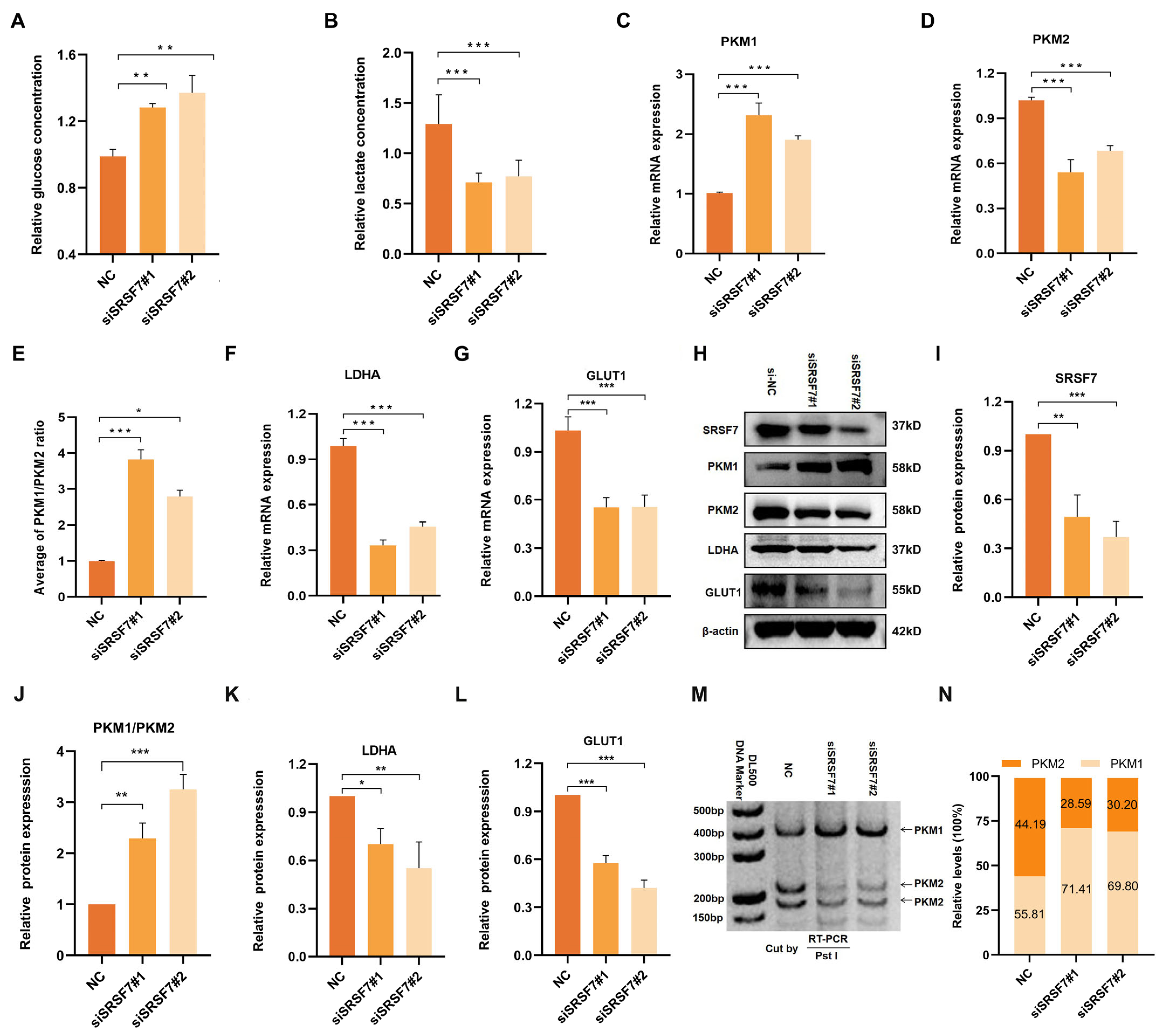
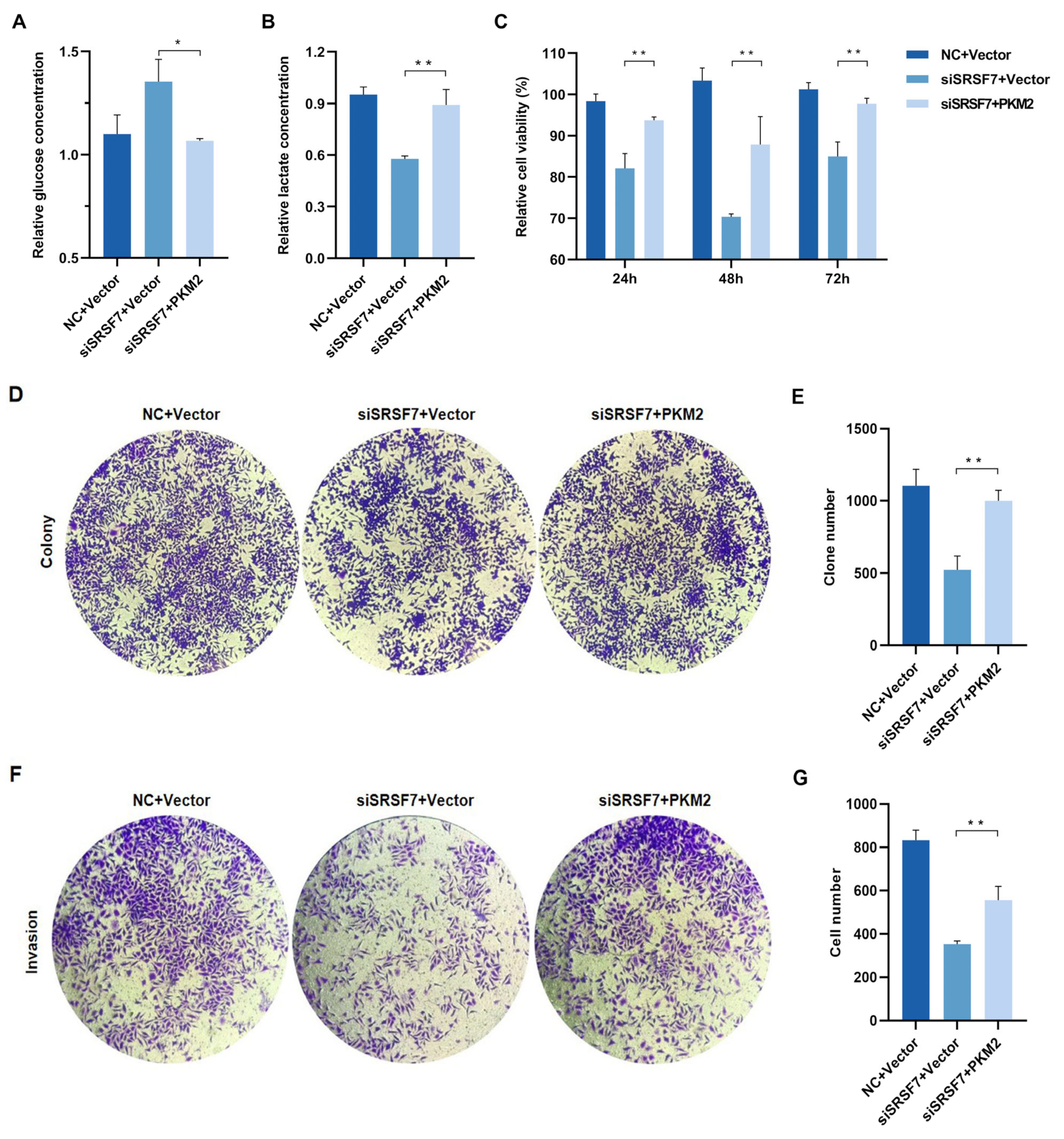
3.3. SRSF7 Knockdown Affects Aerobic Glycolysis in HepG2 Cells
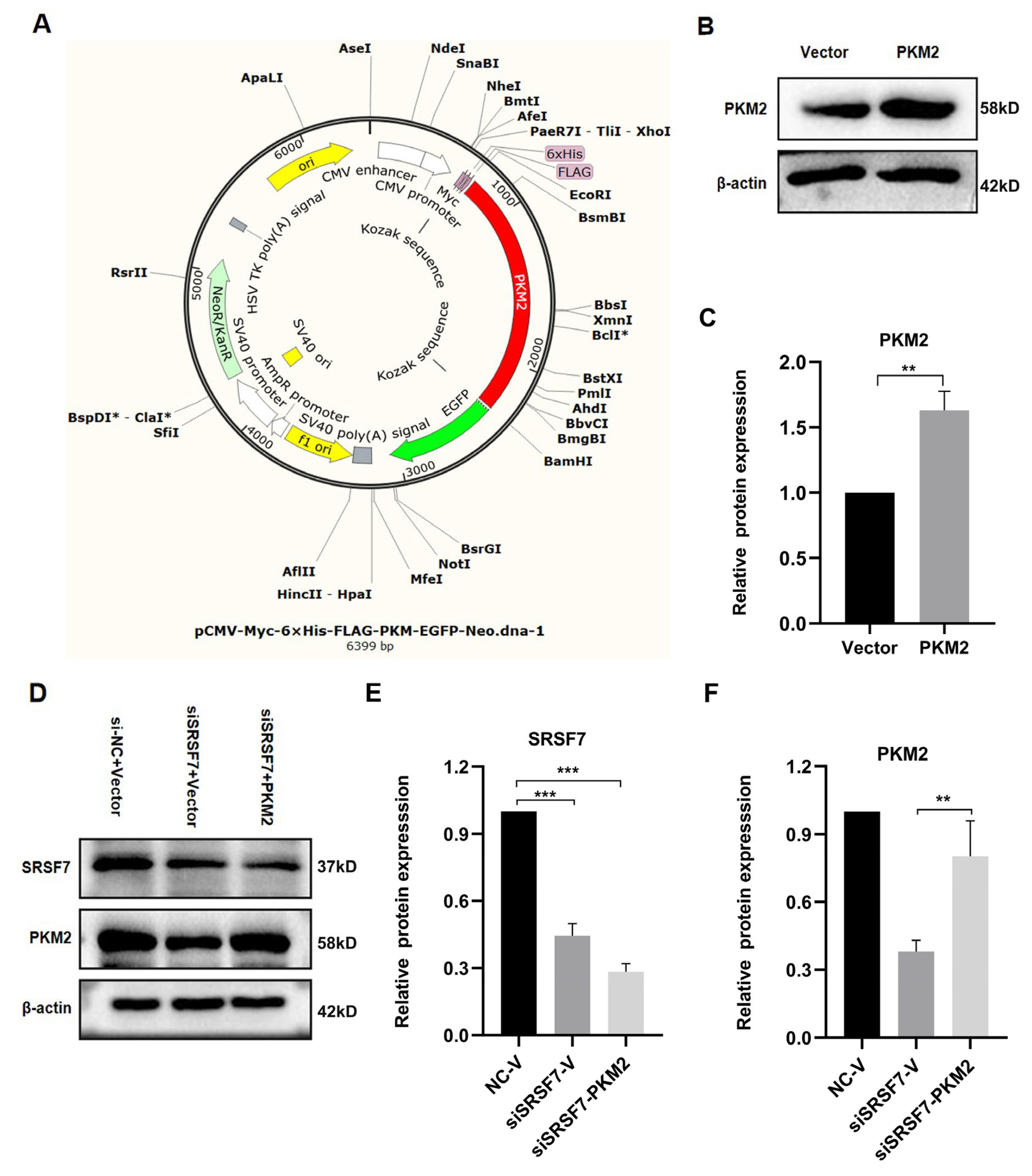
3.4. Verification of PKM2 Overexpression Plasmid and Assessment of Transfection Efficiency
3.5. Verification of PKM2 Overexpression Plasmid and Assessment of Transfection Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg′s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef]
- Allen, A.E.; Locasale, J.W. Glucose Metabolism in Cancer: The Saga of Pyruvate Kinase Continues. Cancer Cell 2018, 33, 337–339. [Google Scholar] [CrossRef]
- Adem, S.; Comakli, V.; Uzun, N. Pyruvate kinase activators as a therapy target: A patent review 2011–2017. Expert. Opin. Ther. Pat. 2018, 28, 61–68. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Sciarrillo, R.; Wojtuszkiewicz, A.; Assaraf, Y.G.; Jansen, G.; Kaspers, G.J.L.; Giovannetti, E.; Cloos, J. The role of alternative splicing in cancer: From oncogenesis to drug resistance. Drug Resist. Updates 2020, 53, 100728. [Google Scholar] [CrossRef]
- Kozlovski, I.; Siegfried, Z.; Amar-Schwartz, A.; Karni, R. The role of RNA alternative splicing in regulating cancer metabolism. Hum. Genet. 2017, 136, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Pal, S.; Gupta, R.; Davuluri, R.V. Alternative transcription and alternative splicing in cancer. Pharmacol. Ther. 2012, 136, 283–294. [Google Scholar] [CrossRef]
- Urbanski, L.M.; Leclair, N.; Anczuków, O. Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip. Rev. RNA 2018, 9, e1476. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Alcedo, K.P.; Kim, H.J.; Snider, N.T. Alternative Splicing in Hepatocellular Carcinoma. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, Z.; Zhao, Y.; Huang, S.; He, X. Transcriptome-wide analysis reveals the landscape of aberrant alternative splicing events in liver cancer. Hepatology 2019, 69, 359–375. [Google Scholar] [CrossRef]
- Li, D.; Yu, W.; Lai, M. Towards understandings of serine/arginine-rich splicing factors. Acta Pharm. Sin. B 2023, 13, 3181–3207. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Van Wijnen, A.J.; Azizi, P.; Abiri, R.; Ashkani, S.; Taheri, S. Towards understanding pre-mRNA splicing mechanisms and the role of SR protein. Gene 2016, 587, 107–119. [Google Scholar] [CrossRef]
- Li, H.; Meng, J.; Wang, Z.; Tang, Y.; Xia, S.; Wang, Y.; Qin, Z.; Luan, Y. Serine/arginine-rich splicing factors: The bridge linking alternative splicing and cancer. Int. J. Biol. Sci. 2020, 16, 2442–2453. [Google Scholar] [CrossRef]
- Anczukow, O.; Krainer, A.R. Splicing-factor alterations in cancers. RNA 2016, 22, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Cavaloc, Y.; Popielarz, M.; Fuchs, J.P.; Gattoni, R.; Stévenin, J. Characterization and cloning of the human splicing factor 9G8: A novel 35 kDa factor of the serine/arginine protein family. EMBO J. 1994, 13, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y. SRSF7 knockdown promotes apoptosis of colon and lung cancer cells. Oncol. Lett. 2018, 15, 5545–5552. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.C.; Preen, D.; Jeffrey, G.P. The evolving epidemiology of hepatocellular carcinoma: A global perspective. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma: New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Silvera, D.; Formenti, S.C.; Schneider, R.J. Translational control in cancer. Nat. Rev. Cancer 2010, 10, 254–266. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, S.; Ragusa, A.; Marisi, G.; De Domenico, S.; Casadei Gardini, A.; Bonafè, M.; Giudetti, A.M. Aberrant Metabolism in Hepatocellular Carcinoma Provides Diagnostic and Therapeutic Opportunities. Oxidative Med. Cell. Longev. 2018, 2018, 7512159. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.Z.; Qu, S.B.; Wang, D.S. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects. World J. Gastroenterol. 2016, 22, 9933–9943. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, Y.; Lin, C.; Lai, S.; Dai, H.; Wang, Z.; Dai, L.; Su, H.; Song, Y.; Zhang, N.; et al. LNCAROD enhances hepatocellular carcinoma malignancy by activating glycolysis through induction of pyruvate kinase isoform PKM2. J. Exp. Clin. Cancer Res. 2021, 40, 299. [Google Scholar] [CrossRef]
- Yu, L.; Kim, J.; Jiang, L.; Feng, B.; Ying, Y.; Ji, K.Y.; Tang, Q.; Chen, W.; Mai, T.; Dou, W.; et al. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat. Commun. 2020, 11, 708. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, R.; Jia, W.; Zhu, Z.; Zhao, X.; Zhao, L.; Huang, G.; Liu, J. Nuclear scaffold protein p54nrb/NONO facilitates the hypoxia-enhanced progression of hepatocellular carcinoma. Oncogene 2021, 40, 4167–4183. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | 5′-GAAATCGTGCGTGACATTAA-3 | 5′-AAGGAAGGCTGGAAGAGTG-3 |
| SRSF7 | 5′-GCGGTACGGAGGAGAAAC-3′ | 5′-TCGGGAGCCACAAATCAC-3′ |
| PKM1 | 5′-TGAAGAACTTGTGCGAGCCT-3′ | 5′-GCCAGACTCCGTCAGAACTA-3′ |
| PKM2 | 5′-TTACCAGCGACCCCACAGAA-3′ | 5′-GACGATTATGGCCCCACTGC-3′ |
| LDHA | 5′-ATGGCAACTCTAAAGGATCAGC-3′ | 5′-CCAACCCCAACAACTGTAATCT-3′ |
| GLUT1 | 5′-TGTGCTCCTGGTTCTGTTCT-3′ | 5′-GCTCCTCGGGTGTCTTGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Yao, X.; Cao, X.; Fu, Y.; Wang, Y. Serine/Arginine-Rich Splicing Factor 7 Knockdown Inhibits Aerobic Glycolysis and Growth in HepG2 Cells by Regulating PKM2 Expression. Curr. Issues Mol. Biol. 2024, 46, 5023-5036. https://doi.org/10.3390/cimb46050301
Shi W, Yao X, Cao X, Fu Y, Wang Y. Serine/Arginine-Rich Splicing Factor 7 Knockdown Inhibits Aerobic Glycolysis and Growth in HepG2 Cells by Regulating PKM2 Expression. Current Issues in Molecular Biology. 2024; 46(5):5023-5036. https://doi.org/10.3390/cimb46050301
Chicago/Turabian StyleShi, Weiye, Xu Yao, Xueyu Cao, Yu Fu, and Yingze Wang. 2024. "Serine/Arginine-Rich Splicing Factor 7 Knockdown Inhibits Aerobic Glycolysis and Growth in HepG2 Cells by Regulating PKM2 Expression" Current Issues in Molecular Biology 46, no. 5: 5023-5036. https://doi.org/10.3390/cimb46050301
APA StyleShi, W., Yao, X., Cao, X., Fu, Y., & Wang, Y. (2024). Serine/Arginine-Rich Splicing Factor 7 Knockdown Inhibits Aerobic Glycolysis and Growth in HepG2 Cells by Regulating PKM2 Expression. Current Issues in Molecular Biology, 46(5), 5023-5036. https://doi.org/10.3390/cimb46050301





