Nerve Growth Factor and the Role of Inflammation in Tumor Development
Abstract
1. Introduction
2. NGF and Inflammation and Tumor Growth
2.1. Nerve Growth Factor and Neurotrophins
- (1)
- TrkA is the high-affinity receptor for NGF; its activation triggers a cascade of intracellular signaling events, including the MAPK/ERK pathway and the PI3K/Akt pathway [35,36]. These pathways play essential roles in cell growth, differentiation, and survival. TrkA is expressed on the surface of neurons and other cell types, enabling NGF to exert its neurotrophic effects;
- (2)
- p75 neurotrophin receptor (p75NTR) has a lower affinity for NGF but plays a modulatory role in NGF signaling [20,37]. It can interact with TrkA and enhance its binding affinity for NGF, influencing the cellular responses elicited by NGF. p75NTR is mostly involved in processes like cell death, survival decisions, and axonal growth.
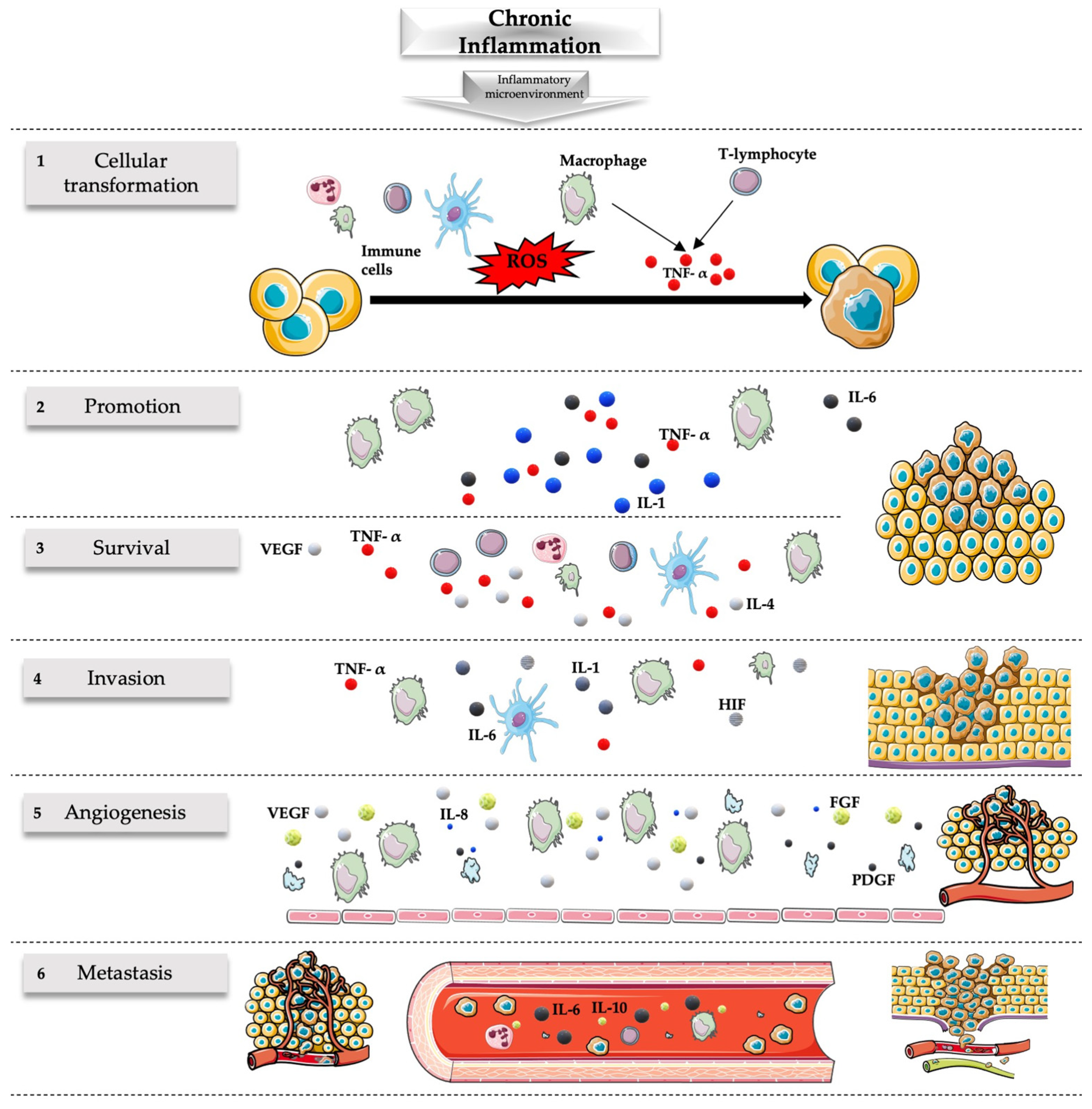
2.2. Inflammation
2.3. Role of NGF in Carcinogenesis
NGF as a Tumor Growth Facilitator or Suppressor
3. NGF and the TME
4. NGF Receptor Expression in Cancer
5. Epigenetics
6. Therapeutic Potential of Neurotrophins and Their Receptors in Tumors
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Dar, A.; Hamid, L.; Nisar, N.; Malik, J.A.; Ali, T.; Nabi Bader, G. Flavonoids as promising molecules in the cancer therapy: An insight. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100167. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.; Fahrner, M. Integration of proteomics in the molecular tumor board. Proteomics 2023, e2300002. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Pawar, V.A.; Srivastava, S.; Tyagi, A.; Kaushik, G.; Shukla, S.K.; Kumar, V. Cancer Vaccines in the Immunotherapy Era: Promise and Potential. Vaccines 2023, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- De Raffele, D.; Ilie, I.M. Unlocking novel therapies: Cyclic peptide design for amyloidogenic targets through synergies of experiments, simulations, and machine learning. Chem. Commun. 2023, 60, 632–645. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Ye, Y.; Ren, Q. Gut microbiota in cancer: Insights on microbial metabolites and therapeutic strategies. Med. Oncol. 2023, 41, 25. [Google Scholar] [CrossRef]
- Liao, C.P.; Booker, R.C.; Brosseau, J.P.; Chen, Z.; Mo, J.; Tchegnon, E.; Wang, Y.; Wade Clapp, D.; Le, L.Q. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J. Clin. Investig. 2018, 128, 2848–2861. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Triaca, V.; Carito, V.; Fico, E.; Rosso, P.; Fiore, M.; Ralli, M.; Lambiase, A.; Greco, A.; Tirassa, P. Cancer stem cells-driven tumor growth and immune escape: The Janus face of neurotrophins. Aging 2019, 11, 11770–11792. [Google Scholar] [CrossRef]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, M.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef]
- Aygun, N. Biological and Genetic Features of Neuroblastoma and Their Clinical Importance. Curr. Pediatr. Rev. 2018, 14, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Karri, D.; Shen, H.; Shao, J.; Dasgupta, S.; Huang, S.; Edwards, D.E.; Ittmann, M.M.; O’Malley, B.W.; Yi, P. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J. Clin. Investig. 2018, 128, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bai, J.; Qin, T.; Wang, Z.; Han, L. NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. J. Cell Mol. Med. 2020, 24, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Guo, Y.; Gan, Z.; Ling, Y.; Xiong, J.; Liang, X.; Cui, J. Nerve Growth Factor (NGF) Encourages the Neuroinvasive Potential of Pancreatic Cancer Cells by Activating the Warburg Effect and Promoting Tumor Derived Exosomal miRNA-21 Expression. Oxid. Med. Cell Longev. 2022, 2022, 8445093. [Google Scholar] [CrossRef]
- Zuo, J.; Yi, C.; Chen, Z.; Zhou, B.; Yang, T.; Lin, J. A novel refined pyroptosis and inflammasome-related genes signature for predicting prognosis and immune microenvironment in pancreatic ductal adenocarcinoma. Sci. Rep. 2022, 12, 18384. [Google Scholar] [CrossRef]
- Anagnostopoulou, V.; Pediaditakis, I.; Alkahtani, S.; Alarifi, S.A.; Schmidt, E.M.; Lang, F.; Gravanis, A.; Charalampopoulos, I.; Stournaras, C. Differential effects of dehydroepiandrosterone and testosterone in prostate and colon cancer cell apoptosis: The role of nerve growth factor (NGF) receptors. Endocrinology 2013, 154, 2446–2456. [Google Scholar] [CrossRef]
- Bono, F.; Lamarche, I.; Bornia, J.; Savi, P.; Della Valle, G.; Herbert, J.M. Nerve growth factor (NGF) exerts its pro-apoptotic effect via the P75(NTR) receptor in a cell cycle-dependent manner. FEBS Lett. 1999, 457, 93–97. [Google Scholar] [CrossRef]
- Molloy, N.H.; Read, D.E.; Gorman, A.M. Nerve growth factor in cancer cell death and survival. Cancers 2011, 3, 510–530. [Google Scholar] [CrossRef]
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef]
- Lykissas, M.G.; Batistatou, A.K.; Charalabopoulos, K.A.; Beris, A.E. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 2007, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Shooter, E.M. Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 2001, 24, 601–629. [Google Scholar] [CrossRef]
- Schramm, A.; Schulte, J.H.; Astrahantseff, K.; Apostolov, O.; Van Limpt, V.; Sieverts, H.; Kuhfittig-Kulle, S.; Pfeiffer, P.; Versteeg, R.; Eggert, A. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005, 228, 143–153. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Cescatti, M.; Rocco, M.L.; Aloe, L.; Lorenzini, L.; Giardino, L.; Calzà, L. Nerve growth factor promotes differentiation and protects the oligodendrocyte precursor cells from in vitro hypoxia/ischemia. Front. Neurosci. 2023, 17, 1111170. [Google Scholar] [CrossRef]
- Ferraguti, G.; Terracina, S.; Micangeli, G.; Lucarelli, M.; Tarani, L.; Ceccanti, M.; Spaziani, M.; D’Orazi, V.; Petrella, C.; Fiore, M. NGF and BDNF in pediatrics syndromes. Neurosci. Biobehav. Rev. 2023, 145, 105015. [Google Scholar] [CrossRef]
- Fahnestock, M.; Yu, G.; Coughlin, M.D. ProNGF: A neurotrophic or an apoptotic molecule? Prog. Brain Res. 2004, 146, 101–110. [Google Scholar] [CrossRef]
- Mohamed, R.; Coucha, M.; Elshaer, S.L.; Artham, S.; Lemtalsi, T.; El-Remessy, A.B. Inducible overexpression of endothelial proNGF as a mouse model to study microvascular dysfunction. Biochim. Biophys. Acta—Mol. Basis Dis. 2018, 1864, 746–757. [Google Scholar] [CrossRef]
- Song, L.; Xu, L.F.; Pu, Z.X.; Wang, H.H. HHIL-10 inhibits apoptosis in brain tissue around the hematoma after ICH by inhibiting proNGF. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; Soligo, M.; Caiello, I.; Prencipe, G.; Manni, L.; Marafon, D.P.; Magni-Manzoni, S.; Manzo, A.; De Benedetti, F.; Gracci-Laudiero, L. ProNGF-p75NTR axis plays a proinflammatory role in inflamed joints: A novel pathogenic mechanism in chronic arthritis. RMD Open 2017, 3, e000441. [Google Scholar] [CrossRef] [PubMed]
- Barcelona, P.F.; Sitaras, N.; Galan, A.; Esquiva, G.; Jmaeff, S.; Jian, Y.; Sarunic, M.V.; Cuenca, N.; Sapieha, P.; Saragovi, H.U. p75NTR and its ligand ProNGF activate paracrine mechanisms etiological to the vascular, inflammatory, and neurodegenerative pathologies of diabetic retinopathy. J. Neurosci. 2016, 36, 8826–8841. [Google Scholar] [CrossRef]
- Capsoni, S.; Brandi, R.; Arisi, I.; D’Onofrio, M.; Cattaneo, A. A Dual Mechanism Linking NGF/proNGF Imbalance and Early Inflammation to Alzheimer’s Disease Neurodegeneration in the AD11 Anti-NGF Mouse Model. CNS Neurol. Disord—Drug Targets 2012, 10, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Descamps, S.; Toillon, R.A.; Adriaenssens, E.; Pawlowski, V.; Cool, S.M.; Nurcombe, V.; Le Bourhis, X.; Boilly, B.; Peyrat, J.P.; Hondermarck, H. Nerve Growth Factor Stimulates Proliferation and Survival of Human Breast Cancer Cells through Two Distinct Signaling Pathways. J. Biol. Chem. 2001, 276, 17864–17870. [Google Scholar] [CrossRef] [PubMed]
- Meco, D.; Di Francesco, A.M.; Melotti, L.; Ruggiero, A.; Riccardi, R. Ectopic nerve growth factor prevents proliferation in glioma cells by senescence induction. J. Cell Physiol. 2019, 234, 6820–6830. [Google Scholar] [CrossRef] [PubMed]
- Chopin, V.; Lagadec, C.; Toillon, R.A.; Le Bourhis, X. Neurotrophin signaling in cancer stem cells. Cell Mol. Life Sci. 2016, 73, 1859–1870. [Google Scholar] [CrossRef]
- Marsland, M.; Dowdell, A.; Jiang, C.C.; Wilmott, J.S.; Scolyer, R.A.; Zhang, X.D.; Hondermarck, H.; Faulkner, S. Expression of NGF/proNGF and Their Receptors TrkA, p75NTR and Sortilin in Melanoma. Int. J. Mol. Sci. 2022, 23, 4260. [Google Scholar] [CrossRef]
- Peach, C.J.; Tonello, R.; Gomez, K.; Calderon-Rivera, A.; Sánchez, M.R.; Maile, L.; Perez-Miller, S.; Manu, A.M.; Hahn, H.; Thomsen, A.R.B.; et al. Neuropilin-1 is a co-receptor for NGF and TrkA-evoked pain. BioRxiv 2023. [Google Scholar] [CrossRef]
- Pond, A.; Roche, F.K.; Letourneau, P.C. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J. Neurobiol. 2002, 51, 43–53. [Google Scholar] [CrossRef]
- Reza, J.N.; Gavazzi, I.; Cohen, J. Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol. Cell Neurosci. 1999, 14, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Claudio Cuello, A.; Pentz, R.; Hall, H. The brain NGF metabolic pathway in health and in Alzheimer’s pathology. Front. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.; Aloe, L. Metabolic syndrome—Neurotrophic hypothesis. Med. Hypotheses 2006, 66, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral Pathol. Med. 2020, 49, 227–234. [Google Scholar] [CrossRef]
- Smith, M.A. Hippocampal vulnerability to stress and aging: Possible role of neurotrophic factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; King, M.A.; Heaton, M.B.; Walker, D.W. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002, 950, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Huang, J.; Luo, H.; Luo, C.; Zhu, X.; Bu, F.; Xiao, H.; Xiao, L.; Zhu, Z. Development of a prognostic index and screening of potential biomarkers based on immunogenomic landscape analysis of colorectal cancer. Aging 2020, 12, 5832–5857. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Vitali, M.; Ceccanti, M.; Chaldakov, G.N.; et al. Nerve Growth Factor, Stress and Diseases. Curr. Med. Chem. 2020, 28, 2943–2959. [Google Scholar] [CrossRef]
- D’Angelo, A.; Ceccanti, M.; Petrella, C.; Greco, A.; Tirassa, P.; Rosso, P.; Ralli, M.; Ferraguti, G.; Fiore, M.; Messina, M.P. Role of neurotrophins in pregnancy, delivery and postpartum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 32–41. [Google Scholar] [CrossRef]
- Fiore, M.; Korf, J.; Antonelli, A.; Talamini, L.; Aloe, L. Long-lasting effects of prenatal MAM treatment on water maze performance in rats: Associations with altered brain development and neurotrophin levels. Neurotoxicol. Teratol. 2002, 24, 179–191. [Google Scholar] [CrossRef]
- Ciafrè, S.; Ferraguti, G.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Greco, A.; Chaldakov, G.N.; Rosso, P.; Fico, E.; Messina, M.P.; et al. Nerve growth factor in the psychiatric brain. Riv. Psichiatr. 2020, 55, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Tarani, L.; Fanfarillo, F.; Tirassa, P.; Ralli, M.; Iannella, G.; Polimeni, A.; Lucarelli, M.; Greco, A.; et al. Nerve Growth Factor and Autoimmune Diseases. Curr. Issues Mol. Biol. 2023, 45, 8950–8973. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, T.; Vagapova, E.; Spirin, P.; Rubtsov, P.; Astashkova, O.; Mikheeva, A.; Sorokin, M.; Vladimirova, U.; Suntsova, M.; Konovalov, D.; et al. Growth factor signaling predicts therapy resistance mechanisms and defines neuroblastoma subtypes. Oncogene 2021, 40, 6258–6272. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Griffin, N.; Rowe, C.W.; Jobling, P.; Lombard, J.M.; Oliveira, S.M.; Walker, M.M.; Hondermarck, H. Nerve growth factor and its receptor tyrosine kinase TrkA are overexpressed in cervical squamous cell carcinoma. FASEB BioAdv. 2020, 2, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Simone, M.D.; Properzi, F. Nerve growth factor: A neurotrophin with activity on cells of the immune system. Microsc. Res. Tech. 1999, 45, 285–291. [Google Scholar] [CrossRef]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Caroen, S.; Reid, T. What Exactly Is Inflammation (and What Is It Not?). Int. J. Mol. Sci. 2022, 23, 14905. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef]
- Tracy, R.P. The five cardinal signs of inflammation: Calor, dolor, rubor, tumor... and penuria (apologies to Aulus Cornelius Celsus, de medicina, c. A.D. 25). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- Dandri, M.; Bertoletti, A.; Lütgehetmann, M. Innate immunity in hepatitis B and D virus infection: Consequences for viral persistence, inflammation, and T cell recognition. Semin. Immunopathol. 2021, 43, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Once upon a time, inflammation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200147. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.; Lohse, A.W.; Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ 2023, 380, e070201. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. Inflammation and liver tumorigenesis. Front. Med. 2013, 7, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Olivieri, J.; Allison, J.J.; Gaffo, A.; Juarez, L.; Kovac, S.H.; Person, S.; Saag, K.G. A group randomized trial to improve safe use of nonsteroidal anti-inflammatory drugs. Am. J. Manag. Care 2005, 11, 537–543. [Google Scholar] [PubMed]
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve growth factor: Role in growth, differentiation and controlling cancer cell development. J. Exp. Clin. Cancer Res. 2016, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A Nerve Growth-Stimulating Factor Isolated from Sarcom As 37 and 180. Proc. Natl. Acad. Sci. USA 1954, 40, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Zeliadt, N. Rita Levi-Montalcini: NGF, the prototypical growth factor. Proc. Natl. Acad. Sci. USA 2013, 110, 4873–4876. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.; Atagi, S.; Amitani, K.; Hobara, N.; Kitamura, Y.; Kawasaki, H. Nerve growth factor suppresses prostate tumor growth. J. Pharmacol. Sci. 2010, 112, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Krygier, S.; Djakiew, D. Neurotrophin receptor p75NTR suppresses growth and nerve growth factor-mediated metastasis of human prostate cancer cells. Int. J. Cancer 2002, 98, 1–7. [Google Scholar] [CrossRef]
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Colleen Karlo, J.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar] [CrossRef]
- Lin, H.; Huang, H.; Yu, Y.; Chen, W.; Zhang, S.; Zhang, Y. Nerve growth factor regulates liver cancer cell polarity and motility. Mol. Med. Rep. 2021, 23, 288. [Google Scholar] [CrossRef]
- Tarani, L.; Ceci, F.M.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Minni, A.; Spaziani, M.; Isidori, A.M.; et al. Neuroimmune Dysregulation in Prepubertal and Adolescent Individuals Affected by Klinefelter Syndrome. Endocr. Metab Immune Disord. Drug Targets 2022, 22, 105–114. [Google Scholar] [CrossRef]
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; de Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Tarani, L.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Messina, M.P.; Rasio, D.; De Luca, E.; Putotto, C.; et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with down Syndrome. J. Immunol. Res. 2020, 2020, 6937154. [Google Scholar] [CrossRef]
- Kritas, S.K.; Saggini, A.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Frydas, S.; Rosati, M.; Tei, M.; Speziali, A.; et al. Neuropeptide NGF mediates neuro-immune response and inflammation through mast cell activation. J. Biol. Regul. Homeost Agents 2014, 28, 177–181. [Google Scholar] [PubMed]
- Bracci-Laudiero, L.; De Stefano, M.E. NGF in early embryogenesis, differentiation, and pathology in the nervous and immune systems. Curr. Top. Behav. Neurosci. 2016, 29, 125–152. [Google Scholar] [CrossRef]
- Vega, J.A.; García-Suárez, O.; Hannestad, J.; Pérez-Pérez, M.; Germanà, A. Neurotrophins and the immune system. J. Anat. 2003, 203, 1–19. [Google Scholar] [CrossRef]
- Seidel, M.F.; Hügle, T.; Morlion, B.; Koltzenburg, M.; Chapman, V.; Maassen Van Den Brink, A.; Lane, N.E.; Perrot, S.; Zieglgänsberger, W. Neurogenic inflammation as a novel treatment target for chronic pain syndromes. Exp. Neurol. 2022, 356, 114108. [Google Scholar] [CrossRef]
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2023, 22, 6–14. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Rizzi, C.; Tiberi, A.; Giustizieri, M.; Marrone, M.C.; Gobbo, F.; Carucci, N.M.; Meli, G.; Arisi, I.; D’Onofrio, M.; Marinelli, S.; et al. NGF steers microglia toward a neuroprotective phenotype. Glia 2018, 66, 1395–1416. [Google Scholar] [CrossRef]
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Singhi, A.D.; Hartman, D.J.; Normolle, D.P.; Albers, K.M.; Davis, B.M. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, N.G.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Tafreshi, A.P. Nerve growth factor prevents demyelination, cell death and progression of the disease in experimental allergic encephalomyelitis. Iran. J. Allergy Asthma Immunol. 2006, 5, 177–181. [Google Scholar]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Castrén, E.; Tanila, H. Neurotrophins and Dementia-Keeping in Touch. Neuron 2006, 51, 1–3. [Google Scholar] [CrossRef][Green Version]
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348. [Google Scholar] [CrossRef]
- Chao, M.Y.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Mierke, C.T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep. Prog. Phys. 2014, 77, 076602. [Google Scholar] [CrossRef]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Bian, X.; Zhang, L.; Lu, L.; Pei, S.; Dong, L.; Shi, W.; Huang, L.; Zhang, X.; et al. Signatures of EMT, immunosuppression, and inflammation in primary and recurrent human cutaneous squamous cell carcinoma at single-cell resolution. Theranostics 2022, 12, 7532–7549. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996, 19, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Romon, R.; Adriaenssens, E.; Lagadec, C.; Germain, E.; Hondermarck, H.; Le Bourhis, X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol. Cancer 2010, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cross talk between the cardiovascular and nervous systems: Neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr. Pharm. Des. 2006, 12, 2609–2622. [Google Scholar] [CrossRef]
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 82. [Google Scholar] [CrossRef]
- Lambert, C.; Mathy-Hartert, M.; Dubuc, J.E.; Montell, E.; Vergés, J.; Munaut, C.; Noel, A.; Henrotin, Y. Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis Res. Ther. 2012, 14, R58. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zhang, X.; Yihe, M.I.N.; Zhao, Y.; Wang, B.; Wei, L.; Mao, S.; Min, W. High-glucose microenvironment promotes perineural invasion of pancreatic cancer via activation of hypoxia inducible factor 1α. Oncol. Rep. 2022, 47, 64. [Google Scholar] [CrossRef]
- Han, S.; Wang, D.; Huang, Y.; Zeng, Z.; Xu, P.; Xiong, H.; Ke, Z.; Zhang, Y.; Hu, Y.; Wang, F.; et al. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J. Exp. Clin. Cancer Res. 2022, 41, 348. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Jung, H.H.; Kim, J.Y.; Cho, E.Y.; Oh, J.M.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; et al. Elevated level of nerve growth factor (Ngf) in serum-derived exosomes predicts poor survival in patients with breast cancer undergoing neoadjuvant chemotherapy. Cancers 2021, 13, 5260. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8, 601738. [Google Scholar] [CrossRef]
- Sun, L.; Chen, S.; Chen, M. Schwann Cells in the Tumor Microenvironment: Need More Attention. J. Oncol. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tetri, L.H.; Kolla, V.; Golden, R.L.; Iyer, R.; Croucher, J.L.; Choi, J.H.; Macfarland, S.P.; Naparaju, K.; Guan, P.; Nguyen, F.; et al. RET receptor expression and interaction with TRK receptors in neuroblastomas. Oncol. Rep. 2020, 44, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yang, H.J.; Ye, M.; Liu, X.; Shim, I.; Chang, Y.T.; Bae, H. Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice. J. Neuroinflamm. 2022, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Shan, Q.; Takabatake, K.; Kawai, H.; Oo, M.W.; Sukegawa, S.; Fujii, M.; Nakano, K.; Nagatsuka, H. Crosstalk between cancer and different cancer stroma subtypes promotes the infiltration of tumor-associated macrophages into the tumor microenvironment of oral squamous cell carcinoma. Int. J. Oncol. 2022, 60, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, C.; Liu, Z.; Sun, Y.; Chen, M.; Guo, Y.; Liu, W.; Zhang, C.; Chen, W.; Sun, J.; et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 2022, 34, 1999–2017.e10. [Google Scholar] [CrossRef] [PubMed]
- Urzua, U.; Tapia, V.; Geraldo, M.P.; Selman, A.; Vega, M.; Romero, C. Nerve growth factor stimulates cellular proliferation of human epithelial ovarian cancer. Horm. Metab. Res. 2012, 44, 656–661. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Wang, X.; Dong, Z.; Chen, F.; Cui, H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. TNF-α/NFκ-B/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef]
- Scalia, P.; Williams, S.J.; Fujita-Yamaguchi, Y.; Giordano, A. Cell cycle control by the insulin-like growth factor signal: At the crossroad between cell growth and mitotic regulation. Cell Cycle 2023, 22, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Tomellini, E.; Touil, Y.; Lagadec, C.; Julien, S.; Ostyn, P.; Ziental-Gelus, N.; Meigna, S.; Lengrand, J.; Adrianseen, E.; Palokowska, R.; et al. Nerve growth factor and prongf simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells 2015, 33, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Cernera, G.; Migliaccio, A.; Castoria, G. Nerve growth factor induces proliferation and aggressiveness in prostate cancer cells. Cancers 2019, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Marsland, M.; Dowdell, A.; Faulkner, S.; Jobling, P.; Rush, R.A.; Gedye, C.; Lynam, J.; Griffin, C.P.; Baker, M.; Marsland, J.; et al. ProNGF Expression and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2023, 24, 1616. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, G.; Liu, Y.; Feng, L.; Wang, M.; Liu, J.; Chen, Y.; Ouyang, L. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 2021, 11, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, E.; Sreter, L.; Schuller, I.; Serve, H.; Koenigsmann, M.; Wiedenmann, B.; Oderberg, D.; Reufi, B.; Thiel, E.; Berdel, W.E. Nerve Growth Factor Stimulates Clonal Growth of Human Lung Cancer Cell Lines and a Human Glioblastoma Cell Line Expressing High-Affinity Nerve Growth Factor Binding Sites Involving Tyrosine Kinase Signaling. Cancer Res. 1995, 55, 2212–2219. [Google Scholar] [PubMed]
- Singer, H.S.; Hansen, B.; Martinie, D.; Karp, C.L. Mitogenesis in glioblastoma multiforme cell lines: A role for NGF and its TrkA receptors. J. Neurooncol. 1999, 45, 1–8. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Krishna, N.; Lawn, S.; Valadez, J.G.; Qu, X.; Fenstermacher, D.A.; Fournier, M.; Potthast, L.; Chinnaiyan, P.; Gibney, G.T.; et al. P75 Neurotrophin Receptor Cleavage By A- and Γ-Secretases Is Required for Neurotrophin-Mediated Proliferation of Brain Tumor-Initiating Cells. J. Biol. Chem. 2014, 289, 8067–8085. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Gong, A.; Wang, C.; Liang, Y.; Chen, Y. Localization of NGF and TrkA at mitotic apparatus in human glioma cell line U251. Biochem. Biophys. Res. Commun. 2005, 337, 68–74. [Google Scholar] [CrossRef]
- Antonelli, A.; Chiaretti, A.; Piastra, M.; Vigneti, E.; Aloe, L. In vitro human ependymoblastoma cells differentiate after exposure to nerve growth factor. J. Neurocytol. 2004, 33, 503–515. [Google Scholar] [CrossRef]
- Engebraaten, O.; Bjerkvig, R.; Pedersen, P.-H.; Laerum, O.D. Effects of EGF, BFGF, NGF and PDGF(bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies In Vitro. Int. J. Cancer 1993, 53, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Fanfarillo, F.; Ferraguti, G.; Lucarelli, M.; Francati, S.; Barbato, C.; Minni, A.; Ceccanti, M.; Tarani, L.; Petrella, C.; Fiore, M. The Impact of ROS and NGF in the Gliomagenesis and their Emerging Implications in the Glioma Treatment. CNS Neurol. Disord. Drug Targets 2023, 23, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Botteri, E.; Gillis, R.D.; Löfling, L.; Le, C.P.; Ziegler, A.I.; Chung, N.C.; Rowe, M.C.; Fabb, S.A.; Hartley, B.J.; et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1147. [Google Scholar] [CrossRef] [PubMed]
- Descamp, S.; Pawlowski, V.; Révillion, F.; Hornez, L.; Hebbar, M.; Boilly, B.; Hondermarck, H.; Peyrat, J.P. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001, 61, 4337–4340. [Google Scholar]
- Jin, M.; Wang, Y.; Zhou, T.; Li, W.; Wen, Q. Norepinephrine/β2-Adrenergic Receptor Pathway Promotes the Cell Proliferation and Nerve Growth Factor Production in Triple-Negative Breast Cancer. J. Breast Cancer 2023, 26, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qiu, X.; Qi, X.; Dong, Z.; Zhao, J.; Huang, J.; Xiang, J. Electroacupuncture promotes apoptosis and inhibits axonogenesis by activating p75 neurotrophin receptor for triple-negative breast xenograft in mice. J. Chem. Neuroanat. 2022, 124, 102133. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Arcuri, D.; Vozzo, F.; Malvaso, A.; Montesanto, A.; Maletta, R. Expression and Signaling Pathways of Nerve Growth Factor (NGF) and Pro-NGF in Breast Cancer: A Systematic Review. Curr. Oncol. 2022, 29, 8103–8120. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; El Yazidi-Belkoura, I.; Adriaenssens, E.; Nurcombe, V.; Hondermarck, H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 2003, 22, 5592–5601. [Google Scholar] [CrossRef]
- Lévêque, R.; Corbet, C.; Aubert, L.; Guilbert, M.; Lagadec, C.; Adriaenssens, E.; Duval, J.; Finetti, P.; Birnbaum, D.; Magné, N.; et al. ProNGF increases breast tumor aggressiveness through functional association of TrkA with EphA2. Cancer Lett. 2019, 449, 196–206. [Google Scholar] [CrossRef]
- Mazaki, A.; Yamauchi, K.; Orita, S.; Inage, K.; Suzuki, M.; Fujimoto, K.; Shiga, Y.; Abe, K.; Inoue, M.; Norimoto, M.; et al. Nerve Growth Factor in Breast Cancer Cells Promotes Axonal Growth and Expression of Calcitonin Gene-related Peptide in a Rat Model of Spinal Metastasis. Anticancer Res. 2022, 42, 581–587. [Google Scholar] [CrossRef]
- Gao, X.; Yang, Z.; Xu, C.; Yu, Q.; Wang, M.; Song, J.; Wu, C.; Chen, M. GeneChip expression profiling identified OLFML2A as a potential therapeutic target in TNBC cells. Ann. Transl. Med. 2022, 10, 274. [Google Scholar] [CrossRef]
- Di Donato, M.; Galasso, G.; Giovannelli, P.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Targeting the Nerve Growth Factor Signaling Impairs the Proliferative and Migratory Phenotype of Triple-Negative Breast Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 676568. [Google Scholar] [CrossRef]
- Johansson, M.; Jonsson, M.; Norrgard, O.; Forsgren, S. New aspects concerning ulcerative colitis and colonic carcinoma: Analysis of levels of neuropeptides, neurotrophins, and TNFalpha/TNF receptor in plasma and mucosa in parallel with histological evaluation of the intestine. Inflamm. Bowel. Dis. 2008, 14, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- De Farias, C.; Stertz, L.; Lima, R.; Kapczinski, F.; Schwartsmann, G.; Roesler, R. Reduced NGF Secretion by HT-29 Human Colon Cancer Cells Treated with a GRPR Antagonist. Protein Pept. Lett. 2009, 16, 650–652. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Rosenberg, R.; Boldis, A.; Yildiz, E.; Kujundzic, K.; Kehl, T.; Dischl, D.; Schuster, T.; Maak, M.; et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res. 2013, 19, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pan, Y.; He, L.; Zhai, H.; Li, X.; Zhao, L.; Sun, L.; Liu, J.; Hong, L.; Song, J.; et al. P75 Neurotrophin Receptor Inhibits Invasion and Metastasis of Gastric Cancer. Mol. Cancer Res. 2007, 5, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Dou, K.F.; Peng, S.Y.; Qian, B.Z.; Xiao, H.S.; Liu, F.; Wang, W.Z.; Guan, W.X.; Gao, Z.Q.; Liu, Y.B.; et al. Expression of NGF family and their receptors in gastric carcinoma: A cDNA microarray study. World J. Gastroenterol. 2003, 9, 1431–1434. [Google Scholar] [CrossRef]
- Dou, N.; Yang, D.; Yu, S.; Wu, B.; Gao, Y.; Li, Y. SNRPA enhances tumour cell growth in gastric cancer through modulating NGF expression. Cell Prolif. 2018, 51, e12484. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Taylor, Y.; Macchini, M.; Middelholff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Noh, S.J.; Kim, K.M.; Jang, K.Y. Individual and co-expression patterns of nerve growth factor and heme oxygenase-1 predict shorter survival of gastric carcinoma patients. Diagn. Pathol. 2017, 12, 48. [Google Scholar] [CrossRef]
- Dudás, J.; Dietl, W.; Romani, A.; Reinold, S.; Glueckert, R.; Schrott-Fischer, A.; Dejaco, D.; Chacko, L.J.; Tuertscher, R.; Schartinger, V.H.; et al. Nerve growth factor (NGF)—Receptor survival axis in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 1771. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Cox, D.P.; Dekker, N.; Schmidt, B.L. Nerve Growth Factor and Tyrosine Kinase A Receptor in Oral Squamous Cell Carcinoma: Is There an Association with Perineural Invasion? J. Oral Maxillofac. Surg. 2010, 68, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, M.; Jiang, Y.; Yang, L.; Lei, D.; Lu, C.; Zhao, Y.; Zhang, P.; Yang, Y.; Li, J. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: Expression patterns and effects on in vitro invasive behavior. J. Oral. Maxillofac. Surg. 2006, 64, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulou, M.; Zolota, V.; Chondrogianni, C.; Gatzounis, G.; Varakis, J. p75NTR and TrkC neurotrophin receptors demonstrate a different immunoreactivity profile in comparison to TrkA and TrkB receptors in human normal pituitary gland and adenomas. Neuroendocrinology 2008, 88, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Roselli, S.; Demont, Y.; Pundavela, J.; Choquet, G.; Leissner, P.; Oldmeadow, C.; Attia, J.; Walker, M.M.; Hondermarck, H. ProNGF is a potential diagnostic biomarker for thyroid cancer. Oncotarget 2016, 7, 28488–28497. [Google Scholar] [CrossRef]
- Griffin, N.; Gao, F.; Jobling, P.; Oldmeadow, C.; Wills, V.; Walker, M.M.; Faulkner, S.; Hondermarck, H. The neurotrophic tyrosine kinase receptor 1 (TrkA) is overexpressed in oesophageal squamous cell carcinoma. Pathology 2021, 53, 470–477. [Google Scholar] [CrossRef]
- Ye, Y.; Dang, D.; Zhang, J.; Viet, C.T.; Lam, D.K.; Dolan, J.C.; Gibbs, J.L.; Schmidt, B.L. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Cancer Ther. 2011, 10, 1667–1676. [Google Scholar] [CrossRef]
- Søland, T.M.; Brusevold, I.J.; Koppang, H.S.; Schenck, K.; Bryne, M. Nerve growth factor receptor (p75NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology 2008, 53, 62–72. [Google Scholar] [CrossRef]
- Alzawi, A.; Iftikhar, A.; Shalgm, B.; Jones, S.; Ellis, I.; Islam, M. Receptor, Signal, Nucleus, Action: Signals That Pass through Akt on the Road to Head and Neck Cancer Cell Migration. Cancers 2022, 14, 2606. [Google Scholar] [CrossRef]
- Herbrich, S.M.; Kannan, S.; Nolo, R.M.; Hornbaker, M.; Chandra, J.; Zweidler-McKay, P.A. Characterization of TRKA signaling in acute myeloid leukemia. Oncotarget 2018, 9, 30092–30105. [Google Scholar] [CrossRef]
- Lebedev, T.D.; Vagapova, E.R.; Popenko, V.I.; Leonova, O.G.; Spirin, P.V.; Prassolov, V.S. Two receptors, two isoforms, two cancers: Comprehensive analysis of kit and trka expression in neuroblastoma and acute myeloid leukemia. Front. Oncol. 2019, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Hillis, J.; O’Dwyer, M.; Gorman, A.M. Neurotrophins and B-cell malignancies. Cell Mol. Life Sci. 2016, 73, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Franzese, O.; Di Francesco, A.M.; Meco, D.; Graziani, G.; Cusano, G.; Levati, L.; Riccardi, R.; Ruggiero, A. hTERT transduction extends the lifespan of primary pediatric low-grade glioma cells while preserving the biological response to NGF. Pathol. Oncol. Res. 2021, 27, 612375. [Google Scholar] [CrossRef] [PubMed]
- Damm, R.; Pech, M.; Cavalli, P.; Haag, F.; Gylstorff, S.; Omari, J.; Thormann, M.; Seidensticker, R.; Ricke, J.; Seidensticker, M.; et al. Correlation of chemokines and growth factors with radiation-induced liver injury after interstitial high dose rate (HDR) brachytherapy of liver metastases. J. Cancer Res. Clin. Oncol. 2022, 148, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Kishibe, K.; Yamada, Y.; Ogawa, K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology 2002, 122, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Morita, M.; Mikami, S.; Shibayama, E.; Uchikoshi, T. Immunohistochemical analysis of TrkA neurotrophin receptor expression in human non-neuronal carcinomas. Pathol. Int. 1998, 48, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Trim, N.; Morgan, S.; Evans, M.; Issa, R.; Fine, D.; Afford, S.; Winkins, B.; Iredale, J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am. J. Pathol. 2000, 156, 1235–1243. [Google Scholar] [CrossRef]
- Rasi, G.; Serafino, A.; Bellis, L.; Lonardo, M.T.; Andreola, F.; Zonfrillo, M.; Vennarecci, G.; Pierimarchi, P.; Sinibaldi Vallebona, P.; Ettorre, G.M.; et al. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 4986–4995. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Giordano, M.; Paladina, I.; Berretta, M.; Cappellani, A.; Malaguarnera, M. Serum markers of hepatocellular carcinoma. Dig. Dis. Sci. 2010, 55, 2744–2755. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, S.G.; Zhu, L.F.; Zhang, F.X.; Li, W.; Zhao, K.; Wen, X.X.; Yu, M.; Zhan, Y.K.; Chen, H.; et al. A selective c-Met and Trks inhibitor Indo5 suppresses hepatocellular carcinoma growth. J. Exp. Clin. Cancer Res. 2019, 38, 130. [Google Scholar] [CrossRef]
- Natri, H.M.; Wilson, M.A.; Buetow, K.H. Distinct molecular etiologies of male and female hepatocellular carcinoma. BMC Cancer 2019, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.M.; Jondal, D.E.; Butters, K.A.; Knudsen, B.E.; Anderson, J.L.; Roberts, L.R.; Callstrom, M.R.; Woodrum, D.A. Heat stress and thermal ablation induce local expression of nerve growth factor inducible (VGF) in hepatocytes and hepatocellular carcinoma: Preclinical and clinical studies. Gene Expr. 2018, 19, 37–47. [Google Scholar] [CrossRef]
- Tokusashi, Y.; Asai, K.; Tamakawa, S.; Yamamoto, M.; Yoshie, M.; Yaginuma, Y.; Miyokawa, N.; Aoki, T.; Kino, S.; Kasai, S.; et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int. J. Cancer 2005, 114, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Owusu Sekyere, S.; Port, K.; Deterding, K.; Cornberg, M.; Wedemeyer, H. Inflammatory patterns in plasma associate with hepatocellular carcinoma development in cured hepatitis C cirrhotic patients. United Eur. Gastroenterol. J. 2021, 9, 486–496. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Y.; Zhou, J.; Xie, M.; Zhang, Z.; Su, C. Influence of Exosomes on Astrocytes in the Pre-Metastatic Niche of Lung Cancer Brain Metastases. Biol. Proced. Online 2023, 25, 5. [Google Scholar] [CrossRef]
- Ricci, A.; Salvucci, C.; Castelli, S.; Carraturo, A.; de Vitis, C.; D’Ascanio, M. Adenocarcinomas of the Lung and Neurotrophin System: A Review. Biomedicines 2022, 10, 2531. [Google Scholar] [CrossRef]
- Gao, F.; Griffin, N.; Faulkner, S.; Rowe, C.W.; Williams, L.; Roselli, S.; Thorne, R.F.; Ferdoshi, A.; Jobling, P.; Walker, M.M.; et al. The neurotrophic tyrosine kinase receptor TrkA and its ligand NGF are increased in squamous cell carcinomas of the lung. Sci. Rep. 2018, 8, 8135. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Capelletti, M.; Le, A.T.; Kako, S.; Butaney, M.; Ercan, D.; Mahale, S.; Davies, K.D.; Aisner, D.L.; Pilling, A.B.; et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 2013, 19, 1469–1472. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, M.; Li, H.; Chen, L. Entrectinib, a new multi-target inhibitor for cancer therapy. Biomed. Pharmacother. 2022, 150, 112974. [Google Scholar] [CrossRef]
- Ricci, A.; Greco, S.; Mariotta, S.; Felici, L.; Bronzetti, E.; Cavazzana, A.; Cardillo, G.; Amenta, F.; Bisetti, A.; Barbolini, G. Neurotrophins and neurotrophin receptors in human lung cancer. Am. J. Respir. Cell Mol. Biol. 2001, 25, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Retamales-Ortega, R.; Oróstica, L.; Vera, C.; Cuevas, P.; Hernández, A.; Hurtado, I.; Vega, M.; Romero, C. Role of nerve growth factor (NGF) and miRNAs in epithelial ovarian cancer. Int. J. Mol. Sci. 2017, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.B.; Fredes, A.N.; Garrido, M.P.; Romero, C. Role of mitochondria in interplay between ngf/trka, mir-145 and possible therapeutic strategies for epithelial ovarian cancer. Life 2022, 12, 8. [Google Scholar] [CrossRef]
- Garrido, M.P.; Salvatierra, R.; Valenzuela-Valderrama, M.; Vallejos, C.; Bruneau, N.; Hernández, A.; Vega, M.; Selman, A.; Quest, A.F.G.; Romero, C. Metformin reduces ngf-induced tumour promoter effects in epithelial ovarian cancer cells. Pharmaceuticals 2020, 13, 315. [Google Scholar] [CrossRef]
- Garrido, M.P.; Torres, I.; Avila, A.; Chnaiderman, J.; Valenzuela-Valderrama, M.; Aramburo, J.; Orostica, L.; Duran-Jara, E.; Lobos-Gonzalez, L.; Romero, C. Ngf/trka decrease mir-145-5p levels in epithelial ovarian cancer cells. Int. J. Mol. Sci. 2020, 21, 7657. [Google Scholar] [CrossRef]
- Wijaya, L.K.; Stumbles, P.A.; Drummond, P.D. A positive feedback loop between alpha1-adrenoceptors and inflammatory cytokines in keratinocytes. Exp. Cell Res. 2020, 391, 112008. [Google Scholar] [CrossRef]
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (trkA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Hou, R.; Nie, Y.; Chen, R. Nerve growth factor and its receptors on onset and diagnosis of ovarian cancer. Oncol. Lett. 2017, 14, 2864–2868. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Minturn, J.E.; Ho, R.; Simpson, A.M.; Iyer, R.; Varela, C.R.; Light, J.E.; Kolla, V.; Evans, A.E. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 2009, 15, 3244–3250. [Google Scholar] [CrossRef]
- Hoehner, J.C.; Olsen, L.; Sandstedt, B.; Kaplan, D.R.; Pahlman, S. Association of neurotrophin receptor expression and differentiation in human neuroblastoma. Am. J. Pathol. 1995, 147, 102–113. [Google Scholar]
- Nakagawara, A.; Arima, M.; Azar, C.G.; Scavarda, N.J.; Brodeur, G.M. Inverse Relationship between trk Expression and N-myc Amplification in Human Neuroblastomas. Cancer Res. 1992, 52, 1364–1368. [Google Scholar]
- Dzieran, J.; Garcia, A.R.; Westermark, U.K.; Henley, A.B.; Sánchez, E.E.; Träger, C.; Johansson, H.J.; Lehtiö, J.; Arsenian-Henriksson, M. MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E1229–E1238. [Google Scholar] [CrossRef]
- Eggert, A.; Ikegaki, N.; Liu, X.G.; Brodeur, G.M. Prognostic and biological role of neurotrophin- receptor TrkA and TrkB in neuroblastoma. Klin. Padiatr. 2000, 212, 200–205. [Google Scholar] [CrossRef]
- Ho, R.; Minturn, J.E.; Simpson, A.M.; Iyer, R.; Light, J.E.; Evans, A.E.; Brodeur, G.M. The effect of P75 on Trk receptors in neuroblastomas. Cancer Lett. 2011, 305, 76–85. [Google Scholar] [CrossRef]
- Cai, S.; Chen, Q.; Xu, Y.; Zhuang, Q.; Ji, S. Atorvastatin inhibits pancreatic cancer cells proliferation and invasion likely by suppressing neurotrophin receptor signaling. Transl. Cancer Res. 2020, 9, 1439–1447. [Google Scholar] [CrossRef]
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking nerve growth factor signaling reduces the neural invasion potential of pancreatic cancer cells. PLoS ONE 2016, 11, e0165586. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Xu, J.B.; Yang, X.C.; Guo, J.H.; Chen, J.Y.; Shang, Y.; Liu, W.J.; Wang, T.-M.; Zou, L. Serum nerve growth factor level indicates therapeutic efficacy of 125I seed implantation in advanced pancreatic adenocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3385–3390. [Google Scholar]
- Zhang, Y.; Dang, C.; Ma, Q.; Shimahara, Y. Expression of nerve growth factor receptors and their prognostic value in human pancreatic cancer. Oncol. Rep. 2005, 14, 161–171. [Google Scholar]
- Dang, C.; Zhang, Y.; Ma, Q.; Shimahara, Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol. 2006, 21, 850–858. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Y.; Jiang, Y.; Sun, Y.; Zhao, X. Expression of nerve growth factor and tyrosine kinase receptor a and correlation with perineural invasion in pancreatic cancer. J. Gastroenterol. Hepatol. 2008, 23, 1852–1859. [Google Scholar] [CrossRef]
- Lanki, M.; Mustonen, H.; Salmi, M.; Jalkanen, S.; Haglund, C.; Seppänen, H. Serum cytokine profiles in patients with pancreatic cancer and chronic pancreatitis. Pancreatology 2023, 23, 657–662. [Google Scholar] [CrossRef]
- Qin, T.; Xiao, Y.; Qian, W.; Wang, X.; Gong, M.; Wang, Q.; An, R.; Han, L.; Duan, W.; Ma, Q.; et al. HGF/c-Met pathway facilitates the perineural invasion of pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis. 2022, 13, 387. [Google Scholar] [CrossRef]
- Baker, D.L.; Molenaar, W.M.; Trojanowski, J.Q.; Evans, A.E.; Ross, A.H.; Rorke, L.B.; Parker, R.J.; Lee, V.M.; Pleasure, D. Nerve growth factor receptor expression in peripheral and central neuroectodermal tumors, other pediatric brain tumors, and during development of the adrenal gland. Am. J. Pathol. 1991, 139, 115–122. [Google Scholar]
- Falsini, B.; Chiaretti, A.; Barone, G.; Piccardi, M.; Pierri, F.; Colosimo, C.; Lazzareschi, I.; Ruggiero, A.; Parisi, V.; Fadda, A.; et al. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: A pilot study including electrophysiology. Neurorehabil. Neural. Repair. 2011, 25, 512–520. [Google Scholar] [CrossRef]
- Al-Ghazzawi, K.; Wessolly, M.; Dalbah, S.; Ketteler, P.; Kiefer, T.; Bechrakis, N.; Leyla, J.; Ting, S.; Biewald, E.; Mairinger, F.D. PDGF, NGF, and EGF as main contributors to tumorigenesis in high-risk retinoblastoma. Front. Oncol. 2023, 13, 1144951. [Google Scholar] [CrossRef]
- Shen, T.; Li, Y.; Wang, D.; Su, Y.; Li, G.; Shang, Z.; Niu, J.; Tan, X. YAP1-TEAD1 mediates the perineural invasion of prostate cancer cells induced by cancer-associated fibroblasts. Biochim. Biophys. Acta—Mol. Basis Dis. 2022, 1868, 166540. [Google Scholar] [CrossRef]
- Di Donato, M.; Giovannelli, P.; Migliaccio, A.; Castoria, G. The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: When the dialogue replaces the monologue. Cell Biosci. 2023, 13, 60. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Guo, S.; Wu, Z.; Zhang, L.; Liu, Y.; Li, X.; Guo, X.; Cao, J.; Yang, C.; et al. FBXO22 Mediates the NGF/TRKA Signaling Pathway in Bone Metastases in Prostate Cancer. Am. J. Pathol. 2023, 193, 1248–1266. [Google Scholar] [CrossRef]
- Di Donato, M.; Cernera, G.; Auricchio, F.; Migliaccio, A.; Castoria, G. Cross-talk between androgen receptor and nerve growth factor receptor in prostate cancer cells: Implications for a new therapeutic approach. Cell Death Discov. 2018, 4, 5. [Google Scholar] [CrossRef]
- Pflug, B.; Djakiew, D. Expression of p75(NTR) in a human prostate epithelial tumor cell line reduces nerve growth factor-induced cell growth by activation of programmed cell death. Mol. Carcinog. 1998, 23, 106–114. [Google Scholar] [CrossRef]
- Pundavela, J.; Demont, Y.; Jobling, P.; Lincz, L.F.; Roselli, S.; Thorne, R.F.; Bond, D.; Bradshaw, R.A.; Walker, M.M.; Hondermarck, H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 2014, 184, 3156–3162. [Google Scholar] [CrossRef]
- Chen-Tsai, C.P.; Colome-Grimmer, M.; Wagner, R.F. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75NGFR, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol. Surg. 2004, 30, 1009–1016. [Google Scholar] [CrossRef]
- Pasini, L.; Re, A.; Tebaldi, T.; Ricci, G.; Boi, S.; Adami, V.; Barbareschi, M.; Quattrone, A. TrkA is amplified in malignant melanoma patients and induces an anti-proliferative response in cell lines. BMC Cancer 2015, 15, 777. [Google Scholar] [CrossRef]
- Chan, M.M.; Tahan, S.R. Low-affinity nerve growth factor receptor (P75 NGFR) as a marker of perineural invasion in malignant melanomas. J. Cutan. Pathol. 2010, 37, 336–343. [Google Scholar] [CrossRef]
- Wehkamp, U.; Stern, S.; Krüger, S.; Weichenthal, M.; Hauschild, A.; Röcken, C.; Egberts, F. Co-expression of NGF and PD-L1 on tumor-associated immune cells in the microenvironment of Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 1301–1308. [Google Scholar] [CrossRef]
- Bröcker, E.B.; Magiera, H.; Herlyn, M. Nerve growth and expression of receptors for nerve growth factor in tumors of melanocyte origin. J. Investig. Dermatol. 1991, 96, 662–665. [Google Scholar] [CrossRef]
- Mirkina, I.; Hadzijusufovic, E.; Krepler, C.; Mikula, M.; Mechtcheriakova, D.; Strommer, S.; Stella, A.; Jensen-Jarolim, E.; Holler, C.; Wacheck, V.; et al. Phenotyping of human melanoma cells reveals a unique composition of receptor targets and a subpopulation co-expressing ErbB4, EPO-R and NGF-R. PLoS ONE 2014, 9, e84417. [Google Scholar] [CrossRef]
- Innominato, P.F.; Libbrecht, L.; van den Oord, J.J. Expression of neurotrophins and their receptors in pigment cell lesions of the skin. J. Pathol. 2001, 194, 95–100. [Google Scholar] [CrossRef]
- Sigal, A.C.; Keenan, M.; Lazova, R. P75 nerve growth factor receptor as a useful marker to distinguish spindle cell melanoma from other spindle cell neoplasms of sun-damaged skin. Am. J. Dermatopathol. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Lazova, R.; Tantcheva-Poor, I.; Sigal, A.C. P75 nerve growth factor receptor staining is superior to S100 in identifying spindle cell and desmoplastic melanoma. J. Am. Acad. Dermatol. 2010, 63, 852–858. [Google Scholar] [CrossRef]
- Truzzi, F.; Marconi, A.; Lotti, R.; Dallaglio, K.; French, L.E.; Hempstead, B.L.; Pincelli, C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Investig. Dermatol. 2008, 128, 2031–2040. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, K.; Chen, D.; Wu, X.; Jiang, S. Targeting tropomyosin receptor kinase for cancer therapy. Eur. J. Med. Chem. 2019, 175, 129–148. [Google Scholar] [CrossRef]
- Ardini, E.; Bosotti, R.; Borgia, A.L.; De Ponti, C.; Somaschini, A.; Cammarota, R.; Amboldi, N.; Raddrizzani, L.; Milani, A.; Magnaghi, P.; et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol. Oncol. 2014, 8, 1495–1507. [Google Scholar] [CrossRef]
- Pinto, A.; Nosé, V.; Rojas, C.; Fan, Y.S.; Gomez-Fernandez, C. Searching for mammary analog secretory carcinoma of salivary gland among its mimics. Mod. Pathol. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Venkat, S.; Fitzpatrick, S.; Drew, P.A.; Bhattacharyya, I.; Cohen, D.M.; Islam, M.N. Secretory Carcinoma of the Oral Cavity: A Retrospective Case Series with Review of Literature. Head Neck Pathol. 2021, 15, 893–904. [Google Scholar] [CrossRef]
- Morerio, C.; Rapella, A.; Rosanda, C.; Tassano, E.; Conte, M.; Gambini, C.; Panarello, C. Differential diagnosis of congenital fibrosarcoma. Cancer Genet. Cytogenet. 2004, 152, 167–168. [Google Scholar] [CrossRef]
- Eguchi, M.; Eguchi-Ishimae, M.; Tojo, A.; Morishita, K.; Suzuki, K.; Sato, Y.; Kudoh, S.; Tanaka, K.; Setoyama, M.; Nagamura, F.; et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood 1999, 93, 1355–1363. [Google Scholar] [CrossRef]
- Eguchi, M.; Eguchi-Ishimae, M. Letter to the editor: Absence of t(12;15) associated ETV6-NTRK3 fusion transcripts in pediatric acute leukemias. Med. Pediatr. Oncol. 2001, 37, 417. [Google Scholar] [CrossRef]
- Créancier, L.; Vandenberghe, I.; Gomes, B.; Dejean, C.; Blanchet, J.C.; Meilleroux, J.; Guimbaud, R.; Selves, J.; Kruczynski, A. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 2015, 365, 107–111. [Google Scholar] [CrossRef]
- Bourgeois, J.M.; Knezevich, S.R.; Mathers, J.A.; Sorensen, P.H.B. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am. J. Surg. Pathol. 2000, 24, 937–946. [Google Scholar] [CrossRef]
- Alam, M.S.; Choi, S.U.; Lee, D.U. Synthesis, anticancer, and docking studies of salicyl-hydrazone analogues: A novel series of small potent tropomyosin receptor kinase A inhibitors. Bioorganic Med. Chem. 2017, 25, 389–396. [Google Scholar] [CrossRef]
- Malekan, M.; Nezamabadi, S.S.; Samami, E.; Mohebalizadeh, M.; Saghazadeh, A.; Rezaei, N. BDNF and its signaling in cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 2621–2636. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: The regulation and intervention. Signal Transduct. Target Ther. 2021, 6, 245. [Google Scholar] [CrossRef]
- Fiore, M.; Messina, M.P.; Petrella, C.; D’Angelo, A.; Greco, A.; Ralli, M.; Ferraguti, G.; Tarani, L.; Ceccanti, M. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: Impact on the Mediterranean diet. J. Funct. Foods 2020, 71, 104012. [Google Scholar] [CrossRef]
- Gasche, J.A.; Hoffmann, J.; Boland, C.R.; Goel, A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int. J. Cancer 2011, 129, 1053–1063. [Google Scholar] [CrossRef]
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin. Immunol. 2018, 196, 59–63. [Google Scholar] [CrossRef]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Ciocan, C.; Gulei, D.; Mehterov, N.; Atanasov, A.G.; Dudea, D.; Berindan-Neagoe, I. Current insights into oral cancer epigenetics. Int. J. Mol. Sci. 2018, 19, 670. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor bdnf, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, H.; Zhang, X.; Tian, X.; Xu, S.; Liu, Y.; Jiang, S.; Liu, X.; Shi, R.; Gong, K.; et al. Involvement of nerve growth factor in mouse hippocampal neuronal cell line (HT22) differentiation and underlying role of DNA methyltransferases. J. Toxicol. Environ. Health Part A 2018, 81, 1116–1122. [Google Scholar] [CrossRef]
- Yuan, H.; Du, S.; Chen, L.; Xu, X.; Wang, Y.; Ji, F. Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPα and contributes to inflammatory hyperalgesia in rats. J. Neuroinflamm. 2020, 17, 34. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Guruprasad, K.P.; Satyamoorthy, K.; Joshi, M.B. Interleukin-6 determines protein stabilization of DNA methyltransferases and alters DNA promoter methylation of genes associated with insulin signaling and angiogenesis. Lab. Investig. 2018, 98, 1143–1158. [Google Scholar] [CrossRef]
- Lian, B.S.X.; Kawasaki, T.; Kano, N.; Ori, D.; Ikegawa, M.; Isotani, A.; Kawai, T. Regulation of Il6 expression by single CpG methylation in downstream of Il6 transcription initiation site. IScience 2022, 25, 104118. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Z.; Tan, B.; Liu, Z.; Zu, Z.; Wu, X.; Bi, Y.; Hu, X. Ibuprofen promotes p75 neurotrophin receptor expression through modifying promoter methylation and N6-methyladenosine-RNA-methylation in human gastric cancer cells. Bioengineered 2022, 13, 14595–14604. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Tarani, L.; Messina, M.P.; Lucarelli, M.; Vitali, M.; De Persis, S.; Greco, A.; Minni, A.; Polimeni, A.; et al. Transgenerational Abnormalities Induced by Paternal Preconceptual Alcohol Drinking. Findings from Humans and Animal Models. Curr. Neuropharmacol. 2021, 19, 1158–1173. [Google Scholar] [CrossRef]
- Daskalakis, M.; Brocks, D.; Sheng, Y.H.; Islam, M.S.; Ressnerova, A.; Assenov, Y.; Milde, T.; Oehme, I.; Witt, O.; Goyal, A.; et al. Reactivation of endogenous retroviral elements via treatment with DNMT-and HDAC-inhibitors. Cell Cycle 2018, 17, 811–822. [Google Scholar] [CrossRef]
- Bellanger, C.; Dubanet, L.; Lise, M.C.; Fauchais, A.L.; Bordessoule, D.; Jauberteau, M.O.; Troutaud, D. Endogenous neurotrophins and Trk signaling in diffuse large B cell lymphoma cell lines are involved in sensitivity to rituximab-induced apoptosis. PLoS ONE 2011, 6, e27213. [Google Scholar] [CrossRef] [PubMed]
- Krytska, K.; Ryles, H.T.; Sano, R.; Raman, P.; Infarinato, N.R.; Hansel, T.D.; Makena, M.R.; Song, M.M.; Patrick Reynolds, C.; Mossé, Y.P. Crizotinib synergizes with chemotherapy in preclinical models of neuroblastoma. Clin. Cancer Res. 2016, 22, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Pacenta, H.L.; Macy, M.E. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug. Des. Dev. Ther. 2018, 12, 3549–3561. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Tan, J.; Tang, L.; Xie, Z.; Chen, G.; Liu, G.; Yuan, L.; Wang, K.-X.; Ding, H.-P.; Qiu, H.; et al. NGF monoclonal antibody DS002 alleviates chemotherapy-induced peripheral neuropathy in rats. Acta Pharmacol. Sin. 2022, 43, 2841–2847. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
| Kind of Tumor | Role of NGF | References |
|---|---|---|
| Brain tumors |
| [81,82,83,84,85,86,87,88,89] |
| Breast Cancer |
| [26,90,91,92,93,94,95,96,97,98,99] |
| Colorectal Cancer |
| [17,47,100,101,102,103] |
| Gastric Cancer |
| [104,105,106,107,108] |
| Head and Neck Cancer |
| [10,44,109,110,111,112,113,114,115,116,117] |
| Leukemias |
| [118,119,120,121] |
| Liver Cancer |
| [80,122,123,124,125,126,127,128,129,130,131,132,133] |
| Lung Cancer |
| [83,134,135,136,137,138,139] |
| Ovarian Cancer |
| [140,141,142,143,144,145,146,147,148,149] |
| Neuroblastoma |
| [24,150,151,152,153,154,155,156] |
| Pancreatic Cancer |
| [13,15,16,157,158,159,160,161,162,163,164,165,166,167] |
| Pediatric tumors |
| [28,36,82,168,169,170] |
| Prostate Cancer |
| [77,171,172,173,174,175,176] |
| Skin tumors |
| [54,177,178,179,180,181,182,183,184,185,186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraguti, G.; Terracina, S.; Tarani, L.; Fanfarillo, F.; Allushi, S.; Caronti, B.; Tirassa, P.; Polimeni, A.; Lucarelli, M.; Cavalcanti, L.; et al. Nerve Growth Factor and the Role of Inflammation in Tumor Development. Curr. Issues Mol. Biol. 2024, 46, 965-989. https://doi.org/10.3390/cimb46020062
Ferraguti G, Terracina S, Tarani L, Fanfarillo F, Allushi S, Caronti B, Tirassa P, Polimeni A, Lucarelli M, Cavalcanti L, et al. Nerve Growth Factor and the Role of Inflammation in Tumor Development. Current Issues in Molecular Biology. 2024; 46(2):965-989. https://doi.org/10.3390/cimb46020062
Chicago/Turabian StyleFerraguti, Giampiero, Sergio Terracina, Luigi Tarani, Francesca Fanfarillo, Sara Allushi, Brunella Caronti, Paola Tirassa, Antonella Polimeni, Marco Lucarelli, Luca Cavalcanti, and et al. 2024. "Nerve Growth Factor and the Role of Inflammation in Tumor Development" Current Issues in Molecular Biology 46, no. 2: 965-989. https://doi.org/10.3390/cimb46020062
APA StyleFerraguti, G., Terracina, S., Tarani, L., Fanfarillo, F., Allushi, S., Caronti, B., Tirassa, P., Polimeni, A., Lucarelli, M., Cavalcanti, L., Greco, A., & Fiore, M. (2024). Nerve Growth Factor and the Role of Inflammation in Tumor Development. Current Issues in Molecular Biology, 46(2), 965-989. https://doi.org/10.3390/cimb46020062












