Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies
Abstract
1. Introduction
2. Ghrelin’s Impact on the GI System
2.1. Synthesis of Ghrelin
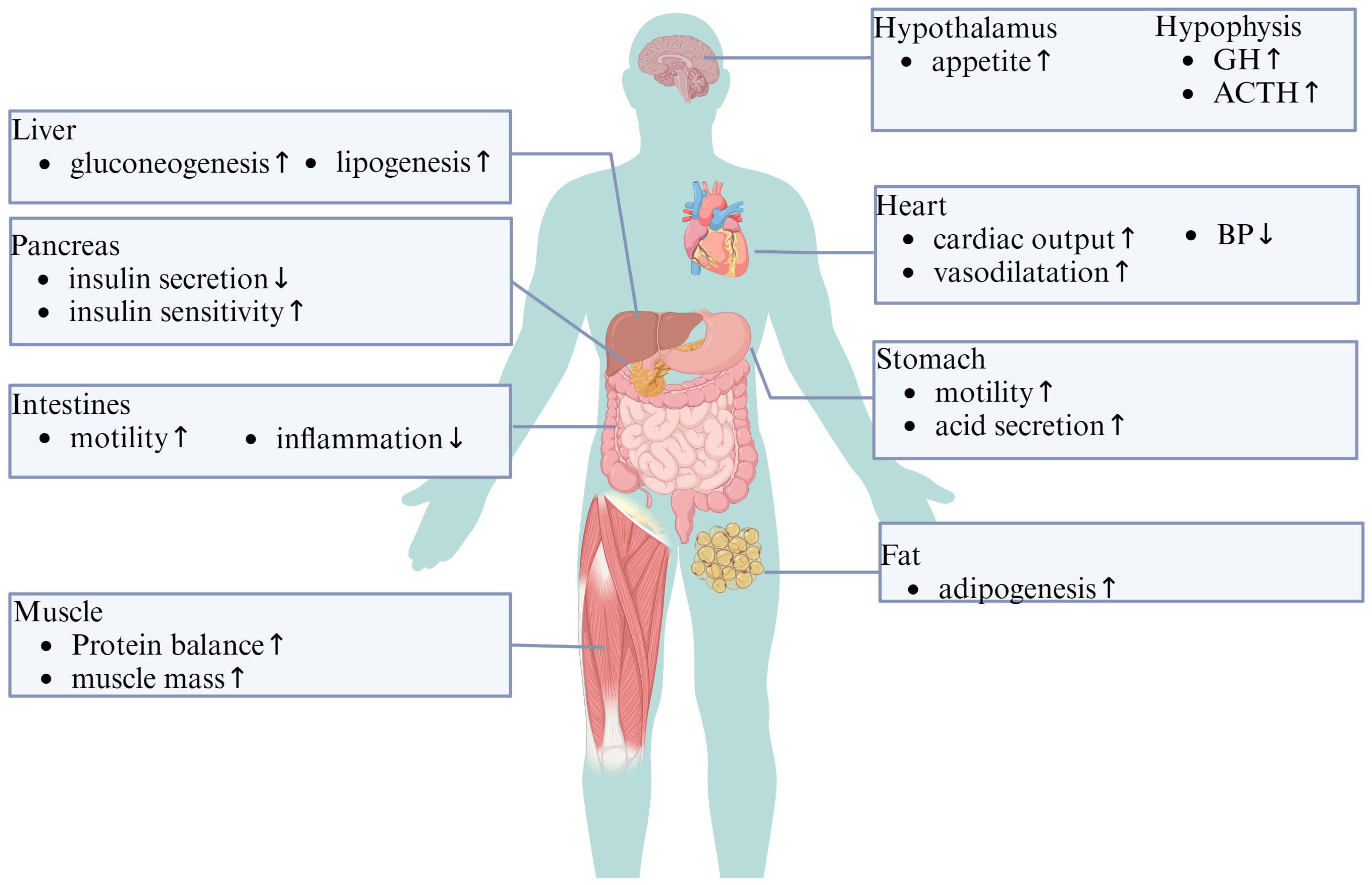
2.2. Physiological Effects of Ghrelin on the GI System
3. The Mechanisms Underlying the Therapeutic Effects of Ghrelin in GI Diseases
3.1. Ghrelin and Autophagy
3.2. Ghrelin and Apoptosis
3.3. Ghrelin and the Inflammatory Response
4. Ghrelin and GI Disorders
4.1. Ghrelin and GI Sepsis
| Main Effect | Mechanism | Ref. |
|---|---|---|
| Tissue perfusion ↑ | Downregulates expression of ET-1 by regulating the NF-κB pathway. | [69] |
| Inflammatory response ↓ | Inhibits sympathetic nerve activity through central nervous system GHS-R. | [70] |
| Inflammatory response ↓ | Inhibits release of HMGB1 by macrophages at the end of sepsis. | [61] |
| Intestinal barrier function ↑ | Activates the vagus nerve through central nervous system GHS-R, which ultimately reduces serum HMGB1. | [62] |
| Autophagy ↑ | Enhances the autophagy of small intestinal epithelial cells. | [36] |
| Intestinal absorption ↑ | 1. Reduces the inflammatory response of intestinal epithelial cells.2. Increases the expression of PepT1. | [71] |
| Immunity ↑ | Increases proliferation of CD4 T cells. | [72] |
| Apoptosis ↓ | Inhibits apoptosis of gastric epithelial cells. | [66] |
| Inflammatory response ↓ | Inhibits inflammation by activating SIRT1 and modulating the KLF4/MMP2 regulatory axis. | [64] |
| Inflammatory response ↓ | Promotes intestinal sepsis by increasing E2F1 and inhibiting the NF-κB pathway. | [63] |
| Intestinal absorption ↑ | Inhibits inflammatory cytokine release by stimulating cholinergic neurons and thus promoting the transcription and translation of PepT1. | [65] |
4.2. Ghrelin and Inflammatory Bowel Disease
4.3. Ghrelin and Gastric Cancer
4.4. Ghrelin and Colorectal Cancer
4.5. Ghrelin and GI Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. An insight into the multifunctional role of ghrelin and structure activity relationship studies of ghrelin receptor ligands with clinical trials. Eur. J. Med. Chem. 2022, 235, 114308. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Mehdar, K.M. The distribution of ghrelin cells in the human and animal gastrointestinal tract: A review of the evidence. Folia Morphol. 2021, 80, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Teive, M.B.; Russi, R.F.; Vieira, D.S.; Teive, A.M.; Costa, A.; d’Acampora, A.J. Quantitative immunohistochemical analysis of duodenal ghrelin cells after sleeve gastrectomy in Wistar rats. Acta Cir. Bras. 2012, 27, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hass, N.; Schwarzenbacher, K.; Breer, H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010, 339, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Nelson, T.A.; Guschina, I.A.; Parsons, L.C.; Lewis, C.L.; Brown, R.C.; Christian, H.C.; Davies, J.S.; Wells, T. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R(1a) activity: Evidence for target cell-induced acylation. Sci. Rep. 2017, 7, 45541. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nakamura, Y.; Shiimura, Y.; Ohgusu, H.; Kangawa, K.; Kojima, M. Structure, regulation and function of ghrelin. J. Biochem. 2012, 151, 119–128. [Google Scholar] [CrossRef]
- Seim, I.; Collet, C.; Herington, A.C.; Chopin, L.K. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genom. 2007, 8, 298. [Google Scholar] [CrossRef]
- Navarro, G.; Aguinaga, D.; Angelats, E.; Medrano, M.; Moreno, E.; Mallol, J.; Cortés, A.; Canela, E.I.; Casadó, V.; McCormick, P.J.; et al. A Significant Role of the Truncated Ghrelin Receptor GHS-R1b in Ghrelin-induced Signaling in Neurons. J. Biol. Chem. 2016, 291, 13048–13062. [Google Scholar] [CrossRef]
- Navarro, G.; Rea, W.; Quiroz, C.; Moreno, E.; Gomez, D.; Wenthur, C.J.; Casadó, V.; Leggio, L.; Hearing, M.C.; Ferré, S. Complexes of Ghrelin GHS-R1a, GHS-R1b, and Dopamine D(1) Receptors Localized in the Ventral Tegmental Area as Main Mediators of the Dopaminergic Effects of Ghrelin. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Furness, J.B. Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol. Rev. 2014, 66, 984–1001. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018, 38, BSR20181061. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, T.; Otani, K.; Miyazato, M.; Kangawa, K. Ghrelin and the heart. Peptides 2019, 111, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, S.; Park, S. Neurogenic Effects of Ghrelin on the Hippocampus. Int. J. Mol. Sci. 2017, 18, 588. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; De Amicis, R.; Pellizzari, M.; Bertoli, S.; Ravella, S.; Battezzati, A. Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter? Biol. Sex Differ. 2022, 13, 25. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef]
- Abot, A.; Cani, P.D.; Knauf, C. Impact of Intestinal Peptides on the Enteric Nervous System: Novel Approaches to Control Glucose Metabolism and Food Intake. Front. Endocrinol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Lin, L.; Nuotio-Antar, A.M.; Ma, X.; Liu, F.; Fiorotto, M.L.; Sun, Y. Ghrelin receptor regulates appetite and satiety during aging in mice by regulating meal frequency and portion size but not total food intake. J. Nutr. 2014, 144, 1349–1355. [Google Scholar] [CrossRef]
- Freeman, J.N.; do Carmo, J.M.; Adi, A.H.; da Silva, A.A. Chronic central ghrelin infusion reduces blood pressure and heart rate despite increasing appetite and promoting weight gain in normotensive and hypertensive rats. Peptides 2013, 42, 35–42. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2011, 62, 139–150. [Google Scholar]
- Date, Y.; Nakazato, M.; Murakami, N.; Kojima, M.; Kangawa, K.; Matsukura, S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem. Biophys. Res. Commun. 2001, 280, 904–907. [Google Scholar] [CrossRef]
- Verbeure, W.; van Goor, H.; Mori, H.; van Beek, A.P.; Tack, J.; van Dijk, P.R. The Role of Gasotransmitters in Gut Peptide Actions. Front. Pharmacol. 2021, 12, 720703. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Porabka, K.; Jaworek, J.; Leja-Szpak, A.; Szklarczyk, J.; Macko, M.; Kot, M.; Mitis-Musioł, M.; Konturek, S.J.; Pawlik, W.W. The effect of luminal ghrelin on pancreatic enzyme secretion in the rat. Regul. Pept. 2007, 143, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Elabadlah, H.; Hameed, R.; D’Souza, C.; Mohsin, S.; Adeghate, E.A. Exogenous Ghrelin Increases Plasma Insulin Level in Diabetic Rats. Biomolecules 2020, 10, 633. [Google Scholar] [CrossRef]
- Kerr, H.L.; Krumm, K.; Lee, I.; Anderson, B.; Christiani, A.; Strait, L.; Breckheimer, B.A.; Irwin, B.; Jiang, A.S.; Rybachok, A.; et al. EXT418, a novel long-acting ghrelin, mitigates Lewis lung carcinoma induced cachexia in mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Higuchi, J.; Honda, N.; Komura, N. Pharmacological profile and clinical efficacy of anamorelin HCl (ADLUMIZ®Tablets), the first orally available drug for cancer cachexia with ghrelin-like action in Japan. Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 2021, 156, 370–381. [Google Scholar] [CrossRef]
- Wang, T.; Liu, K.; Wen, L.; Yang, Y.; Yin, X.; Liu, K.; Chen, Y.; He, Y.; Yang, M.; Wei, Y.; et al. Autophagy and Gastrointestinal Diseases. Adv. Exp. Med. Biol. 2020, 1207, 529–556. [Google Scholar] [CrossRef]
- Liu, H.; Lv, W.; Ouyang, L.; Xu, L. Ghrelin alleviates hypoxia/reoxygenation-induced H9C2 injury by activating autophagy and AMPK/ULK1 pathway. Cell. Mol. Biol. 2023, 69, 139–245. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L. Ghrelin inhibits NLRP3 inflammasome activation by upregulating autophagy to improve Alzheimer’s disease. Vitr. Cell. Dev. Biol. Anim. 2023, 59, 665–673. [Google Scholar] [CrossRef]
- Ezquerro, S.; Frühbeck, G.; Rodríguez, A. Ghrelin and autophagy. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 402–408. [Google Scholar] [CrossRef]
- Thein, W.; Po, W.W.; Choi, W.S.; Sohn, U.D. Autophagy and Digestive Disorders: Advances in Understanding and Therapeutic Approaches. Biomol. Ther. 2021, 29, 353–364. [Google Scholar] [CrossRef]
- Song, B.; Yan, X.; Li, R.; Zhang, H. Ghrelin ameliorates chronic obstructive pulmonary disease-associated infllammation and autophagy. Biotechnol. Appl. Biochem. 2021, 68, 356–365. [Google Scholar] [CrossRef]
- Lu, W.; Cai, H.; Chen, Y.; Liao, X.; Zhang, L.; Ma, T.; Sun, H.; Qi, Y. Ghrelin inhibited pressure overload-induced cardiac hypertrophy by promoting autophagy via CaMKK/AMPK signaling pathway. Peptides 2021, 136, 170446. [Google Scholar] [CrossRef]
- Bonfili, L.; Cuccioloni, M.; Cecarini, V.; Mozzicafreddo, M.; Palermo, F.A.; Cocci, P.; Angeletti, M.; Eleuteri, A.M. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis Int. J. Program. Cell Death 2013, 18, 1188–1200. [Google Scholar] [CrossRef]
- Wan, S.X.; Shi, B.; Lou, X.L.; Liu, J.Q.; Ma, G.G.; Liang, D.Y.; Ma, S. Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed. Pharmacother. 2016, 83, 1315–1320. [Google Scholar] [CrossRef]
- Jiang, M.; Wan, S.; Dai, X.; Ye, Y.; Hua, W.; Ma, G.; Pang, X.; Wang, H.; Shi, B. Protective effect of ghrelin on intestinal I/R injury in rats. Open Med. 2022, 17, 1308–1317. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, G.; Zhao, M.; Wang, S. Ghrelin Mitigates High-Glucose-Induced Oxidative Damage and Apoptosis in Lens Epithelial Cells. J. Diabetes Res. 2022, 2022, 1373533. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Lv, T.; Liu, J.; Ren, Y.; Zhang, J.; Zhang, Y. Effects of Ghrelin on the Apoptosis of Rheumatoid Arthritis Fibroblast-Like Synoviocyte MH7A Cells. Biol. Pharm. Bull. 2019, 42, 158–163. [Google Scholar] [CrossRef]
- Konturek, P.C.; Burnat, G.; Rau, T.; Hahn, E.G.; Konturek, S. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig. Dis. Sci. 2008, 53, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ercan, S.; Basaranlar, G.; Gungor, N.E.; Kencebay, C.; Sahin, P.; Celik-Ozenci, C.; Derin, N. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 56, 154–161. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Slomiany, A. Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediat. Inflamm. 2010, 2010, 280464. [Google Scholar] [CrossRef][Green Version]
- Jiang, M.; Gao, P.F.; Li, H.Q.; Tian, P.Y.; Fan, X.M. Ghrelin inhibition of ethanol-induced gastric epithelial cell apoptosis is mediated by miR-21. Int. J. Clin. Exp. Pathol. 2015, 8, 4662–4672. [Google Scholar] [PubMed]
- Zhang, L.; Cheng, J.; Shen, J.; Wang, S.; Guo, C.; Fan, X. Ghrelin Inhibits Intestinal Epithelial Cell Apoptosis Through the Unfolded Protein Response Pathway in Ulcerative Colitis. Front. Pharmacol. 2021, 12, 661853. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, S.; Zhou, L.; Tang, T.; Cheng, Y.; Ao, X.; Tan, L. Diagnostic Accuracy of Plasma Ghrelin Concentrations in Pediatric Sepsis-Associated Acute Respiratory Distress Syndrome: A Single-Center Cohort Study. Front. Pediatr. 2021, 9, 664052. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Wu, C.S.; DeLuca, J.A.A.; Devaraj, S.; Jayaraman, A.; Alaniz, R.C.; Tan, X.D.; Allred, C.D.; Sun, Y. Novel Role of Ghrelin Receptor in Gut Dysbiosis and Experimental Colitis in Aging. Int. J. Mol. Sci. 2022, 23, 2219. [Google Scholar] [CrossRef]
- Francisco, V.; Tovar, S.; Conde, J.; Pino, J.; Mera, A.; Lago, F.; González-Gay, M.A.; Dieguez, C.; Gualillo, O. Levels of the Novel Endogenous Antagonist of Ghrelin Receptor, Liver-Enriched Antimicrobial Peptide-2, in Patients with Rheumatoid Arthritis. Nutrients 2020, 12, 1006. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, D.G.; Li, R.S. Ghrelin attenuates inflammation in diabetic lung disease by TLR4 pathway in vivo and in vitro. BMJ Open Diabetes Res. Care 2023, 11, e003027. [Google Scholar] [CrossRef]
- Chang, R.J.; Wang, H.L.; Qin, M.B.; Liang, Z.H.; He, J.P.; Wei, Y.L.; Fu, H.Z.; Tang, G.D. Ghrelin inhibits IKKβ/NF-κB activation and reduces pro-inflammatory cytokine production in pancreatic acinar AR42J cells treated with cerulein. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 366–375. [Google Scholar] [CrossRef]
- Shao, X.F.; Li, B.; Shen, J.; Wang, Q.F.; Chen, S.S.; Jiang, X.C.; Qiang, D. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int. Immunopharmacol. 2020, 79, 106175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, R.; Qu, Y.; Zhao, J.; Tong, L.; Ye, S.; Qin, Y. Ghrelin ameliorates transformation of hepatic ischemia-reperfusion injury to liver fibrosis by blocking Smad and ERK signalling pathways, and promoting anti-inflammation and anti-oxidation effects. Transpl. Immunol. 2022, 73, 101597. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, H.; Wang, L. Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-κB pathway. Mol. Med. Rep. 2016, 14, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.Q.; Wu, S.V.; Wang, L.; Taché, Y. The ghrelin agonist, HM01 activates central vagal and enteric cholinergic neurons and reverses gastric inflammatory and ileus responses in rats. Neurogastroenterol. Motil. 2023, 35, e14561. [Google Scholar] [CrossRef]
- Mathur, N.; Mehdi, S.F.; Anipindi, M.; Aziz, M.; Khan, S.A.; Kondakindi, H.; Lowell, B.; Wang, P.; Roth, J. Ghrelin as an Anti-Sepsis Peptide: Review. Front. Immunol. 2020, 11, 610363. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Maier, C.; Riedl, M.; Nowotny, P.; Ludvik, B.; Luger, A.; Clodi, M. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J. Clin. Endocrinol. Metab. 2007, 92, 3930–3934. [Google Scholar] [CrossRef]
- Nikitopoulou, I.; Kampisiouli, E.; Jahaj, E.; Vassiliou, A.G.; Dimopoulou, I.; Mastora, Z.; Tsakiris, S.; Perreas, K.; Tzanela, M.; Routsi, C.; et al. Ghrelin alterations during experimental and human sepsis. Cytokine 2020, 127, 154937. [Google Scholar] [CrossRef]
- Koch, A.; Sanson, E.; Helm, A.; Voigt, S.; Trautwein, C.; Tacke, F. Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit. Care 2010, 14, R94. [Google Scholar] [CrossRef]
- Wu, J.; Lyu, B.; Gan, T.; Wang, L.; Zhu, M. Electroacupuncture improves acute bowel injury recovery in rat models. Exp. Ther. Med. 2017, 14, 4655–4662. [Google Scholar] [CrossRef]
- Wu, J.N.; Wu, W.; Jiang, R.L.; Zhu, M.F.; Lei, S.; Lu, B. Effect of electro-acupuncture at zusanli (ST36) on the expression of ghrelin and HMGB1 in the small intestine of sepsis rats. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2014, 34, 1113–1117. [Google Scholar]
- Chorny, A.; Anderson, P.; Gonzalez-Rey, E.; Delgado, M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J. Immunol. 2008, 180, 8369–8377. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dong, W.; Qiang, X.; Wang, H.; Blau, S.A.; Ravikumar, T.S.; Wang, P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit. Care Med. 2009, 37, 2421–2426. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Zhu, L.; Cao, Y.; Dou, Z.; Yu, Q. HDAC5 promotes intestinal sepsis via the Ghrelin/E2F1/NF-κB axis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21368. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dou, Z.; Zhang, L.; Zhu, L.; Cao, Y.; Yu, Q. Ghrelin Alleviates Intestinal Dysfunction in Sepsis Through the KLF4/MMP2 Regulatory Axis by Activating SIRT1. Front. Immunol. 2021, 12, 646775. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Hong, J.; Chen, M.; Zheng, Y.; Lin, Z.; Yang, X.; Sun, R.; Liu, J. Role of cholinergic anti-inflammatory pathway in Ghrelin regulation of peptide transporter 1 expression in small intestinal epithelium of septic rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2022, 34, 1132–1137. [Google Scholar] [CrossRef]
- Li, B.; Lin, Q.; Guo, H.; Liu, L.; Li, Y. Ghrelin regulates sepsis-induced rat acute gastric injury. Mol. Med. Rep. 2019, 19, 5424–5432. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Y.; Zuo, S.; Zhao, W.; Wu, Y.; Ma, X. Enteral nutrition alleviated lipopolysaccharides-induced hypercatabolism through ghrelin/GHS-R1α-POMC. Biochem. Biophys. Res. Commun. 2022, 597, 122–127. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, M.; Xu, Y.; Tang, S.; Li, X.; Chen, Y.; Lu, H.; Gao, T.; Yu, W. Comparison of the effects of different calorie amounts of enteral nutrition in hypercatabolism associated with ghrelin-POMC in endotoxemic rats. Nutr. Metab. 2022, 19, 28. [Google Scholar] [CrossRef]
- Wu, R.; Dong, W.; Zhou, M.; Cui, X.; Hank Simms, H.; Wang, P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc. Res. 2005, 68, 318–326. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, M.; Das, P.; Dong, W.; Ji, Y.; Yang, D.; Miksa, M.; Zhang, F.; Ravikumar, T.S.; Wang, P. Ghrelin inhibits sympathetic nervous activity in sepsis. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1697–E1702. [Google Scholar] [CrossRef]
- Liu, J.; Shi, B.; Shi, K.; Ma, G.; Zhang, H.; Lou, X.; Liu, H.; Wan, S.; Liang, D. Ghrelin upregulates PepT1 activity in the small intestine epithelium of rats with sepsis. Biomed. Pharmacother. 2017, 86, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Aziz, M.; Ochani, M.; Yang, W.L.; Sharma, A.; Wang, P. The protective role of human ghrelin in sepsis: Restoration of CD4 T cell proliferation. PLoS ONE 2018, 13, e0201139. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Babajani, N.; Amirsardari, Z.; Saeedian, B.; Peiman, S.; Berger, N.A.; Behnoush, A.H. Unveiling the Ghrelin and Obestatin Roles in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis Assessing Their Pathogenic Implications and Biomarker Utility. Inflamm. Bowel Dis. 2023, izad202. [Google Scholar] [CrossRef]
- Hosomi, S.; Oshitani, N.; Kamata, N.; Sogawa, M.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Watanabe, T.; Fujiwara, Y.; Maeda, K.; et al. Phenotypical and functional study of ghrelin and its receptor in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- De Smet, B.; Thijs, T.; Moechars, D.; Colsoul, B.; Polders, L.; Ver Donck, L.; Coulie, B.; Peeters, T.L.; Depoortere, I. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2009, 21, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Wang, W.G.; Li, Q.; Tang, M.; Li, J.; Wu, W.T.; Wan, Y.H.; Wang, Z.G.; Bao, S.S.; Fei, J. Growth hormone secretagogue receptor is important in the development of experimental colitis. Cell Biosci. 2015, 5, 12. [Google Scholar] [CrossRef]
- Meister, A.L.; Burkholder, C.R.; Doheny, K.K.; Travagli, R.A. Ghrelin ameliorates the phenotype of newborn rats induced with mild necrotizing enterocolitis. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13682. [Google Scholar] [CrossRef]
- Muthyala, S.; Chapkin, R.S.; Wu, C.; Wu, C.S. Ghrelin Alleviates Experimental Ulcerative Colitis in Old Mice and Modulates Colonocyte Metabolism via PPARγ Pathway. Int. J. Mol. Sci. 2022, 24, 565. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 41–47. [Google Scholar]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Dai, W.; Mao, Y.; Li, S.; Wang, J.; Li, H.; Guo, C.; Fan, X. Ghrelin ameliorates intestinal barrier dysfunction in experimental colitis by inhibiting the activation of nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 2015, 458, 140–147. [Google Scholar] [CrossRef]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 875–885. [Google Scholar]
- Tuchaai, E.; Endres, V.; Jones, B.; Shankar, S.; Klemashevich, C.; Sun, Y.; Wu, C.S. Deletion of ghrelin alters tryptophan metabolism and exacerbates experimental ulcerative colitis in aged mice. Exp. Biol. Med. 2022, 247, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, A.; Yazdanbod, A.; Lee, Y.Y.; Boreiri, M.; Samadi, F.; Alizadeh, B.Z.; Islami, F.; Fyfe, V.; Babaei, M.; Namazi, M.J.; et al. Serum ghrelin; a new surrogate marker of gastric mucosal alterations in upper gastrointestinal carcinogenesis. PLoS ONE 2013, 8, e74440. [Google Scholar] [CrossRef]
- Pritchett, N.R.; Maziarz, M.; Shu, X.O.; Kamangar, F.; Dawsey, S.M.; Fan, J.H.; Ji, B.T.; Gao, Y.T.; Xiang, Y.B.; Qiao, Y.L.; et al. Serum ghrelin and esophageal and gastric cancer in two cohorts in China. Int. J. Cancer 2020, 146, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Yu, J.; Zhang, Q.; Wang, Y.; Xia, Y.; Dong, J. Circulating ghrelin levels in patients with gastric cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1255112. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.W.; Byun, J.; Jeong, J.B.; Kim, B.G.; Lee, K.L. Plasma ghrelin level and plasma ghrelin/obestatin ratio are related to intestinal metaplasia in elderly patients with functional dyspepsia. PLoS ONE 2017, 12, e0175231. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Joukar, F.; Baghaee, M.; Sepehrimanesh, M.; Hojati, A. Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer. Biomol. Concepts 2019, 10, 82–90. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Xie, Y. GHRL as a prognostic biomarker correlated with immune infiltrates and progression of precancerous lesions in gastric cancer. Front. Oncol. 2023, 13, 1142017. [Google Scholar] [CrossRef]
- An, J.Y.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Jin, D.K.; Kim, S. Clinical significance of ghrelin concentration of plasma and tumor tissue in patients with gastric cancer. J. Surg. Res. 2007, 143, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mottershead, M.; Karteris, E.; Barclay, J.Y.; Suortamo, S.; Newbold, M.; Randeva, H.; Nwokolo, C.U. Immunohistochemical and quantitative mRNA assessment of ghrelin expression in gastric and oesophageal adenocarcinoma. J. Clin. Pathol. 2007, 60, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Ye, B.; Wu, F.; Wang, P. High expression of ghrelin and obestatin prepropeptide in tumor tissues predicted adverse overall survival in gastric carcinoma patients. Medicine 2020, 99, e20635. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Feng, L. Ghrelin Regulates Cyclooxygenase-2 Expression and Promotes Gastric Cancer Cell Progression. Comput. Math. Methods Med. 2021, 2021, 5576808. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, L.; Hu, D.; Ji, J. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol. Cell. Biochem. 2013, 382, 163–172. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, V.; Leoncini, R.; Carli, R.; Santoro, A.; Giglioni, S.; Sorbellini, F.; Marzocca, G.; Bernini, A.; Campagna, S.; Marinello, E.; et al. Circulating gastrin and ghrelin levels in patients with colorectal cancer: Correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed. Pharmacother. 2007, 61, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zygulska, A.L.; Furgala, A.; Krzemieniecki, K.; Kaszuba-ZwoiNska, J.; Thor, P. Enterohormonal disturbances in colorectal cancer patients. Neoplasma 2017, 64, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, D.; Theocharis, S.; Moutsios-Rentzos, A.; Kouraklis, G.; Kostakis, A. The role of serum total ghrelin level elevation in colon cancer patients. J. B.U.ON. Off. J. Balk. Union Oncol. 2014, 19, 388–393. [Google Scholar]
- Huang, Q.; Fan, Y.Z.; Ge, B.J.; Zhu, Q.; Tu, Z.Y. Circulating ghrelin in patients with gastric or colorectal cancer. Dig. Dis. Sci. 2007, 52, 803–809. [Google Scholar] [CrossRef]
- Larsson, S.C.; Höijer, J.; Sun, J.; Li, X.; Burgess, S.; Michaëlsson, K. Genome-Wide Association and Two-Sample Mendelian Randomization Analyses of Plasma Ghrelin and Gastrointestinal Cancer Risk. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2023, 32, 1771–1776. [Google Scholar] [CrossRef]
- Stojsavljevic-Shapeski, S.; Virovic-Jukic, L.; Tomas, D.; Duvnjak, M.; Tomasic, V.; Hrabar, D.; Kralj, D.; Budimir, I.; Barsic, N.; Ljubicic, N. Expression of adipokine ghrelin and ghrelin receptor in human colorectal adenoma and correlation with the grade of dysplasia. World J. Gastrointest. Surg. 2021, 13, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Role of the Ghrelin System in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 5380. [Google Scholar] [CrossRef]
- Coppedè, F.; Stoccoro, A.; Lazzarotti, A.; Spisni, R.; Migliore, L. Investigation of GHSR and GHRL methylation in colorectal cancer. Epigenomics 2018, 10, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kanemaru, A.; Fukushima, T.; Yamamoto, K.; Tanaka, H.; Haruyama, Y.; Itoh, H.; Matsumoto, N.; Kangawa, K.; Nakazato, M.; et al. Ghrelin administration suppresses inflammation-associated colorectal carcinogenesis in mice. Cancer Sci. 2015, 106, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Farahani, H.; Mahmoudi, T.; Tabaeian, S.P.; Rezamand, G.; Mohammadbeigi, A.; Dabiri, R.; Nobakht, H.; Rezvan, S.; Mohammadi, F. Circulating Ghrelin Levels and Susceptibility to Colorectal Câncer. Arq. Gastroenterol. 2021, 58, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Huang, C.; Xu, J.; Cai, X. Lentivirus-mediated shRNA interference of ghrelin receptor blocks proliferation in the colorectal cancer cells. Cancer Med. 2016, 5, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.S.; Lin, C.H.; Yang, Y.L.; Wu, M.S.; Chen, B.C. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur. J. Pharmacol. 2016, 776, 124–131. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ashley, S.W.; Robinson, M.K. Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides 2014, 52, 113–121. [Google Scholar] [CrossRef]
- Perboni, S.; Bowers, C.; Kojima, S.; Asakawa, A.; Inui, A. Growth hormone releasing peptide 2 reverses anorexia associated with chemotherapy with 5-fluoruracil in colon cancer cell-bearing mice. World J. Gastroenterol. 2008, 14, 6303–6305. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, S.; Lin, Y.; Ke, Z. Acylated and unacylated ghrelin inhibit apoptosis in myoblasts cocultured with colon carcinoma cells. Oncol. Rep. 2018, 39, 1387–1395. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Sakai, H.; Oka, K.; Sakagami, J.; Okuda, T.; Hattori, C.; Taniguchi, M.; Hara, T.; Tsuji, T.; Komaki, T.; et al. Predictors of response to anamorelin in gastrointestinal cancer patients with cachexia: A retrospective study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2023, 31, 115. [Google Scholar] [CrossRef] [PubMed]
- McInnis, K.; Brown, J.L.; Finlayson, G.; Dent, R.; Doucet, É. Appetite Changes in Weight Regain and Weight Maintenance after Roux-en-Y Gastric Bypass. Obes. Surg. 2022, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Probst, P.; Haselbeck-Köbler, M.; Brandenburg, J.M.; Kalkum, E.; Störzinger, D.; Kessler, J.; Simon, J.J.; Friederich, H.C.; Angelescu, M.; et al. The Problem of Appetite Loss After Major Abdominal Surgery: A Systematic Review. Ann. Surg. 2022, 276, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Gounitsioti, I.S.; Poulimeneas, D.; Grammatikopoulou, M.G.; Kotzamanidis, C.; Gkiouras, K.; Nigdelis, M.P.; Tsolakidis, D.; Papanikolaou, A.; Tarlatzis, B.C.; Bogdanos, D.P.; et al. Objective and Subjective Appetite Assessment in Patients with Gynecological Cancer: A Pre- and Post-Operative Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 10322. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Wang, X.; Wang, C.; Yang, J.; Guan, B. Change in Adipokines and Gastrointestinal Hormones After Bariatric Surgery: A Meta-analysis. Obes. Surg. 2023, 33, 789–806. [Google Scholar] [CrossRef]
- Li, P.; Rao, Z.; Laing, B.; Bunner, W.P.; Landry, T.; Prete, A.; Yuan, Y.; Zhang, Z.T.; Huang, H. Vertical sleeve gastrectomy improves liver and hypothalamic functions in obese mice. J. Endocrinol. 2019, 241, 135–147. [Google Scholar] [CrossRef]
- Zhang, D.H.; Fan, Y.H.; Zhang, Y.Q.; Cao, H. Neuroendocrine and neuroimmune mechanisms underlying comorbidity of pain and obesity. Life Sci. 2023, 322, 121669. [Google Scholar] [CrossRef]
- Upton, K.R.; Riley, L.G. Acute stress inhibits food intake and alters ghrelin signaling in the brain of tilapia (Oreochromis mossambicus). Domest. Anim. Endocrinol. 2013, 44, 157–164. [Google Scholar] [CrossRef]
- Fonseca, D.C.; Sala, P.; Singer, J.; Singer, P.; Torrinhas, R.S.; Waitzberg, D.L. Upregulation of Ghrelin Gene Expression in the Excluded Stomach of Obese Women with Type 2 Diabetes After Roux-en-Y Gastric Bypass in the SURMetaGIT Study. Obes. Surg. 2018, 28, 877–880. [Google Scholar] [CrossRef]
- De Bandt, D.; Rives-Lange, C.; Frigout, Y.; Bergerot, D.; Blanchard, A.; Le Gall, M.; Lacorte, J.M.; Chevallier, J.M.; Czernichow, S.; Poghosyan, T.; et al. Similar Gut Hormone Secretions Two Years after One Anastomosis Gastric Bypass and Roux-en-Y Gastric Bypass: A Pilot Study. Obes. Surg. 2022, 32, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Asaoka, T.; Eguchi, H.; Fukuda, Y.; Iwagami, Y.; Yamada, D.; Miyazaki, Y.; Noda, T.; Takahashi, T.; Gotoh, K.; et al. Plasma ghrelin suppression as an early predictor for postoperative complications after pancreatoduodenectomy. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2018, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tsubouchi, H.; Hamada, T.; Imamura, N.; Hiyoshi, M.; Yano, K.; Kangawa, K.; Nakazato, M.; Nanashima, A. Plasma desacyl ghrelin-to-acyl ghrelin ratio is a predictor of postoperative complications and prognosis after pancreaticoduodenectomy. Oncol. Lett. 2019, 18, 4974–4983. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, S.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Hosoda, H.; Kangawa, K.; Mori, M.; et al. Impact of synthetic ghrelin administration for patients with severe body weight reduction more than 1 year after gastrectomy: A phase II clinical trial. Surg. Today 2016, 46, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Falkén, Y.; Webb, D.L.; Abraham-Nordling, M.; Kressner, U.; Hellström, P.M.; Näslund, E. Intravenous ghrelin accelerates postoperative gastric emptying and time to first bowel movement in humans. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2013, 25, 474–480. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, W.S.; Shao, X.M.; Yang, A.M.; Zhang, F.F.; Fang, J.Q. Effect of Transcutaneous Electrical Acupoint Stimulation on Post-surgical Gastrointestinal Function, Autonomic Nerve Activities and Plasma Brain-gut Peptide Levels in Patients Undergoing Gastrointestinal Surgery. Zhen Ci Yan Jiu Acupunct. Res. 2016, 41, 240–246. [Google Scholar]
- Bianchi, E.; Boekelheide, K.; Sigman, M.; Lamb, D.J.; Hall, S.J.; Hwang, K. Ghrelin ameliorates adhesions in a postsurgical mouse model. J. Surg. Res. 2016, 201, 226–234. [Google Scholar] [CrossRef]
- Yang, C.G.; Yu, S.; Wang, Z.G.; Zheng, Q. Effect of the expression of ghrelin receptors on the postoperative underpowered small intestinal motility in rats. Zhonghua Wei Chang Wai Ke Za Zhi Chin. J. Gastrointest. Surg. 2011, 14, 455–458. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Zhu, L.; Dou, Z.; Hou, Q.; Wang, S.; Yuan, Z.; Li, B. Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies. Curr. Issues Mol. Biol. 2024, 46, 948-964. https://doi.org/10.3390/cimb46010061
Wu W, Zhu L, Dou Z, Hou Q, Wang S, Yuan Z, Li B. Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies. Current Issues in Molecular Biology. 2024; 46(1):948-964. https://doi.org/10.3390/cimb46010061
Chicago/Turabian StyleWu, Wei, Lei Zhu, Zhimin Dou, Qiliang Hou, Sen Wang, Ziqian Yuan, and Bin Li. 2024. "Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies" Current Issues in Molecular Biology 46, no. 1: 948-964. https://doi.org/10.3390/cimb46010061
APA StyleWu, W., Zhu, L., Dou, Z., Hou, Q., Wang, S., Yuan, Z., & Li, B. (2024). Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies. Current Issues in Molecular Biology, 46(1), 948-964. https://doi.org/10.3390/cimb46010061





