The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. The Quantitative HPLC Analysis
2.3. Writhing Test
2.4. Cell Viability and NO Production in RAW 264.7 Cells
2.5. Inflammation-Related Cytokine Production Analysis in RAW264.7
2.6. Western Blot Analysis
2.7. Animals and Experimental Design
2.8. Weight-Bearing Index and Arthritis Clinical Index Analysis
2.9. Gait Analysis
2.10. Serum Biochemical Analysis
2.11. Micro-CT Analysis
2.12. Histological Analysis
2.13. Statistical Analysis
3. Results
3.1. The Quantitative HPLC Analysis
3.2. Writhing Test
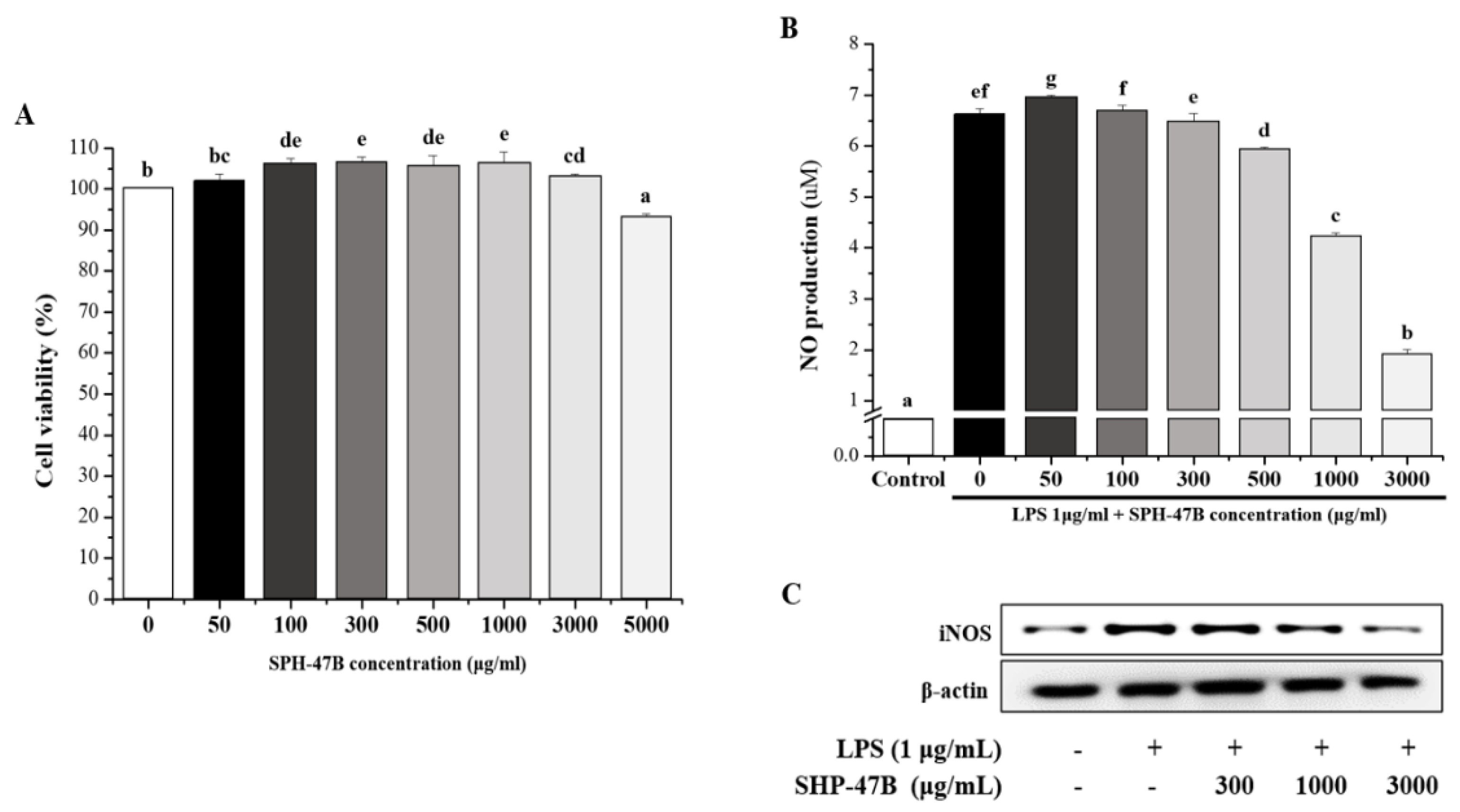
3.3. Cell Viability and NO Production in RAW 264.7 Cells
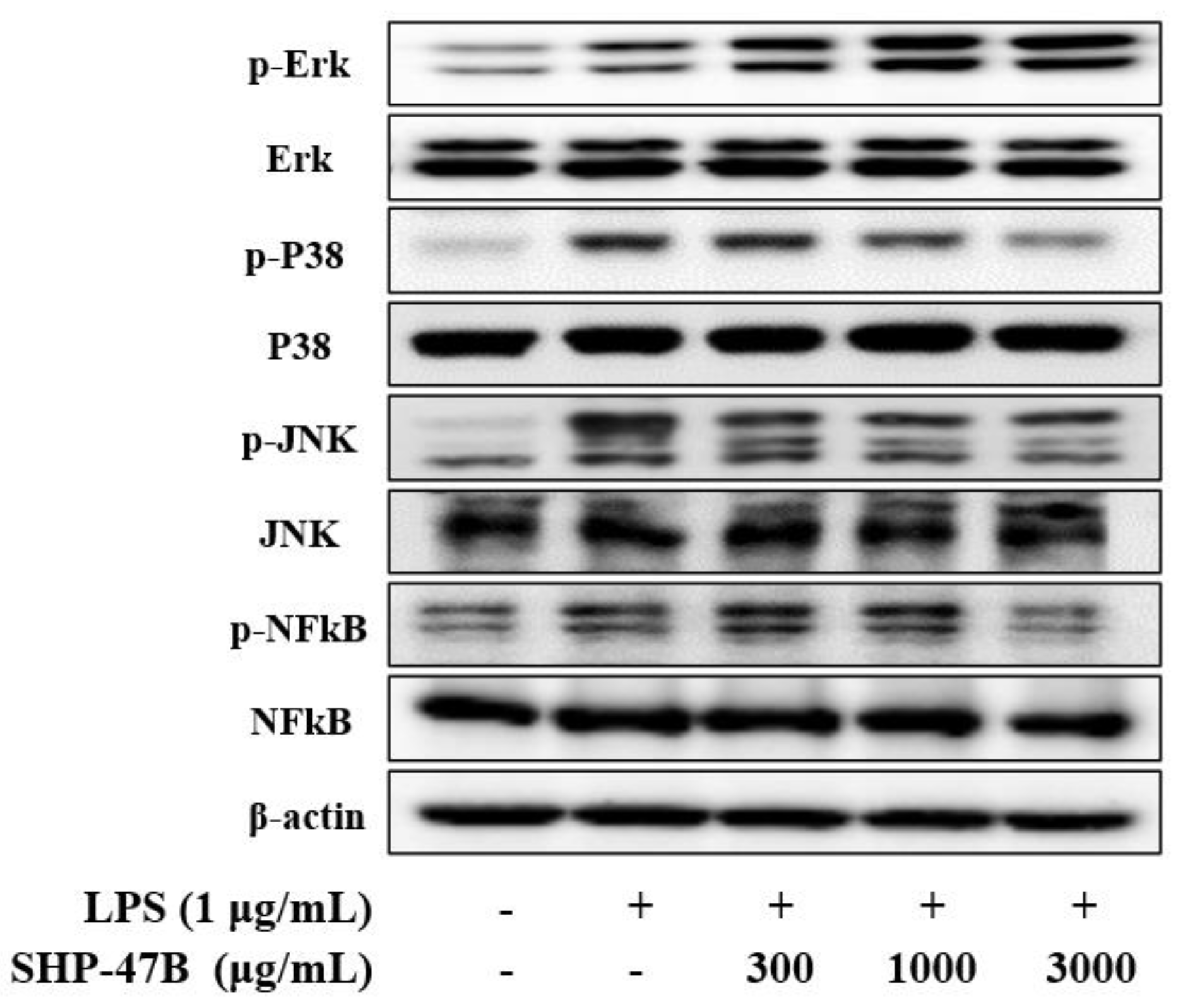
3.4. Western Blot Analysis
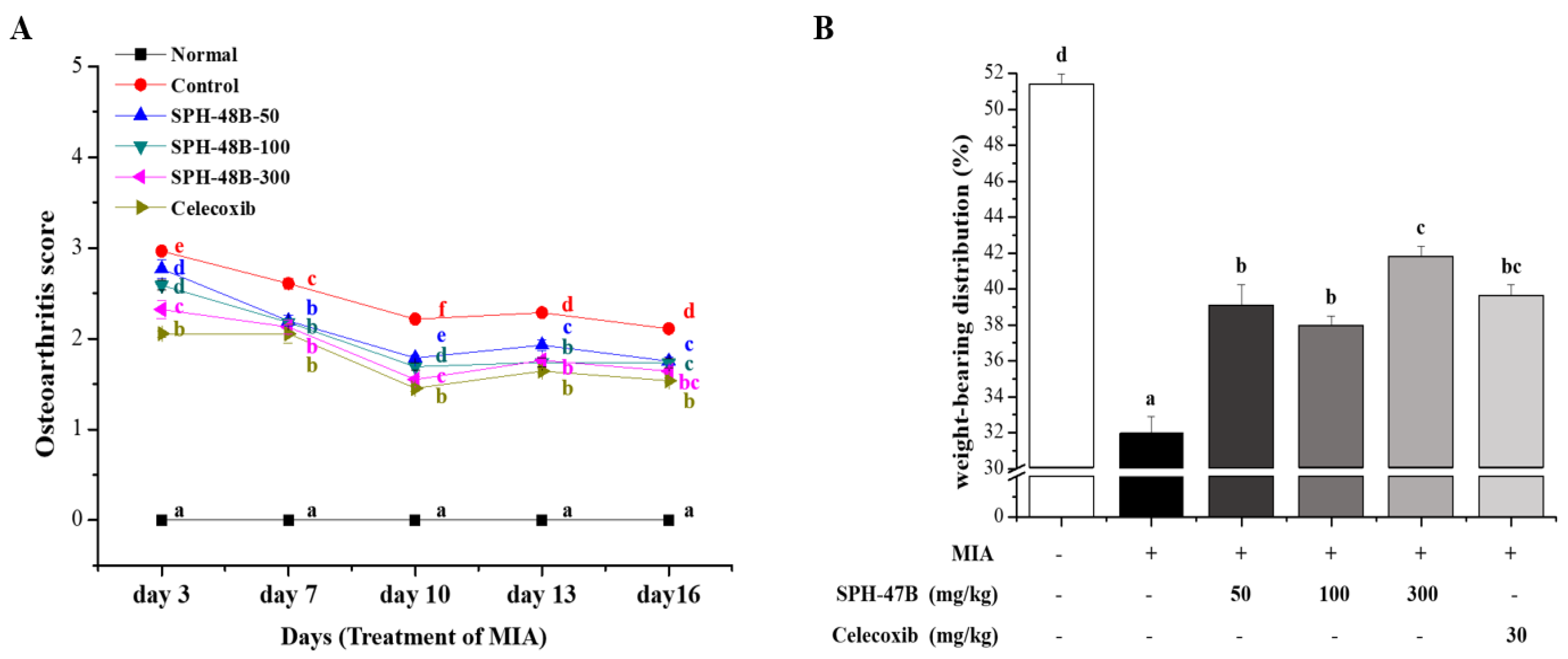
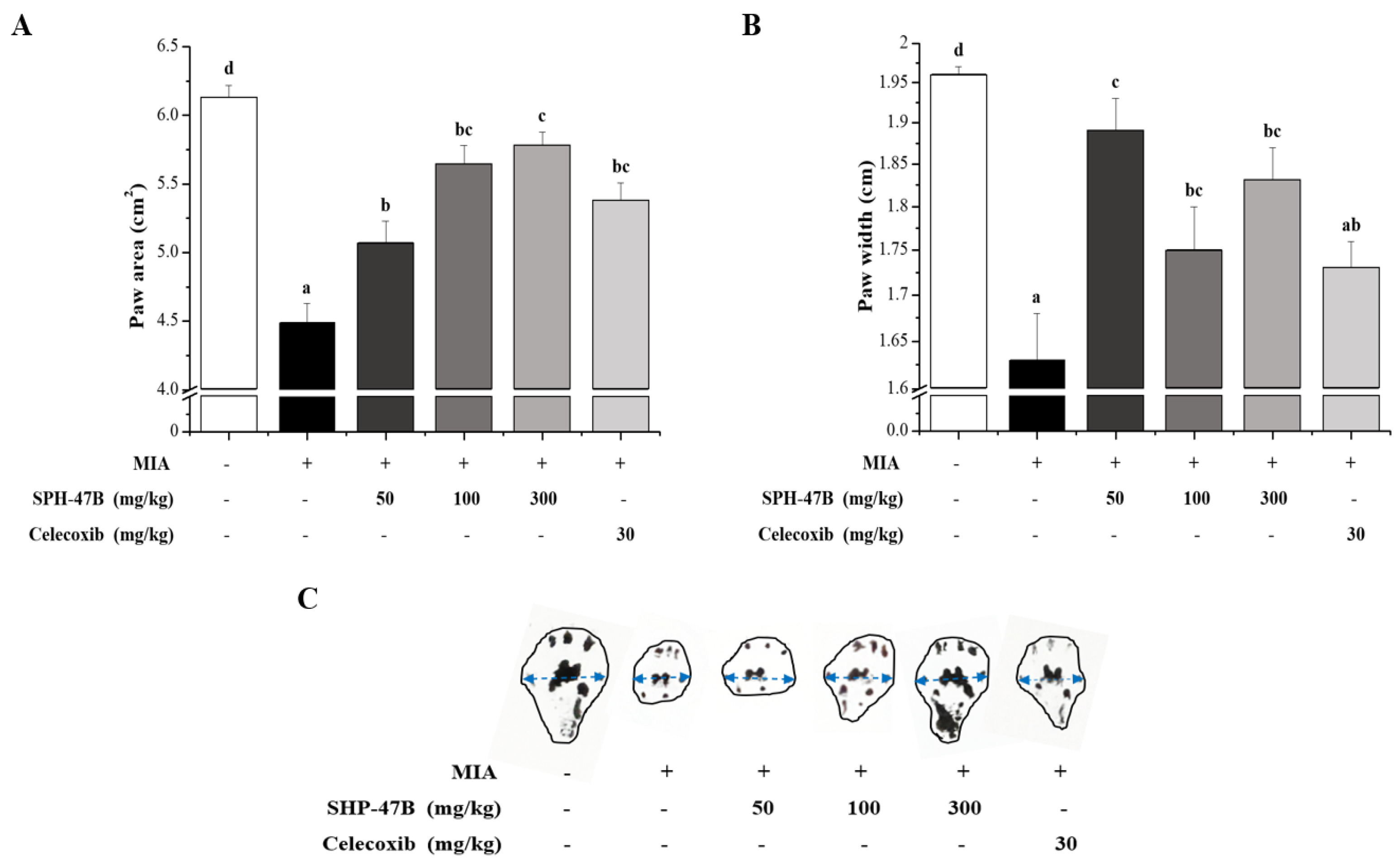
3.5. Weight-Bearing Index and Arthritis Clinical Index Analysis
3.6. Gait Analysis
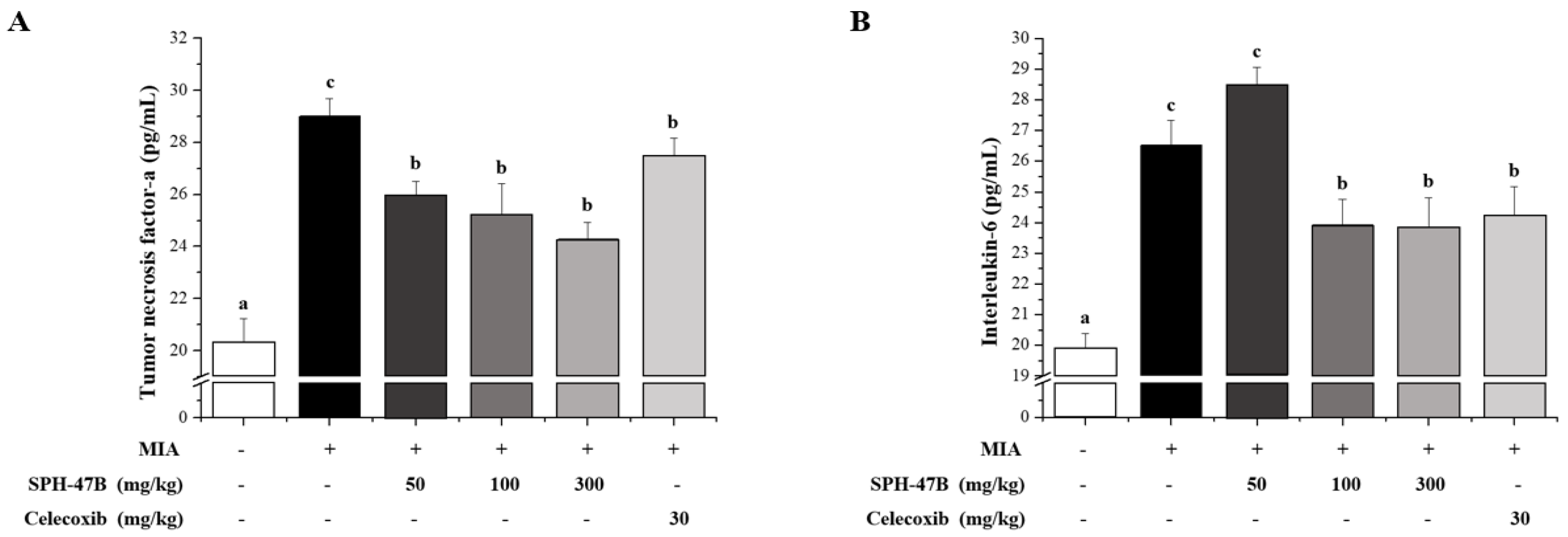
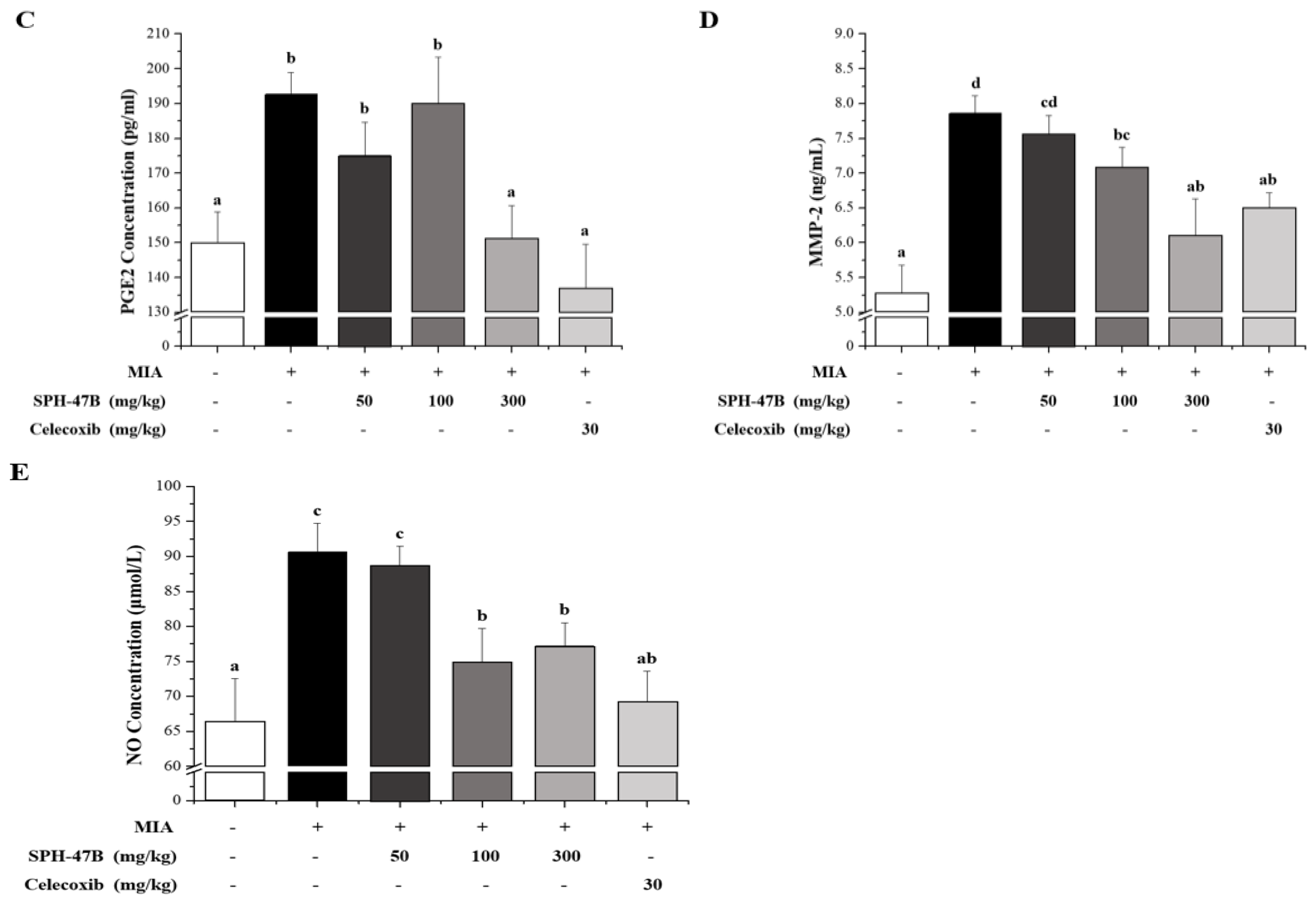
3.7. Serum Biochemical Analysis
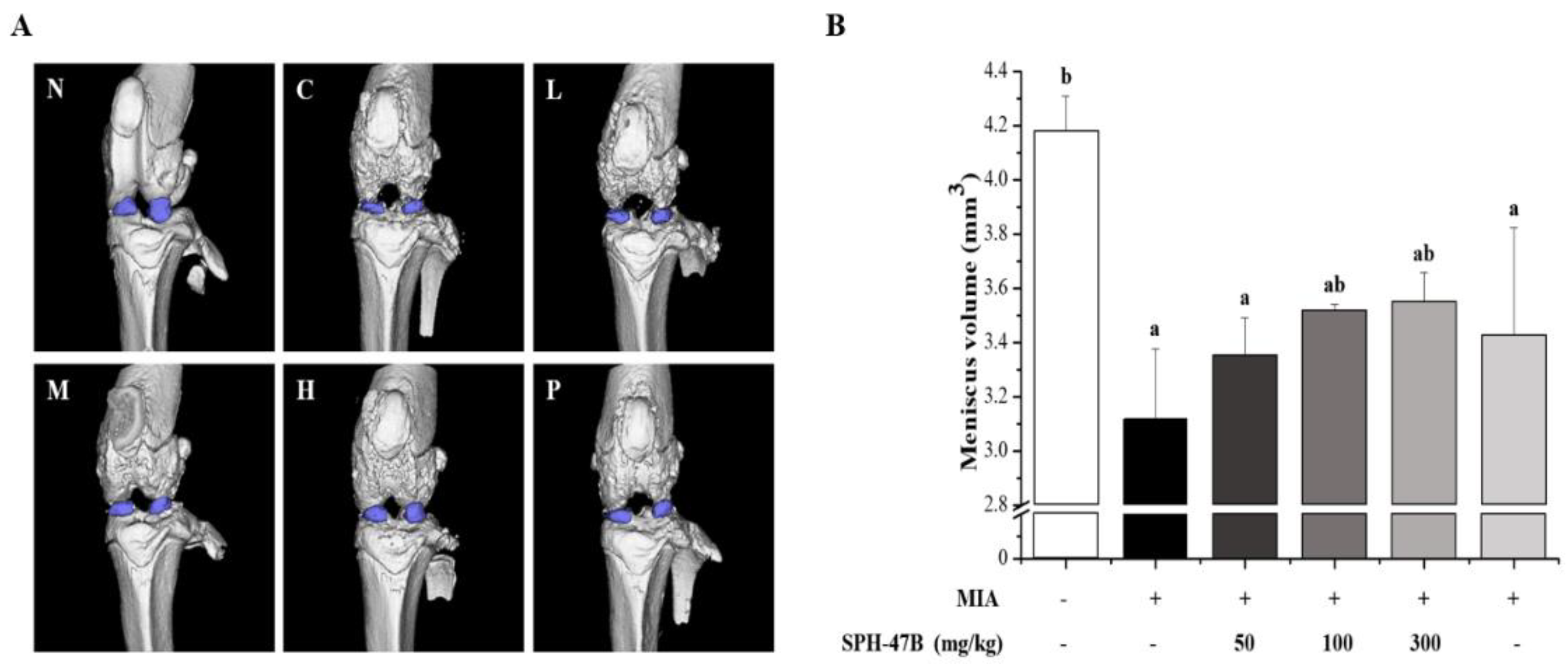
3.8. Micro-CT Analysis
3.9. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, J.C.; Keith, A.; Rice, S.J.; Shepherd, C.; Agarwal, V.; Loughlin, J.; Shendure, J. Functional testing of thousands of osteoarthritis-associated variants for regulatory activity. Nat. Commun. 2019, 10, 2434. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Hu, P.; Chen, P.; Xue, X.; Li, Z.; He, F.; Qiu, Z.; Cheng, J.; Huang, F. Huoxuezhitong capsule ameliorates MIA-induced osteoarthritis of rats through suppressing PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2020, 129, 110471. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Ravaud, J.-F.; Papelard, A.; Ravaud, P.; Poiraudeau, S. The burden of musculoskeletal conditions. Osteoarthr. Cartil. 2014, 9, S218–S219. [Google Scholar] [CrossRef][Green Version]
- Nielsen, L.A.; Schepman, P.; Blakeman, K.H.; Wilhelm, S.; Robinson, R.; Beck, C.; Hansen, J.L.; Rolfson, O. Prescription patterns and predictors of unmet pain relief in patients with difficult-to-treat osteoarthritis in the Nordics: Analyses from the BISCUITS study. Scand. J. Pain 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Grynpas, M.D.; Alpert, B.; Katz, I.; Lieberman, I.; Pritzker, K.P.H. Subchondral bone in osteoarthritis. Calcif. Tissue Int. 1991, 49, 20–26. [Google Scholar] [CrossRef]
- Camacho-Encina, M.; Balboa-Barreiro, V.; Rego-Perez, I.; Picchi, F.; VanDuin, J.; Qiu, J.; Fuentes, M.; Oreiro, N.; LaBaer, J.; Ruiz-Romero, C.; et al. Discovery of an autoantibody signature for the early diagnosis of knee osteoarthritis: Data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 2019, 78, 1699–1705. [Google Scholar] [CrossRef]
- Orlowsky, E.W.; Kraus, V.B. The Role of Innate Immunity in Osteoarthritis: When Our First Line of Defense Goes on the Offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Brown, T.D. Joint Injury, Repair, and Remodeling: Roles in post-traumatic osteoarthritis. Clin. Orthop. Relat. Res. 2004, 423, 7–16. [Google Scholar] [CrossRef]
- Fang, Z.; Li, X.; Lei, S.; Feng, S.; Zhou, C.; Tong, X.; Han, R. Protective effects of Pudilan Tablets against osteoarthritis in mice induced by monosodium iodoacetate. Sci. Rep. 2023, 13, 2760. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Park, K.H.; Kim, M.J.; Yoon, S.M.; Kim, K.N.; Yang, H.J.; Kim, M.J.; Choi, S.-C.; et al. Potential Effect of Enzymatic Porcine Placental Hydrolysate (EPPH) to Improve Alcoholic Liver Disease (ALD) by Promoting Lipolysis in the Liver. Biology 2022, 11, 1012. [Google Scholar] [CrossRef]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-B Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Rosshirt, N.; Reiner, T.; Schiltenwolf, M.; Moradi, B. Inflammation and osteoarthritis-related pain. Schmerz 2019, 33, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiolo-gy. Biorheology 2002, 39, 237–246. [Google Scholar]
- Choi, S.-C.; Lee, I.-A. Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection. Fishes 2021, 6, 34. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar]
- Van Men, C.; Jang, Y.S.; Lee, K.J.; Lee, J.H.; Quang, T.H.; Van Long, N.; Van Luong, H.; Kim, Y.H.; Kang, J.S. Multiple component quantitative analysis for the pattern recognition and quality evaluation of Kalopanacis Cortex using HPLC. Arch. Pharmacal Res. 2011, 34, 2065–2071. [Google Scholar] [CrossRef]
- Edwards, M.H.; van der Pas, S.; Denkinger, M.D.; Parsons, C.; Jameson, K.A.; Schaap, L.; Zambon, S.; Castell, M.-V.; Herbolsheimer, F.; Nasell, H.; et al. Relationships between physical performance and knee and hip osteoarthritis: Findings from the European Project on Osteoarthritis (EPOSA). Age Ageing 2014, 43, 806–813. [Google Scholar] [CrossRef] [PubMed]
- White, D.K.; Niu, J.; Zhang, Y. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis Care Res. 2012, 65, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2017, 56, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.-B.; Kim, J.-H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ju, M.-K.; Kim, H. Commiphora Extract Mixture Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Nutrients 2020, 12, 1477. [Google Scholar] [CrossRef]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Quicke, J.G.; Foster, N.E.; Croft, P.R.; Ogollah, R.O.; Holden, M.A. Change in physical activity level and clinical outcomes in older adults with knee pain: A secondary analysis from a randomised controlled trial. BMC Musculoskelet. Disord. 2018, 19, 59. [Google Scholar] [CrossRef]
- Sano, K.; Sanada, S.; Ida, Y.; Shoji, J. Studies on the constituents of the bark of Kalopanax pictus Nakai. Chem. Pharm. Bull. 1991, 39, 865–870. [Google Scholar] [CrossRef]
- Shao, C.-J.; Kasai, R.; Ohtani, K.; Xu, J.-D.; Tanaka, O. Saponins from leaves of Kalopanax septemlobus (Thunb.) Koidz.: Structures of Kalopanax-saponins La, Lb and Lc. Chem. Pharm. Bull. 1989, 37, 3251–3254. [Google Scholar] [CrossRef]
- Choi, J.; Huh, K.; Kim, S.-H.; Lee, K.-T.; Park, H.-J.; Han, Y.N. Antinociceptive and anti-rheumatoidal effects of Kalopanax pictus extract and its saponin components in experimental animals. J. Ethnopharmacol. 2002, 79, 199–204. [Google Scholar] [CrossRef]
- Um, M.S. The evaluation of Anti-oxidation, Anti-inflammation and Anti-wrinkle activity of Jeju Native Achy-ranthes japonica Nakai. J. Korean Appl. Sci. Technol. 2021, 38, 1209–1218. [Google Scholar]
- Kim, C.S.; Kim, H. Morphometrics and distribution of Achyranthes bidentata complex. Korean J. Med. Crop Sci. 2011, 28, 86–87. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, Y.K. The therapeutic effect of Achyranthis Radix on the collagen-induced arthritis in mice. Korea J. Herbol. 2010, 25, 129–135. [Google Scholar]
- Kim, J.-C.; Choi, G.J.; Lee, S.-W.; Chung, K.Y.; Cho, K.Y. Screening extracts of Achyranthes japonica and Rumex crispus for activity against various plant pathogenic fungi and control of powdery mildew. Pest Manag. Sci. 2004, 60, 803–808. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef]
- Jung, J.I.; Lee, H.S.; Kim, R.; Kim, E.J. Anti-Osteoarthritis Effect of Enriched Boswellia serrata Gum Resin Extract in SW1353 Chondrocytes. J. Korean Soc. Food Sci. Nutr. 2023, 52, 460–472. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, M.R.; Choi, H.J.; Park, H.J.; Choi, H.W.; Kim, H.Y.; Roh, S.S. 3-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia serrata Attenuates Monosodium lodoacetate-induced Osteoarthritis by Chondroprotective and Anti-inflammatory Effect. Kor. J. Herbol. 2022, 37, 27–35. [Google Scholar]
- Lee, N.-K.; Ku, J.-E.; Han, H.S. Cytoprotective and Anti-inflammatory Effects of 6-Shogaol on Human Dermal Fibroblasts. Asian J. Beauty Cosmetol. 2017, 15, 367–376. [Google Scholar] [CrossRef]
- Ministry of Ministry of Food and Drug Safety. Korean Pharmacopoeia, 12th ed.; Ministry of Ministry of Food and Drug Safety: Seoul, Republic of Korea, 2019.
- Jang, W.H.; Lee, W.Y.; Lee, B.J.; Kim, J.M.; Park, S.J. Validation of Simultaneous Analysis Method of Standard Compounds in Femented Kalopanax pictus Nakai by Bioconversion. Korean J. Food Nutr. 2019, 32, 148–154. [Google Scholar]
- Kim, M.Y.; Yoo, Y.M.; Nam, J.H.; Choi, J.W.; Park, H.J. Quantitative Determination on the Constituents of the Stem Bark and the Leaf Shoot of Kalopanax pictus by HPLC Analysis. Korean J. Pharmacogn. 2007, 38, 270–276. [Google Scholar]
- Lee, Y.M.; Son, E.; Kim, S.-H.; Kim, D.-S. Effect of Alpinia oxyphylla extract in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model. Phytomedicine 2019, 65, 153095. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-Iodoacetate-Induced Histologic Changes in Subchondral Bone and Articular Cartilage of Rat Femorotibial Joints: An Animal Model of Osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Jeong, S.Y.; Moon, N.C.; Son, K.H.; Son, J.K.; Woo, M.H. High performance liquid chromatography used for quality control of Achyranthis Radix. Arch. Pharmacal Res. 2012, 35, 1449–1455. [Google Scholar] [CrossRef]
- Boo, K.H.; Lee, D.; Van Nguyen, Q.; Jin, S.B.; Kang, S.; Viet, C.D.; Park, S.P.; Lee, D.-S.; Riu, K.Z. Fluctuation of 20-hydroxyecdysone in individual organs of Achyranthes japonica during reproductive growth stage and its accumulation into seed. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 335–338. [Google Scholar] [CrossRef]
- Boo, K.H.; Lee, D.; Jeon, G.L.; Ko, S.H.; Cho, S.K.; Kim, J.H.; Park, S.P.; Hong, Q.; Lee, S.-H.; Lee, D.-S.; et al. Distribution and Biosynthesis of 20-Hydroxyecdysone in Plants of Achyranthes japonica Nakai. Biosci. Biotechnol. Biochem. 2010, 74, 2226–2231. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Gottschalk, R.A.; Martins, A.J.; Angermann, B.R.; Dutta, B.; Ng, C.E.; Uderhardt, S.; Tsang, J.S.; Fraser, I.D.; Meier-Schellersheim, M.; Germain, R.N. Distinct NF-κB and MAPK Activation Thresholds Uncouple Steady-State Microbe Sensing from Anti-pathogen Inflammatory Responses. Cell Syst. 2016, 2, 378–390. [Google Scholar] [CrossRef]
- Asthana, C.; Peterson, G.M.; Shastri, M.; Patel, R.P. Development and validation of a novel high performance liquid chromatography-coupled with Corona charged aerosol detector method for quantification of glucosamine in dietary supplements. PLoS ONE 2019, 14, e0216039. [Google Scholar] [CrossRef]
- Kumavat, R.; Kumar, V.; Malhotra, R.; Pandit, H.; Jones, E.; Ponchel, F.; Biswas, S. Biomarkers of Joint Damage in Osteoarthritis: Current Status and Future Directions. Mediat. Inflamm. 2021, 2021, 5574582. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Im, G.-I. Intracellular Signal Transduction Pathways and Transcription Factors for Osteogenesis. J. Korean Rheum. Assoc. 2008, 15, 1–10. [Google Scholar] [CrossRef][Green Version]
- Na, J.-Y.; Song, K.-B.; Kim, S.; Kwon, Y.-B.; Kim, D.-G.; Lee, J.-K.; Jo, H.-K.; Kwon, J. Effects of HPL-04 on Degenerative Osteoarthritis. J. Korean Soc. Food Sci. Nutr. 2014, 43, 30–39. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, B.R.; Lee, D.Y.; Choi, S.I.; Lee, Y.S.; Kim, G.S. Inhibitory Effect of Mixed Extracts Obtained from Astragali Radix and Lithospermi Radix on Matrix Metalloproteinases in IL-1β-induced SW1353 Cells and Quantitative Analysis of Active Compounds. Korean J. Med. Crop. Sci. 2019, 27, 247–258. [Google Scholar] [CrossRef]
- Janusz, M.; Hookfin, E.; Heitmeyer, S.; Woessner, J.; Freemont, A.; Hoyland, J.; Brown, K.; Hsieh, L.; Almstead, N.; De, B.; et al. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthr. Cartil. 2001, 9, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Kong, X.; Wang, X.; Tang, Y.; Wang, C.; Wang, M.; Zhang, L.; Liu, Y.; Fei, C.; Xue, F.; et al. Tizoxanide Inhibits Inflammation in LPS-Activated RAW264.7 Macrophages via the Suppression of NF-κB and MAPK Activation. Inflammation 2019, 42, 1336–1349. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, J.; Guo, L.; Xu, Y.; Hu, L.; Mao, H.; Miao, L.; Zhang, H.; Chai, L. Neobavaisoflavone ameliorates LPS-induced RAW264.7 cell inflammations by suppressing the activation of NF-κB and MAPKs signaling pathways. Iran. J. Basic. Med. Sci. 2022, 25, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Son, E.; Kim, S.-H.; Kim, D.-S. Anti-Inflammatory and Analgesic Effects of Schisandra chinensis Leaf Extracts and Monosodium Iodoacetate-Induced Osteoarthritis in Rats and Acetic Acid-Induced Writhing in Mice. Nutrients 2022, 14, 1356. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2020, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Pustovrh, M.C.; Jawerbaum, A.; White, V.; Capobianco, E.; Higa, R.; Martiínez, N.; Loípez-Costa, J.J.; Gonzaílez, E. The role of nitric oxide on matrix metalloproteinase 2 (MMP2) and MMP9 in placenta and fetus from diabetic rats. Reproduction 2007, 134, 605–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radu, A.F.; Bungau, S.G.; Tit, D.M.; Behl, T.; Uivaraseanu, B.; Marcu, M.F. Highlighting the Benefits of Rehabilitation Treatments in Hip Osteoarthritis. Medicina 2022, 58, 494. [Google Scholar] [CrossRef]




| Group | Dose (mg/kg, P.O) | Average No. of Writhing | Decrease in Writhing | Inhibition (%) | |
|---|---|---|---|---|---|
| Control | A | - | 59.00 ± 7.23 de | 0.00 ab | 0 ab |
| HPS-3C | 100 | 54.33 ± 0.88 cde | 4.67 abc | 7.91 abc | |
| SHP-40 | 100 | 35.67 ± 8.19 bc | 23.33 cd | 39.55 cd | |
| SHP-41 | 100 | 43.00 ± 5.13 bcd | 16.00 bcd | 27.12 bcd | |
| SHP-42 | 100 | 47.00 ± 1.15 bcde | 12.00 abcd | 20.34 abcd | |
| SHP-43 | 100 | 15.33 ± 8.41 a | 43.67 e | 74.01 e | |
| SHP-44 | 100 | 58.67 ± 4.37 de | 0.33 ab | 0.56 ab | |
| SHP-45 | 100 | 68.33 ± 6.39 e | −9.33 a | -15.82 a | |
| SHP-46 | 100 | 54.33 ± 10.84 cde | 4.67 abc | 7.91 abc | |
| SHP-47 | 100 | 11.67 ± 7.22 a | 47.33 e | 80.23 e | |
| SHP-48 | 100 | 51.33 ± 6.36 cde | 7.67 abc | 12.99 abc | |
| Indomethacin | 10 | 27.00 ± 3.21 ab | 32.00 de | 54.24 de | |
| SHP-47A | B | 100 | 36.67 ± 0.88 | ||
| SHP-47B | 30.33 ± 4.48 | ||||
| SHP-47C | 32.33 ± 1.20 | ||||
| SHP-47D | 43.00 ± 7.55 | 32.00 de | 54.24 de | ||
| Group | ×103 cell/μL | % of WBC | |||||
|---|---|---|---|---|---|---|---|
| WBC | GRA | LYM | MID | GRA | LYM | MID | |
| Normal | 8.49 ± 0.48 b | 2.03 ± 0.18 c | 6.21 ± 0.51 abc | 0.23 ± 0.02 b | 22.63 ± 1.39 d | 70.67 ± 1.52 a | 2.46 ± 0.17 |
| Control | 7.73 ± 0.34 ab | 1.28 ± 0.05 a | 6.20 ± 0.30 abc | 0.17 ± 0.01 a | 15.21 ± 0.50 a | 80.10 ± 0.95 d | 2.16 ± 0.06 |
| SPH-47B-50 | 8.27 ± 0.38 b | 1.51 ± 0.11 ab | 6.33 ± 0.32 bc | 0.20 ± 0.01 ab | 17.24 ± 1.33 ab | 75.40 ± 1.03 bc | 2.27 ± 0.15 |
| SPH-47B-100 | 8.96 ± 0.56 b | 1.61 ± 0.12 ab | 7.08 ± 0.50 c | 0.20 ± 0.01 ab | 16.61 ± 0.71 ab | 77.69 ± 1.01 cd | 2.17 ± 0.13 |
| SPH-47B-300 | 6.80 ± 0.31 a | 1.41 ± 0.10 ab | 5.14 ± 0.19 a | 0.17 ± 0.02 a | 19.37 ± 0.94 bc | 74.96 ± 0.96 bc | 2.27 ± 0.07 |
| Celecoxib | 7.73 ± 0.29 ab | 1.67 ± 0.05 b | 5.74 ± 0.27 ab | 0.19 ± 0.01 ab | 20.93 ± 0.84 cd | 73.61 ± 0.92 b | 2.44 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Park, Y.-M.; Hwang, H.-M.; Shin, D.-Y.; Jeong, H.-N.; Kim, J.-G.; Park, H.-Y.; Kim, D.-S.; Yoo, J.-J.; Kim, M.-S.; et al. The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model. Curr. Issues Mol. Biol. 2023, 45, 6395-6414. https://doi.org/10.3390/cimb45080404
Lee H-Y, Park Y-M, Hwang H-M, Shin D-Y, Jeong H-N, Kim J-G, Park H-Y, Kim D-S, Yoo J-J, Kim M-S, et al. The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model. Current Issues in Molecular Biology. 2023; 45(8):6395-6414. https://doi.org/10.3390/cimb45080404
Chicago/Turabian StyleLee, Hak-Yong, Young-Mi Park, Hai-Min Hwang, Dong-Yeop Shin, Han-Na Jeong, Jae-Gon Kim, Hyo-Yeon Park, Dae-Sung Kim, Jin-Joo Yoo, Myung-Sunny Kim, and et al. 2023. "The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model" Current Issues in Molecular Biology 45, no. 8: 6395-6414. https://doi.org/10.3390/cimb45080404
APA StyleLee, H.-Y., Park, Y.-M., Hwang, H.-M., Shin, D.-Y., Jeong, H.-N., Kim, J.-G., Park, H.-Y., Kim, D.-S., Yoo, J.-J., Kim, M.-S., Kim, M.-J., Yang, H.-J., Choi, S.-C., & Lee, I.-A. (2023). The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model. Current Issues in Molecular Biology, 45(8), 6395-6414. https://doi.org/10.3390/cimb45080404






