Biomarker Profiles Associated with COVID-19 Severity and Mortality
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Pulmonary Function Testing
2.3. Chest HRCT Scanning
2.4. Cytokine Analysis
2.5. YKL-40 and KL-6 Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Cytokine Expression Profiles
3.3. Levels of Pulmonary Fibrosis Biomarkers
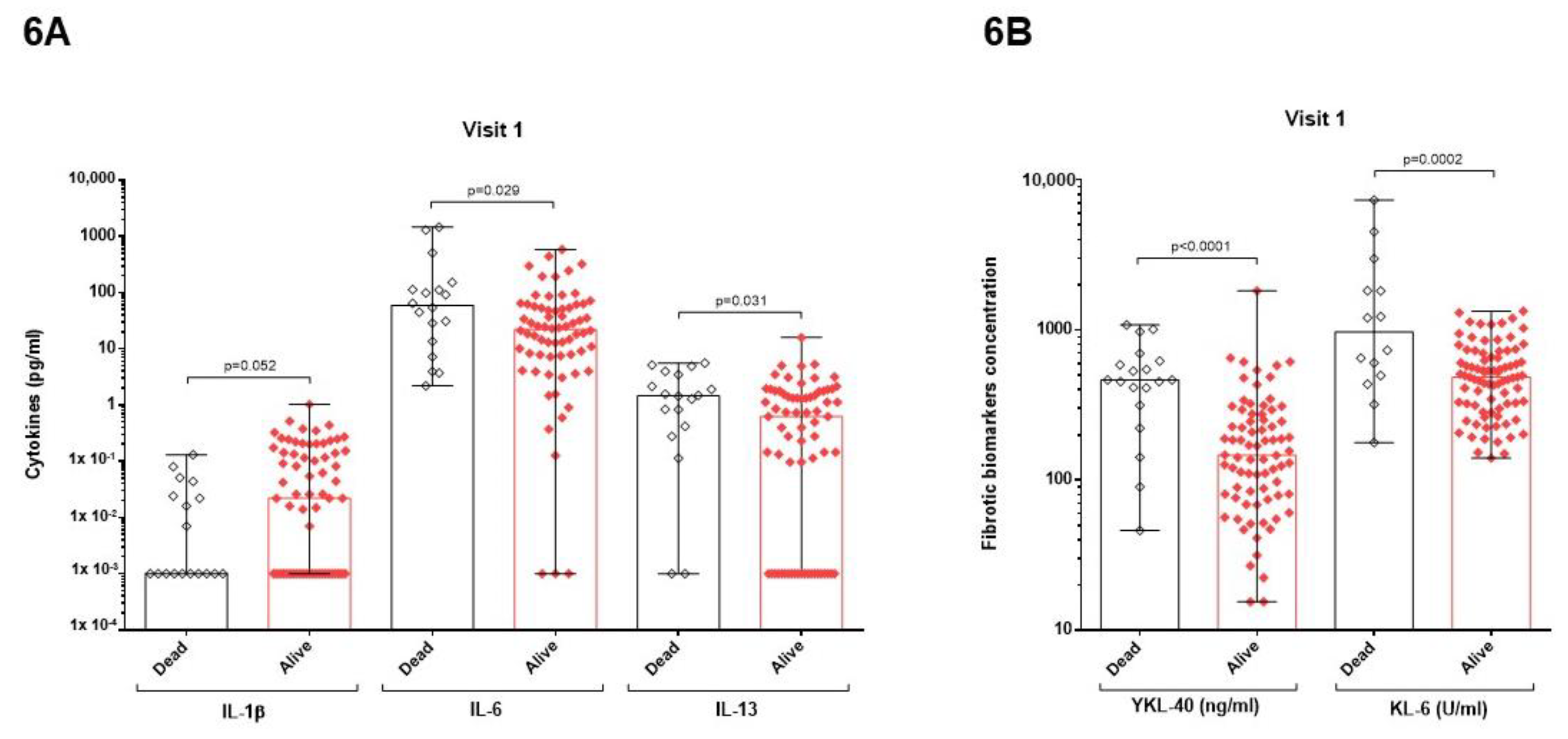
3.4. Relation between Mortality and Cytokines or Pulmonary Fibrotic Biomarkers
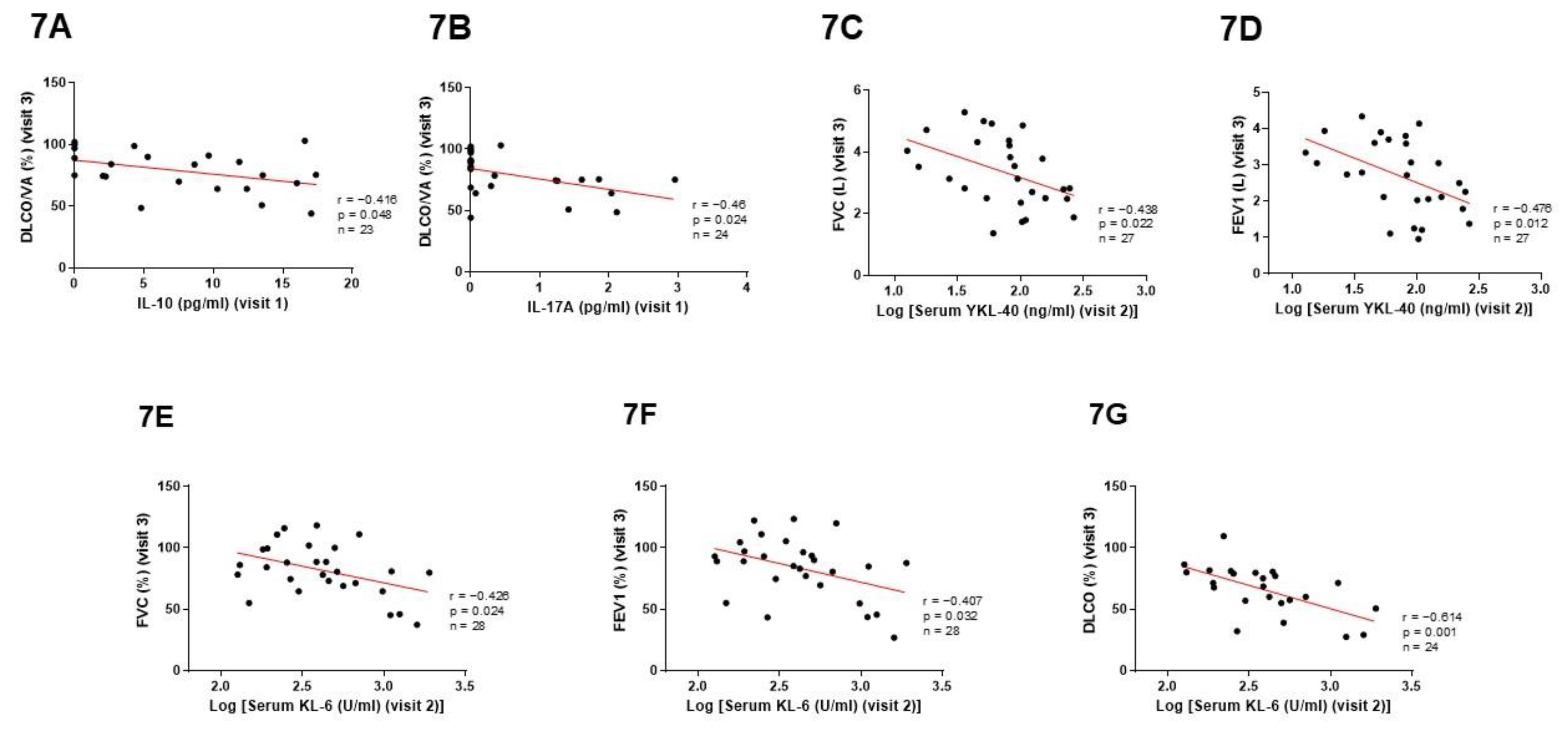
3.5. Predictive Value of Cytokines and Pulmonary Fibrotic Biomarkers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef]
- Qu, L.; Chen, C.; Yin, T.; Fang, Q.; Hong, Z.; Zhou, R.; Tang, H.; Dong, H. Ace2 and innate immunity in the regulation of SARS-CoV-2-induced acute lung injury: A review. Int. J. Mol. Sci. 2021, 22, 11483. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Cameli, P.; Otranto, A.; Luzzi, S.; Bianchi, F.; Bargagli, E. Cytokine profiles in the detection of severe lung involvement in hospitalized patients with COVID-19: The IL-8/IL-32 axis. Cytokine 2022, 151, 155804. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Puzyrenko, A.; Felix, J.C.; Ledeboer, N.A.; Sun, Y.; Rui, H.; Sheinin, Y. Cytotoxic CD8-positive T-lymphocyte infiltration in the lungs as a histological pattern of SARS-CoV-2 pneumonitis. Pathology 2021, 54, 404–408. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef] [PubMed]
- Mardi, A.; Meidaninikjeh, S.; Nikfarjam, S.; Majidi Zolbanin, N.; Jafari, R. Interleukin-1 in COVID-19 Infection: Immunopathogenesis and Possible Therapeutic Perspective. Viral Immunol. 2021, 34, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.-M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society Statement on Long COVID-19 Follow-Up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. 2020, 26, 928996. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2006, 27, 337–349. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; Miller, M.R.; et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, E70–E88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Schett, G.; Sticherling, M.; Neurath, M.F. COVID-19: Risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020, 20, 271–272. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Costa, P.R.; Correia, C.A.; Marmorato, M.P.; de Carvalho Dias, J.Z.; Thomazella, M.V.; da Silva, A.C.; de Oliveira, A.C.S.; Gusmão, A.F.; Ferrari, L.; Freitas, A.C.; et al. Humoral and cellular immune responses to CoronaVac assessed up to one year after vaccination. medRxiv Prepr. Serv. Health Sci. 2022, 3, 22272513. [Google Scholar]
- Roncati, L.; Nasillo, V.; Lusenti, B.; Riva, G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020, 99, 1419–1420. [Google Scholar] [CrossRef]
- MacKall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- John, A.E.; Joseph, C.; Jenkins, G.; Tatler, A.L. COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts. Immunol. Rev. 2021, 302, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of Chitin and Chitinase/Chitinase-Like Proteins in Inflammation, Tissue Remodeling, and Injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef]
- Di Rosa, M.; Distefano, G.; Zorena, K.; Malaguarnera, L. Chitinases and immunity: Ancestral molecules with new functions. Immunobiology 2016, 221, 399–411. [Google Scholar] [CrossRef]
- Lee, C.G.; Hartl, D.; Lee, G.R.; Koller, B.; Matsuura, H.; Da Silva, C.A.; Sohn, M.H.; Cohn, L.; Homer, R.J.; Kozhich, A.A.; et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13–induced tissue responses and apoptosis. J. Exp. Med. 2009, 206, 1149–1166. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Kohno, N.; Yokoyama, A.; Inoue, Y.; Abe, M.; Hiwada, K. KL-6, a Human MUC1 Mucin, Is Chemotactic for Human Fibroblasts. Am. J. Respir. Cell Mol. Biol. 1997, 17, 501–507. [Google Scholar] [CrossRef]
- Ohshimo, S.; Yokoyama, A.; Hattori, N.; Ishikawa, N.; Hirasawa, Y.; Kohno, N. KL-6, a human MUC1 mucin, promotes proliferation and survival of lung fibroblasts. Biochem. Biophys. Res. Commun. 2005, 338, 1845–1852. [Google Scholar] [CrossRef]
- Scotto, R.; Pinchera, B.; Perna, F.; Atripaldi, L.; Giaccone, A.; Sequino, D.; Zappulo, E.; Sardanelli, A.; Moriello, N.S.; Stanziola, A.; et al. Serum KL-6 could represent a reliable indicator of unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Environ. Res. Public Health 2021, 18, 2078. [Google Scholar] [CrossRef]
- Yamaya, T.; Hagiwara, E.; Baba, T.; Kitayama, T.; Murohashi, K.; Higa, K.; Sato, Y.; Otoshi, R.; Tabata, E.; Shintani, R.; et al. Serum Krebs von den Lungen-6 levels are associated with mortality and severity in patients with coronavirus disease 2019. Respir. Investig. 2021, 59, 596–601. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Cameli, P.; Refini, R.M.; Bergantini, L.; Alonzi, V.; Lanzarone, N.; Bennett, D.; Rana, G.D.; Montagnani, F.; Scolletta, S.; et al. Serum KL-6 concentrations as a novel biomarker of severe COVID-19. J. Med. Virol. 2020, 92, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Schoneveld, L.; Ladang, A.; Henket, M.; Frix, A.N.; Cavalier, E.; Guiot, J.; Schoneveld, L.; Ladang, A.; Henket, M.; Frix, A.; et al. YKL-40 as a new promising prognostic marker of severity in COVID infection. Crit. Care 2021, 25, 66. [Google Scholar] [CrossRef]
- Ye, L.; Yao, G.; Lin, S.; Fang, Y.; Chen, X.; Wang, L.; He, S. The Investigation of Pulmonary Function Changes of COVID-19 Patients in Three Months. J. Healthc. Eng. 2022, 2022, 9028835. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Prediletto, I.; Bensai, S.; Betti, S.; Daniele, F.; Di Scioscio, V.; Modolon, C.; Rimondi, M.R.; De Molo, C.; Serra, C.; et al. Spontaneous Evolution of COVID-19 Lung Sequelae: Results from a Double-Step Follow-Up. Respiration 2022, 101, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Eksombatchai, D.; Wongsinin, T.; Phongnarudech, T.; Thammavaranucupt, K.; Amornputtisathaporn, N.; Sungkanuparph, S. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: A prospective cohort study. PLoS One 2021, 16, e0257040. [Google Scholar]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Laguna-Goya, R.; Utrero-Rico, A.; Talayero, P.; Lasa-Lazaro, M.; Ramirez-Fernandez, A.; Naranjo, L.; Segura-Tudela, A.; Cabrera-Marante, O.; Rodriguez de Frias, E.; Garcia-Garcia, R.; et al. IL-6–based mortality risk model for hospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020, 146, 799–807.e9. [Google Scholar] [CrossRef]
- Donlan, A.N.; Sutherland, T.E.; Marie, C.; Preissner, S.; Bradley, B.T.; Carpenter, R.M.; Sturek, J.M.; Ma, J.Z.; Moreau, G.B.; Donowitz, J.R.; et al. IL-13 is a driver of COVID-19 severity. JCI Insight 2021, 6, 150107. [Google Scholar] [CrossRef]
- Deimel, L.P.; Li, Z.; Ranasinghe, C. Interleukin-13 as a target to alleviate severe coronavirus disease 2019 and restore lung homeostasis. J. Clin. Transl. Res. 2021, 7, 116–120. [Google Scholar]
- Zhao, Y.; Qin, L.; Zhang, P.; Li, K.; Liang, L.; Sun, J.; Xu, B.; Dai, Y.; Li, X.; Zhang, C.; et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020, 5, 139834. [Google Scholar] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; Almeida de Jesus, A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6, 144455. [Google Scholar] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [PubMed]
- Maslennikov, R.; Ivashkin, V.; Vasilieva, E.; Chipurik, M.; Semikova, P.; Semenets, V.; Russkova, T.; Levshina, A.; Grigoriadis, D.; Magomedov, S.; et al. Interleukin 17 antagonist netakimab is effective and safe in the new coronavirus infection (COVID-19). Eur. Cytokine Netw. 2021, 32, 8–14. [Google Scholar] [PubMed]
- Avdeev, S.N.; Trushenko, N.V.; Tsareva, N.A.; Yaroshetskiy, A.I.; Merzhoeva, Z.M.; Nuralieva, G.S.; Nekludova, G.V.; Chikina, S.Y.; Gneusheva, T.Y.; Suvorova, O.A.; et al. Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study. Cytokine 2021, 146, 155627. [Google Scholar] [CrossRef] [PubMed]
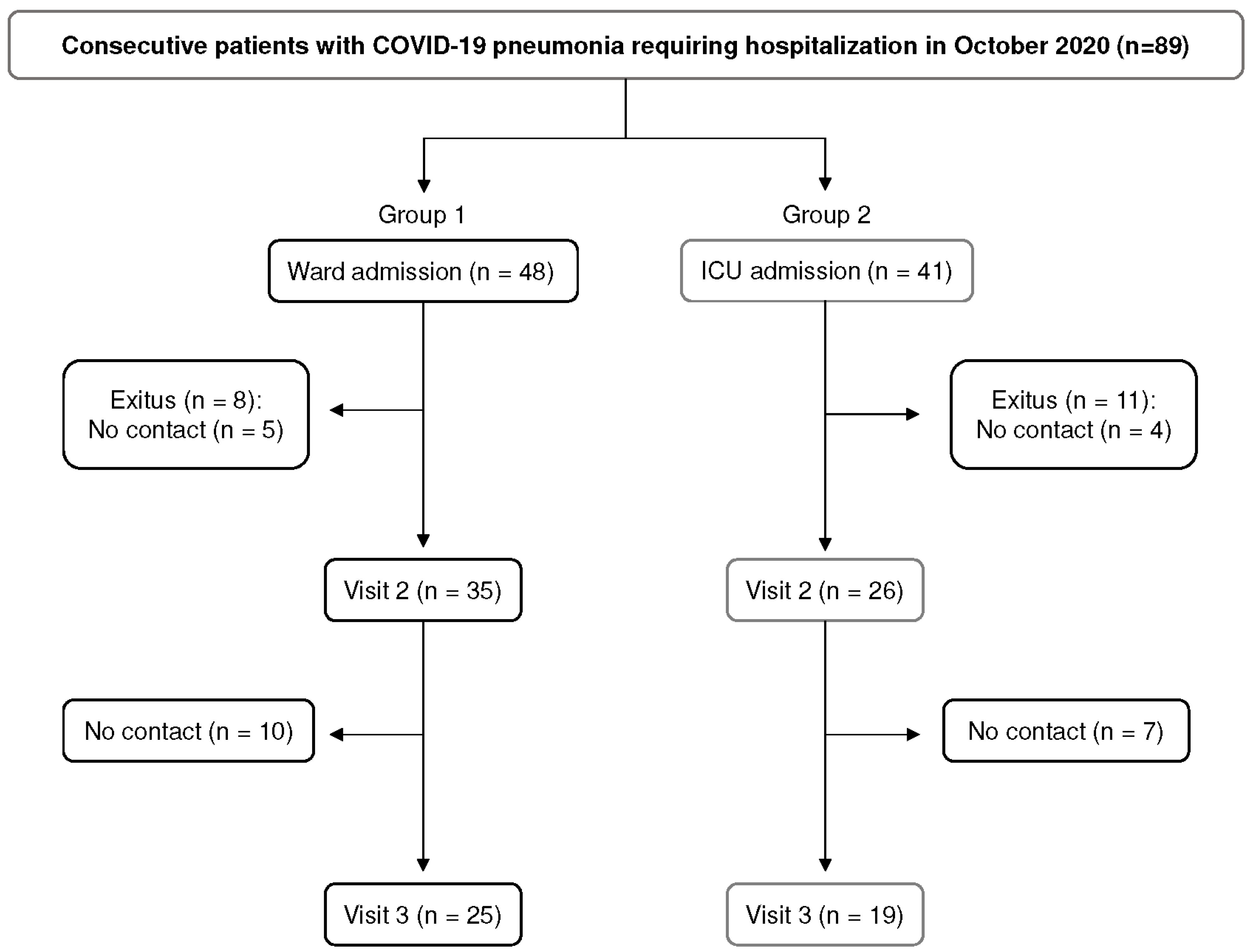
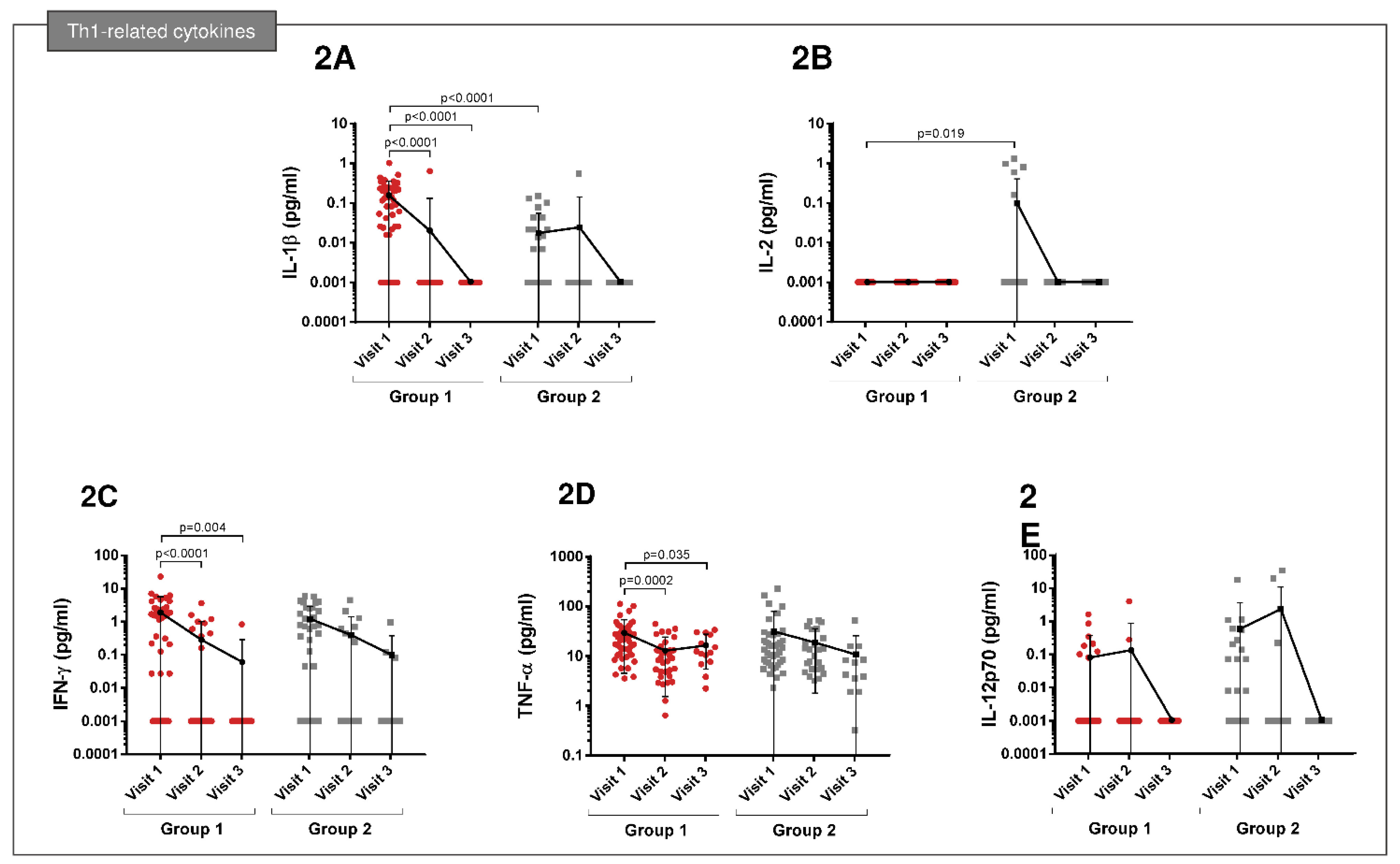
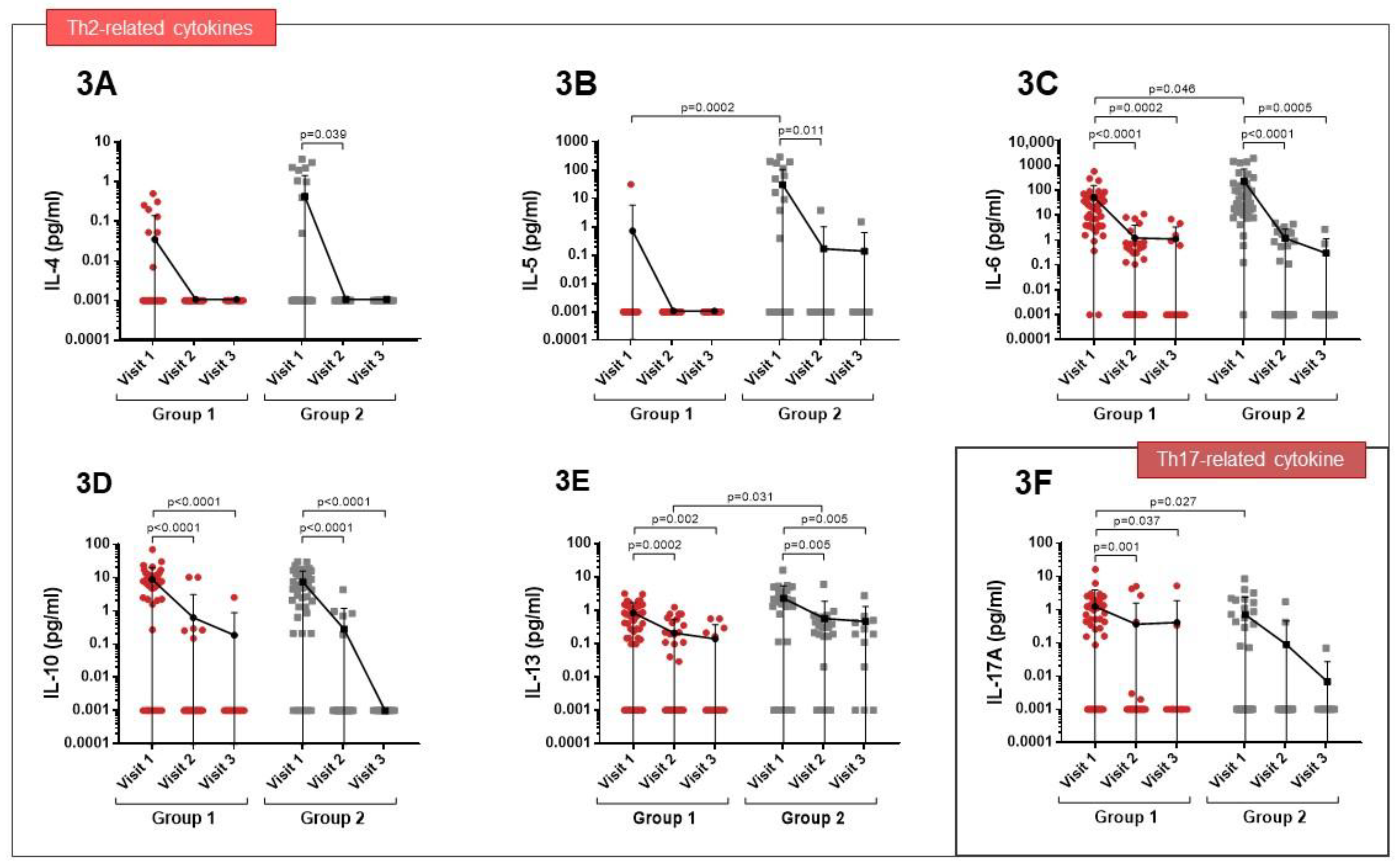
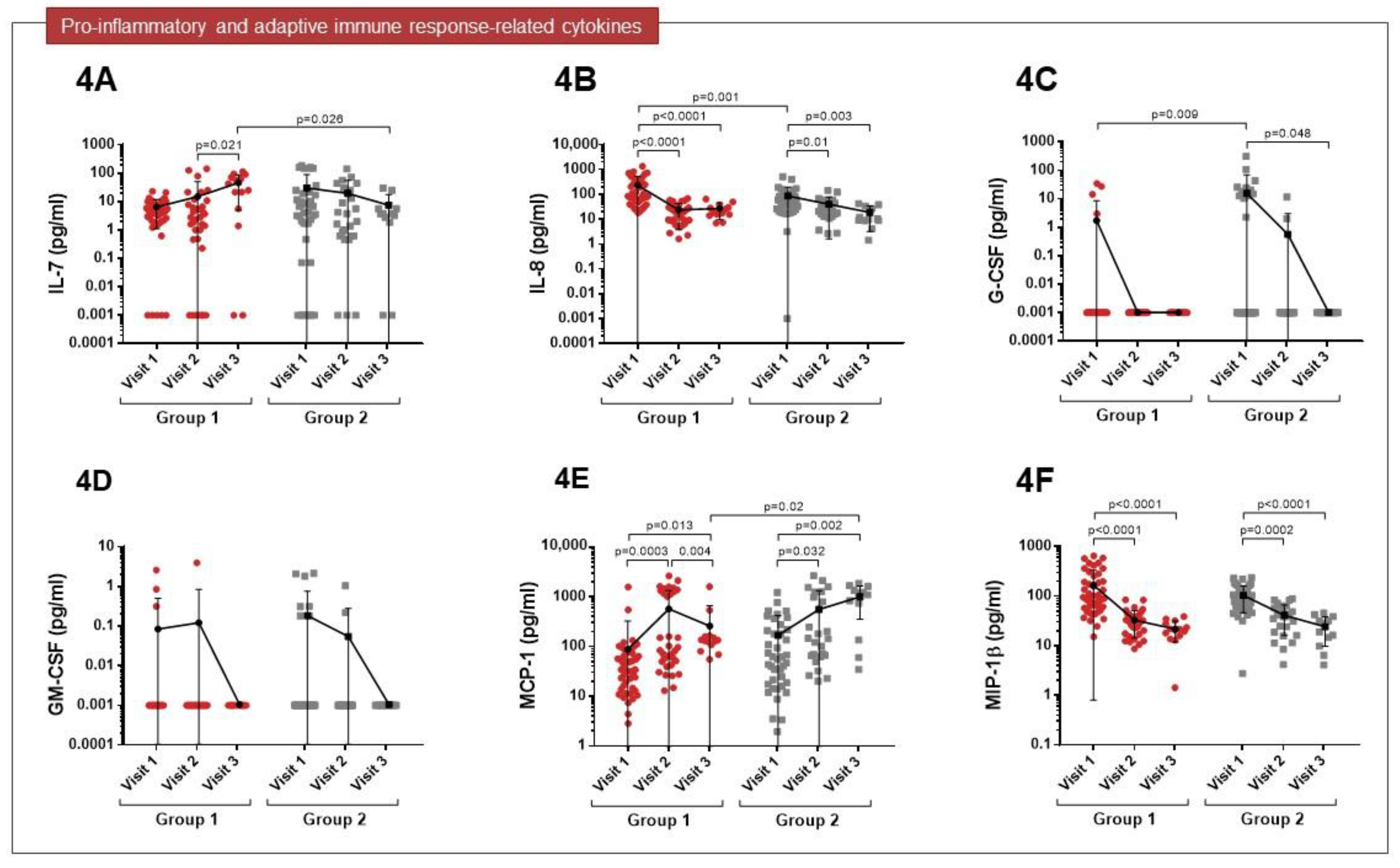

| Group 1 (n = 48) | Group 2 (n = 41) | p Value | |
|---|---|---|---|
| Sociodemographic and clinical data | |||
| Age (years), median (range) | 66 (25–90) | 63 (40–76) | 0.403 |
| Sex (male), n (%) | 31 (64) | 33 (80) | 0.106 |
| BMI (Kg/m2), median (range) | 30 (15–41) | 32.9 (25–33) | 0.499 |
| BMI > 30 (Kg/m2), n (%) | 18 (38) | 18 (44) | 0.665 |
| Smoking habit, n (%) | 0.041 | ||
| Smoker | 4 (8) | 11 (27) | 0.025 |
| Former smoker | 21 (44) | 11 (27) | 0.123 |
| Non-smoker | 21 (44) | 16 (39) | 0.673 |
| Survival, n (%) | 40 (83) | 30 (73) | 0.303 |
| Respiratory comorbidities, n (%) | |||
| Asthma | 2 (4) | 0 (0) | 0.497 |
| Obstructive sleep apneas | 5 (10) | 0 (0) | 0.059 |
| Chronic obstructive pulmonary disease | 5 (10) | 0 (0) | 0.059 |
| Bronchiectasis | 0 | 1 (2) | 0.461 |
| Pulmonary arterial hypertension | 2 (4) | 0 (0) | 0.497 |
| Pulmonary fibrosis | 1 (2) | 0 (0) | 1.000 |
| Other interstitial diseases | 2 (4) | 0 (0) | 0.497 |
| Lung transplant | 2 (4) | 0 (0) | 0.497 |
| Non-respiratory comorbidities, n (%) | |||
| Arterial hypertension | 24 (50) | 20 (49) | 1.000 |
| Obesity | 18 (38) | 18 (44) | 0.665 |
| Diabetes | 12 (25) | 7 (17) | 0.441 |
| Chronic renal insufficiency | 6 (12) | 3 (7) | 0.498 |
| Immunosuppression | 3 (6) | 2 (5) | 1.000 |
| Ischemic heart disease | 7 (15) | 1 (2) | 0.065 |
| Heart failure | 1 (2) | 0 (0) | 1.000 |
| Atrial fibrillation | 3 (6) | 0 (0) | 0.246 |
| Other cardiac diseases | 4 (8) | 2 (5) | 0.683 |
| Solid cancer | 13 (27) | 2 (5) | 0.009 |
| Cured | 6 (46) | 2 (100) | 0.467 |
| Leukemia | 3 (6) | 0 (0) | 0.246 |
| Psychiatric disease | 5 (10) | 3 (7) | 0.721 |
| Gastroesophageal reflux | 0 (0) | 1 (2) | 1.000 |
| Ventilatory support, n (%) | (n = 47) | (n = 40) | |
| Yes | 21 (45) | 40 (100) | <0.0001 |
| Type of support, n (%) | |||
| High-flow nasal cannula (HFNC) | 18 (86) | 26 (65) | 0.133 |
| Non-invasive mechanical ventilation (NIMV) | 3 (14) | 2 (5) | 0.329 |
| Invasive mechanical ventilation (IMV) | 13 (62) | 37 (93) | 0.005 |
| Extracorporeal membrane oxygenation | 1 (5) | 4 (10) | 0.651 |
| Oxygen therapy 100% | 2 (10) | 3 (8) | 1.000 |
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Pulmonary function parameters | Visit 2 (n = 35) | Visit 3 (n = 25) | p Value | Visit 2 (n = 26) | Visit 3 (n = 19) | p Value |
| FVC (% predicted), median (range) | 87 (41–118) | 84 (45–118) | 0.533 | 85 (53–137) | 81 (37–111) | 0.143 |
| FVC < 85%, n (%) | 13 (37) | 9 (36) | 1.000 | 10 (38) | 7 (37) | 1.000 |
| FEV1 (% predicted), median (range) | 91 (42–134) | 89 (43–124) | 0.089 | 91 (56–139) | 88 (27–122) | 0.653 |
| FEV1 < 80%, n (%) | 7 (20) | 6 (24) | 0.758 | 6 (23) | 3 (16) | 0.712 |
| FEV1/FVC (% predicted), median (range) | 81 (52–100) | 82 (43–99) | 0.109 | 84 (76–98) | 86 (56–90) | 0.25 |
| FEV1/FVC < 70%, n (%) | 3 (9) | 2 (8) | 1.000 | 0 (0) | 1 (5) | 0.422 |
| DLCO (% predicted), median (range) | 70 (23–94) | 73 (27–86) | 0.457 | 62 (25–106) | 57 (29–110) | 0.044 |
| DLCO < 80%, n (%) | 22 (63) | 10 (40) | 0.116 | 16 (62) | 8 (42) | 0.237 |
| DLCO/VA (% predicted), median (range) | 75 (31–123) | 75 (44–100) | 0.649 | 78 (42–109) | 85 (19–103) | 0.097 |
| HRCT, n (%) | Visit 2 (n = 33) | Visit 3 (n = 11) | p Value | Visit 2 (n = 20) | Visit 3 (n = 8) | p Value |
| Pathological findings | ||||||
| Yes | 28 (85) | 10 (90) | 1.000 | 19 (95) | 7 (88) | 0.497 |
| Location | ||||||
| Unilateral | 1 (4) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Bilateral | 27 (96) | 10 (100) | 1.000 | 19 (100) | 7 (100) | 1.000 |
| Distribution | ||||||
| Central | 1 (4) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Peripheral | 25 (89) | 10 (100) | 0.552 | 19 (100) | 7 (100) | 1.000 |
| Central and peripheral | 2 (7) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Type of injury | ||||||
| Simple | 1 (4) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Multiple | 1 (4) | 0 (0) | 1.000 | 0 (0) | 1 (14) | 0.269 |
| Diffuse | 26 (92) | 10 (100) | 1.000 | 19 (100) | 6 (86) | 0.269 |
| Main imaging features | ||||||
| Ground glass | 5 (18) | 4 (40) | 0.205 | 1 (5) | 5 (71) | 0.002 |
| Consolidation | 2 (7) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Linear opacities | 0 (0) | 1 (10) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Reticulation | 3 (11) | 0 (0) | 0.552 | 5 (26) | 0 (0) | 0.278 |
| Mixed pattern | 15 (54) | 5 (50) | 1.000 | 11 (58) | 2 (29) | 0.378 |
| Interstitial changes | 6 (21) * | 0 (0) | 0.168 | 10 (53) * | 2 (29) | 0. 391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Díez, S.; Gómez-Ollés, C.; Cruz, M.-J.; de Homdedeu, M.; Espejo, D.; Ferrer, J.; Roca, O.; Pacheco, A.; Muñoz, X. Biomarker Profiles Associated with COVID-19 Severity and Mortality. Curr. Issues Mol. Biol. 2023, 45, 1998-2012. https://doi.org/10.3390/cimb45030128
Sánchez-Díez S, Gómez-Ollés C, Cruz M-J, de Homdedeu M, Espejo D, Ferrer J, Roca O, Pacheco A, Muñoz X. Biomarker Profiles Associated with COVID-19 Severity and Mortality. Current Issues in Molecular Biology. 2023; 45(3):1998-2012. https://doi.org/10.3390/cimb45030128
Chicago/Turabian StyleSánchez-Díez, Silvia, Carlos Gómez-Ollés, María-Jesús Cruz, Miquel de Homdedeu, David Espejo, Jaume Ferrer, Oriol Roca, Andrés Pacheco, and Xavier Muñoz. 2023. "Biomarker Profiles Associated with COVID-19 Severity and Mortality" Current Issues in Molecular Biology 45, no. 3: 1998-2012. https://doi.org/10.3390/cimb45030128
APA StyleSánchez-Díez, S., Gómez-Ollés, C., Cruz, M.-J., de Homdedeu, M., Espejo, D., Ferrer, J., Roca, O., Pacheco, A., & Muñoz, X. (2023). Biomarker Profiles Associated with COVID-19 Severity and Mortality. Current Issues in Molecular Biology, 45(3), 1998-2012. https://doi.org/10.3390/cimb45030128







