Abstract
Hypoxic preconditioning has been demonstrated to increase the resistance of neural stem cells (NSCs) to hypoxic conditions, as well as to improve their capacity for differentiation and neurogenesis. Extracellular vesicles (EVs) have recently emerged as critical mediators of cell–cell communication, but their role in this hypoxic conditioning is presently unknown. Here, we demonstrated that three hours of hypoxic preconditioning triggers significant neural stem cell EV release. Proteomic profiling of EVs from normal and hypoxic preconditioned neural stem cells identified 20 proteins that were upregulated and 22 proteins that were downregulated after hypoxic preconditioning. We also found an upregulation of some of these proteins by qPCR, thus indicating differences also at the transcript level within the EVs. Among the upregulated proteins are CNP, Cyfip1, CASK, and TUBB5, which are well known to exhibit significant beneficial effects on neural stem cells. Thus, our results not only show a significant difference of protein cargo in EVs consequent to hypoxic exposure, but identify several candidate proteins that might play a pivotal role in the cell-to-cell mediated communication underlying neuronal differentiation, protection, maturation, and survival following exposure to hypoxic conditions.
1. Introduction
Neural stem cells (NSCs) are multipotent precursor cells that exist in special regions of the fetal and adult central nervous system (CNS). They have self-renewal ability and can generate neurons, astrocytes, and oligodendrocytes when needed [1]. Transplantation of neural stem cells has shown great potential in regenerative medicine. However, the therapeutic efficacy is often limited due to high cell death, low differentiation, and reduced function after cell transplantation. Various methods have been used to modify NSCs in order to obtain the most stable and functional NSCs after transplantation [2]. For example, preconditioning using various conditions, encapsulation, and genetic modulation of cells prior to transplantation have been tested. Recently, different preconditioning protocols using cytokines and hypoxic preconditioning (HP) have been examined [3,4]. In particular, decreased oxygen pre-treatments have been demonstrated to increase the resistance of NSCs in hypoxic and ischemic conditions [5]. Previously, it was shown that EVs are involved in preconditioning [6]. HP of NSCs has also been reported to benefit NSCs and promote neuronal cell survival, neural differentiation, and neurogenesis [5,7,8,9,10,11,12,13,14,15]. However, the mechanism by which HP exerts these benefits is still unclear.
Previous evidence has shown that almost all living cells release extracellular vesicles (EVs), including microvesicles, apoptotic bodies, and exosomes. EVs exist in a variety of sizes, contents, and origins [16]. Communication between cells via EVs is more complex than through secretion of chemokines and cytokines because of the EV packaging of the biomaterials [17,18]. Based on the genetic materials and proteins that the EVs carry, they can develop a certain capability to interact with different cells or initiate different chemical cascades within the recipient cells [19]. Indeed, it has been now shown that EVs play an important role in many biological processes in both healthy and abnormal cells [16]. Hence, we hypothesized that EVs might be involved in the HP effect on NSCs, and thus a detailed examination of their protein cargo may be useful in identifying crucial proteins that may restore the function of other brain cells or aid in their rescue after hypoxia.
2. Results
2.1. NSCs Characterization, Identification, and Differentiation
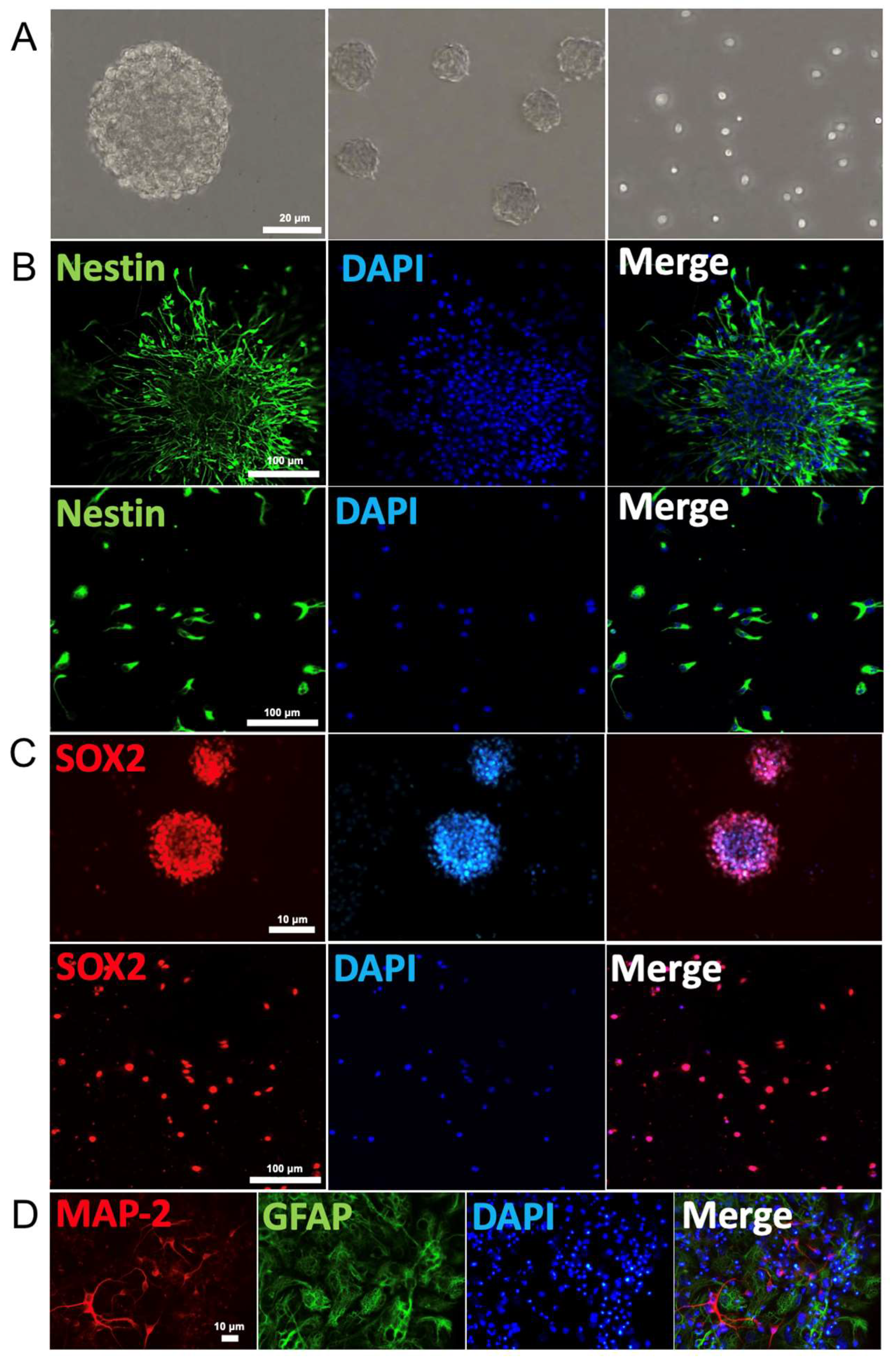
We first demonstrated that NSCs cultured in growth medium proliferated into approximately uniformly sized neurospheres with typical shape as well as single cells (Figure 1A). Both neurospheres and free single cells immuno-stained positively for the stem cells markers nestin (green fluorescence) and Sox2 (red fluorescence, Figure 1B,C). These NSCs were able to differentiate into neurons and glial cells after removal of growth factor from growth cell medium over six days, as judged by the immuno-labelling of MAP-2 (red fluorescence) and GFAP (green fluorescence), respectively (Figure 1D). Thus, these results confirmed that these NSCs exhibit the typically observed biomarkers for the characterization of NSCs. Negative control was not incubated with primary antibody (Supplementary Figure S1).
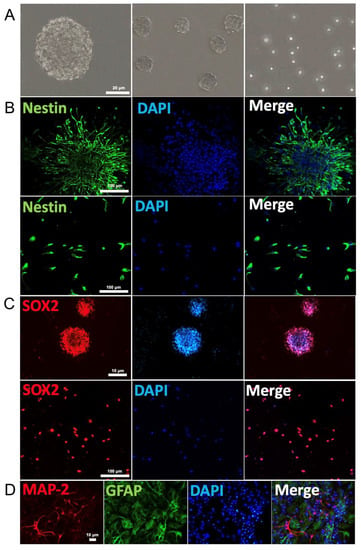
Figure 1.
Neural stem cell identification: (A) Neurospheres and free single cells representing typical morphological feature of NSCs. (B) Immunofluorescence of neurospheres (top row) and single cells (bottom row) show expression of the NSC markers nestin (green). Cell nuclei were counterstained with DAPI (blue). (C) Immunofluorescence of neurospheres (top row) and single cells (bottom row) for SOX2 (red) expression and nuclei counterstained with DAPI (blue). (D) Immunostaining identification of the primary mouse cortex NSCs. Neural dendrites and axons were identified by anti-MAP-2 (red) and astrocytes by GFAP (green). The nuclei of all cells were identified by DAPI (blue).
2.2. Hypoxic Preconditioning Affects NSC Viability
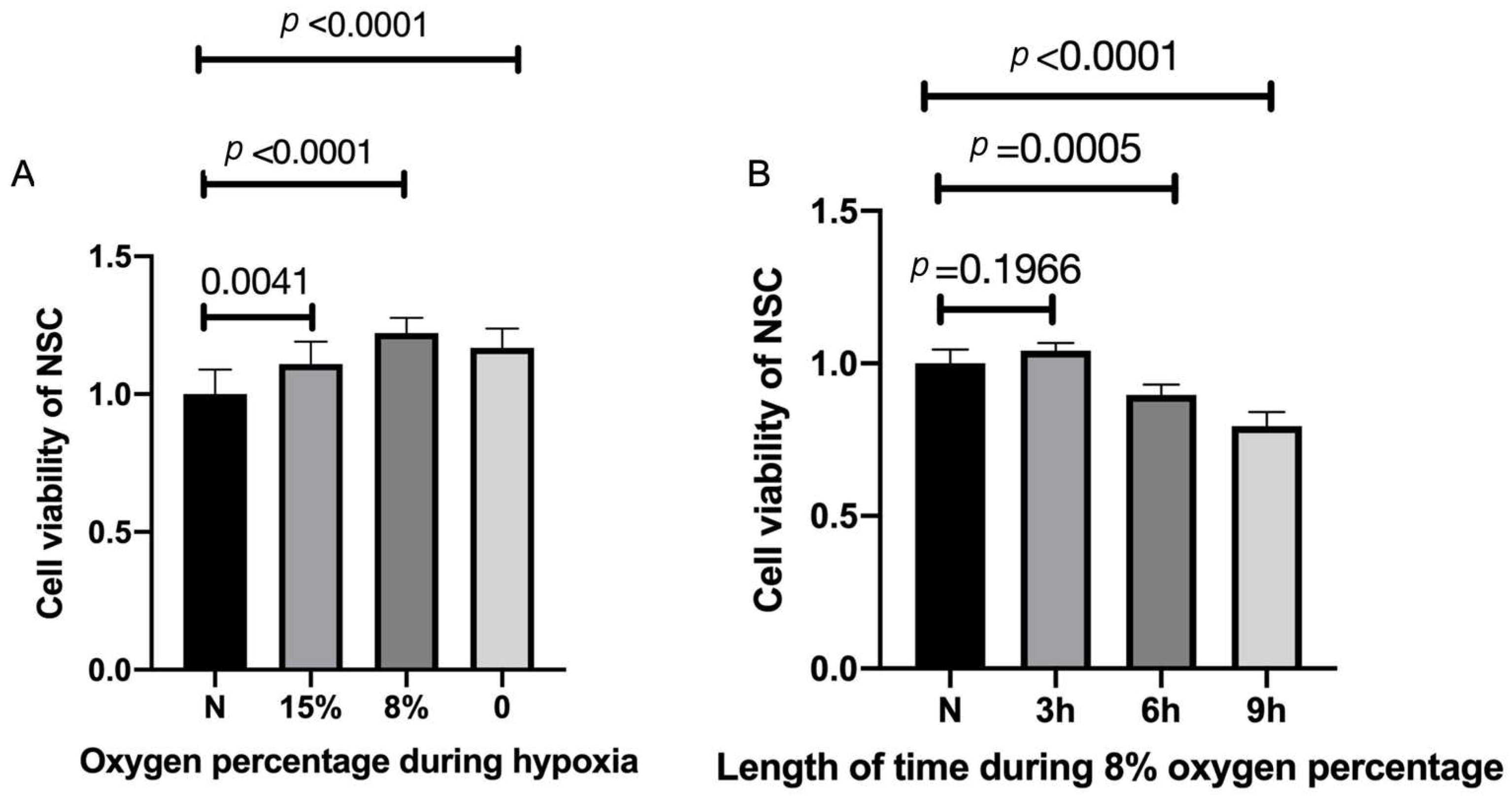
To examine for optimal low-oxygen growth conditions, NSCs were exposed to 0%, 8%, 15%, and normal levels (20%) of oxygen for three hours. Three hours of HP of 15% oxygen exposure made a significant difference in cell viability compared with normal levels and the viability increased significantly after three hours of HP with the exposure of 8% oxygen (Figure 2A). Treatment with 0% resulted in slightly less viability than at 15%. Thus, we examined the 8% levels on cell viability for various periods of time (Figure 2B). Three hours of HP with 8% oxygen improved cell viability compared with the control group, with longer incubation periods resulting in lower viability (Figure 2B). Thus, these results suggest that 8% oxygen for three hours is optimal under our conditions to study the triggering of NSCs via HP (Figure 2B)
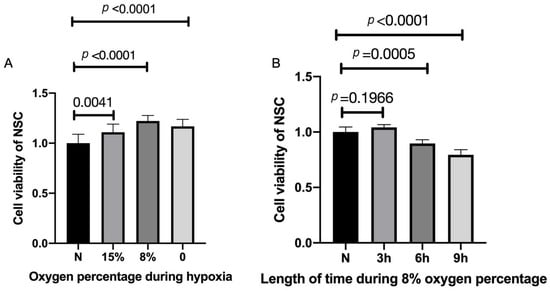
Figure 2.
Increased cell viability after hypoxic preconditioning. (A) NSCs were exposed to 0%, 8%, 15%, and normal percentage of oxygen for three hours. n = 10, p < 0.05, each oxygen percentage group vs. normal group. (B) Three hours of hypoxic preconditioning of 15% and 0% oxygen exposure made no difference in cell viability, but the viability significantly increased after three hours of hypoxic preconditioning with the exposure of 8% oxygen. n = 10 cell culture wells per group, p < 0.05 each HP period of time group vs. normal group. Cell viabilities are all expressed as means ± SD.
2.3. Characterization of Normal vs. Hypoxic Preconditioned NSC-Derived EVs
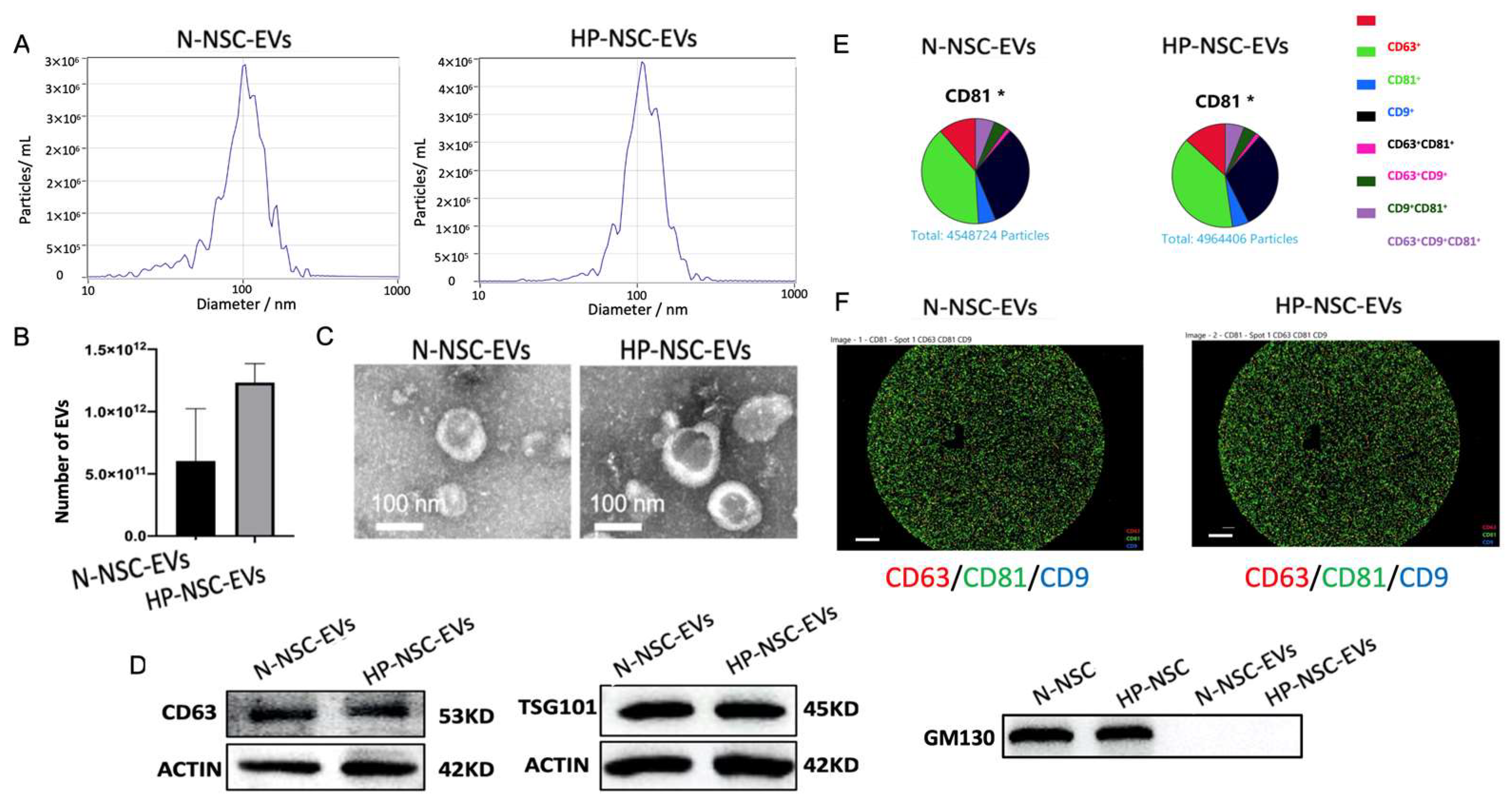
NSCs were split into two groups: a group of HP incubated with 8% oxygen for 3 h and a control group kept under normal oxygen conditions. EVs were isolated from the culture medium supernatant of both groups after second passaging using ultracentrifugation. Purified NSC EVs were then characterized by nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), Western blotting, and ExoView (Figure 3A–F). NTA results demonstrated that the number of EVs was significantly greater in the HP-NSC-EVs group (Figure 3B). Co-expressions of EV markers were measured by probing captured EVs with the indicated secondary fluorescence-labelled antibody. We found that both normal and HP-NSC-EVs were all positive for the three EV markers, CD63, CD9, and CD81 (Figure 3D–F). Overall, we found that the size, morphology, and presence of surface markers were fairly similar in both groups.
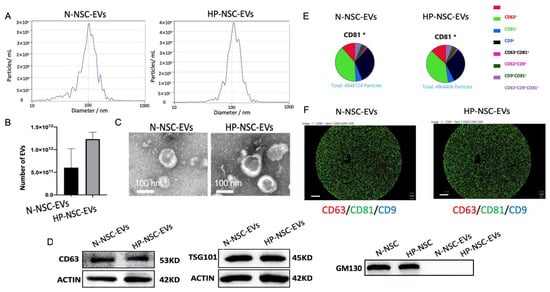
Figure 3.
Characterization of normal vs. hypoxic preconditioned NSC-derived extracellular vesicles. (A). Particle size distribution measured by nanoparticle tracking analysis (NTA). (B) Concentration (particles/mL) of EVs measured by NTA. n = 4 NSCs isolated from mice per group, p = 0.0712 HP-NSC-EVs vs. N-NSC-EVs and number of EVs are expressed as means ± SD. (C) Normal vs. hypoxic preconditioned NSC EVs morphology identified by transmission electron microscopy (TEM). (D) Western blot analysis of EV surface markers, CD63, TSG101, and non-EV marker, GM130 (N = 4). (E) Both normal and HP-NSC-EVs were all positive for the three EV markers, CD63, CD9, and CD81. Co-expressions of EV markers were measured by probing captured EVs with the indicated secondary fluorescence-labelled antibody. (F) The specific EV markers from both normal and HP NSCs were analyzed by NanoSight NS300 and ExoView®. Scale bar = 20 μm.
2.4. Proteomic Profiling of Normal vs. Hypoxic Preconditioned NSC-Derived EVs
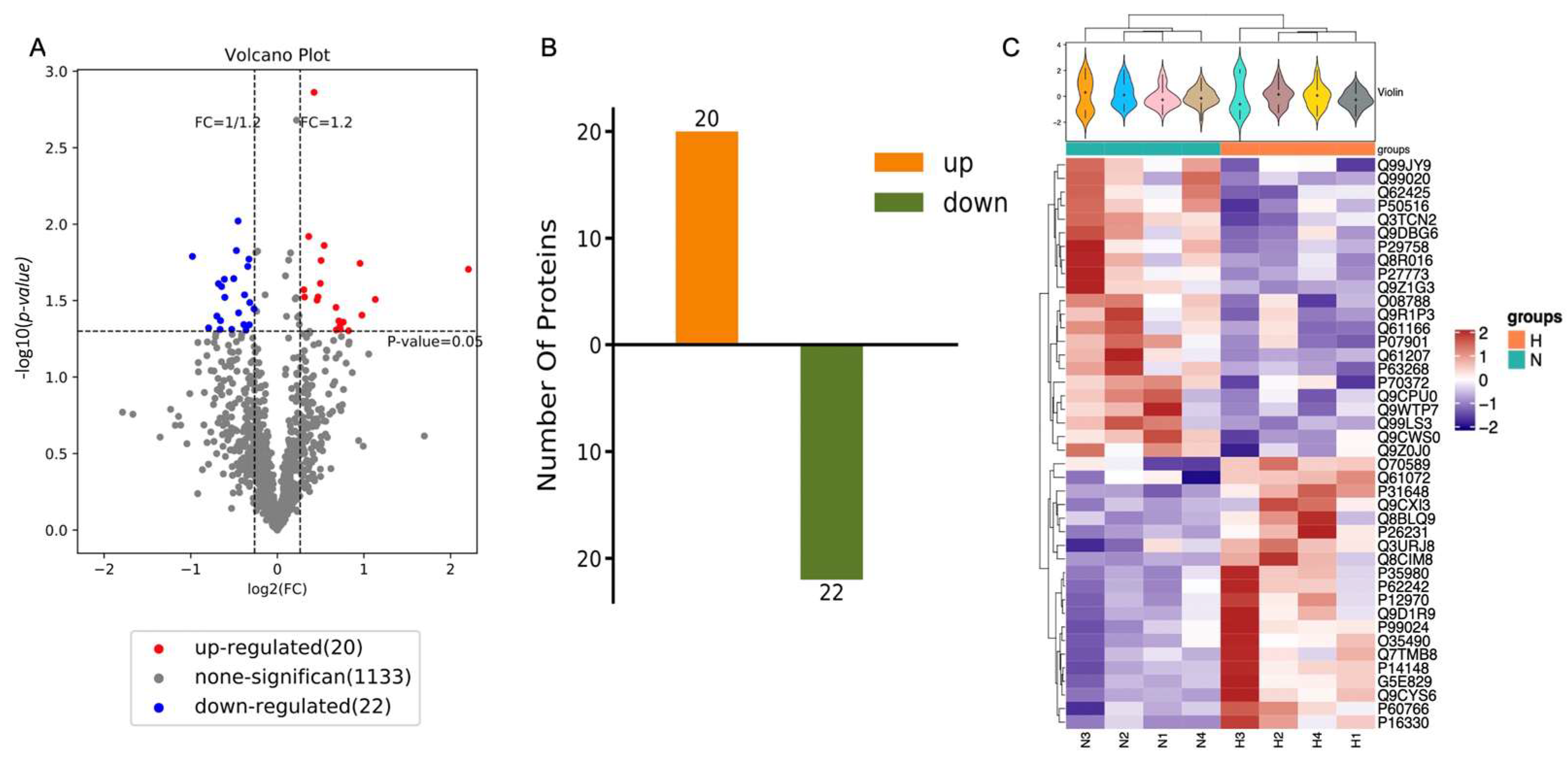
The purified EVs from both the HP and normal group were next sequenced for their protein composition (n = 6 NSC isolated from mice per group) using the iTRAQ method. Overall, out of 2018 proteins, 42 vesicular proteins were identified to be differentially expressed: 20 proteins were identified to be overexpressed and 22 proteins were downregulated after HP (Figure 4A,B). These differences were evident by examination of each individual sample as well (Figure 4C, Table 1).
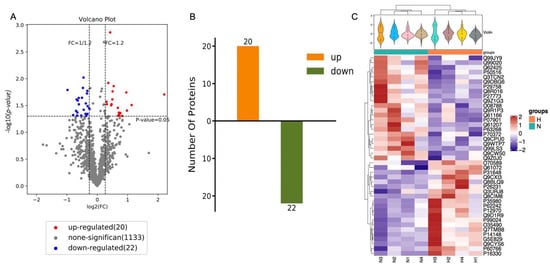
Figure 4.
Identification of differentially expressed proteins. (A) Volcano differentially expressed proteins of NSC-EVs under normal condition comparing to hypoxic condition. Volcano plot indicates the differentially expressed proteins. Fold change > 1.2 or < 1/1.2 and p < 0.05 is considered to be a significant differentially expressed protein. Red for upregulated proteins, blue for downregulated proteins, and grey for no differentially expressed proteins. (B) The number of differentially expressed proteins (N-NSC-EVs vs. HP-NSC-EVs). n = 6. (C) Heat maps of identified proteins in N-NSC-EVs and HP-NSC-EVs.

Table 1.
The expression levels of differentially expressed proteins.
2.5. Differentially Expressed Proteins Enriched in Specific Biological Pathways
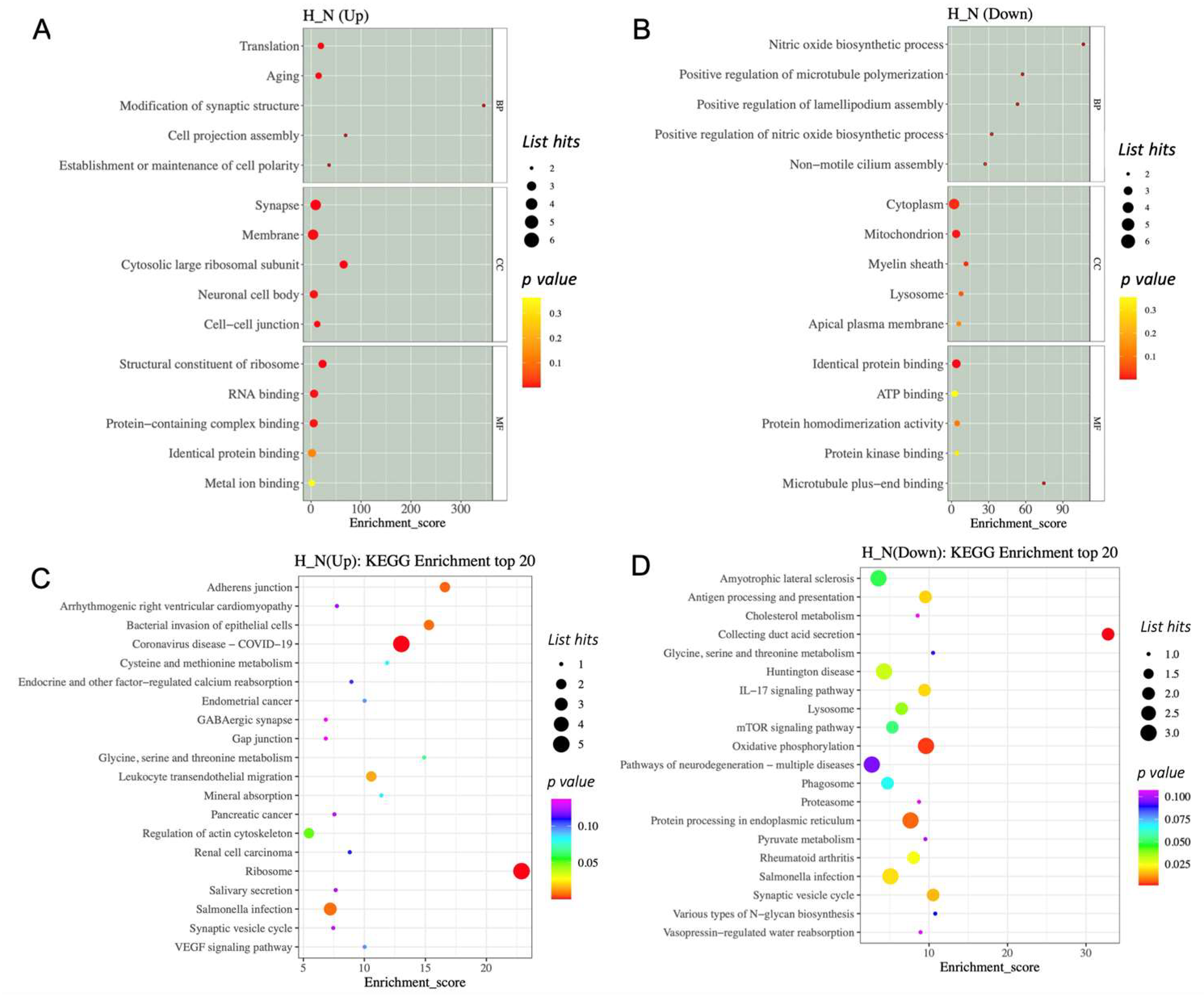
To get a better understanding of the protein functions and signalling pathways of hypoxia-related differentially expressed proteins (DEPs), we performed GO and KEGG pathway enrichment analysis. The top 15 enriched terms are shown in Figure 5. For the hypoxia-related upregulated DEP, the GO analysis revealed that the HP-EVs were highly enriched in translation and aging processes (Figure 5A). Further, in terms of cellular components, there was a significant enrichment of synapse, membrane, cytosolic large ribosomal subunits, neuronal cell body, and cell–cell junction complexes, while in terms of molecular functions, there was enrichment of ribosome components, as well as RNA binding and protein-containing complexes (Figure 5A). As for the hypoxia-related downregulated DEP, there was an enrichment of proteins involved in cytoplasm and mitochondrion (Figure 5A,B). In particular, for the hypoxia-related upregulated DEPs, there was an enrichment of ribosome processes as well as components involved in synaptic vesicles and cell–cell adherens and gap junctions (Figure 5D), while proteins involved in processing within the endoplasmic reticulum were enriched in the hypoxia-related downregulated DEP (Figure 5C). Interestingly, this analysis also identified proteins involved in Huntington disease, amyotrophic lateral sclerosis, and pathways of neurodegeneration among the hypoxia-related downregulated DEP. Thus, this analysis revealed a significant enrichment of specific proteins within HP-EVs that play a direct role in normal neuronal cellular functions, as well as a decrease in components known to be important in neuronal diseases.
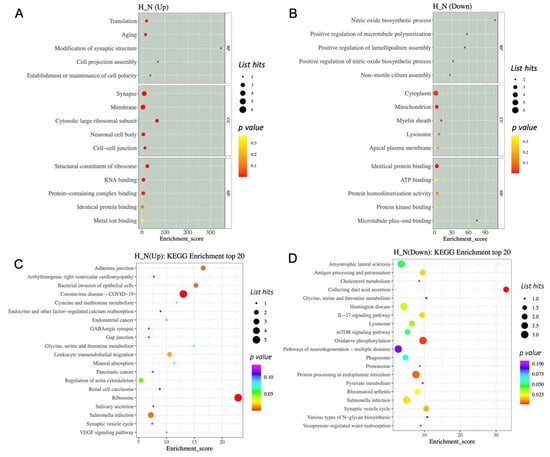
Figure 5.
The GO and KEGG enrichment analysis of differentially expressed proteins. (A,B) GO enrichment analysis top 15 (screening the GO entries that correspond to the number of differential proteins greater than 1 in the three categories). (C,D) The KEGG enrichment analysis of differentially expressed proteins.
2.6. Detection of Elevated mRNA Transcripts of Upregulated Proteins within HP-EVs
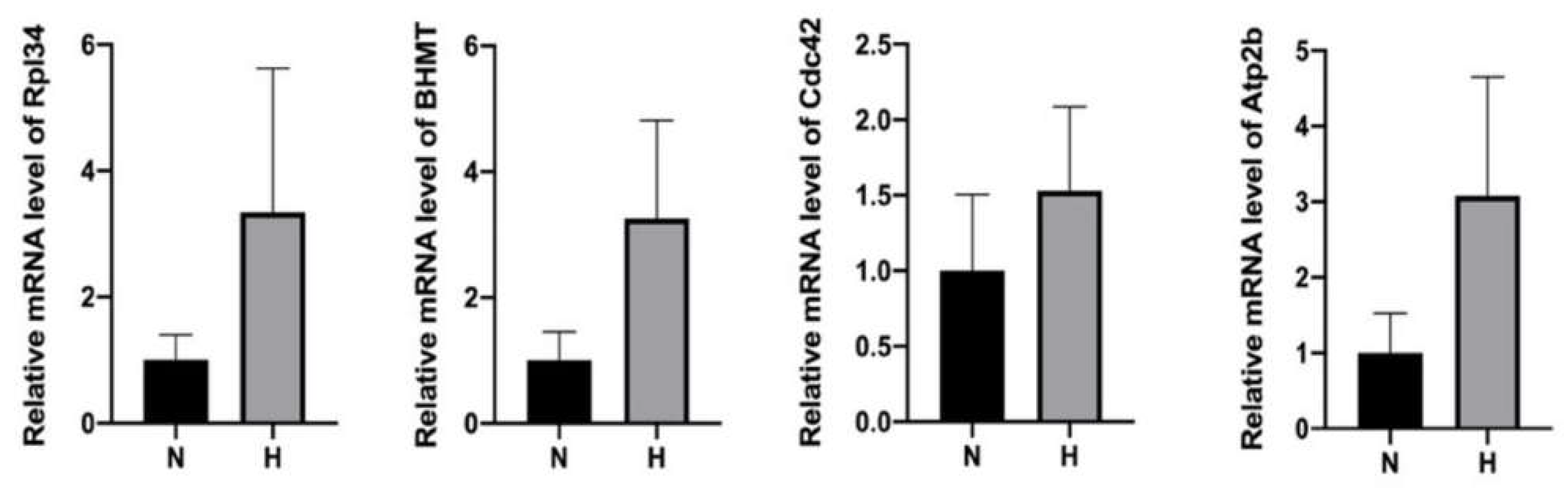
We speculated that the presence of the upregulated proteins in the HP-EVs may be a consequence of enhanced expression in the HP-treated cells, and thus suspected that the HP-EVs might also contain an enrichment of the transcripts of these upregulated proteins as well. To test this, we determined the extent of the transcripts of select upregulated proteins by qRT-PCR in both normal and HP EVs (primer sequences are in Supplementary Table S1). Indeed, we found that the levels of mRNA of Rpl34, BHMT, Cdc42, and Atp2b were all higher in the HP-EVs exosomes than those in N-EVs (Figure 6). Thus, after the entrance of HP-EVs into recipient cells, it would result in the transfer of EV-specific proteins to recipient cells. In addition, based on our results, since the levels of some of those corresponding mRNAs also increased, the delivery of those mRNAs may also impact the protein synthesis in the recipient cells.
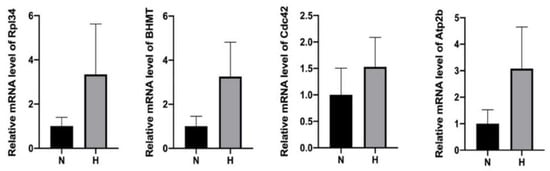
Figure 6.
qPCR analysis revealed an enhancement of mRNA in HP-EVs of select upregulated proteins within HP-EVs. n = 4, p = 0.1143 HP group vs. normal group for Rpl34 mRNAs, p = 0.2363 HP group vs. normal group for BHMT, p = 0.3322 HP group vs. normal group for Cdc42, p = 0.1560 HP group vs. normal group for Atp2b. MRNA levels are all expressed as means ± SD.
3. Discussion
EVs are well known to play important roles in both disease progression and prevention. Although some discoveries have been made, many unknown changes of the EVs under various conditions are yet poorly characterized. Based on previous studies, cells load various cargos in their EVs and release them to communicate to other cells in both balanced and imbalanced conditions. Hence, the exploration of EV cargos can help us to understand the role of EVs, their cargos, and their potential mechanisms of intercellular communication. In fact, it is highly demanding to map and compare the composition of EVs from different cells at different conditions to obtain a clear idea of the role of EVs in initiating, regulating, and suppressing diseases.
Numerous studies have been conducted regarding the RNA content of EVs. However, proteins are cardinal for final biological function; therefore, we decided to map and compare the protein cargo of NSC-EVs after HP. Despite recent efforts in understanding the role of extracellular vesicles in NSCs after hypoxia, additional studies are required to focus on the effect of hypoxia on NSC-EV secretion, production, and various signalling and biological molecules, and to distinguish the rescuing versus damaging EV cargos after hypoxia in the brain microenvironment. We successfully developed suitable conditions to induce hypoxia as a trigger for preconditioning NSCs. Previous studies demonstrated that HP could be beneficial for NSC survival, cell viability, and neurogenesis [11,20]. As previously reported, hypoxia can help maintain the proliferation and self-renewal of NSCs that could be related to an increase in HIF-1α expression [2,21,22,23]. In addition, under hypoxia, the high levels of HIF-1α elevate the level of angiogenesis-related factors such as VEGF, EPO, and GLUT which are related to neurogenesis [11].
We identified optimal conditions of 8% oxygen for 3 h to study the effects of low-oxygen exposure on NSCs (Figure 1). Previously, other studies have performed HP on NSCs with various oxygen levels and periods of time. A study found that 1% oxygen for 3 h was beneficial to NSC [12]. Similar to another study that examined preconditioning of NSCs with oxygen–glucose deprivation, 0% oxygen was found to be beneficial to NSC for 2 h and by increasing the length of time, the cell viability was lowered and cells started to be injured [20]. By contrast, in our study, our aim was to slightly trigger NSC with a lower amount of oxygen. Our result identified 8% oxygen to be the most beneficial and least devastating to NSCs. We also found that after HP, NSC increased the quantity of EV secretion, similar to many previous reports regarding various kind of cells [24].
Our work clearly showed that hypoxia influenced the protein composition within NSC-EVs. As previously reported, hypoxia influenced the efficiency of NSC treatment in various CNS diseases. We found that 42 proteins were differentially expressed after HP in NSC-EVs (Table 1). Among the 42 proteins, 20 proteins were upregulated. We also found that the transcripts of select upregulated proteins (Rpl34, Bhmt, Cdc42, and Atp2b) were also enhanced within the NSC-EVs. Interestingly, it was previously reported that BHMT could affect the nucleus to repair epigenetic control and accelerate neuroprotection [25]. Another study found that functional recovery was improved with ascorbic acid and sodium–vitamin C cotransporter 2 promoted NSCs’ migration through Cdc42 activation to facilitate F-actin assembly, which increased the therapeutic effect of ascorbic acid and NSC migration after brain injury [26]. Further, axon repair and nerve regeneration were promoted after cerebral ischemia through Netrin-1/Rac1/Cdc42 signalling pathways [25]. Acyl ghrelin improved neurite growth after oxygen glucose deficiency injury via Cdc42 [27]. We also noted that knockdown of TDP-43 in Neuro-2a cells inhibited neurite outgrowth and induced cell death. In the knockdown cells, the Rho family members RhoA, Rac1, and Cdc42 GTPases were inactivated; thus, TDP-43 may play a role in neuronal survival through protein regulation of those proteins [28]. By causing Cdc42 succinylation, ischemic buildup of succinate lowers the activity of Cdc42 GTPase, which reduces the proliferation of neural stem cells and exacerbates cerebral ischemia/reperfusion injury [29]. EGFR degradation and autophagy interact functionally during embryonic neurogenesis, and Ccd42b and ACK govern neuronal differentiation and can offer fresh insights into this interaction [30]. Wnt5a’s changed activation of Cdc42 on FIBER provides proof that the scaffold topography can affect how differently cells respond to their microenvironments [31]. N-WASP-Arp2/3 signalling-mediated development of Purkinje cell dendrites requires the upstream activator of N-WASP, Cdc42 [32]. Rat primary hippocampal neurons undergo axonogenesis in response to cdc42’s inhibition of GSK-3 activity [33]. Axon development and exocytosis are regulated by Cdc42b downstream of its activator Arhgef7 [34]. Patients with acute ischemic stroke are regularly monitored for disease progression and recurrence risk using the biomarker Cdc42 [35]. In addition to Cdc42, de novo mutations in Atp2b1 are thought to cause a monogenic form of neurodevelopmental disability, according to genetic discoveries, the probands’ overlapping phenotypes, and functional investigations [36]. Generally, Cdc42 and Atp2b may be beneficial for neuronal survival, homeostasis, and regulation [37,38,39,40,41,42,43]. Cadm2 protein, which was also one of the upregulated proteins in NSC-EVs after HP, was also reported to be involved in neuron cell–cell adhesion, molecular signalling in neurons, axon–axon interactions, and other complex sets of interactions in the nervous system [44,45,46,47]. The possibility of Rpl34 as a novel prognostic biomarker and therapeutic target for ischemic stroke was also reported previously [48,49]. Noteworthily, other overexpressed proteins such as Cnp, Cyfip1, Cask, and Tubb5 that were also shown to be overexpressed in our proteomics result are found to be involved in neuronal regulation, protection, maturation, and development [50,51,52,53,54,55,56,57,58,59,60,61,62].
Several proteins among the 22 downregulated proteins out of 42 total changes in proteins were previously reported to be involved in neurodegenerative diseases and have various functions in neurons in both healthy and disease situations.
Some of these downregulated proteins were previously tested and found to be involved in neurodegeneration and neurodegenerative diseases. Deficiency of the Npc2 protein and downregulation of ACTG2 were reported to be involved in the pathogenesis of Niemann–Pick Type C (NPC) disease [63,64]. Blmh protein is expressed in the brain and may be involved in Huntington disease [65].
Regarding Alzheimer’s disease (AD), Blmh plays role in the metabolism of homocysteine and has a connection to AD [66]. A study reported that a unique strategy to slow AD progression may be to boost DDAH1 activity in neural cells [67]. In addition, experimental autoimmune encephalomyelitis showed that increasing Ddah1 expression improved the ability to remyelinate. By modifying Ddah1 activity, these findings offer a unique therapeutic strategy for demyelinating disorders [68]. The expression of the heat shock proteins Hsp90 was significantly increased in the cells of Alzheimer’s disease patients, according to Western blot analysis [69]. Ndufa4 was also shown to be linked to mitochondrial dysfunction in the etiology of AD [70]. Alterations in Pdia3 levels also appeared to be age- and/or pathology-dependent, corroborating the ER chaperone’s involvement in AD pathology, and supporting the Pdia3 protein as a potential novel therapeutic target for the treatment of AD [71]. P-Tau protein accumulation may be influenced by decreasing Psap levels and their interactions in neurons [72]. Other studies have suggested that Psap may play a role in Alzheimer’s disease [73,74,75]. Psap has also been linked to neurotrophic and cell-damaging effects [76,77,78].
In Parkinson’s disease (PD) brain tissue samples, numerous dysregulated microRNAs have been found by recent human and animal investigations; Psmb2 is one of the downstream target proteins impacted by those dysregulated microRNAs [79]. Genetic evidence from previous studies also shows the involvement of the Psap saposin D domain in PD [80]. Dctn1 mutations may play a role in a variety of neurodegenerative illnesses, such as familial motor neuron disease, parkinsonism, and frontotemporal atrophy [81].
Various dysregulated proteins from our study were reported to be involved in amyotrophic lateral sclerosis (ALS). REEP1 cooperates with Ndufa4 in a mechanism that protects the integrity of mitochondrial complex IV, which is linked to ALS [82,83,84,85]. Bunina bodies are found in ALS, showing that Psap may also be a part of Bunina bodies. The interaction of Psap with other proteins may change how they both operate, resulting in motor neuron degeneration in ALS [86]. Several studies have linked the Dctn1 protein to ALS and Perry syndrome [87,88,89,90,91].
Atp6v1a, dctn1, Psap, and Ddah1 have also been mentioned as being involved in neurodegeneration and other neurodegenerative diseases [92,93,94,95,96].
Atp6v1a, Ndufa4, Dctn1, and Hsp90aa1 were also linked with abnormal CNS developments such as neural tube deficiency and Dandy-Walker malformation diseases [97,98,99,100].
To conclude, our work supports the idea that HP-NSC-EVs contain important proteins that contribute to the increased survival of NSCs following HP. Many of the proteins that we found were either downregulated or overexpressed in HP-EVs. Previous research showed that some of the overexpressed proteins in our study are well known to play many roles in neuronal cell function and thus may indeed be involved in neuronal survival, regulation, protection, maturation, and differentiation. Interestingly, many of the downregulated proteins in our study were previously tested to be involved in CNS degenerative diseases. Our work further underscores the potential critical role that EVs can play in changing cellular behavior as well as their usefulness as a potential target in the diagnosis of CNS diseases.
4. Methods and Materials
4.1. Primary NSC Isolation, Culture, and Passaging
Neural stem cells were obtained from the embryonic ICR mouse cortex under approval from the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China. Primary NSCs were isolated from embryonic day 14.5 ICR mice and cultured in DMEM/F12 medium (Gibco, Carlsbad, CA, USA) containing 2% B27 (Gibco, Carlsbad, CA, USA), 20 ng/mL fibroblast growth factor (bFGF, PeproTech, Rockhill, NJ, USA), 20 ng/mL epidermal growth factor (EGF, PeproTech), 2 nM L-glutamine (Gibco), and 1% penicillin–streptomycin (Gibco) as neurospheres in incubator at 37 °C in humidified condition containing 5% CO2. The cultured medium was changed every 3 days and floating neurospheres were passaged every 5–7 days using acutase (Gibco). After passaging, floating or seeded single cells with the density of 1 × 105 cells/mL were used for further experiments.
4.2. Immunohistochemistry
PFA-fixed NSC cells were washed three times with PBS for 5 min. Then, they were incubated with 0.3% Triton X-100 for 10 min and blocked with 5% bovine serum albumin for 1 h at room temperature. Afterwards, cells were incubated with primary antibodies at 4 °C overnight. The primary antibodies were used as follows: anti-GFAP antibody 1:100 (AB5804; Millipore Corporation, Danvers, MA, USA), anti-nestin (1:100, ab81462, Abcam, Cambridge, MA, USA), anti-MAP2 (1:200, MAB3418), and anti-SOX-2 (1:200 sc-365823, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After rinsing the samples with PBS, the samples were then incubated with corresponding secondary antibodies for 1 h at room temperature. Nuclei were stained using 4,6-diamidino-2-phenylindole (DAPI, 1:1000; Life Technologies, Mulgrave, VIC, Australia).
4.3. Hypoxic Preconditioning
Cultured NSCs were exposed to various levels of oxygen concentration in a hermetically sealed chamber at 37 °C for various periods of time. After that, the cells were returned back to normal condition. NSCs cultured in normal situation of oxygen concentration level were regarded as normal control.
4.4. NSC Proliferation and Cell Viability Assay
NSCs were seeded in PDL-coated 96-well plates at a density of 20 × 104 cell/mL, then placed in a normal condition as a control group or treated with different levels of oxygen at different periods of time for HP. The NSC cell viability was tested by the cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) 24 h after treatment according to the kit instructions.
4.5. Extracellular Vesicle Isolation
EVs were isolated from both control and hypoxia-preconditioned NSC culture supernatant using serial, sequential centrifugation, and ultracentrifugation [101].
EV protein concentration was tested by BCA protein assay (Thermo Scientific, Waltham, MA, USA).
4.6. Characterization of EVs
EVs were stained with 1% uranyl acetate and detected by transmission electron microscope (TEM, Thermo Scientific) for morphology characterization. EV particle number and size distribution were evaluated by nanoparticle tracking analysis (NTA, Brookhaven, NY, USA) according to the NTA device instruction.
4.7. Western Blotting
EV markers of CD63 and tumor of susceptibility gene 101 (TSG 101) were detected by Western blot. Western blot analysis of EV proteins of total 20 µg per sample was conducted as previously described [101]. The primary antibodies used were:
Anti-TSG-101 (1:1000, ab125011, Abcam), anti-β-actin (1:5000, 60008, Proteintech, Chicago, IL, USA), and anti-CD63 (1:1000, sc-5275, Santa Cruz Biotechnology).
4.8. EV Analysis with ExoView
The ExoView Tetraspanin chip (ExoView, Boston, MA, USA) was used to identify EVs. It was arrayed with antibodies against the proteins CD81, CD63, and CD9. As a negative control, mouse IgG1 was utilized. The chip surface received 35 µL of sample, which was then incubated for a whole night. After being washed, the chips were exposed to ExoView Tetraspanin Labelling ABs (EV-TC-AB-01), which included CD9/ALEXA 488, CD81/ALEXA 555, and CD63/ALEXA 647, for co-localization tests to identify EVs. The ExoView R100 reader (ExoView) was then used to image the chips utilizing the single particle interferometric reflectance imaging sensor (SP-IRIS) technology and ExoScan v0.998 (ExoView) acquisition software. With sizing thresholds set between 50 and 200 nm in diameter, the data were examined using ExoViewer v0.998.
4.9. Extracellular Vesicle Proteome Profiling
Both groups of normal and HP-NSC-EVs were sampled for protein extraction and sequencing. The samples underwent tandem MS and liquid chromatography analysis after being labelled with iTRAQ labelling reagents (ABSCIEX, 4,381,663). Relative iTRAQ quantification and protein analysis were carried out by Shanghai OE Biotechnology Limited Company. The basic process of bioinformatics analysis for proteomics is to search the database for qualitative and quantitative data and then, after quality assessment and preprocessing, perform expression level analysis and functional analysis, respectively. Functional annotator analysis of identified proteins was performed using multiple common databases. GO and KEGG analyses were performed on the screened differential proteins. At the same time, correlation analysis, expression pattern clustering heat map, venn analysis, etc., were performed on the data of the differential comparison group. In addition, according to the data situation, the relevant or interesting parts were explored, such as selecting the key proteins and their functions or pathways [102].
4.10. EV RNA Isolation
Total RNA was extracted from both control and HP-purified NSC EVs using miRNeasy Mini kit (Qiagen, Hilden, Germany), Kit. Extracted EVs were washed and centrifuged by buffer XBP and XWP, and mixed with suitable volume of QIAzol lysate stored at room temperature for 5 min. Chloroform was added according to the protocol to separate lysate, then the upper aqueous layer was transferred to a new collection tube. After addition of proper volume of 100% ethanol, the mixture was transferred into RNAeasy MinElute spin column in a collection tube. After centrifugation, RNA in the membrane was washed with buffer RWT and PRE. DNAase/RNAse-free water was added and centrifuged at 12,000× g for 5 min to dilute the RNA in the collection tube. The concentration and purity of EV RNAs were checked by Thermo micro spectrophotometer NanoDrop2000.
4.11. RT-qPCR
EV RNA was transcribed into cDNA-containing mixtures (10 μL) by miRCURY SYBER green PCR Kit (Qiagen, GER) according to the kit instructions. Then, SYBR Green Master Mix (11203ES08, Yeason, Shanghai, China) was used to perform real-time PCR. A two-stage amplification reaction was carried out under 95 °C for 5 min, followed by 40 cycles at 95 °C for 10 s, and at 60 °C for 30 s.
4.12. Statistical Analysis
All information was reported as mean and standard deviation (SD). The unpaired Student’s t test was used to compare means between two groups, and one-way ANOVA followed by the Tukey post hoc test were used to compare means between multiple groups in GraphPad Prism 6. (GraphPad Software, San Diego, CA, USA). A p value of 0.05 or lower was regarded as statistically significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb45030127/s1, Figure S1: Negative control for neural stem cell identification; Table S1: Primer sequence.
Author Contributions
T.G. conceived and designed the experiment, carried out most of the experiments, and wrote the manuscript. C.L. helped in carrying out some of the experimental results together with H.K., G.-Y.Y., Y.T. and Z.Z. contributed to the discussion of ideas, helped in the correction, and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Scientific and Technological Innovation Act Program of Shanghai Science and Technology Commission (20JC1411900 [GYY]). National Key R&D Program of China (2019YFA0112000 [YT], 2022YFA1603600 [ZZ]), the National Natural Science Foundation of China (82071284 [YT], 81974179 [ZZ], 82271320 [ZZ]), Scientific Research and Innovation Program of Shanghai Education Commission (2019-01-07-00-02-E00064 [GYY]), Shanghai Rising Star (21QA1405200 [YT]).
Institutional Review Board Statement
The protocol was approval by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China (No. 2019052).
Informed Consent Statement
Not applicable.
Data Availability Statement
The proteomic data has been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD037966.
Acknowledgments
We thank members of the Med-X Research Institute for their collective and kind support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CNS, central nervous system; EV, extracellular vesicle; NSC, neural stem cell; HP, hypoxic preconditioning; DEP, differentially expressed proteins.
References
- Boese, A.C.; Le, Q.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem. Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Ma, T.; Li, Y. Preconditioning stem cells for in vivo delivery. BioRes. Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Narasimhan, P.; Maier, C.M.; Nishiyama, Y.; Chan, P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012, 32, 3462–3473. [Google Scholar] [CrossRef]
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012, 135 Pt 11, 3298–3310. [Google Scholar] [CrossRef]
- Theus, M.H.; Wei, L.; Cui, L.; Francis, K.; Hu, X.; Keogh, C.; Yu, S.P. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp. Neurol. 2008, 210, 656–670. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Femmino, S.; Ravera, F.; Angelini, F.; Caccioppo, A.; Franchin, L.; Grosso, A.; Comita, S.; Cavallari, C.; Penna, C.; et al. Extracellular vesicles from patients with Acute Coronary Syndrome impact on ischemia-reperfusion injury. Pharmacol. Res. 2021, 170, 105715. [Google Scholar] [CrossRef] [PubMed]
- Francis, K.R.; Wei, L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010, 1, e22. [Google Scholar] [CrossRef]
- Wakai, T.; Narasimhan, P.; Sakata, H.; Wang, E.; Yoshioka, H.; Kinouchi, H.; Chan, P.H. Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 2016, 36, 2134–2145. [Google Scholar] [CrossRef]
- Mung, K.L.; Tsui, Y.P.; Tai, E.W.; Chan, Y.S.; Shum, D.K.; Shea, G.K. Rapid and efficient generation of neural progenitors from adult bone marrow stromal cells by hypoxic preconditioning. Stem. Cell Res. Ther. 2016, 7, 146. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Qu, Z.; Li, D.; Si, P.; Qiao, Q.; Zhang, W.; Xue, Y.; Zhen, J.; Wang, W. Chronic mild hypoxia promotes hippocampal neurogenesis involving Notch1 signaling in epileptic rats. Brain Res. 2019, 1714, 88–98. [Google Scholar] [CrossRef]
- Huang, L.; Wan, Y.; Dang, Z.; Yang, P.; Yang, Q.; Wu, S. Hypoxic preconditioning ameliorated neuronal injury after middle cerebral artery occlusion by promoting neurogenesis. Brain Behav. 2020, 10, e01804. [Google Scholar] [CrossRef] [PubMed]
- Ara, J.; De Montpellier, S. Hypoxic-preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem. Cell Res. 2013, 11, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.L.; Guan, Y.J.; Ma, D.D.; Du, H.M. Optimal concentration and time window for proliferation and differentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours. Neural Regen. Res. 2015, 10, 1516–1522. [Google Scholar] [CrossRef]
- Fan, W.L.; Liu, P.; Wang, G.; Pu, J.G.; Xue, X.; Zhao, J.H. Transplantation of hypoxic preconditioned neural stem cells benefits functional recovery via enhancing neurotrophic secretion after spinal cord injury in rats. J. Cell. Biochem. 2018, 119, 4339–4351. [Google Scholar] [CrossRef] [PubMed]
- Motomura, A.; Shimizu, M.; Kato, A.; Motomura, K.; Yamamichi, A.; Koyama, H.; Ohka, F.; Nishikawa, T.; Nishimura, Y.; Hara, M.; et al. Remote ischemic preconditioning protects human neural stem cells from oxidative stress. Apoptosis 2017, 22, 1353–1361. [Google Scholar] [CrossRef]
- Gharbi, T.; Zhang, Z.; Yang, G.Y. The Function of Astrocyte Mediated Extracellular Vesicles in Central Nervous System Diseases. Front. Cell Dev. Biol. 2020, 8, 568889. [Google Scholar] [CrossRef]
- Rooj, A.K.; Mineo, M.; Godlewski, J. MicroRNA and extracellular vesicles in glioblastoma: Small but powerful. Brain Tumor Pathol. 2016, 33, 77–88. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Wang, Y. Enhanced differentiation of neural stem cells to neurons and promotion of neurite outgrowth by oxygen-glucose deprivation. Int. J. Dev. Neurosci. 2015, 43, 50–57. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Zhu, Y.B.; Zhang, J.Y.; McCrary, M.R.; Wang, S.; Zhang, Y.B.; Yu, S.P.; Wei, L. Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy. Chin. Med. J. 2017, 130, 2361–2374. [Google Scholar] [PubMed]
- De Filippis, L.; Delia, D. Hypoxia in the regulation of neural stem cells. Cell. Mol. Life Sci. 2011, 68, 2831–2844. [Google Scholar] [CrossRef]
- Abati, E.; Bresolin, N.; Comi, G.P.; Corti, S. Preconditioning and Cellular Engineering to Increase the Survival of Transplanted Neural Stem Cells for Motor Neuron Disease Therapy. Mol. Neurobiol. 2019, 56, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hsiang, F.; Chang, J.H.; Yao, X.Q.; Zhao, H.; Zou, H.Y.; Wang, L.; Zhang, Q.X. Houshiheisan and its components promote axon regeneration after ischemic brain injury. Neural Regen. Res. 2018, 13, 1195–1203. [Google Scholar]
- Yang, Y.; Zhang, K.; Chen, X.; Wang, J.; Lei, X.; Zhong, J.; Xian, J.; Quan, Y.; Lu, Y.; Huang, Q.; et al. SVCT2 Promotes Neural Stem/Progenitor Cells Migration Through Activating CDC42 After Ischemic Stroke. Front Cell Neurosci. 2019, 13, 429. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Dong, R.; Sun, C.; Li, S.; Zhu, S. Ghrelin Promotes Cortical Neurites Growth in Late Stage After Oxygen-Glucose Deprivation/Reperfusion Injury. J. Mol. Neurosci. 2019, 68, 29–37. [Google Scholar] [CrossRef]
- Iguchi, Y.; Katsuno, M.; Niwa, J.I.; Yamada, S.I.; Sone, J.; Waza, M.; Adachi, H.; Tanaka, F.; Nagata, K.I.; Arimura, N.; et al. TDP-43 depletion induces neuronal cell damage through dysregulation of Rho family GTPases. J. Biol. Chem. 2009, 284, 22059–22066. [Google Scholar] [CrossRef]
- Huang, L.Y.; Ma, J.Y.; Song, J.X.; Xu, J.J.; Hong, R.; Fan, H.D.; Cai, H.; Wang, W.; Wang, Y.L.; Hu, Z.L.; et al. Ischemic accumulation of succinate induces Cdc42 succinylation and inhibits neural stem cell proliferation after cerebral ischemia/reperfusion. Neural Regen. Res. 2023, 18, 1040–1045. [Google Scholar]
- Endo, M.; Cerione, R.A. The brain-specific splice variant of the CDC42 GTPase works together with the kinase ACK to downregulate the EGF receptor in promoting neurogenesis. J. Biol. Chem. 2022, 298, 102564. [Google Scholar] [CrossRef]
- Rahman, S.U.; Kim, W.J.; Chung, S.H.; Woo, K.M. Nanofibrous topography-driven altered responsiveness to Wnt5a mediates the three-dimensional polarization of odontoblasts. Mater. Today Bio 2022, 17, 100479. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Matsui, T.K.; Kondo, J.; Kuwako, K.I. N-WASP-Arp2/3 signaling controls multiple steps of dendrite maturation in Purkinje cells in vivo. Development 2022, 149, dev201214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Chen, F.Z.; Chen, W.; Zhu, H.M.; Chen, Y.; Li, Z.L.; Yan, F.; Liu, Z.Y.; Dong, W.R.; Zhang, L.; et al. Cdc42 Promotes Axonogenesis of Primary Hippocampal Neurons by Inhibiting Glycogen Synthase Kinase-3beta. J. Integr. Neurosci. 2022, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Puschel, A.W. An isoform-specific function of Cdc42 in regulating mammalian Exo70 during axon formation. Life Sci. Alliance 2023, 6, e202201722. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ye, J.; Zhang, X.; Meng, K. Longitudinal Variations of CDC42 in Patients with Acute Ischemic Stroke during 3-Year Period: Correlation with CD4(+) T Cells, Disease Severity, and Prognosis. Front. Neurol. 2022, 13, 848933. [Google Scholar] [CrossRef]
- Rahimi, M.J.; Urban, N.; Wegler, M.; Sticht, H.; Schaefer, M.; Popp, B.; Gaunitz, F.; Morleo, M.; Nigro, V.; Maitz, S.; et al. De novo variants in ATP2B1 lead to neurodevelopmental delay. Am. J. Hum. Genet. 2022, 109, 944–952. [Google Scholar] [CrossRef]
- Linseman, D.A.; Laessig, T.; Meintzer, M.K.; McClure, M.; Barth, H.; Aktories, K.; Heidenreich, K.A. An essential role for Rac/Cdc42 GTPases in cerebellar granule neuron survival. J. Biol. Chem. 2001, 276, 39123–39131. [Google Scholar] [CrossRef]
- Govek, E.E.; Newey, S.E.; Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005, 19, 1–49. [Google Scholar] [CrossRef]
- Kalpachidou, T.; Spiecker, L.; Kress, M.; Quarta, S. Rho GTPases in the Physiology and Pathophysiology of Peripheral Sensory Neurons. Cells 2019, 8, 591. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Rojo, D.R.; Prough, D.S.; Falduto, M.T.; Boone, D.R.; Micci, M.A.; Kahrig, K.M.; Crookshanks, J.M.; Jimenez, A.; Uchida, T.; Cowart, J.C.; et al. Influence of stochastic gene expression on the cell survival rheostat after traumatic brain injury. PLoS ONE 2011, 6, e23111. [Google Scholar] [CrossRef] [PubMed]
- Krey, J.F.; Dolmetsch, R.E. Molecular mechanisms of autism: A possible role for Ca2+ signaling. Curr. Opin. Neurobiol. 2007, 17, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Verpelli, C.; Dvoretskova, E.; Vicidomini, C.; Rossi, F.; Chiappalone, M.; Schoen, M.; Di Stefano, B.; Mantegazza, R.; Broccoli, V.; Bockers, T.M.; et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J. Biol. Chem. 2011, 286, 34839–34850. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Verbaas, C.A.; Bressler, J.; Debette, S.; Schuur, M.; Smith, A.V.; Bis, J.C.; Davies, G.; Trompet, S.; Smith, J.A.; Wolf, C.; et al. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol. Psychiatry 2016, 21, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, J.; Chen, C.; Xu, J.; Bell, R.L.; Hall, F.S.; Koob, G.F.; Volkow, N.D.; Qing, H.; Lin, Z. Epistatic evidence for gender-dependant slow neurotransmission signalling in substance use disorders: PPP1R12B versus PPP1R1B. EBioMedicine 2020, 61, 103066. [Google Scholar] [CrossRef] [PubMed]
- Frei, J.A.; Andermatt, I.; Gesemann, M.; Stoeckli, E.T. The SynCAM synaptic cell adhesion molecules are involved in sensory axon pathfinding by regulating axon-axon contacts. J. Cell Sci. 2014, 127 Pt 24, 5288–5302. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, F.; Gerber, A.; Bauer, C.; Ballivet, M.; Ossipow, V. The adhesion molecule Necl-3/SynCAM-2 localizes to myelinated axons, binds to oligodendrocytes and promotes cell adhesion. BMC Neurosci. 2007, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Q.; Lu, Y.P.; Sun, H.L.; Gao, L.N.; Song, P.P.; Feng, Z.J.; You, C.G. Sex Difference of Ribosome in Stroke-Induced Peripheral Immunosuppression by Integrated Bioinformatics Analysis. BioMed Res. Int. 2020, 2020, 3650935. [Google Scholar] [CrossRef]
- Wei, X.Y.; Zhang, T.Q.; Suo, R.; Qu, Y.Y.; Chen, Y.; Zhu, Y.L. Long non-coding RNA RPL34-AS1 ameliorates oxygen-glucose deprivation-induced neuronal injury via modulating miR-223-3p/IGF1R axis. Hum. Cell 2022, 35, 1785–1796. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, L. C-type natriuretic peptide functions as an innate neuroprotectant in neonatal hypoxic-ischemic brain injury in mouse via natriuretic peptide receptor 2. Exp. Neurol. 2018, 304, 58–66. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, K.; Tian, K.Y.; Zhang, X.Y.; Liu, W.; Wang, J.; Zhong, C.P.; Qiu, J.H.; Zha, D.J. Atrial Natriuretic Peptide Promotes Neurite Outgrowth and Survival of Cochlear Spiral Ganglion Neurons in vitro through NPR-A/cGMP/PKG Signaling. Front. Cell Dev. Biol. 2021, 9, 681421. [Google Scholar] [CrossRef] [PubMed]
- Haan, N.; Westacott, L.J.; Carter, J.; Owen, M.J.; Gray, W.P.; Hall, J.; Wilkinson, L.S. Haploinsufficiency of the schizophrenia and autism risk gene Cyfip1 causes abnormal postnatal hippocampal neurogenesis through microglial and Arp2/3 mediated actin dependent mechanisms. Transl. Psychiatry 2021, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Habela, C.W.; Yoon, K.J.; Kim, N.S.; Taga, A.; Bell, K.; Bergles, D.E.; Maragakis, N.J.; Ming, G.L.; Song, H. Persistent Cyfip1 Expression Is Required to Maintain the Adult Subventricular Zone Neurogenic Niche. J. Neurosci. 2020, 40, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Harony-Nicolas, H.; Buxbaum, J.D.; Bozdagi-Gunal, O.; Benson, D.L. Cyfip1 Regulates Presynaptic Activity during Development. J. Neurosci. 2016, 36, 1564–1576. [Google Scholar] [CrossRef]
- Yoon, K.J.; Nguyen, H.N.; Ursini, G.; Zhang, F.; Kim, N.S.; Wen, Z.; Makri, G.; Nauen, D.; Shin, J.H.; Park, Y.; et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 2014, 15, 79–91. [Google Scholar] [CrossRef]
- Biembengut, I.V.; Silva, I.L.Z.; Souza, T.; Shigunov, P. Cytoplasmic FMR1 interacting protein (CYFIP) family members and their function in neural development and disorders. Mol. Biol. Rep. 2021, 48, 6131–6143. [Google Scholar] [CrossRef]
- Kim, G.H.; Zhang, Y.; Kang, H.R.; Lee, S.H.; Shin, J.; Lee, C.H.; Kang, H.; Ma, R.; Jin, C.; Kim, Y.; et al. Altered presynaptic function and number of mitochondria in the medial prefrontal cortex of adult Cyfip2 heterozygous mice. Mol. Brain 2020, 13, 123. [Google Scholar] [CrossRef]
- Atasoy, D.; Schoch, S.; Ho, A.; Nadasy, K.A.; Liu, X.; Zhang, W.; Mukherjee, K.; Nosyreva, E.D.; Fernandez-Chacon, R.; Missler, M.; et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc. Natl. Acad. Sci. USA 2007, 104, 2525–2530. [Google Scholar] [CrossRef]
- Mori, T.; Kasem, E.A.; Suzuki-Kouyama, E.; Cao, X.; Li, X.; Kurihara, T.; Uemura, T.; Yanagawa, T.; Tabuchi, K. Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B. Mol. Psychiatry 2019, 24, 1079–1092. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Chen, Y.; Zhu, S.; Mi, J.; Wang, J.; Zhang, M. Structural Basis for the High-Affinity Interaction between CASK and Mint1. Structure 2020, 28, 664–673.e3. [Google Scholar] [CrossRef]
- Ngo, L.; Haas, M.; Qu, Z.; Li, S.S.; Zenker, J.; Teng, K.S.; Gunnersen, J.M.; Breuss, M.; Habgood, M.; Keays, D.A.; et al. TUBB5 and its disease-associated mutations influence the terminal differentiation and dendritic spine densities of cerebral cortical neurons. Hum. Mol. Genet. 2014, 23, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Breuss, M.; Heng, J.I.; Poirier, K.; Tian, G.; Jaglin, X.H.; Qu, Z.; Braun, A.; Gstrein, T.; Ngo, L.; Haas, M.; et al. Mutations in the beta-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2012, 2, 1554–1562. [Google Scholar] [CrossRef]
- Walkley, S.U.; Suzuki, K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim. Biophys. Acta 2004, 1685, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Hetmanczyk-Sawicka, K.; Iwanicka-Nowicka, R.; Fogtman, A.; Ciesla, J.; Wlodarski, P.; Zyzynska-Granica, B.; Filocamo, M.; Dardis, A.; Peruzzo, P.; Bednarska-Makaruk, M.; et al. Changes in global gene expression indicate disordered autophagy, apoptosis and inflammatory processes and downregulation of cytoskeletal signalling and neuronal development in patients with Niemann-Pick C disease. Neurogenetics 2020, 21, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Ratovitski, T.; Chighladze, E.; Waldron, E.; Hirschhorn, R.R.; Ross, C.A. Cysteine proteases bleomycin hydrolase and cathepsin Z mediate N-terminal proteolysis and toxicity of mutant huntingtin. J. Biol. Chem. 2011, 286, 12578–12589. [Google Scholar] [CrossRef]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H. Hyperhomocysteinemia and bleomycin hydrolase modulate the expression of mouse brain proteins involved in neurodegeneration. J. Alzheimers Dis. 2014, 40, 713–726. [Google Scholar] [CrossRef]
- Luo, Y.; Yue, W.; Quan, X.; Wang, Y.; Zhao, B.; Lu, Z. Asymmetric dimethylarginine exacerbates Abeta-induced toxicity and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic. Biol. Med. 2015, 79, 117–126. [Google Scholar] [CrossRef]
- Uyeda, A.; Quan, L.; Kato, Y.; Muramatsu, N.; Tanabe, S.; Sakai, K.; Ichinohe, N.; Kawahara, Y.; Suzuki, T.; Muramatsu, R. Dimethylarginine dimethylaminohydrolase 1 as a novel regulator of oligodendrocyte differentiation in the central nervous system remyelination. Glia 2021, 69, 2591–2604. [Google Scholar] [CrossRef]
- Lopez-Toledo, G.; Silva-Lucero, M.D.; Herrera-Diaz, J.; Garcia, D.E.; Arias-Montano, J.A.; Cardenas-Aguayo, M.D. Patient-Derived Fibroblasts with Presenilin-1 Mutations, That Model Aspects of Alzheimer’s Disease Pathology, Constitute a Potential Object for Early Diagnosis. Front. Aging Neurosci. 2022, 14, 921573. [Google Scholar] [CrossRef]
- Shim, J.S.; Song, M.Y.; Yim, S.V.; Lee, S.E.; Park, K.S. Global analysis of ginsenoside Rg1 protective effects in beta-amyloid-treated neuronal cells. J. Ginseng Res. 2017, 41, 566–571. [Google Scholar] [CrossRef]
- Cassano, T.; Giamogante, F.; Calcagnini, S.; Romano, A.; Lavecchia, A.M.; Inglese, F.; Paglia, G.; Bukke, V.N.; Romano, A.D.; Friuli, M.; et al. PDIA3 Expression Is Altered in the Limbic Brain Regions of Triple-Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3005. [Google Scholar] [CrossRef] [PubMed]
- Mendsaikhan, A.; Tooyama, I.; Serrano, G.E.; Beach, T.G.; Walker, D.G. Loss of Lysosomal Proteins Progranulin and Prosaposin Associated with Increased Neurofibrillary Tangle Development in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2021, 80, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Callizot, N.; Estrella, C.; Burlet, S.; Henriques, A.; Brantis, C.; Barrier, M.; Campanari, M.L.; Verwaerde, P. AZP2006, a new promising treatment for Alzheimer’s and related diseases. Sci. Rep. 2021, 11, 16806. [Google Scholar] [CrossRef] [PubMed]
- Mendsaikhan, A.; Tooyama, I.; Bellier, J.P.; Serrano, G.E.; Sue, L.I.; Lue, L.F.; Beach, T.G.; Walker, D.G. Characterization of lysosomal proteins Progranulin and Prosaposin and their interactions in Alzheimer’s disease and aged brains: Increased levels correlate with neuropathology. Acta Neuropathol. Commun. 2019, 7, 215. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, L.; Bracko, O.; Choi, J.W.; Jia, Y.; Nana, A.L.; Brady, O.A.; Hernandez, J.C.C.; Nishimura, N.; Seeley, W.W.; et al. Impaired prosaposin lysosomal trafficking in frontotemporal lobar degeneration due to progranulin mutations. Nat. Commun. 2017, 8, 15277. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Hu, Y.; Liao, A.; Zheng, W.; Wang, X.; Lan, J.; Shen, J.; Wang, S.; Yang, F.; et al. Progranulin improves neural development via the PI3K/Akt/GSK-3beta pathway in the cerebellum of a VPA-induced rat model of ASD. Transl. Psychiatry 2022, 12, 114. [Google Scholar] [CrossRef]
- Sikora, J.; Harzer, K.; Elleder, M. Neurolysosomal pathology in human prosaposin deficiency suggests essential neurotrophic function of prosaposin. Acta Neuropathol. 2007, 113, 163–175. [Google Scholar] [CrossRef]
- Troncoso, M.; Bannoud, N.; Carvelli, L.; Asensio, J.; Seltzer, A.; Sosa, M.A. Hypoxia-ischemia alters distribution of lysosomal proteins in rat cortex and hippocampus. Biol. Open 2018, 7, bio036723. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs in Parkinson’s disease and emerging therapeutic targets. Neural Regen. Res. 2017, 12, 1945–1959. [Google Scholar]
- Oji, Y.; Hatano, T.; Ueno, S.I.; Funayama, M.; Ishikawa, K.I.; Okuzumi, A.; Noda, S.; Sato, S.; Satake, W.; Toda, T.; et al. Variants in saposin D domain of prosaposin gene linked to Parkinson’s disease. Brain 2020, 143, 1190–1205. [Google Scholar] [CrossRef]
- Araki, E.; Tsuboi, Y.; Daechsel, J.; Milnerwood, A.; Vilarino-Guell, C.; Fujii, N.; Mishima, T.; Oka, T.; Hara, H.; Fukae, J.; et al. A novel DCTN1 mutation with late-onset parkinsonism and frontotemporal atrophy. Mov. Disord. 2014, 29, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; You, P.; Yu, H.; Su, B. REEP1 Preserves Motor Function in SOD1(G93A) Mice by Improving Mitochondrial Function via Interaction with NDUFA4. Neurosci. Bull. 2022, 1–18. [Google Scholar] [CrossRef]
- Finsterer, J.; Burgunder, J.M. Recent progress in the genetics of motor neuron disease. Eur. J. Med. Genet. 2014, 57, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Henty-Ridilla, J.L. Multiple roles for the cytoskeleton in ALS. Exp. Neurol. 2022, 355, 114143. [Google Scholar] [CrossRef]
- Castellanos-Montiel, M.J.; Chaineau, M.; Durcan, T.M. The Neglected Genes of ALS: Cytoskeletal Dynamics Impact Synaptic Degeneration in ALS. Front. Cell Neurosci. 2020, 14, 594975. [Google Scholar] [CrossRef]
- Wada, Y.; Nagai, A.; Sheikh, A.M.; Onoda, K.; Terashima, M.; Shiota, Y.; Araki, A.; Yamaguchi, S. Co-localization of cystatin C and prosaposin in cultured neurons and in anterior horn neurons with amyotrophic lateral sclerosis. J. Neurol. Sci. 2018, 384, 67–74. [Google Scholar] [CrossRef]
- Gustavsson, E.K.; Trinh, J.; Guella, I.; Szu-Tu, C.; Khinda, J.; Lin, C.H.; Wu, R.M.; Stoessl, J.; Appel-Cresswell, S.; McKeown, M.; et al. DCTN1 p.K56R in progressive supranuclear palsy. Park. Relat. Disord. 2016, 28, 56–61. [Google Scholar] [CrossRef]
- Deshimaru, M.; Kinoshita-Kawada, M.; Kubota, K.; Watanabe, T.; Tanaka, Y.; Hirano, S.; Ishidate, F.; Hiramoto, M.; Ishikawa, M.; Uehara, Y.; et al. DCTN1 Binds to TDP-43 and Regulates TDP-43 Aggregation. Int. J. Mol. Sci. 2021, 22, 3985. [Google Scholar] [CrossRef]
- Mishima, T.; Fujioka, S.; Tomiyama, H.; Yabe, I.; Kurisaki, R.; Fujii, N.; Neshige, R.; Ross, O.A.; Farrer, M.J.; Dickson, D.W.; et al. Establishing diagnostic criteria for Perry syndrome. J. Neurol. Neurosurg. Psychiatry 2018, 89, 482–487. [Google Scholar] [CrossRef]
- Deshimaru, M.; Mishima, T.; Watanabe, T.; Kubota, K.; Hosoi, M.; Kinoshita-Kawada, M.; Yuasa-Kawada, J.; Ikeda, M.; Mori, M.; Murata, Y.; et al. Behavioral profile in a Dctn1(G71A) knock-in mouse model of Perry disease. Neurosci. Lett. 2021, 764, 136234. [Google Scholar] [CrossRef]
- Honda, H.; Sasagasako, N.; Shen, C.; Shijo, M.; Hamasaki, H.; Suzuki, S.O.; Tsuboi, Y.; Fujii, N.; Iwaki, T. DCTN1 F52L mutation case of Perry syndrome with progressive supranuclear palsy-like tauopathy. Park. Relat. Disord. 2018, 51, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, A.; Sekiya, M.; Beckmann, N.D.; Quan, X.; Schrode, N.; Fernando, M.B.; Yu, A.; Zhu, L.; Cao, J.; et al. Transformative Network Modeling of Multi-omics Data Reveals Detailed Circuits, Key Regulators, and Potential Therapeutics for Alzheimer’s Disease. Neuron 2021, 109, 257–272.e14. [Google Scholar] [CrossRef] [PubMed]
- Dulski, J.; Konno, T.; Wszolek, Z. DCTN1-Related Neurodegeneration. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Wang, N.; Ma, Q.; Peng, P.; Yu, Y.; Xu, S.; Wang, G.; Ying, Z.; Wang, H. Autophagy and Ubiquitin-Proteasome System Coordinate to Regulate the Protein Quality Control of Neurodegenerative Disease-Associated DCTN1. Neurotox. Res. 2020, 37, 48–57. [Google Scholar] [CrossRef]
- Yoneshige, A.; Suzuki, K.; Suzuki, K.; Matsuda, J. A mutation in the saposin C domain of the sphingolipid activator protein (Prosaposin) gene causes neurodegenerative disease in mice. J. Neurosci. Res. 2010, 88, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, M.; Dou, Y.; Du, K.; Liu, X.; Zhao, Y. DDAH1/ADMA Regulates Adiponectin Resistance in Cerebral Ischemia via the ROS/FOXO1/APR1 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2350857. [Google Scholar] [CrossRef]
- Fassio, A.; Esposito, A.; Kato, M.; Saitsu, H.; Mei, D.; Marini, C.; Conti, V.; Nakashima, M.; Okamoto, N.; Olmez Turker, A.; et al. De novo mutations of the ATP6V1A gene cause developmental encephalopathy with epilepsy. Brain 2018, 141, 1703–1718. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Li, Y.; Li, R.; Lei, T.Y.; Wang, D.; Yang, X.; Han, J.; Pan, M.; Zhen, L.; Ou, Y.M.; et al. NDUFA4 enhances neuron growth by triggering growth factors and inhibiting neuron apoptosis through Bcl-2 and cytochrome C mediated signaling pathway. Am. J. Transl. Res. 2018, 10, 164–174. [Google Scholar]
- Yu, J.; Mu, J.; Guo, Q.; Yang, L.; Zhang, J.; Liu, Z.; Yu, B.; Zhang, T.; Xie, J. Transcriptomic profile analysis of mouse neural tube development by RNA-Seq. IUBMB Life 2017, 69, 706–719. [Google Scholar] [CrossRef]
- Guan, Z.; Liang, Y.; Wang, X.; Zhu, Z.; Yang, A.; Li, S.; Yu, J.; Niu, B.; Wang, J. Unraveling the Mechanisms of Clinical Drugs-Induced Neural Tube Defects Based on Network Pharmacology and Molecular Docking Analysis. Neurochem. Res. 2022, 47, 3709–3722. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).