The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases
Abstract
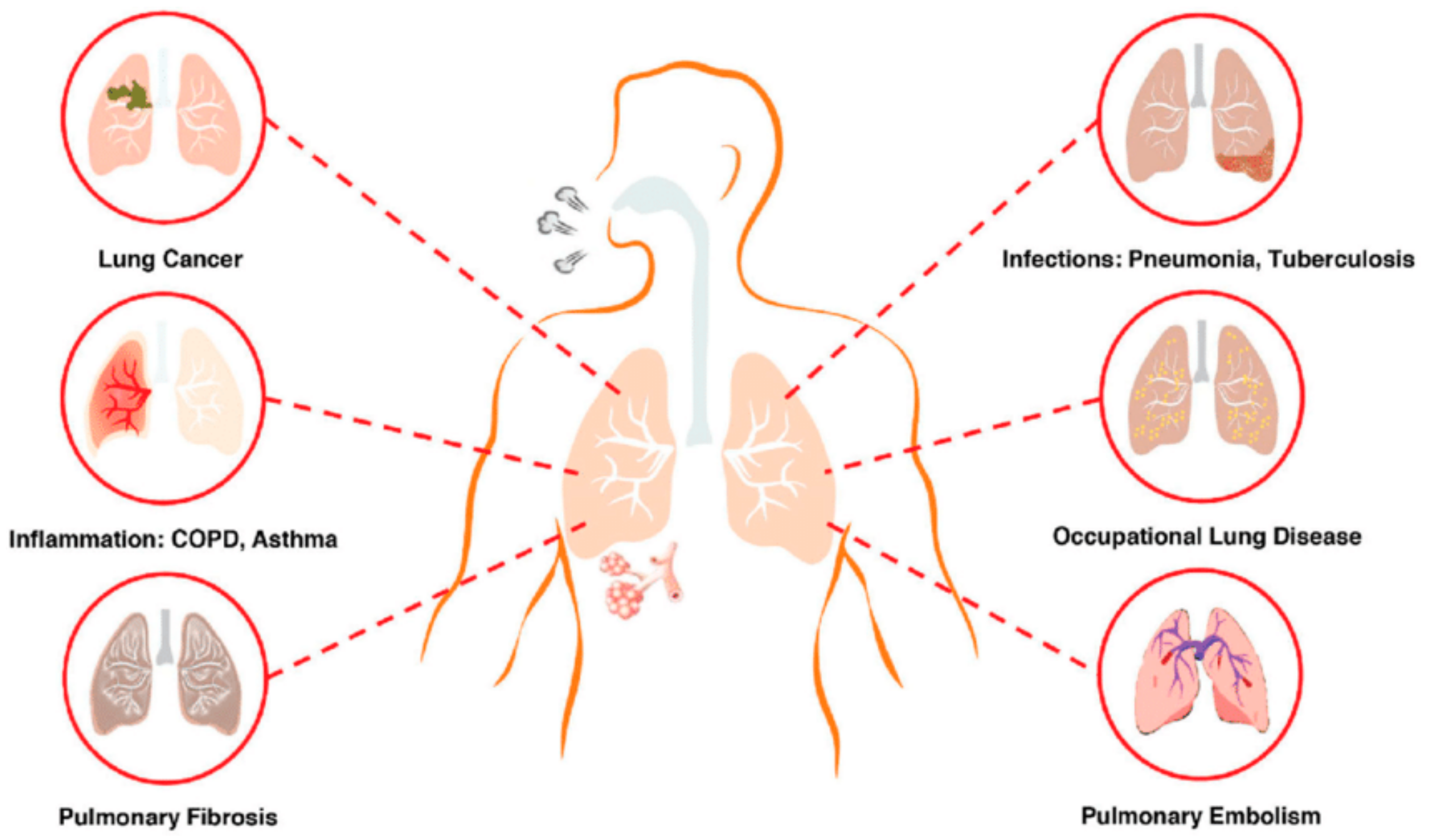
:1. Introduction
2. Conventional Herbal Application in the Treatment of Pulmonary Diseases
2.1. Chronic Obstructive Pulmonary Disorder (COPD)
2.2. Tuberculosis
2.3. Asthma
3. The Contribution of Various Phytochemicals to Respiratory Health
3.1. Alkaloids: Bridging Bronchodilation and Immune Modulation
3.2. Saponins: Surfactant Enhancement and Immunomodulation
3.3. Lignans: Hormonal and Antioxidant Potential
3.4. Terpenoids: Anti-Inflammatory and Antimicrobial Allies
3.5. Flavonoids: Antioxidant Guardians and Inflammatory Modulators
4. Physiological and Anatomical Factors Influencing Pulmonary Drug Delivery
4.1. Lung Anatomy
4.2. Mucociliary Clearance
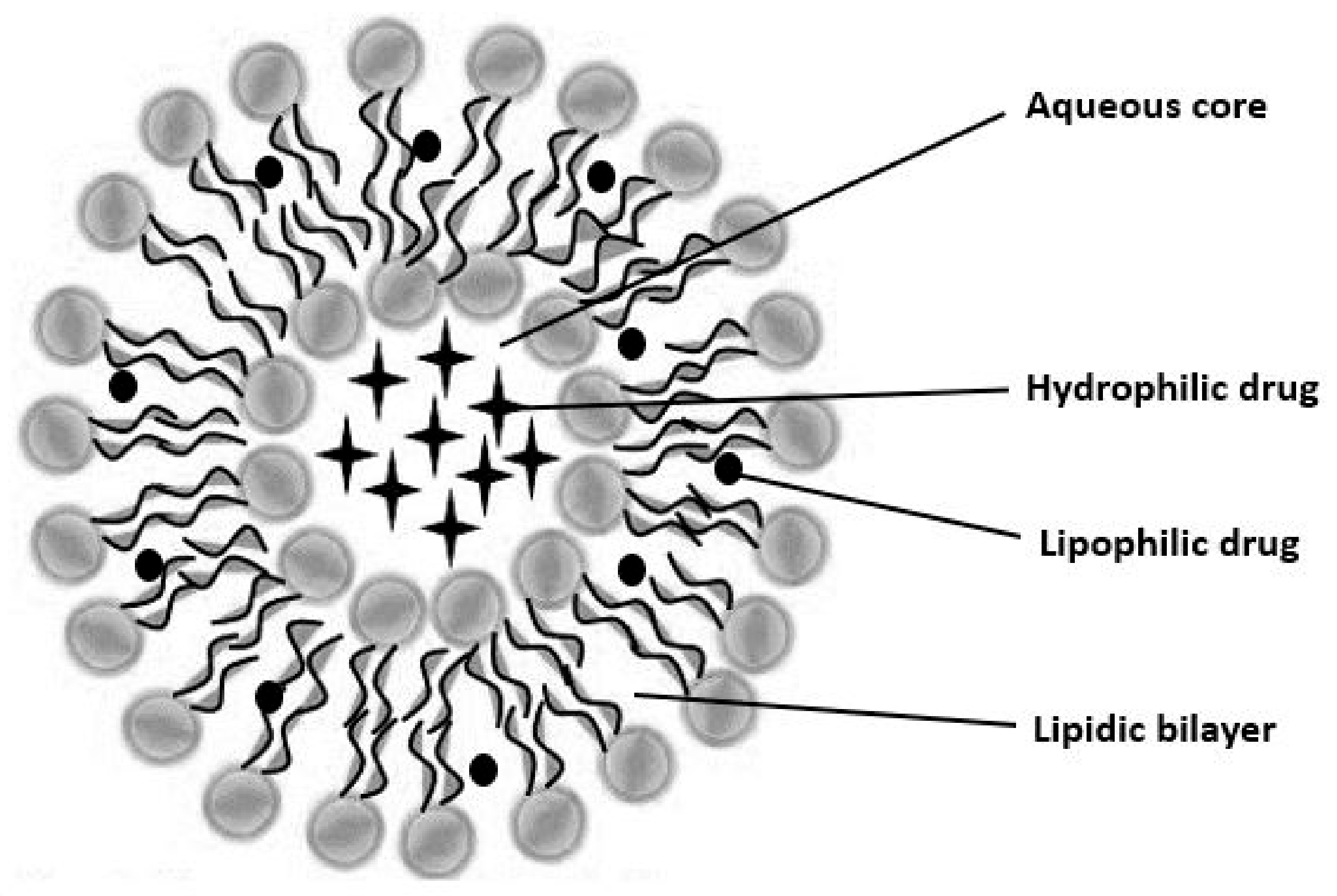
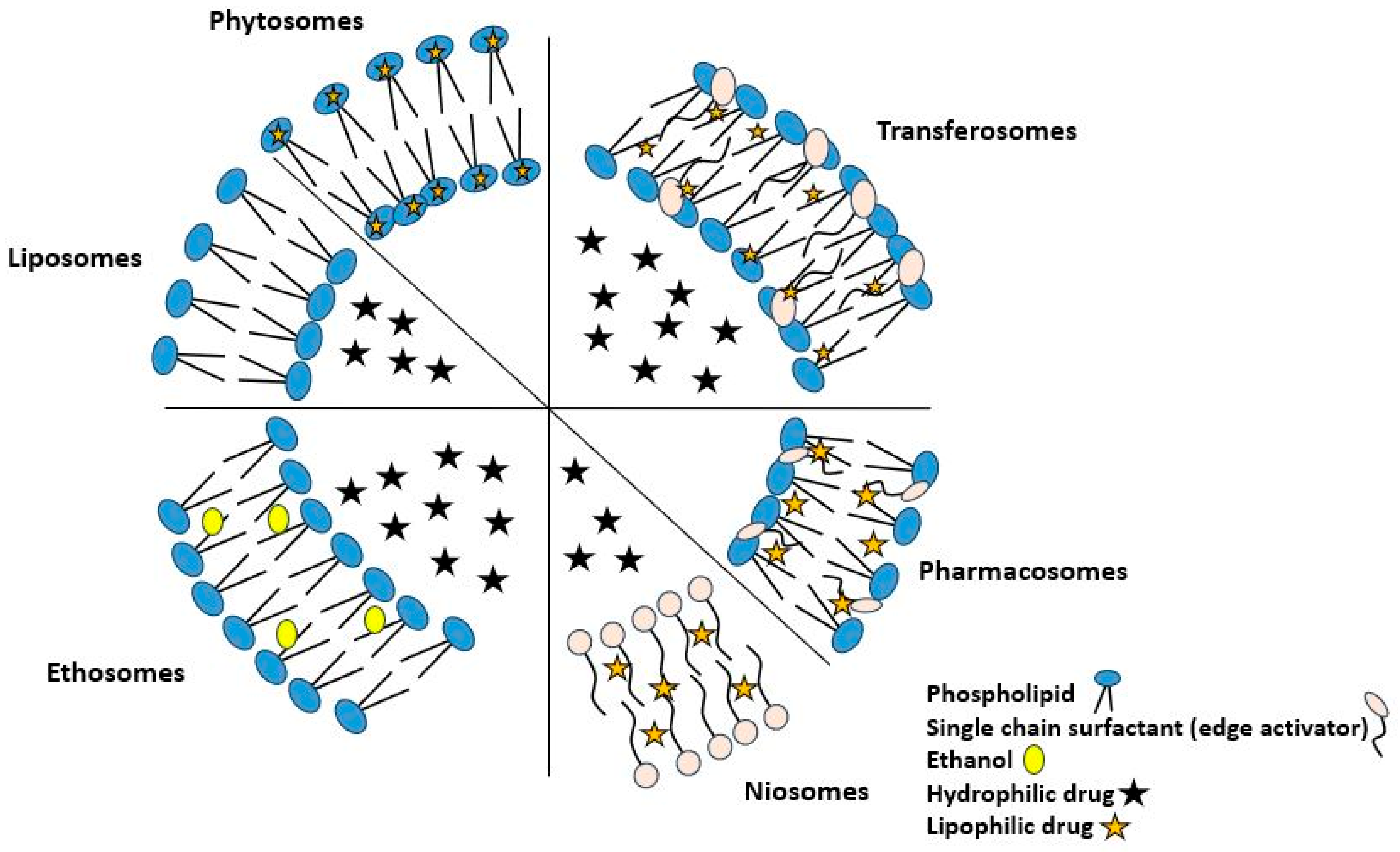
5. Vesicular Drug Delivery Systems (VDDS)
6. General Preparation and Characterization Techniques of Plant-Material-Loaded VDDS
6.1. Preparation of Multilamellar Vesicles (MLV)
6.1.1. Solvent Evaporation
6.1.2. Transmembrane pH Gradient Drug Uptake
6.2. Preparation of Small Unilamellar Vesicles (SUV)
6.2.1. Microfluidics
6.2.2. Sonication
6.3. Preparation of Large Unilamellar Vesicles
6.3.1. Solvent Ether-Injection Process
6.3.2. Reversed-Phase Evaporation (REV)
6.4. Miscellaneous Techniques
6.4.1. Emulsion Formation
6.4.2. Lipid Injection
6.5. Characterization of VDDS
6.5.1. Particle Size, Particle Shape, and Particle Size Distribution
Dynamic Light Scattering
Zeta Potential (ZP)
Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Cryo-Electron Microscopy (Cryo-EM), Atomic Force Microscopy (AFM) and Fluorescent Microscopy
6.5.2. Surface Charge
6.5.3. Solubility and Partition Coefficient
6.5.4. Encapsulation Efficiency (EE)
6.5.5. In Vitro Drug Release
6.5.6. Lamellarity
6.5.7. Crystallinity and Thermal Stability
Differential Scanning Calorimetry (DSC)
X-ray Diffraction (XRD)
6.5.8. Chemical Composition
Fourier Transform Infrared Spectroscopy (FTIR)
Raman Spectroscopy
7. Plant-Based Vesicular Delivery System and Applications in Respiratory Diseases
7.1. Lung Cancer
7.2. Asthma
- Naringenin-Loaded Phytosomes for Lung Injury Treatment:
- Gingerol-Loaded Phytosomes for Respiratory Infections:
- Boswellia serrata Phytosomes for Asthma:
- Curcumin Liposomes for Asthma:
- a-Tocopherol Liposomes for Lung Injury:
- Paclitaxel Liposomes for Lung Cancer:
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.T.; Morais-Almeida, M.; Sousa, C.S.; Bousquet, J. The “Big Five” Lung Diseases in CoViD-19 Pandemic—A Google Trends Analysis. Pulmonology 2021, 27, 71–72. [Google Scholar] [CrossRef]
- Bouazza, B.; Hadj-Said, D.; Pescatore, K.A.; Chahed, R. Are Patients with Asthma and Chronic Obstructive Pulmonary Disease Preferred Targets of COVID-19? Tuberc. Respir. Dis. 2020, 84, 22. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Sharma, N.; Vyas, M.; Khurana, N.; Maurya, P.K.; Singh, H.; de Jesus, T.P.A.; Dureja, H.; Chellappan, D.K.; Gupta, G.; et al. Interactions with the Macrophages: An Emerging Targeted Approach Using Novel Drug Delivery Systems in Respiratory Diseases. Chem.-Biol. Interact. 2019, 304, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691. [CrossRef]
- Chronic Respiratory Diseases. Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 17 November 2022).
- Hansen-Flaschen, J.; Bates, D.V.; Dixon, L.; Rodriguez, E.; Rogers, K.; Setia, V.; Young, G. Respiratory Disease. In Definition, Causes, & Major Types; Britannica: Edinburgh, UK, 2023. [Google Scholar]
- WHO EMRO|Chronic Obstructive Pulmonary Disease (COPD)|Health Topics. Available online: https://www.emro.who.int/health-topics/chronic-obstructive-pulmonary-disease-copd/index.html (accessed on 28 February 2023).
- WHO Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 28 February 2023).
- Johns Hopkins Medicine|Chronic Bronchitis. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/chronic-bronchitis (accessed on 27 March 2023).
- Barnes, P.J. A New Approach to the Treatment of Asthma. N. Engl. J. Med. 2010, 128, 408–418. [Google Scholar] [CrossRef]
- Sears, M.R. Trends in the Prevalence of Asthma. Am. Coll. Chest 2014, 145, 219–225. [Google Scholar] [CrossRef]
- Lai, C.K.W.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S. Global Variation in the Prevalence and Severity of Asthma Symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; La Grutta, S. The Burden of Pediatric Asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef]
- Marquis, J.F.; Nantel, A.; LaCourse, R.; Ryan, L.; North, R.J.; Gros, P. Fibrotic Response as a Distinguishing Feature of Resistance and Susceptibility to Pulmonary Infection with Mycobacterium tuberculosis in Mice. Infect. Immun. 2008, 76, 78–88. [Google Scholar] [CrossRef]
- Floyd, K.; Glaziou, P.; Zumla, A.; Raviglione, M. The Global Tuberculosis Epidemic and Progress in Care, Prevention, and Research: An Overview in Year 3 of the End TB Era. Lancet Respir. Med. 2018, 6, 299–314. [Google Scholar] [CrossRef]
- Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 17 November 2022).
- Shrestha, J.; Razavi Bazaz, S.; Aboulkheyr, H.; Azari, D.; Thierry, B.; Ebrahimi Warkiani, M.; Ghadiri, M. Lung-on-a-Chip: The Future of Respiratory Disease Models and Pharmacological Studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern Morocco (Errachidia Province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–774. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal Plants: Past History and Future Perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability Enhancers of Herbal Origin: An Overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Harwansh, R.; Mirza, A.; Hussain, S.; Hussain, A. Oral Lipid Based Drug Delivery System (LBDDS): Formulation, Characterization and Application: A Review. Curr. Drug Deliv. 2011, 8, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Bioavailability of Herbal Products: Approach Toward Improved Pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 217–245. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Shilakari Asthana, G.; Sharma, P.K.; Asthana, A. In Vitro and In Vivo Evaluation of Niosomal Formulation for Controlled Delivery of Clarithromycin. Scientifica 2016, 2016, 6492953. [Google Scholar] [CrossRef]
- Faheem, A.M.; Abdelkader, D.H. Novel Drug Delivery Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081025482. [Google Scholar]
- Devi, V.K.; Jain, N.; Valli, K.S. Importance of Novel Drug Delivery Systems in Herbal Medicines. Pharmacogn. Rev. 2010, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, A. Drug Delivery Aspects of Herbal Medicines; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809444-0. [Google Scholar]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Pharmaceutics Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Asadbeigi, M.; Mohammadi, T.; Rafieian-Kopaei, M.; Saki, K.; Bahmani, M.; Delfan, M. Traditional Effects of Medicinal Plants in the Treatment of Respiratory Diseases and Disorders: An Ethnobotanical Study in the Urmia. Asian Pac. J. Trop. Med. 2014, 7, S364–S368. [Google Scholar] [CrossRef]
- Clarke, R.; Lundy, F.L.; McGarvey, L. Herbal Treatment in Asthma and COPD—Current Evidence. Clin. Phytosci. 2015, 1, 4. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Stojanović-Radić, Z.Z.; Fokou, P.V.T.; Sharifi-Rad, M.; Mahady, G.B.; Sharifi-Rad, M.; Masjedi, M.R.; Lawal, T.O.; Ayatollahi, S.A.; et al. Medicinal Plants Used in the Treatment of Tuberculosis—Ethnobotanical and Ethnopharmacological Approaches. Biotechnol. Adv. 2020, 44, 107629. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/ (accessed on 1 November 2023).
- Pleasants, R.A.; Riley, I.L.; Mannino, D.M. Defining and Targeting Health Disparities in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 2475–2496. [Google Scholar] [CrossRef] [PubMed]
- Raherison, C.; Girodet, P.O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef]
- Petty, T.L. The History of COPD. Int. J. Chron. Obs. Pulmon. Dis. 2006, 1, 3–14. [Google Scholar] [CrossRef]
- Ram, A.; Balachandar, S.; Vijayananth, P.; Singh, V.P. Medicinal Plants Useful for Treating Chronic Obstructive Pulmonary Disease (COPD): Current Status and Future Perspectives. Fitoterapia 2011, 82, 141–151. [Google Scholar] [CrossRef]
- Celli, B.R.; Snider, G.L.; Heffner, J.; Tiep, B.; Ziment, I.; Make, B.; Braman, S.; Olsen, G.; Phillips, Y. American Thoracic Society Standards for the Diagnosis and Care of Patients with Chronic Obstructive Pulmonary Disease. This official statement of the American Thoracic Society was approved by the ATS Board of Directors, March 1995. Am. J. Respir. Crit. Care Med. 1995, 152, S77–S120. [Google Scholar]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A Natural Arsenal against Bacterial Pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Lei, X.-P.; Dong, W.-B. Resveratrol as a Potential Therapeutic Drug for Respiratory System Diseases. Drug Des. Dev. Ther. 2017, 11, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Açıkara, Ö.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and Anti-Inflammatory Therapeutic Potential of Gingerols and Their Nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax Genus and Their Biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Arzanlou, M.; Bohlooli, S. Introducing of Green Garlic Plant as a New Source of Allicin. Food Chem. 2010, 120, 179–183. [Google Scholar] [CrossRef]
- Dwivedi, V.P.; Bhattacharya, D.; Singh, M.; Bhaskar, A.; Kumar, S.; Fatima, S.; Sobia, P.; Kaer, L.V.; Das, G. Allicin Enhances Antimicrobial Activity of Macrophages during Mycobacterium tuberculosis Infection. J. Ethnopharmacol. 2019, 243, 111634. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Bhattacharya, D.; Kar, S.; Ranganathan, A.; Van Kaer, L.; Das, G. Curcumin Nanoparticles Enhance Mycobacterium Bovis BCG Vaccine Efficacy by Modulating Host Immune Responses. Infect. Immun. 2019, 87, e00291-19. [Google Scholar] [CrossRef] [PubMed]
- Chitra, M.; Devi, C.S.S.; Sukumar, E. Antibacterial Activity of Embelin. Fitoterapia 2003, 74, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Han, S.; Park, E.; Yim, D.; Lee, S.; Lee, C.-K.; Cho, K.; Kim, K. Immunomodulatory Activity of Betulinic Acid by Producing Pro-Inflammatory Cytokines and Activation of Macrophages. Arch. Pharm. Res. 2003, 26, 1087–1095. [Google Scholar] [CrossRef]
- Wijaya, V.; Janďourek, O.; Křoustková, J.; Hradiská-Breiterová, K.; Korábečný, J.; Sobolová, K.; Kohelová, E.; Hošťálková, A.; Konečná, K.; Šafratová, M.; et al. Alkaloids of Dicranostigma franchetianum (Papaveraceae) and Berberine Derivatives as a New Class of Antimycobacterial Agents. Biomolecules 2022, 12, 844. [Google Scholar] [CrossRef]
- Ozturk, M.; Chia, J.E.; Hazra, R.; Saqib, M.; Maine, R.A.; Guler, R.; Suzuki, H.; Mishra, B.B.; Brombacher, F.; Parihar, S.P. Evaluation of Berberine as an Adjunct to TB Treatment. Front. Immunol. 2021, 12, 656419. [Google Scholar] [CrossRef]
- 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 1 November 2023).
- Asthma: Types, Causes, Symptoms, Diagnosis & Treatment. Available online: https://my.clevelandclinic.org/health/diseases/6424-asthma (accessed on 22 November 2022).
- Khurana, S.; Jarjour, N.N. Systematic Approach to Asthma of Varying Severity. Clin. Chest Med. 2019, 40, 59–70. [Google Scholar] [CrossRef]
- Asthma—What Is Asthma?|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health/asthma (accessed on 26 March 2023).
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the Potential Effect on Allergic Diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Rahman, I. Antioxidant therapeutic advances in COPD. Ther. Adv. Respir. Dis. 2008, 2, 351–374. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a Potential Antiinflammatory Agent: An Overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Sang, L.; Liu, H.; Xu, Q.; Liu, Z. Boswellic Acid Attenuates Asthma Phenotypes by Downregulation of GATA3 via pSTAT6 Inhibition in a Murine Model of Asthma. Int. J. Clin. Exp. Pathol. 2015, 8, 236–243. [Google Scholar]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.H.G.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.; Prado, C.M. Structure-Activity Association of Flavonoids in Lung Diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M.; Kaur, S.; Garcia-Villar, E.; Molasky, N.; Belli, S.; Sitek, J.D.; Gionfra, F.; Pedersen, J.Z.; Incerpi, S. Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants 2020, 9, 382. [Google Scholar] [CrossRef]
- Shang, X.F.; Yang, C.J.; Morris-Natschke, S.L.; Li, J.C.; Yin, X.D.; Liu, Y.Q.; Guo, X.; Peng, J.W.; Goto, M.; Zhang, J.Y.; et al. Biologically Active Isoquinoline Alkaloids Covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The Amazing and Mighty Ginger. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-0713-2. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Dezube, R. Control of Breathing—Lung and Airway Disorders—MSD Manual Consumer Version. Available online: https://www.msdmanuals.com/home/lung-and-airway-disorders/biology-of-the-lungs-and-airways/control-of-breathing (accessed on 8 August 2023).
- Ghorani-Azam, A.; Riahi-Zanjani, B.; Balali-Mood, M. Effects of Air Pollution on Human Health and Practical Measures for Prevention in Iran. J. Res. Med. Sci. 2016, 21, 65. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.; Barros, L.; Ferreira, I.C. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Arshad, L.; Septama, A.W.; Haque, M.A.; Mohamed-Hussein, Z.; Govender, N.T. Antiviral Effects of Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 and Their Mechanisms of Action: A Review. Phytother. Res. 2022, 37, 1036–1056. [Google Scholar] [CrossRef]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Gonçalves, P.B.; Romeiro, N.C. Multi-Target Natural Products as Alternatives against Oxidative Stress in Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Med. Chem. 2019, 163, 911–931. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary Flaxseed Prevents Radiation-Induced Oxidative Lung Damage, Inflammation and Fibrosis in a Mouse Model of Thoracic Radiation Injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef]
- Tatli Cankaya, I.I.; Somuncuoglu, E.I. Potential and Prophylactic Use of Plants Containing Saponin-Type Compounds as Antibiofilm Agents against Respiratory Tract Infections. Evid.-Based Complement. Altern. Med. 2021, 2021, e6814215. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Joshee, N., Dhekney, S., Parajuli, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Shina, S. A Review of natural products chemistry-their distribution, effects and usage to man. Dutse J. Pure Appl. Sci. (DUJOPAS) 2018, 2, 2. [Google Scholar]
- Bhardwaj, N.; Pathania, A.; Kumar, P. Naturally Available Nitrogen-Containing Fused Heterocyclics as Prospective Lead Molecules in Medicinal Chemistry. Curr. Tradit. Med. 2021, 7, 5–27. [Google Scholar] [CrossRef]
- Mathias, C.B. Respiratory Disorders of the Immune System and Their Pharmacological Treatment. In Pharmacology of Immunotherapeutic Drugs; Mathias, C.B., McAleer, J.P., Szollosi, D.E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 99–140. ISBN 978-3-030-19922-7. [Google Scholar]
- Schudt, C.; Hatzelmann, A.; Beume, R.; Tenor, H. Phosphodiesterase Inhibitors: History of Pharmacology. In Phosphodiesterases as Drug Targets; Francis, S.H., Conti, M., Houslay, M.D., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–46. ISBN 978-3-642-17969-3. [Google Scholar]
- Kunatsa, Y.; Katerere, D.R. Checklist of African Soapy Saponin—Rich Plants for Possible Use in Communities’ Response to Global Pandemics. Plants 2021, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Campos, F.; Clares, B.; Rodríguez-Lagunas, M.J.; Jauregui, O.; Casals, I.; Calpena, A.C. Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration. Pharmaceutics 2019, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Palanisamy, A.; Dhama, K.; Mal, G.; Singh, B.; Singh, K.P. Exploring the Possible Use of Saponin Adjuvants in COVID-19 Vaccine. Hum. Vaccin. Immunother. 2020, 16, 2944–2953. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Secondary metabolites: Secondary metabolic products consisting of C and H; C, H, and O; N, S, and P elements; and O/N heterocycles. In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2: Phytochemistry and Bioactive Compounds; Alamgir, A.N.M., Ed.; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2018; pp. 165–309. ISBN 978-3-319-92387-1. [Google Scholar]
- Jang, W.Y.; Kim, M.Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Santos, S.; Barata, P.; Charmier, A.; Lehmann, I.; Rodrigues, S.; Melosini, M.M.; Pais, P.J.; Sousa, A.P.; Teixeira, C.; Santos, I.; et al. Cannabidiol and Terpene Formulation Reducing SARS-CoV-2 Infectivity Tackling a Therapeutic Strategy. Front. Immunol. 2022, 13, 841459. [Google Scholar] [CrossRef]
- Janecki, M.; Graczyk, M.; Lewandowska, A.A.; Pawlak, Ł. Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 4170. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.N.M. Pharmacognostical Botany: Classification of Medicinal and Aromatic Plants (MAPs), Botanical Taxonomy, Morphology, and Anatomy of Drug Plants. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacognosy; Alamgir, A.N.M., Ed.; Progress in Drug Research; Springer International Publishing: Cham, Switzerland, 2017; pp. 177–293. ISBN 978-3-319-63862-1. [Google Scholar]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Coombs, M.R.P. Editorial: Immune Modulation by Flavonoids. Front. Immunol. 2022, 13, 899577. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Drug Delivery to the Lungs: Challenges and Opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary Drug Delivery. Part I: Physiological Factors Affecting Therapeutic Effectiveness of Aerosolized Medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling Medicines: Delivering Drugs to the Body through the Lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary Drug Delivery Strategies: A Concise, Systematic Review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Raza, F.; Evans, L.; Motallebi, M.; Zafar, H.; Pereira-Silva, M.; Saleem, K.; Peixoto, D.; Rahdar, A.; Sharifi, E.; Veiga, F.; et al. Liposome-Based Diagnostic and Therapeutic Applications for Pancreatic Cancer. Acta Biomater. 2023, 157, 1–23. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-Based Drug Co-Delivery Systems in Cancer Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef]
- Atmakuri, L.R.; Dathi, S. Current Trends in Herbal Medicines. J. Pharm. Res. 2009, 3, 109–113. [Google Scholar]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A Vesicular Transdermal Delivery System for Enhanced Drug Permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Khadra, I.; Aljabali, A.A.A.; Amawi, H.; Ferro, V.A. Characterisation of Niosome Nanoparticles Prepared by Microfluidic Mixing for Drug Delivery. Int. J. Pharm. X 2022, 4, 100137. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Bheemidi, V.S.; Tiruckovela, M.; Varanasi, P. An Imperative Note on Novel Drug Delivery Systems. J. Nanomed. Nanotechnol. 2011, 2, 1000125. [Google Scholar] [CrossRef]
- Terreno, E.; Castelli, D.D.; Cabella, C.; Dastrù, W.; Sanino, A.; Stancanello, J.; Tei, L.; Aime, S. Paramagnetic Liposomes as Innovative Contrast Agents for Magnetic Resonance (MR) Molecular Imaging Applications. Chem. Biodivers. 2011, 5, 1901–1912. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A Future of Targeted Drug Delivery Systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Sharma, P. Pharmacosomes: An Emerging Novel Vesicular Drug Delivery System for Poorly Soluble Synthetic and Herbal Drugs. ISRN Pharm. 2013, 2013, 348186. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Rawat, B.S.; Singh, D.; Rawat, M. Development and Evaluation of Pharmacosomes of Aceclofenac. Indian J. Pharm. Sci. 2010, 72, 576. [Google Scholar] [CrossRef]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A Novel Vesicular Carrier, Transethosome, for Enhanced Skin Delivery of Voriconazole: Characterization and in Vitro/in Vivo Evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M.; et al. Nanostructed Cubosomes as Advanced Drug Delivery System. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Health 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, B.; Kumar Singh, S.; Kaur, B.; Singh, S. A review on phytosomes: Novel approach for herbal phytochemicals. Asian J. Pharm. Clin. Res. 2017, 10, 41–47. [Google Scholar] [CrossRef]
- Dhase, A.S.; Saboo, S.S. Preparation and Evaluation of Phytosomes Containing Methanolic Extract of Leaves of Aegle marmelos (Bael). Int. J. PharmTech Res. 2015, 8, 231–240. [Google Scholar]
- Semalty, A.; Semalty, M.; Rawat, B.S.; Singh, D.; Rawat, M.S.M. Pharmacosomes: The Lipid-Based New Drug Delivery System. Expert Opin. Drug Deliv. 2009, 6, 599–612. [Google Scholar] [CrossRef]
- Kidd, P. Phosphatidylcholine: A Superior Protectant against Liver Damage. Altern. Med. Rev. 2009, 14, 248–257. [Google Scholar]
- Maddrey, W.C. Milk Thistle, Silymarin, and Liver Disease. Am. J. Med. 2004, 117, 548–549. [Google Scholar]
- Islam, N.; Irfan, M.; Hussain, T.; Mushtaq, M.; Khan, I.U.; Yousaf, A.M.; Ghori, M.U.; Shahzad, Y. Piperine Phytosomes for Bioavailability Enhancement of Domperidone. J. Liposome Res. 2021, 32, 172–180. [Google Scholar] [CrossRef]
- Hua, S.; Wu, S.Y. The Use of Lipid-Based Nanocarriers for Targeted Pain Therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable Liposomes and Ethosomes as Carriers for Skin Delivery of Ketotifen. Pharmazie 2007, 62, 133–137. [Google Scholar] [PubMed]
- Moghassemi, S.; Hadjizadeh, A. Nano-Niosomes as Nanoscale Drug Delivery Systems: An Illustrated Review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and Skin: From Drug Delivery to Model Membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef]
- Podili, C.; Firoz, S. A review on transferosomes for transdermal drug delivery. J. Glob. Trends Pharm. Sci. 2014, 5, 2118–2127. [Google Scholar]
- Sharma, S.; Kumari, D.; Khan, S.; Pathak, P.; Katiyar, D.; Imam, S.S. An expedient approach to treat asthma through non-steroidal, natural transferosomes aerosol system. Innovare J. Med. Sci. 2022, 10, 7–11. [Google Scholar] [CrossRef]
- Benson, H.A.E. Transfersomes for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef]
- Patel, R.H.; Lyles, K.W. Senile Osteoporosis as a Geriatric Syndrome. In Osteoporosis in Older Persons; Duque, G., Kiel, D.P., Eds.; Springer: London, UK, 2009; pp. 71–81. [Google Scholar] [CrossRef]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, Optimization and Evaluation of Transferosomal Gel for Transdermal Insulin Delivery. Saudi Pharm. J. 2012, 20, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G. Isothermal Lipid Phase Transitions. Chem. Phys. Lipids 1991, 57, 293–307. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of Charged and Non-Ionic Membrane Additives on Physicochemical Properties and Stability of Niosomes. AAPS PharmSciTech 2008, 9, 851–859. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Muzzalupo, R.; Celia, C.; Citraro, R.; Caponio, D.; Picci, N.; Fresta, M. Novel PEG-Coated Niosomes Based on Bola-Surfactant as Drug Carriers for 5-Fluorouracil. Biomed. Microdevices 2009, 11, 1115–1125. [Google Scholar] [CrossRef]
- Paolino, D.; Muzzalupo, R.; Ricciardi, A.; Celia, C.; Picci, N.; Fresta, M. In Vitro and in Vivo Evaluation of Bola-Surfactant Containing Niosomes for Transdermal Delivery. Biomed. Microdevices 2007, 9, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Baillie, A.J.; Florence, A.T.; Hume, L.R.; Muirhead, G.T.; Rogerson, A. The Preparation and Properties of Niosomes–Non-Ionic Surfactant Vesicles. J. Pharm. Pharmacol. 1985, 37, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Vyas, S.P. Non-Ionic Surfactant Based Vesicles (Niosomes) in Drug Delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Supraja, B.; Mulangi, S. An Updated Review on Pharmacosomes, a Vesicular Drug Delivery System. J. Drug Deliv. Ther. 2019, 9, 393–402. [Google Scholar] [CrossRef]
- Shinde, R.R.; Chaudhari, B.P.; Velhal, A.B.; Redasani, V.K. Pharmacosome as a Vesicular Drug Delivery System. Asian J. Res. Pharm. Sci. 2022, 12, 291–296. [Google Scholar] [CrossRef]
- Bhingare, U.; Khadabadi, S.S.; Shinde, N. Pharmacosomes: A Novel Drug Delivery System. Int. J. Pharm. Res. Allied Sci. 2014, 3, 14–20. [Google Scholar]
- Bagheri, A.; Chu, B.S.; Yaakob, H. Niosomal Drug Delivery Systems: Formulation, Preparation and Applications. World Appl. Sci. J. 2014, 32, 1671–1685. [Google Scholar] [CrossRef]
- Repka, M.; Reo, J.; Felton, L.; Howard, S.; Tan, Q.; Liu, S.; Chen, X.; Wu, M.; Wang, H.; Yin, H.; et al. Theme: Advanced Technologies for Oral Controlled Release Design and Evaluation of a Novel Evodiamine-Phospholipid Complex for Improved Oral Bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef]
- Habbu, P.; Madagundi, S.; Kulkarni, R.; Jadav, S.; Vanakudri, R.; Kulkarni, V. Preparation and Evaluation of Bacopa-Phospholipid Complex for Antiamnesic Activity in Rodents. Drug Invent. Today 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Yue, P.F.; Yuan, H.L.; Li, X.Y.; Yang, M.; Zhu, W.F. Process Optimization, Characterization and Evaluation in Vivo of Oxymatrine-Phospholipid Complex. Int. J. Pharm. 2010, 387, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S.; et al. Phytosomes Loaded with Mitomycin C-Soybean Phosphatidylcholine Complex Developed for Drug Delivery. Mol. Pharm. 2013, 10, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Damle, M.; Mallya, R. Development and Evaluation of a Novel Delivery System Containing Phytophospholipid Complex for Skin Aging. AAPS PharmSciTech 2015, 17, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.; Builders, P.; Nzekwe, I.; Kunle, O.; Adikwu, M.; Attama, A. Formulation and in Vitro Evaluation of pH-Responsive Ethosomes for Vaginal Delivery of Metronidazole. J. Drug Deliv. Sci. Technol. 2014, 24, 565–571. [Google Scholar] [CrossRef]
- Mayer, L.D.; Bally, M.B.; Hope, M.J.; Cullis, P.R. Uptake of Antineoplastic Agents into Large Unilamellar Vesicles in Response to a Membrane Potential. Biochim. Biophys. Acta (BBA) Biomembr. 1985, 816, 294–302. [Google Scholar] [CrossRef]
- Niosomes: A Controlled and Novel Drug Delivery System. Available online: https://www.jstage.jst.go.jp/article/bpb/34/7/34_7_945/_article (accessed on 16 November 2023).
- Evans, S.M.; Litzenberger, A.L.; Ellenberger, A.E.; Maneval, J.E.; Jablonski, E.L.; Vogel, B.M. A Microfluidic Method to Measure Small Molecule Diffusion in Hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Mujoriya, R.; Bodla, R.B.; Dhamande, K.; Singh, D.; Patle, L. Niosomal Drug Delivery System: The Magic Bullet. J. Appl. Pharm. Sci. 2011, 1, 20–23. Available online: https://japsonline.com/abstract.php?article_id=254 (accessed on 16 November 2023).
- Mujoriya, R.Z.; Bodla, D.R.B. Niosomes—Challenge in preparation for pharmaceutical scientist. Int. J. Appl. Pharm. 2011, 3, 11–15. [Google Scholar]
- Saraf, S.; Khan, J.; Alexander, A.; Ajazuddin; Saraf, S. Recent Advances and Future Prospects of Phyto-Phospholipid Complexation Technique for Improving Pharmacokinetic Profile of Plant Actives. J. Control. Release Off. J. Control. Release Soc. 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Procedure for Preparation of Liposomes with Large Internal Aqueous Space and High Capture by Reverse-Phase Evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, F.; Li, N.; Yang, Y.; Li, K. Studies on a High Encapsulation of Colchicine by a Niosome System. Int. J. Pharm. 2002, 244, 73–80. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable Lipid Emulsions-Advancements, Opportunities and Challenges. AAPS PharmSciTech 2010, 11, 1526–1540. [Google Scholar] [CrossRef]
- Jadhav, S.M.; Morey, P.; Karpe, M.M.; Kadam, V. Novel Vesicular System: An Overview. J. Appl. Pharm. Sci. 2012, 2, 193–202. [Google Scholar]
- Gupta, N.K.; Dixit, V.K. Development and Evaluation of Vesicular System for Curcumin Delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Olusanya, T.O.B.; Lamprou, D.A. Characterization of Drug Delivery Vehicles Using Atomic Force Microscopy: Current Status. Expert Opin. Drug Deliv. 2018, 15, 1211–1221. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Goda, Y.; Izutsu, K.; Sakai-Kato, K. Instrument-Dependent Factors Affecting the Precision in the Atomic Force Microscopy Stiffness Measurement of Nanoscale Liposomes. Chem. Pharm. Bull. 2020, 68, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Charact. Nanopart. Intend. Drug Deliv. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.D.; Kwon, Y.M.; Omidi, Y.; Speth, R.C. Nanoparticle Approaches for the Renin—Angiotensin System. Heliyon 2023, 9, e16951. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta Potential and Surface Charge of DPPC and DOPC Liposomes in the Presence of PLC Enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low Level LED Photodynamic Therapy Using Curcumin Loaded Tetraether Liposomes. Eur. J. Pharm. Biopharm. 2018, 126, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral Delivery of Vancomycin by Tetraether Lipid Liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Aljarbou, A.N.; Aldebasi, Y.H.; Alorainy, M.S.; Rahmani, A.H.; Younus, H.; Khan, A. Liposomal Formulation of Glycosphingolipids from Sphingomonas paucimobilis Induces Antitumour Immunity in Mice. J. Drug Target 2018, 26, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kotyńska, J.; Figaszewski, Z.A. Binding of Trivalent Metal Ions (Al3+, In3+, La3+) with Phosphatidylcholine Liposomal Membranes Investigated by Microelectrophoresis. Eur. Phys. J. E Soft Matter. 2018, 41, 70. [Google Scholar] [CrossRef]
- Dolder, N.; von Ballmoos, C. Bifunctional DNA Duplexes Permit Efficient Incorporation of pH Probes into Liposomes. ChemBioChem 2020, 21, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. (Ed.) Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration [Electronic Resource]; Springer: Berlin, Germany, 2016; pp. 1–172. [Google Scholar] [CrossRef]
- Goodhew, P.J.; Humphreys, J. Electron Microscopy and Analysis, 3rd ed.; CRC Press: London, UK, 2014. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Springer: New York, NY, USA, 2007; pp. 1–40. [Google Scholar] [CrossRef]
- Lučić, V.; Leis, A.; Baumeister, W. Cryo-Electron Tomography of Cells: Connecting Structure and Function. Histochem. Cell Biol. 2008, 130, 185–196. [Google Scholar] [CrossRef]
- Vahabi, S.; Nazemi Salman, B.; Javanmard, A. Atomic Force Microscopy Application in Biological Research: A Review Study. Iran J. Med. Sci. 2013, 38, 76–83. [Google Scholar] [PubMed]
- Gangadaran, P.; Hong, C.M.; Ahn, B.-C. An Update on In Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-Phytosome as a Potential Food-Grade Delivery System. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- Fang, C.-L.; Al-Suwayeh, S.A.; Fang, J.-Y. Nanostructured Lipid Carriers (NLCs) for Drug Delivery and Targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.D.; Raghav, N. Nano-Crystalline Cellulose: Preparation, Modification and Usage as Sustained Release Drug Delivery Excipient for Some Non-Steroidal Anti-Inflammatory Drugs. Int. J. Biol. Macromol. 2020, 147, 921–930. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Kabedev, A.; Parrow, A.; Bergström, C.A.S.; Larsson, P. Molecular Simulation as a Computational Pharmaceutics Tool to Predict Drug Solubility, Solubilization Processes and Partitioning. Eur. J. Pharm. Biopharm. 2019, 137, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Nele, V.; Holme, M.N.; Kauscher, U.; Thomas, M.R.; Doutch, J.J.; Stevens, M.M. Effect of Formulation Method, Lipid Composition, and PEGylation on Vesicle Lamellarity: A Small-Angle Neutron Scattering Study. Langmuir 2019, 35, 6064–6074. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Das, M.K.; Kalita, B. Design and Evaluation of Phyto-Phospholipid Complexes (Phytosomes) of Rutin for Transdermal Application. J. Appl. Pharm. Sci. 2014, 4, 051–057. [Google Scholar] [CrossRef]
- Hao, H.; Jia, Y.; Han, R. Phytosomes: An Effective Approach to Enhance the Oral Bioavailability of Active Constituents Extracted from Plantss. J. Chin. Pharm. Sci. 2013, 22, 385–392. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Weissig, V. (Eds.) Liposomes: A Practical Approach, 2nd ed.; Practical Approach Series; Oxford University Press: Oxford, NY, USA, 2003; ISBN 978-0-19-963654-9. [Google Scholar]
- Tripathy, S.; Patel, D.K.; Baro, L.; Nair, S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Ther. 2011, 2013, 147–152. [Google Scholar]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaerf, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Boulesbaa, M. Spectroscopic Ellipsometry and FTIR Characterization of Annealed SiOxNy:H Films Prepared by PECVD. Opt. Mater. 2021, 122, 111693. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Ferreira, D.D.S.; Lopes, S.C.d.A.; Franco, M.S.; Oliveira, M.C. pH-Sensitive Liposomes for Drug Delivery in Cancer Treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Badria, F.A.; Abdelaziz, A.E.; Hassan, A.H.; Elgazar, A.A.; Mazyed, E.A. Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules 2020, 25, 5668. [Google Scholar] [CrossRef]
- Qian, H.; Shao, X.; Zhang, H.; Wang, Y.; Liu, S.; Pan, J.; Xue, W. Diagnosis of Urogenital Cancer Combining Deep Learning Algorithms and Surface-Enhanced Raman Spectroscopy Based on Small Extracellular Vesicles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121603. [Google Scholar] [CrossRef]
- Carlomagno, C.; Giannasi, C.; Niada, S.; Bedoni, M.; Gualerzi, A.; Brini, A.T. Raman Fingerprint of Extracellular Vesicles and Conditioned Media for the Reproducibility Assessment of Cell-Free Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 640617. [Google Scholar] [CrossRef]
- Rodà, F.; Picciolini, S.; Mangolini, V.; Gualerzi, A.; Seneci, P.; Renda, A.; Sesana, S.; Re, F.; Bedoni, M. Raman Spectroscopy Characterization of Multi-Functionalized Liposomes as Drug-Delivery Systems for Neurological Disorders. Nanomaterials 2023, 13, 699. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.; Lyu, Z.; Shan, K.; Chen, Z.; Chen, B.; Chen, Y.; Hu, X.; Dou, B.; Zhang, J.; et al. Medicinal Plant-Based Drug Delivery System for Inflammatory Bowel Disease. Front. Pharmacol. 2023, 14, 1158945. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.Á.; Gámez-Méndez, A.; Martínez-Ruiz, M.; Nájera-Martínez, E.F.; Morales-Flores, B.A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Nanostructures for Drug Delivery in Respiratory Diseases Therapeutics: Revision of Current Trends and Its Comparative Analysis. J. Drug Deliv. Sci. Technol. 2022, 70, 103219. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A Roadmap to Pulmonary Delivery Strategies for the Treatment of Infectious Lung Diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef]
- Mehta, P.P.; Ghoshal, D.; Pawar, A.P.; Kadam, S.S.; Dhapte-Pawar, V.S. Recent Advances in Inhalable Liposomes for Treatment of Pulmonary Diseases: Concept to Clinical Stance. J. Drug Deliv. Sci. Technol. 2020, 56, 101509. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation Strategy and Use of Excipients in Pulmonary Drug Delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, L.; Li, M.; Hu, Y.; Zhang, E.; Jiang, Q.; Han, G.; Jin, Y. Inhalable Andrographolide-β-Cyclodextrin Inclusion Complexes for Treatment of Staphylococcus aureus Pneumonia by Regulating Immune Responses. Mol. Pharm. 2017, 14, 1718–1725. [Google Scholar] [CrossRef]
- Mao, J.T.; Smoake, J.; Park, H.K.; Lu, Q.Y.; Xue, B. Grape Seed Procyanidin Extract Mediates Antineoplastic Effects against Lung Cancer via Modulations of Prostacyclin and 15-HETE Eicosanoid Pathways. Cancer Prev. Res. 2016, 9, 925–932. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary Prevention and Management of Asthma with Quercetin Phytosome: A Pilot Registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef]
- Morimoto, Y.; Adachi, Y. Pulmonary Uptake of Liposomal Phosphatidylcholine upon Intratracheal Administration to Rats. Chem. Pharm. Bull. 1982, 30, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.G.; Taylor, G.; Kellaway, I.W.; Stevens, J. The Influence of Liposomal Encapsulation on Sodium Cromoglycate Pharmacokinetics in Man. Pharm. Res. 1989, 6, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Wang, C.H. Pulmonary Delivery of Insulin by Liposomal Carriers. J. Control. Release 2006, 113, 9–14. [Google Scholar] [CrossRef]
- Turrens, J.F.; Crapo, J.D.; Freeman, B.A. Protection against Oxygen Toxicity by Intravenous Injection of Liposome-Entrapped Catalase and Superoxide Dismutase. J. Clin. Investig. 1984, 73, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, R.V.; Gudapaty, R.; Liener, I.E.; Schwartz, B.A.; Hoidal, J.R. Protection against Pulmonary Oxygen Toxicity in Rats by the Intratracheal Administration of Liposome-Encapsulated Superoxide Dismutase or Catalase. Am. Rev. Respir. Dis. 1985, 132, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Shek, P.N.; Suntres, Z.E.; Brooks, J.I. Liposomes in Pulmonary Applications: Physicochemical Considerations, Pulmonary Distribution and Antioxidant Delivery. J. Drug Target. 1994, 2, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, X.; Chen, H.; Zhu, L. Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 194–204. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phytosome Complexed with Chitosan for Gingerol Delivery in the Treatment of Respiratory Infection: In Vitro and In Vivo Evaluation. Eur. J. Pharm. Sci. 2018, 122, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, T.; De Vincentiis, G.; Di Pierro, F. Functional Study on Boswellia Phytosome as Complementary Intervention in Asthmatic Patients. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3757–3762. [Google Scholar]
- Ng, Z.Y.; Wong, J.Y.; Panneerselvam, J.; Madheswaran, T.; Kumar, P.; Pillay, V.; Hsu, A.; Hansbro, N.; Bebawy, M.; Wark, P.; et al. Assessing the Potential of Liposomes Loaded with Curcumin as a Therapeutic Intervention in Asthma. Colloids Surf. B Biointerfaces 2018, 172, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Hepworth, S.R.; Shek, P.N. Pulmonary Uptake of Liposome-Associated Alpha-Tocopherol Following Intratracheal Instillation in Rats. J. Pharm. Pharmacol. 1993, 45, 514–520. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Waldrep, J.C.; Roberts, L.E.; Golunski, E.; Melton, S.; Knight, V. Paclitaxel Liposome Aerosol Treatment Induces Inhibition of Pulmonary Metastases in Murine Renal Carcinoma Model1. Clin. Cancer Res. 2001, 7, 3258–3262. [Google Scholar] [PubMed]

| Compound | Structure | Significant Structural Qualities | Ref. |
|---|---|---|---|
| Curcumin | 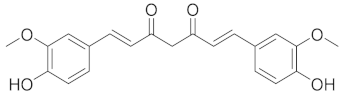 |
| [73] |
| Allicin | 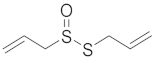 |
| [74] |
| Quercetin | 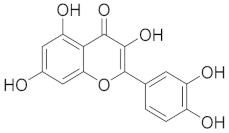 |
| [75] |
| Embellin | 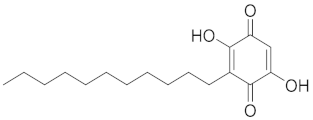 |
| [76] |
| Berberine |  |
| [77] |
| Gingerol | 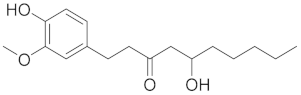 |
| [78] |
| Epigallocatechin gallate (EGCG) | 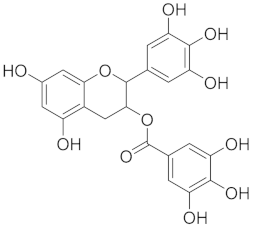 |
| [72] |
| Resveratrol | 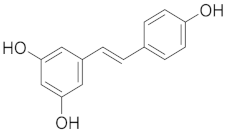 |
| [79] |
| VDDS | Description | Advantage | Material Composition | Refs. |
|---|---|---|---|---|
| Phytosome | Novel phospholipid-bound plant-derived drug delivery formulation that provides an envelope-like coating for the pharmaceutical ingredient; the main component of herbal extracts remains protected from degradation by digestive secretions and bacteria. | Phytosomal drug delivery systems have distinctive attributes, viz., small size, which enables phytocomponents to reach the target site or receptor by passing through the vesicular membrane of phytosomes, and physiochemical properties that include amphiphilicity, biocompatibility, and potential for targeted and controlled payload release. |
| [134,135,136,137,138,139] |
| Liposome | Colloidal drug delivery device with a lipid layer surrounding an aqueous center. Drug distribution depends on solubility in the hydrophilic core or lipid layer. | Because of its excellent biocompatibility, simplicity of manufacture, and chemical diversity, molecules that are hydrophilic, amphiphilic, and lipophilic can be loaded. |
| [129,140,141,142,143,144] |
| Transferosome | An artificial vesicle called a transferosome carrier is made to resemble a cell vesicle or a cell that is exocytosing, making it appropriate for regulated and possibly targeted drug administration. | When applied non-occlusively, transferosome increase transdermal medication delivery. Squeezing along the stratum corneum’s inter-cellular sealing lipid helps transferosomes permeate. They distribute low- and high-molecular-weight medicines. |
| [122,145,146,147,148,149,150] |
| Niosome | Multilamellar vesicular structure of nonionic surfactants, comparable to liposomes, but without phospholipids. Hydrophilic medications can be encapsulated in the core aqueous compartment and hydrophobic drugs in the lipid membrane. | The entrapping of a greater number of compounds, a greater degree of stability, the elimination of the requirement for special handling or storage conditions, as well as the accessibility and low cost of produced materials are all advantages of this method. |
| [123,151,152,153,154,155] |
| Pharmacosome | These lipid-based drug delivery systems utilize colloidal dispersions of covalent, amphiphilic medicinal agents to facilitate the translocation of drugs across membranes, tissues, and cellular barriers within the organism. | Amphiphilic substances dissolve better in GI fluid and absorb better via lipophilic membranes. This enhances medication absorption. Complexing both types of drugs improve biological characteristics. |
| [136,155,156,157,158] |
| Ethosome | Ethosomes are delivery carriers that are soft, pliable, and non-invasive. They improve the delivery of active medicines to the systemic circulation by enhancing the distribution of these agents. | Ethosomes are capable of completely penetrating the skin, which allows them to significantly improve the transport of drugs via the skin. Ethosomes also have a high degree of deformability and trapping efficiency. |
| [124,159] |
| VDDS | Encapsulated Plant Material | Critical Quality Attributes (CQA) | Application | Outcomes | Ref. |
|---|---|---|---|---|---|
| Phytosomes | Naringenin | ZP = 20.97 ± 0.55 mV PS = 150.8 ± 6.9 nm %EE = 92.1% ± 1.87% | Lung injury | A significant reduction in cytokine expression was observed with naringenin-loaded DPPC phytosomes for dry powder inhalation (NPDPIs) in the treatment of pulmonary edemas. | [231] |
| Phytosome | Gingerol | ZP = −13.11 mV PS = 254.01 ± 0.05 nm %EE = 86.02 ± 0.18% | Respiratory infection | Pharmacodynamic parameters showed a sustained antibacterial action as well as considerable anti-inflammatory effects against both Gram-positive and Gram-negative bacteria responsible for respiratory infections. | [232] |
| Phytosome | Boswellia serrata | Asthma | Patients receiving phytosome needed fewer inhalations. Insomnia and nausea were the only mild-moderate adverse events associated with phytosome treatment. | [233] | |
| Liposome | Curcumin | ZP = −61.0 mV PS = 271.3 ± 3.06 nm %EE = 81.1% | Asthma | Curcumin liposome formulation suppressed pro-inflammatory markers (IL-6, IL-8, IL-1β, and TNF-α) in BCi-NS1.1 cell line. | [234] |
| Liposome | a-tocopherol | Lung injury | It has been demonstrated that liposomal a-tocopherol formulation is highly effective at preventing oxidative lung damage. | [235] | |
| Liposome | Paclitaxel | Lung cancer | Decreased number of observable tumor foci on the lung surfaces, increased medication effectiveness, and longer survival. | [236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukhele, B.S.; Bassey, K.; Witika, B.A. The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases. Curr. Issues Mol. Biol. 2023, 45, 9985-10017. https://doi.org/10.3390/cimb45120624
Lukhele BS, Bassey K, Witika BA. The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases. Current Issues in Molecular Biology. 2023; 45(12):9985-10017. https://doi.org/10.3390/cimb45120624
Chicago/Turabian StyleLukhele, Bongani Sannyboy, Kokoette Bassey, and Bwalya Angel Witika. 2023. "The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases" Current Issues in Molecular Biology 45, no. 12: 9985-10017. https://doi.org/10.3390/cimb45120624
APA StyleLukhele, B. S., Bassey, K., & Witika, B. A. (2023). The Utilization of Plant-Material-Loaded Vesicular Drug Delivery Systems in the Management of Pulmonary Diseases. Current Issues in Molecular Biology, 45(12), 9985-10017. https://doi.org/10.3390/cimb45120624







