The Cytotoxicity of Cotyledon orbiculata Aqueous Extract and the Biogenic Silver Nanoparticles Derived from the Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Determination of IC50
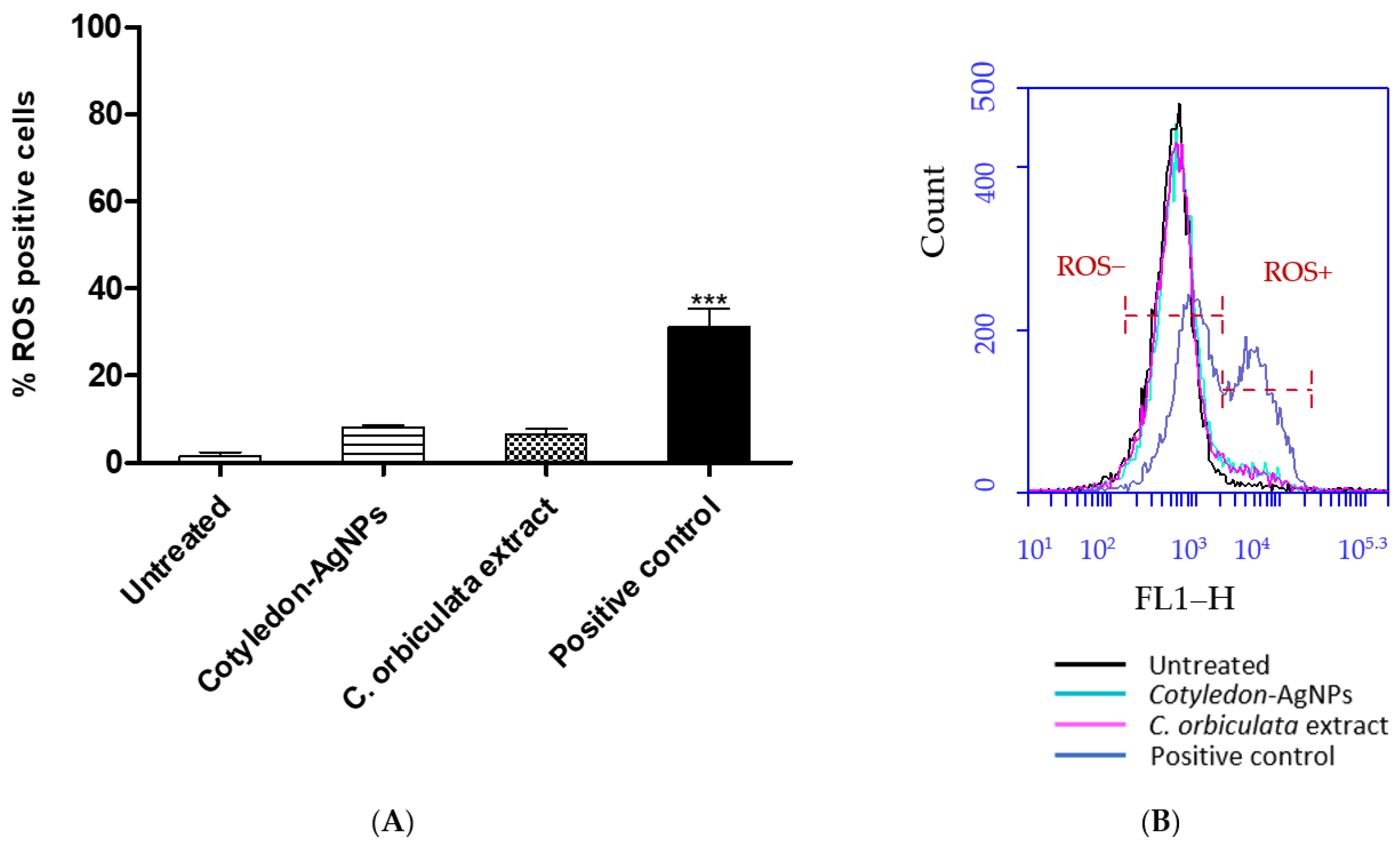
2.3. ROS Assay
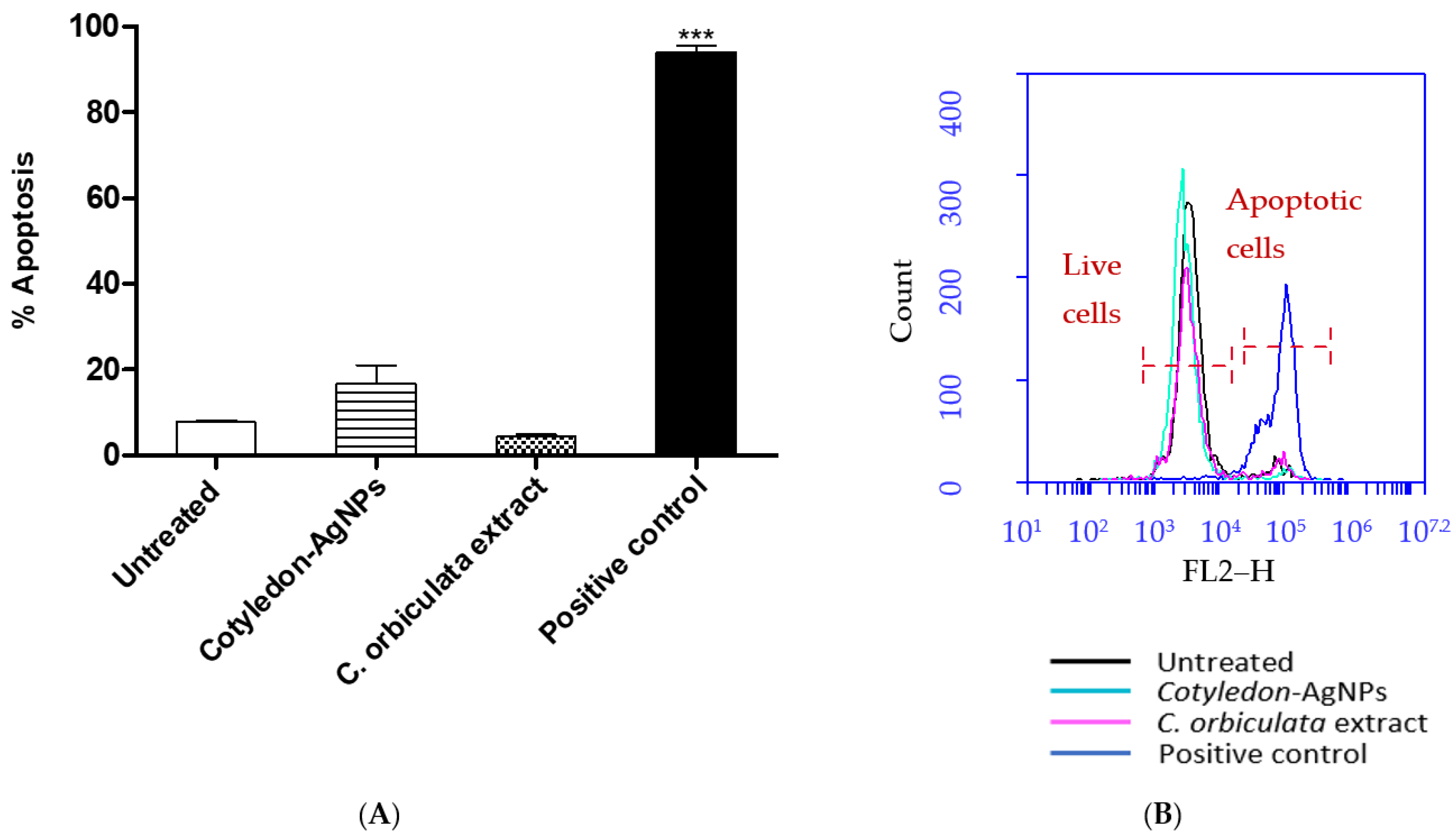
2.4. APOPercentageTM Assay
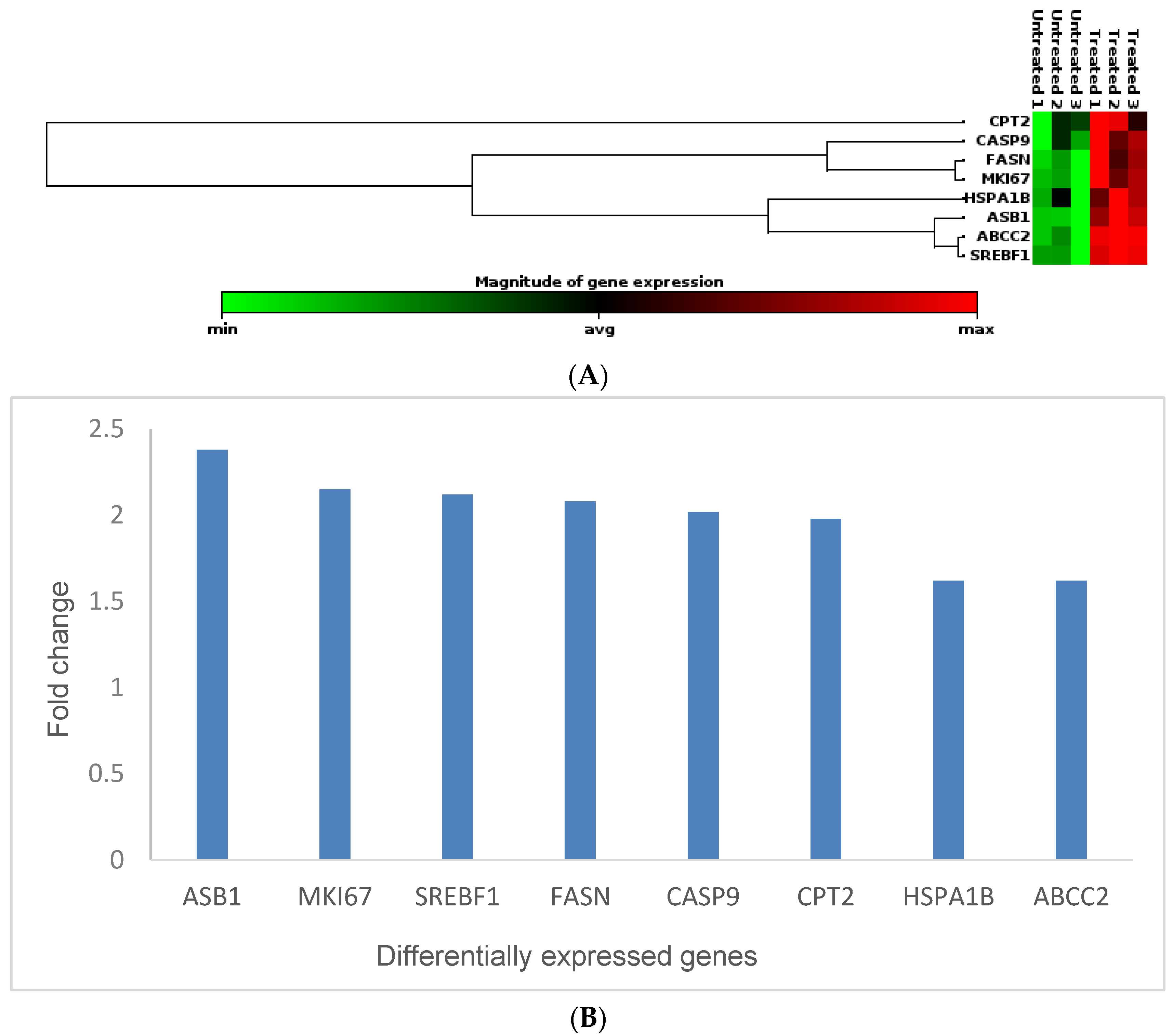
2.5. Gene Expression Studies Using the Human Molecular Toxicology PathwayFinder RT2 Profiler PCR Array
3. Results and Discussion
3.1. Synthesis of Cotyledon-AgNPs
3.2. The Cytotoxicity of C. orbiculata Extracts and Cotyledon-AgNPs
3.3. Effects of C. orbiculata Extracts and Cotyledon-AgNPs on Cellular ROS Levels
3.4. Effects of C. orbiculata Extracts and Cotyledon-AgNPs on Apoptosis
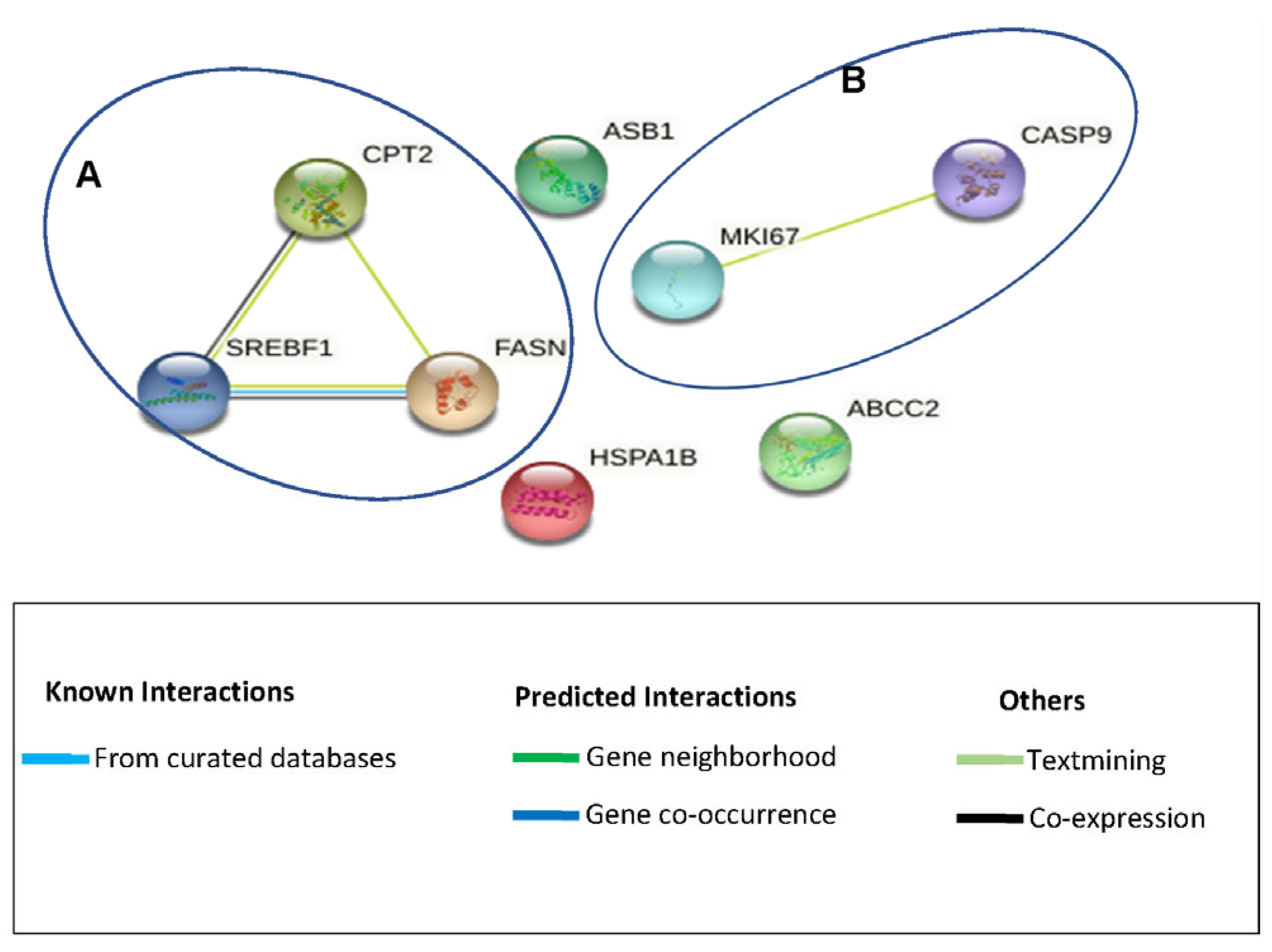
3.5. Effects of Cotyledon-AgNPs on the Expression Genes Involved in Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Jahromi, A.S.; Ghasemi, Y. Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med. Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef]
- Naik, K.; Kowshik, M. The Silver Lining: Towards the Responsible and Limited Usage of Silver. J. Appl. Microbiol. 2017, 123, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowska, A.; Zyro, D.; Ochocki, J.; Jurowski, P. Clinical Application and Efficacy of Silver Drug in Ophthalmology: A Literature Review and New Formulation of EYE Drops with Drug Silver (I) Complex of Metronidazole with Improved Dosage Form. Biomedicines 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview. J. Nanomater. 2016, 2016, 7614753. [Google Scholar] [CrossRef]
- Wang, S.; Tang, T.W.; Wu, E.; Wang, D.; Liao, Y. Anionic Surfactant-Facilitated Coating of Antimicrobial Peptide and Antibiotic Reduces Biomaterial-Associated Infection. ACS Biomater. Sci. Engnieering 2020, 6, 4561–4572. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Hawsawi, N.M.; Hamad, A.M.; Rashid, S.N.; Alshehri, F.; Sharaf, M.; Zakai, S.A.; Yousef, S.A.A.; Ali, A.M.; Abou-Elnour, A.; Alkhudhayri, A.; et al. Biogenic Silver Nanoparticles Eradicate of Pseudomonas Aeruginosa and Staphylococcus Aureus (MRSA) Isolated from the Sputum of COVID-19 Patients. Front. Microbiol. 2023, 14, 1142646. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.; Al-askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Origanum Majorana with Potential Bioactivity against Multidrug Resistant Bacterial Strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Meyer, M.; Meyer, S. Cellular and Molecular Events of Wound Healing and the Potential of Silver Based Nanoformulations as Wound Healing Agents. Bioengineering 2022, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Huang, J.; Chen, C.; Wang, Z.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon Orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Meyer, M.; Wusu, A.D.; Madiehe, A.M.; Meyer, S. The Antioxidant and In Vitro Wound Healing Activity of Cotyledon Orbiculata Aqueous Extract and the Synthesized Biogenic Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 16094. [Google Scholar] [CrossRef]
- Maroyi, A. A Review of Botany, Medicinal Uses, Phytochemistry and Biological Activities of Cotyledon Orbiculata. J. Pharm. Sci. Res. 2019, 11, 3491–3496. [Google Scholar]
- Badmus, J.A.; Ekpo, O.E.; Sharma, J.R.; Sibuyi, N.R.S.; Meyer, M.; Hussein, A.A.; Hiss, D.C. An Insight into the Mechanism of Holamine- and Funtumine-Induced Cell Death in Cancer Cells. Molecules 2020, 25, 5716. [Google Scholar] [CrossRef]
- Meyer, M.; Essack, M.; Kanyanda, S.; Rees, J.G. A Low-Cost Flow Cytometric Assay for the Detection and Quantification of Apoptosis Using an Anionic Halogenated Fluorescein Dye. BioTechniques 2008, 45, 317–320. [Google Scholar] [CrossRef]
- Wusu, A.D.; Sibuyi, N.R.S.; Moabelo, K.L.; Goboza, M.; Madiehe, A.; Meyer, M. Citrate-Capped Gold Nanoparticles with a Diameter of 14 Nm Alter the Expression of Genes Associated with Stress Response, Cytoprotection and Lipid Metabolism in CaCo-2 Cells. Nanotechnology 2022, 33, 105101. [Google Scholar] [CrossRef] [PubMed]
- González-Larraza, P.G.; López-Goerne, T.M.; Padilla-Godínez, F.J.; González-López, M.A.; Hamdan-Partida, A.; Gómez, E. IC50Evaluation of Platinum Nanocatalysts for Cancer Treatment in Fibroblast, HeLa, and DU-145 Cell Lines. ACS Omega 2020, 5, 25381–25389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Ski. Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver Nanoparticles Induce Toxicity in A549 Cells via ROS-Dependent and ROS-Independent Pathways. Toxicol. In Vitr. 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of Silver Nanoparticles-Nanoparticle or Silver Ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 1–17. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative Stress-Dependent Toxicity of Silver Nanoparticles in Human Hepatoma Cells. Toxicol. In Vitr. 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Gopinath, P.; Gogoi, S.K.; Sanpui, P.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Signaling Gene Cascade in Silver Nanoparticle Induced Apoptosis. Colloids Surf. B Biointerfaces 2010, 77, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative Assessment of the Apoptotic Potential of Silver Nanoparticles Synthesized by Bacillus Tequilensis and Calocybe Indica in MDA-MB-231 Human Breast Cancer Cells: Targeting P53 for Anticancer Therapy. Int. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [PubMed]
- Plackal Adimuriyil George, B.; Kumar, N.; Abrahamse, H.; Ray, S.S. Apoptotic Efficacy of Multifaceted Biosynthesized Silver Nanoparticles on Human Adenocarcinoma Cells. Sci. Rep. 2018, 8, 14368. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 575415. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; You, C.P.; Zhao, R.R.; Liu, S.J.; Zhang, Z.H.; Zhang, C.; Liu, Y. Mitochondria in Human Diseases and Animal Evolution. Curr. Mol. Med. 2014, 14, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Qureshi, R.; Munazir, M.; Maqsood, M.; Munir, M.; Shah, S.S.H.; Rahim, B.Z. Application of Green Synthesized Silver Nanoparticles in Cancer Treatment—A Critical Review. Mater. Res. Express 2021, 8, 92001. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver Nanoparticles Induce Oxidative Cell Damage in Human Liver Cells through Inhibition of Reduced Glutathione and Induction of Mitochondria-Involved Apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the Aquatic Environment: An EU-USA Perspective on the Status of Ecotoxicity Testing, Research Priorities and Challenges Ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067. [Google Scholar] [CrossRef]
- Bajak, E.; Fabbri, M.; Ponti, J.; Gioria, S.; Ojea-jiménez, I.; Collotta, A.; Mariani, V.; Gilliland, D.; Rossi, F.; Gribaldo, L. Changes in Caco-2 Cells Transcriptome pro Fi Les upon Exposure to Gold Nanoparticles. Toxicol. Lett. 2015, 233, 187–199. [Google Scholar] [CrossRef]
- Schroeder, B.; Steen, T.V.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z. Fatty Acid Synthase (FASN) Regulates the Mitochondrial Priming of Cancer Cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef]
- Mayas, M.D.; Ortega, F.J.; Macías-gonzález, M.; Bernal, R.; Gómez-huelgas, R.; Fernández-real, J.M.; Tinahones, F.J. Inverse Relation between FASN Expression in Human Adipose Tissue and the Insulin Resistance Level. Nutr. Metab. 2010, 7, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veigel, D.; Wagner, R.; Stubiger, G.; Wuczkowski, M.; Filipits, M.; Horvat, R.; Benham, B.; Lopez-Rodrıguez, M.L.; Leisser, A.; Valent, P.; et al. Fatty Acid Synthase Is a Metabolic Marker of Cell Proliferation Rather than Malignancy in Ovarian Cancer and Its Precursor Cells. Int. J. Cancer 2015, 136, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Yang, Q.; Jiang, Y.-Y.; Yang, W.; Jiang, Y.; Li, X.; Hazawa, M.; Zhou, B.; Huang, G.-W.; Xu, X.-E.; et al. Interplay and Cooperation between SREBF1 and Master Transcription Factors Regulate Lipid Metabolism and Tumor-Promoting Pathways in Squamous Cancer. Nat. Commun. 2021, 12, 4362. [Google Scholar] [CrossRef] [PubMed]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Sal, G. Del Sterol Regulatory Element Binding Protein 1 Couples Mechanical Cues and Lipid Metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-In Fl Ammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Liu, S.; Li, J.; Wu, L.; Lv, X.; Xu, J.; Chen, B.; Zhao, S.; Yang, H. CPT2 Down-Regulation Promotes Tumor Growth and Metastasis through Inducing ROS/NF ? B Pathway in Ovarian Cancer. Transl. Oncol. 2021, 14, 101023. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.; Fang, X. Fatty Acid Oxidation: An Emerging Facet of Metabolic Transformation in Cancer. Cancer Lett. 2019, 435, 92–100. [Google Scholar] [CrossRef]
- Uxa, S.; Castillo-Binder, P.; Kohler, R.; Stangner, K.; Müller, G.A.; Engeland, K. Ki-67 Gene Expression. CDDpress 2021, 28, 3357–3370. [Google Scholar] [CrossRef]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P. Caspase-9: Structure, Mechanisms and Clinical Application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Ryoo, H.D.; Bergmann, A. The Role of Apoptosis-Induced Proliferation for Regeneration and Cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef] [PubMed]
- Emeny, R.T.; Baumert, J.; Zannas, A.S.; Kunze, S.; Wahl, S.; Iurato, S.; Arloth, J.; Erhardt, A.; Balsevich, G.; Schmidt, M.V.; et al. Anxiety Associated Increased CpG Methylation in the Promoter of Asb1: A Translational Approach Evidenced by Epidemiological and Clinical Studies and a Murine Model. Neuropsychopharmacology 2017, 43, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Radhakrishnan, J.; Knight, J.C. Genetic Determinants of HSP70 Gene Expression Following Heat Shock. Hum. Mol. Genet. 2010, 19, 4939–4947. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The Human HSP70 Family of Chaperones: Where Do We Stand ? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Leak, R.K. Heat Shock Proteins in Neurodegenerative Disorders and Aging. J. Cell Commun. Signal. 2014, 8, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.; Vogel, L.K.; Kopp, T.I.; Sæb, M.; Nonboe, A.W.; Hamfjord, J.; Kure, E.H.; Vogel, U. High ABCC2 and Low ABCG2 Gene Expression Are Early Events in the Colorectal Adenoma- Carcinoma Sequence. PLoS ONE 2015, 10, e0119255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, H.; Yang, S.; Su, D. Increased ABCC2 Expression Predicts Cisplatin Resistance in Non-Small Cell Lung Cancer. Cell Biochem. Funct. 2021, 39, 277–286. [Google Scholar] [CrossRef]
- Kielar, D.; Kaminski, W.E.; Liebisch, G.; Piehler, A.; Wenzel, J.J.; Christoph, M.; Heimerl, S.; Langmann, T.; Friedrich, S.O.; Alfred, B.; et al. Adenosine Triphosphate Binding Cassette (ABC) Transporters Are Expressed and Regulated During Terminal Keratinocyte Di¡erentiation: A Potential Role for ABCA7 in Epidermal Lipid Reorganization. J. Investig. Dermatol. 2003, 121, 465–474. [Google Scholar] [CrossRef]
| IC50 (µg/mL) | |||
|---|---|---|---|
| Treatment | KMST-6 | HaCaT | CHO |
| C. orbiculata extract | 296 ± 27.34 | >1000 | >1000 |
| Cotyledon-AgNPs | 122 ± 19.30 | 40.55 ± 2.68 | 21.08 ± 0.76 |
| Gene Name | Symbol | Function |
|---|---|---|
| Fatty acid synthase | FASN | Steatosis |
| Sterol regulatory element-binding transcription factor 1 | SREBF1 | Steatosis |
| Carnitine palmitoyl transferase II | CPT2 | Fatty Acid Metabolism (β-Oxidation) |
| Ankyrin repeat and SOCS box protein 1 | ASB1 | Mitochondrial Energy Metabolism |
| Heat Shock Protein Family A (Hsp70) Member 1B | HSPA1B | Mitochondrial Energy Metabolism |
| ATP Binding Cassette Subfamily C Member 2 | ABCC2 | Cholestasis |
| Caspase-9 | CASP9 | Apoptosis |
| Marker Of Proliferation Ki-67 | MKI67 | Immunotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyavambiza, C.; Meyer, M.; Wusu, A.D.; Madiehe, A.; Meyer, S. The Cytotoxicity of Cotyledon orbiculata Aqueous Extract and the Biogenic Silver Nanoparticles Derived from the Extract. Curr. Issues Mol. Biol. 2023, 45, 10109-10120. https://doi.org/10.3390/cimb45120631
Tyavambiza C, Meyer M, Wusu AD, Madiehe A, Meyer S. The Cytotoxicity of Cotyledon orbiculata Aqueous Extract and the Biogenic Silver Nanoparticles Derived from the Extract. Current Issues in Molecular Biology. 2023; 45(12):10109-10120. https://doi.org/10.3390/cimb45120631
Chicago/Turabian StyleTyavambiza, Caroline, Mervin Meyer, Adedoja Dorcas Wusu, Abram Madiehe, and Samantha Meyer. 2023. "The Cytotoxicity of Cotyledon orbiculata Aqueous Extract and the Biogenic Silver Nanoparticles Derived from the Extract" Current Issues in Molecular Biology 45, no. 12: 10109-10120. https://doi.org/10.3390/cimb45120631
APA StyleTyavambiza, C., Meyer, M., Wusu, A. D., Madiehe, A., & Meyer, S. (2023). The Cytotoxicity of Cotyledon orbiculata Aqueous Extract and the Biogenic Silver Nanoparticles Derived from the Extract. Current Issues in Molecular Biology, 45(12), 10109-10120. https://doi.org/10.3390/cimb45120631






