1. Introduction
Natural nanoparticles (NP) are organic or inorganic, intracellular or extracellular, with various functions. Intracellular vesicles transport both molecules, supramolecular complexes and nanoparticles from the cell surface to the interior. Over 50 proteins take part in the formation of clathrin-coated endocytic vesicles [
1]. Extracellular vesicles including exosomes are released by most animal, plant and bacterial cultivable cells in vitro and some cells such as mesenchymal stem cells, B cells, endothelial, dendritic and mast cells, adipocytes, neurons, as well as all transformed tumour cells in vivo [
2,
3]. Lipoproteins are self-assembling structures consisting of lipids and apolipoproteins, capable of transferring lipids in both invertebrates and vertebrates. Spherical or discoidal lipoprotein NP of 7–80 nm have a core consisting of non-polar lipids, triacylglycerols and esterified cholesterol with shells from apolipoproteins, phospholipids and non-esterified cholesterol [
3]. Other natural protein-based NP include lectins and ferritin. Lectins are proteins of non-immune origin able to bind specifically certain sugars on cellular membranes. Ferritin NP in bacteria, archaea and eukaryotes store iron oxides. In eukaryotes, ferritin is composed of 24 subunits with a four-helical bundle forming a hollow. Magnetotactic bacteria possess a specialized organelle—the magnetosome of 50–70 nm, consisting of lipids and containing iron-containing minerals [
3].
After the first approval of NP for drug delivery in the 1990s artificial nanomaterials, spanning from inorganic and synthetic polymeric NP to protein, RNA, or lipid NP are widely used in biomedicine for prophylactic, diagnostic and therapeutic purposes, cosmetics, food processing, electronics, buildings and aeronautics. In comparison to free molecules, NP have improved bioavailability, increased intracellular delivery and important features to overcome cellular, local and systemic barriers. NP may serve as a depot for vaccine antigen storage and prolonged immune response. However, the number of NP-based drugs approved by the FDA and the European market does not correspond to the high expectations associated with nanomedicine [
4,
5]. The translational gap between research and clinical implementation may result from unavailable data on NP stability in living cells and organisms, distribution among organs and possible pathological sequelae. Stability, cellular uptake, accumulation in organs, biodegradation and excretion of NP in biological fluids and extracellular matrix are important for biomedical applications [
6]. Despite the majority of artificial NP being biocompatible and biodegradable, some of them exhibit toxic side effects. Potential side effects and possible immunological deterioration may be caused by both NP physical sizes, shapes, and charges, as well as chemical composition and especially surface decoration [
7]. Acute and chronic toxicity, biocompatibility [
6,
7], in vivo kinetics and the ability to escape through the reticuloendothelial system hamper the clinical implementation of NP.
Living organisms possess defense mechanisms against foreign nanomaterials including coating of NP with biocorona in biological fluids [
8], cellular endocytosis with subsequent NP biodegradation in lysosomes, innate and adaptive immunity induction, reactive oxygen species (ROS) formation, neutrophil extracellular traps (NETs) and tunneling nanotubes [
9,
10]. Biomolecular corona on NP surfaces can be transient “soft” with high rates of both association and dissociation (in particular, a the coating consisting of albumin and fibrinogen) or “hard” with long-term stability (shells from opsonins including immunoglobulins) [
8]. Surface layers consisting of biopolymers or nonionic surfactants may enhance steric hindrances and electrostatic repulsion.
Bio-reactivity, cellular uptake and distribution among organs of natural, synthetic and environmental nanomaterials including both organic and inorganic NP depend on surface proteins due to biocorona formation immediately after the addition of biorelevant media or administration into organisms. Protein conformations can be changed on the surfaces of nanostructures. Additionally, coating with foreign proteins may induce innate and adaptive immune response [
9]. The surface polyethylene glycol layer does not allow intracellular delivery of nanomaterials resulting in their circulation in blood [
11]. Delivery of nanomaterials to brain tumours is mediated by NP biocorona in the bloodstream; leaky blood vessels at the tumour site; enhanced penetration and retention (EPR) in transformed cells; inhibition of drug efflux in endothelial and cancer cells; and active targeting by means of specific ligands to receptors at the blood–brain barrier [
12]. Thus, apolipoprotein E directs NP to the brain [
13,
14].
Among proteins all albumins are poorly immunogenic with induction of anti-inflammatory response in macrophages [
15] and able to act as extracellular antioxidants providing competitive protection from free radicals and other harmful chemicals. Albumins provide oncotic pressure within capillaries, transport fatty acids, bilirubin, minerals and hormones, and function as anticoagulants. Many drugs and endogenous molecules are known to bind with albumins [
9]. The ability of albumins of different origins to transfer drugs is used for drug delivery in nanomedicine. Albumin was the first protein approved by the Food and Drug Administration (FDA) of the USA for nanomedicines. Currently, albumin-based drugs include Abraxane (Celgene) (paclitaxel loaded albumin NP), optison (GE Healthcare, Chicago, Illinois, United States) (human serum albumin stabilized perflutren microspheres as an ultrasound contrast agent) and albumin-bound anti-cancer drugs (ABI-009 (Aadi with Celgene)—albumin-bound rapamycin and ABI-011 (NantBioScience, Los Angeles, California, United States)—albumin-bound thiocolchicine analog (IDN 5405)) [
9].
Biodistribution and biodegradation of foreign NP depend on the protection systems of organisms including both innate and subsequent adaptive immune response. Inflammation is a key reaction following exposure to any foreign solid material, including NP. Induction of pro-inflammatory cytokines was shown for several nanomaterials in vitro and some cytokines can bind to nanomaterials [
9,
16].
Oxidative stress is responsible for NP-induced injury [
17]. The kidney is susceptible to reactive oxygen species (ROS)-induced injury [
17].
Non-invasive painless delivery of nanomedicines across the biological barriers of the mucosal surfaces may induce innate and adaptive systemic and mucosal immunity to prevent respiratory and intestinal infections. At present oral administration is considered preferable in comparison with other routes of NP administration including intravenous and intranasal.
Our research was aimed at the protein NP stability and distribution in organs of mice and rabbits after non-invasive intranasal and peroral administrations.
4. Discussion
BSA, a globular non-glycosylated protein with a molecular weight close to 66,430 Da, was used for NP fabrication due to its high solubility in water and fluoroalcohols as well as stability in a wide range of pH (4–9) and temperatures (up to 60 °C for 10 hours).
To monitor inorganic nanomaterials and especially metallic NP a number of methods have been developed and widely used whereas artificial protein NP remain undetectable within living cells and cannot be isolated because of ubiquitous natural solid NP and vesicles. Therefore, fluorescent labelling was necessary to reveal the biodistribution of BSA NP among organs of laboratory animals by using quantitative spectrofluorometry and fluorescent microscopy. The rhodamine dyes are stable in a wide range of pH in the presence of high concentrations of proteins. RhoB fluorescence emission maximum at 580 nm is far from the autofluorescence of aromatic amino acid residues of proteins from living cells. Consequently, the fluorescence emission spectra of the rhodamine dye RhoB and cellular proteins do not overlap. To reduce the risk of conformational changes of BSA, the molar ratio RhoB/BSA did not exceed 5. BSA conformational and antigenic stability was confirmed by ELISA with polyclonal antibodies against BSA as earlier described [
18].
Validation of the spectrofluorometric and microscopic methods with necessary controls including the fluorescent dye RhoB solution with known concentrations and molar extinction coefficient, the fluorescent protein RhoB-BSA and the corresponding nanoparticles were previously described in detailsed [
9,
18]. Despite covalent binding between BSA and RhoB during fluorescent labelling and subsequent gel chromatography purification, the free dye molecules cannot be completely excluded from consideration because of possible degradation of the RhoB-BSA inside cells [
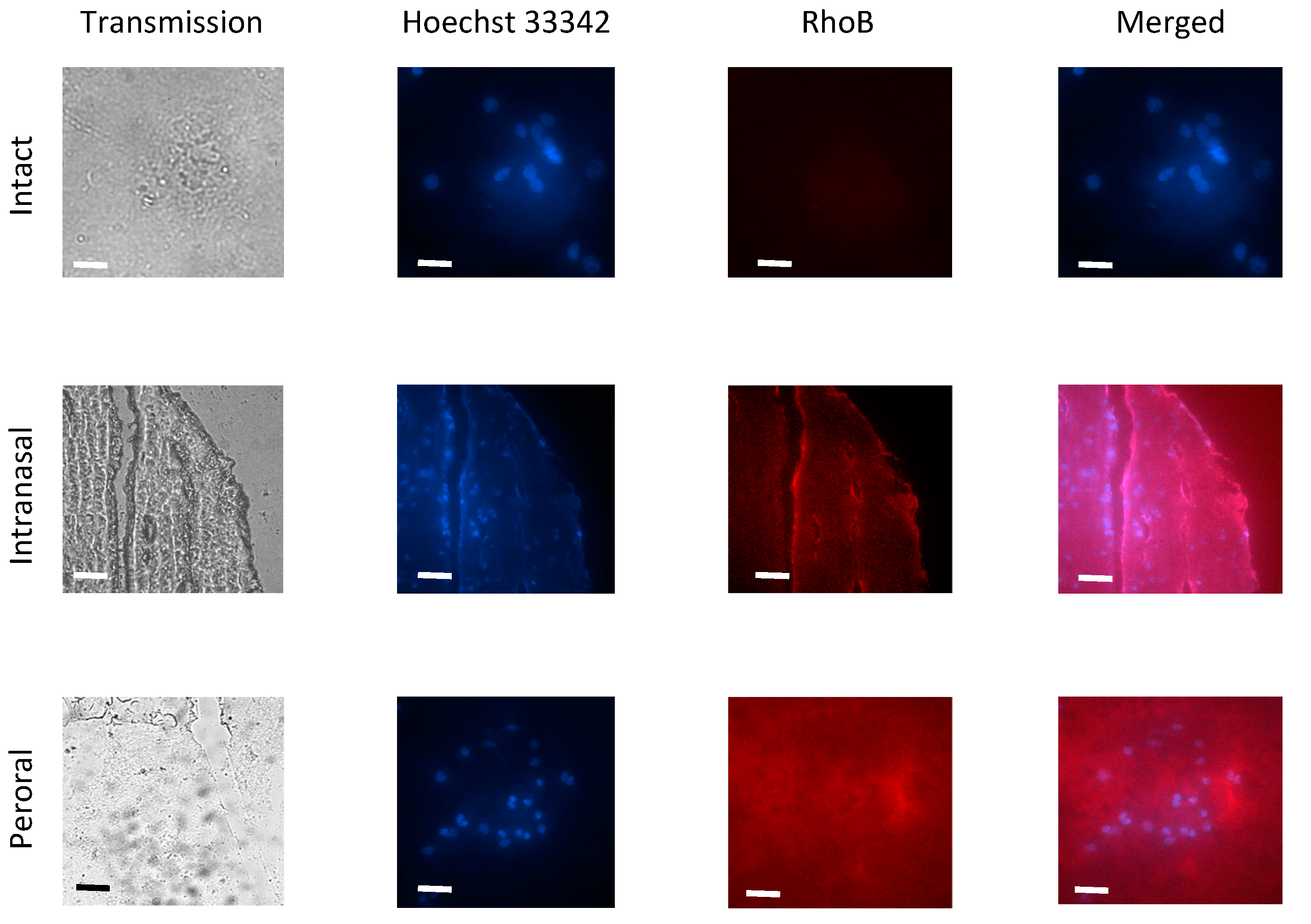
9]. Autofluorescence of organs of intact mice and rabbits in the range near 580 nm was minimal as shown by means of fluorescent microscopy (
Figure 4).
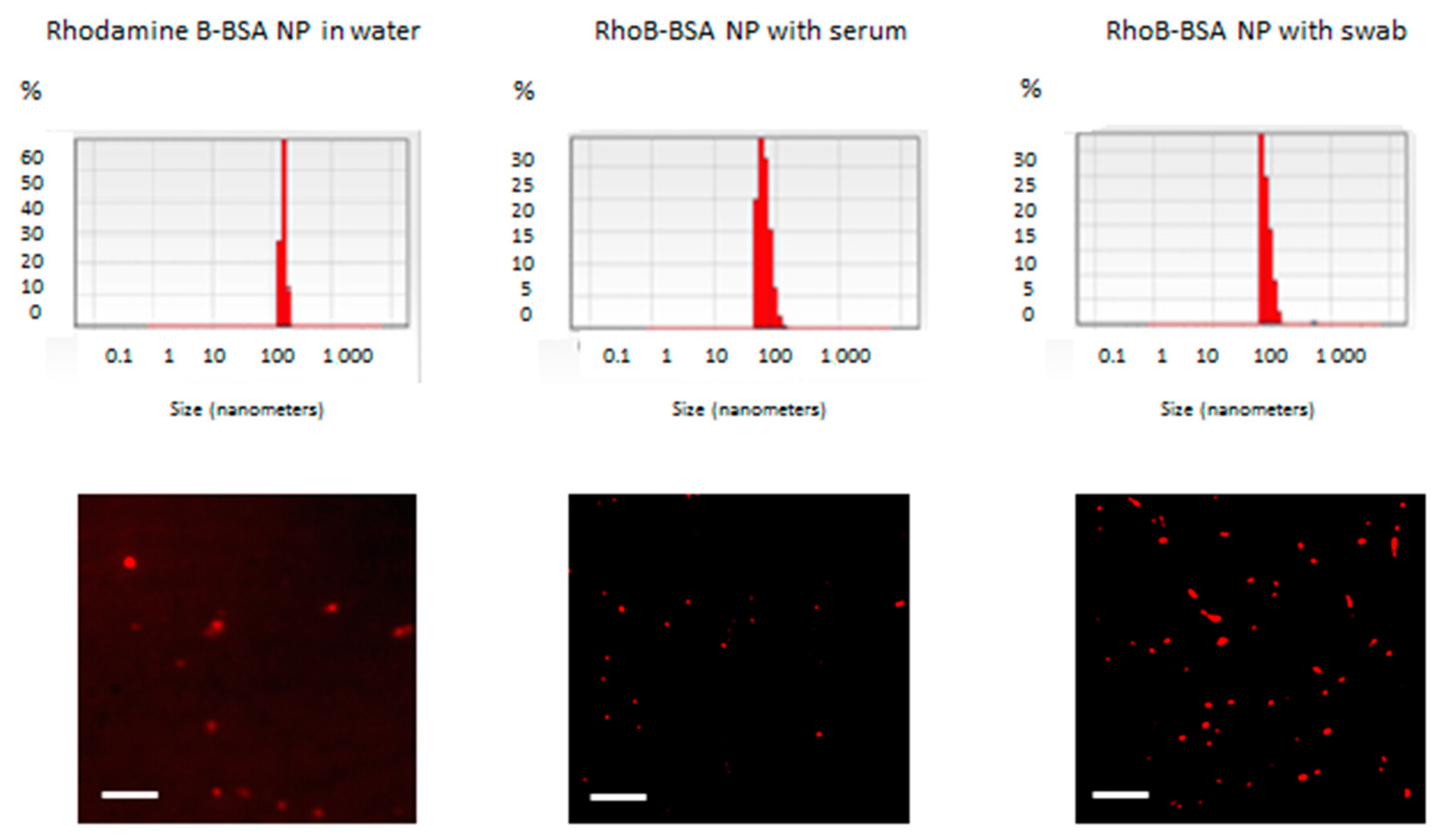
RhoB-BSA were stable in water at +4 °C during the whole period of observation for 3 years [
9,
18], whereas in the presence of human or fetal bovine blood sera and nasopharyngeal swabs with saliva for 5 days at +37 °C (
Figure 1); however, they were hydrolyzed with trypsin [
18]. In addition to high total protein concentrations in blood and possible competitive inhibition between blood proteins and the fluorescent protein NP, protease inhibitors encompassing nearly 10% of all blood proteins may be responsible for the NP stability [
19]. Most inhibitors are highly specific for single or several related proteinases whereas the α-2-macroglobulin and the alpha 1-proteinase (α-1-antitrypsin) have a broader specificity [
19]. Mucosal swabs and saliva also contain a number of proteins, including mucin, histatin, cystatin, statherin, amylase, lingual lipase, secretory immunoglobulins and proline-rich protein [
20]. These proteins maintain tooth and mucosal integrity, mucosal immunity and are involved in digestion, lubrication, buffering and antibacterial activity [
20]. Complex regulation of proteins with enzymatic, immunomodulation and antimicrobial properties in saliva is mediated by a balance between proteases mostly derived from white blood cells and bacteria in the oral cavity and their inhibitors. Protease inhibitors of saliva include Kunitz inhibitors, serpins and cystatins. Salivary protease inhibitory proteins include antileukoproteinase and cysteine protease inhibitors, cystatin-B, -C, -D, -S, -SA and –SN [
20]. All the secreted protease inhibitors may determine protein NP stability in the presence of mucosal swabs (
Figure 1). Limited stability of BSA NP during incubation in blood sera and with swabs and saliva for several days at +37 °C (
Figure 1) can be caused by from high total protein concentrations in NP in comparison with available proteases in the biological fluids, competitive inhibition of protease activity with other proteins derived from the biological samples, protease inhibitors in blood and saliva [
19,
20] and slow layer-by-layer degradation of surface proteins in NP with possible induction of innate immunity [
9,
10]. Available data on the stability of protein NP in vitro [
9,
18] (
Figure 1) permit to suggest their implementation as diagnostic tools and potential drug delivery vehicles.
RhoB-BSA NP detection, quantitation, biodistribution and degradation in living organisms in vivo and in eukaryotic cells in vitro [
9] were based on quantitative spectrofluorometry and fluorescent microscopy since direct visualization of unlabeled protein NP or their isolation from cells are hardly possible due to ubiquitous natural solid NP and vesicles. However, the stability of the fluorescent rhodamine dye RhoB in lysosomes in an acidic environment and oxidation in the presence of ROS cannot be excluded from consideration. Similar gradual uptake of the RhoB-labelled BSA NP in human immortal tissue cultures and primary blood leukocytes for 2 days and following decline up to 5 days allowed us to suggest that intracellular delivery and biodegradation of the protein NP are based on common mechanisms [
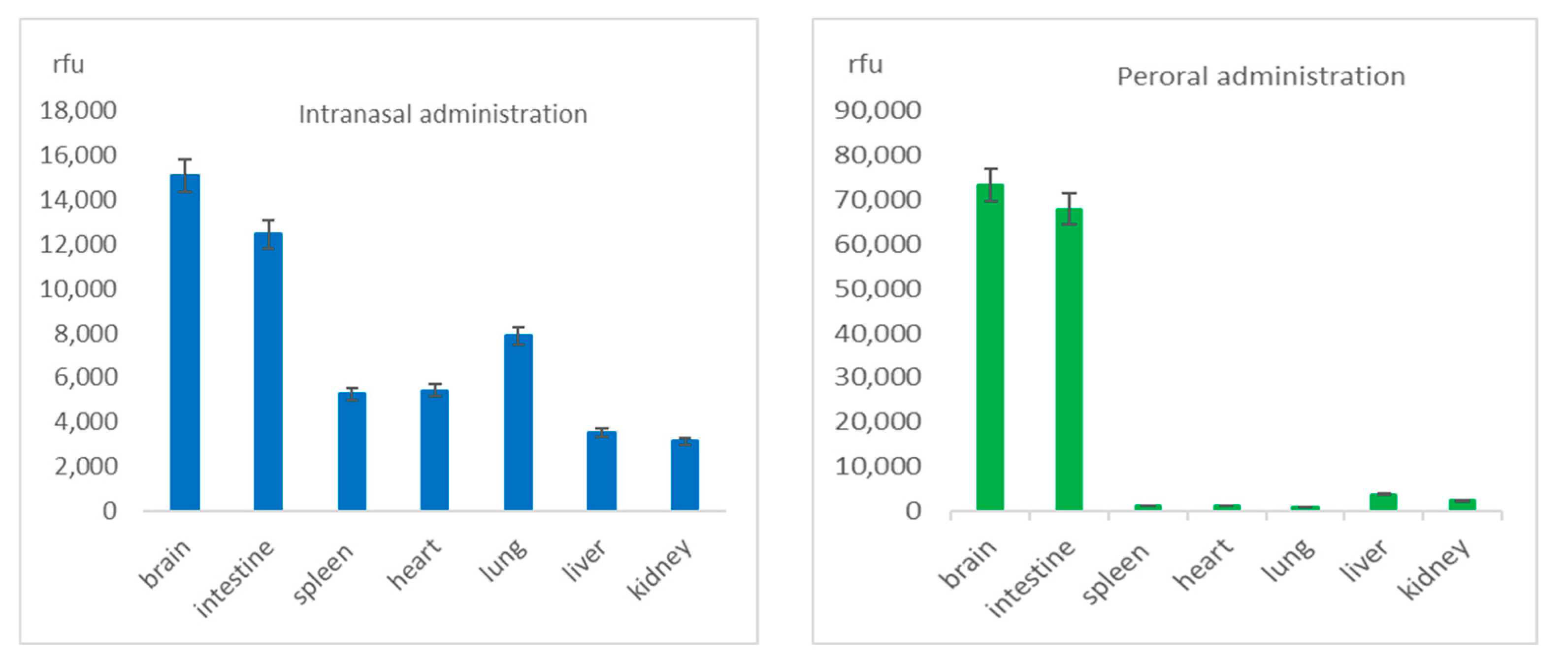
9]. The maximal levels of the RhoB-BSA NP were detected in 1–2 days after both intranasal or oral instillation (
Figure 2) and coincided with the dynamics of their cellular uptake in vitro [
9]. However, after a single intravenous injection via the mouse tail vein, two different protein-based NP rapidly transferred within 15 min in lung-associated lymph nodes [
21]. More than half (57–73%) of these protein NP excreted in 24 h after intravenous administration [
21]. Evidently, noninvasive mucosal instillations showed slower dynamics of the protein NP biodistribution (
Figure 2 and
Figure 3) compared to intravenous injections [
21] due to the required additional stage of NP penetration into blood or lymph.
Protein-based NP extensively translocated throughout different mouse organs and tissues after mucosal instillations via nose or mouth (
Figure 2 and
Figure 3) and after intravenous injection [
21]. The protein-based were found in the circulatory and lymphatic endothelial cells, pulmonary epithelial cells, the interstitium of the lung, the outer capsule and perilymphoid zones of the spleen, the sinusoids of the liver, the convoluted tubules of the kidney, and draining lymph nodes [
21]. The broad distribution of protein NP among different mouse organs and tissues suggested their translocation from the vascular system to interstitial areas, in the lymphatics, and interstitial areas of organs [
21]. Similarly, RhoB-BSA NP after intranasal administration rapidly traversed the airway epithelium and entered in endothelial cells of blood vessels by means of transcytosis via cells or through intercellular tight junctions. Surprisingly, after intravenous administration, the protein-based were found in all organs, except the brain [
21], whereas after intranasal and oral administration the RhoB-BSA NP accumulation significantly differed for different organs with prevalent accumulation in the brain (
Figure 2 and
Figure 3). The gelatin NP appeared to accumulate in macrophages, thus reaching macrophage-rich organs and crossing the blood–brain barrier (BBB) [
22].
Whereas microparticles >2000 nm accumulate within the spleen, liver, and lung capillaries, NP of 100–200 nm could escape filtration by the liver and spleen. The broad distribution of the fluorescent protein NP in mouse organs (
Figure 2 and
Figure 3) [
21,
22], relative stability for a few days (
Figure 2), penetration into the circulatory system after mucosal administration and absence of accumulation in liver and spleen (
Figure 2 and
Figure 3) may serve as evidence of the NP escape from reticuloendothelial system in spite of mucosa-associated lymphoid tissue (MALT) is known as an important part of the system.
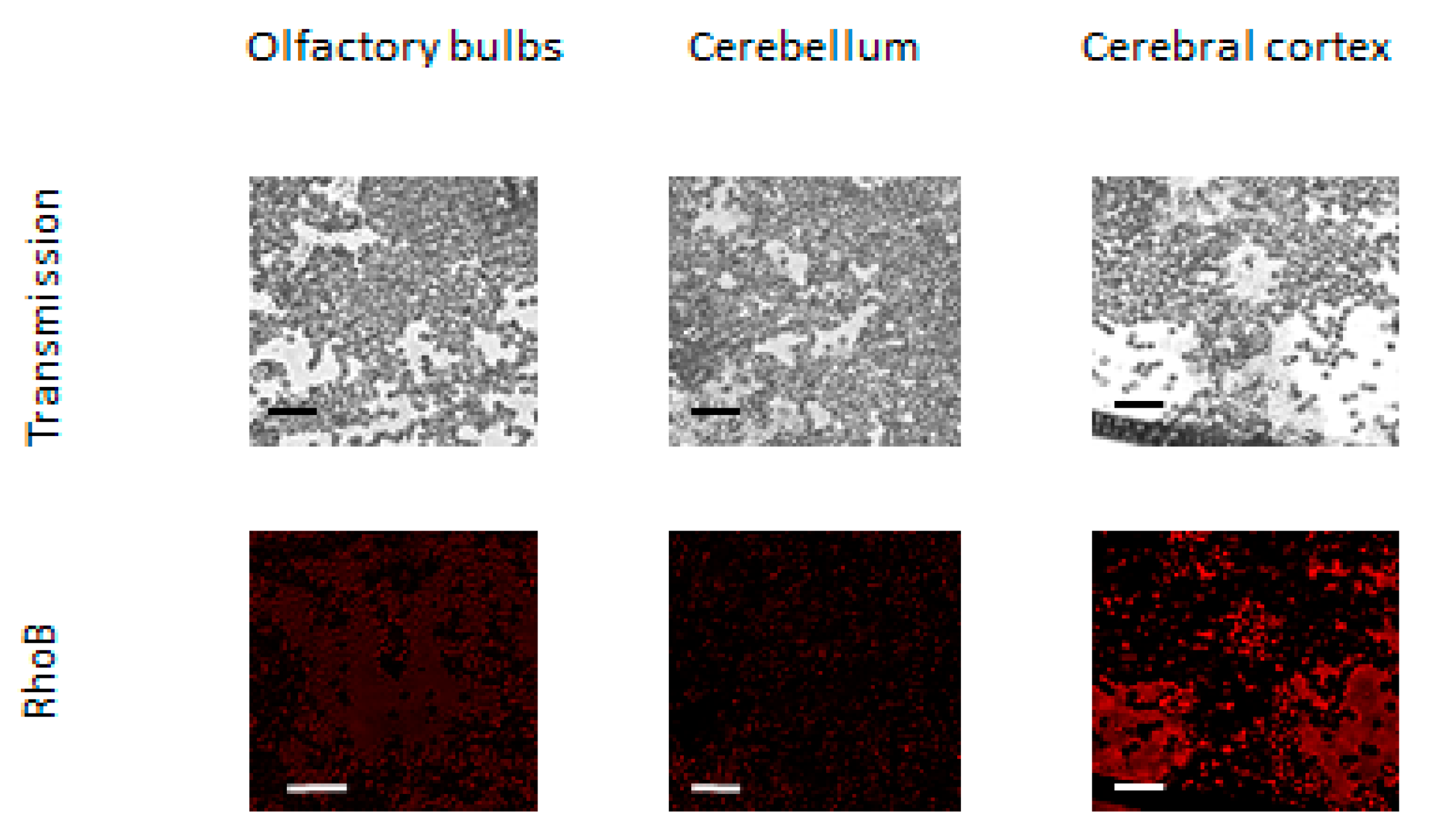
Prevalent accumulation of BSA NP in the brain could be caused by penetration through olfactory bulbs where BBB is absent. BBB has the least permeable capillaries due to intercellular tight junctions. Therefore, the BBB is the obstacle or a rate-limiting factor for the intracerebral delivery of drugs [
23]. Currently, most drugs, including recombinant proteins, antibodies or other biopolymers, are not capable of crossing the BBB. Small molecules of molecular weight < 600 Da including peptides with less than 6 amino acid residues and hydrophobic molecules soluble in lipids can penetrate the brain. The BBB also restricts more than 98% of small drugs. Nasal or transcranial administration, infused hypertonic agents, and lipidation of small-molecule drugs were used to transfer medicines through the BBB [
23]. Free diffusion across the BBB is known for several liposoluble small molecules (MW < 400 Da). The chemical methods to overcome BBB include: (1) chemical modification of the drug to form a more lipophilic prodrug; (2) coupling the drugs with mannitol or aromatic substances (such as borneol and musk), because mannitol could induce a high osmotic pressure to opening the BBB temporarily and aromatic substances could cross the BBB as the resuscitation medicine; (3) using appropriate chemical drug delivery system or drug carrier with the ability to cross BBB. Protein NP were suggested to overcome the limitations of free low-molecular-weight drugs and to cross systemic, microenvironmental and cellular barriers.
Although the majority of large blood molecules were prevented from penetration into the brain by the tight junctions in the BBB, the specific and, nonspecific transcytotic mechanisms exist to transport large molecules and complexes across the BBB. The passive penetrations in brains inside leukocytes and the transcellular lipophilic pathway common for lipid agents could be excluded from mechanisms of RhoB-BSA NP localization in the brain. Mechanisms of active NP uptake such as tight junction modifications and modulation, carrier-mediated transcytosis and receptor-mediated transcytosis seem unlikely. Consequently, the protein NP adsorption and unspecific endocytosis with subsequent transcytosis may be responsible for the RhoB-BSA NP distribution in different parts of mouse and rabbit brains (
Figure 4 and
Figure 5).
Nanocarriers can favour the delivery of chemotherapeutics to brain tumours owing to different strategies, including chemical stabilization of the drug in the bloodstream; passive targeting (because of the leaky vascularization at the tumour site); inhibition of drug efflux mechanisms in endothelial and cancer cells; and active targeting by exploiting carriers and receptors overexpressed at the blood–brain tumour barrier. Within this concern, a suitable nanomedicine-based therapy for gliomas should not be limited to cytotoxic agents, but also target the most important pathogenetic mechanisms, including cell differentiation pathways and angiogenesis. Moreover, the combinatorial approach of cell therapy plus nanomedicine strategies can open new therapeutical opportunities. The major part of attempted preclinical approaches on animal models involves active targeting with protein ligands, but, despite encouraging results, a small number of nanomedicines reached clinical trials, and most of them include drug-loaded nanocarriers free of targeting ligands, also because of safety and scalability concerns.
BBB breakdown in vivo by altering the permeability of both cell membranes and intercellular tight junctions for foreign NP could induce brain edema formation [
24]. oteworthy that the leakage of Evans blue dye from NP was observed largely in the ventral surface of the brain and the proximal frontal cortex but the dorsal surfaces of the cerebellum showed mild to moderate staining [
24].
Detection of the fluorescent protein NP in the intestine was not self-evident due to mucosal barriers after oral and nasal instillations, a large pH gradient ranging from pH 1–2.5 in the stomach to pH 7–8 in the colon, proteolytic enzymes (pepsin in the stomach as well as trypsin and chymotrypsin in the duodenum) [
25], tight junctions between neighboring epithelial cells, the enterocytes of the intestinal epithelium and the subepithelial tissue [
26]. The fluorescent rhodamine dye RhoB appeared to remain stable in the intestine (
Figure 2 and
Figure 3) despite the aggressive conditions of the gastrointestinal tract. The weak innate immunity induction after intranasal and peroral RhoB-BSA NP administration did not correspond to Th1 polarized innate immunity in human cell lines and blood mononuclear cells [
9] probably due to minimal amounts of NP in blood and prevalent accumulation in the brain and intestine with isolated immunity systems of the central nervous system and mucosa. Taken together, intranasal and peroral administration of RhoB-BSA NP did not induce systemic innate immunity that suggested negligible specific cellular and humoral response. Therefore, the fluorescent rhodamine RhoB-BSA NP elimination from living organisms could not be mediated by the immune system.