Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Byproduct Materials
2.3. Qualitative Analysis by ATR-FTIR
2.4. Extraction Procedure
2.5. Determination of Phenol Content
2.6. Determination of Flavonoid Content
2.7. Determination of Antioxidant Activity through DPPH, CUPRAC, FRAP, and ABTS Methods
2.8. Photochemiluminescence Assay
2.9. Statistical Analysis
3. Results and Discussion
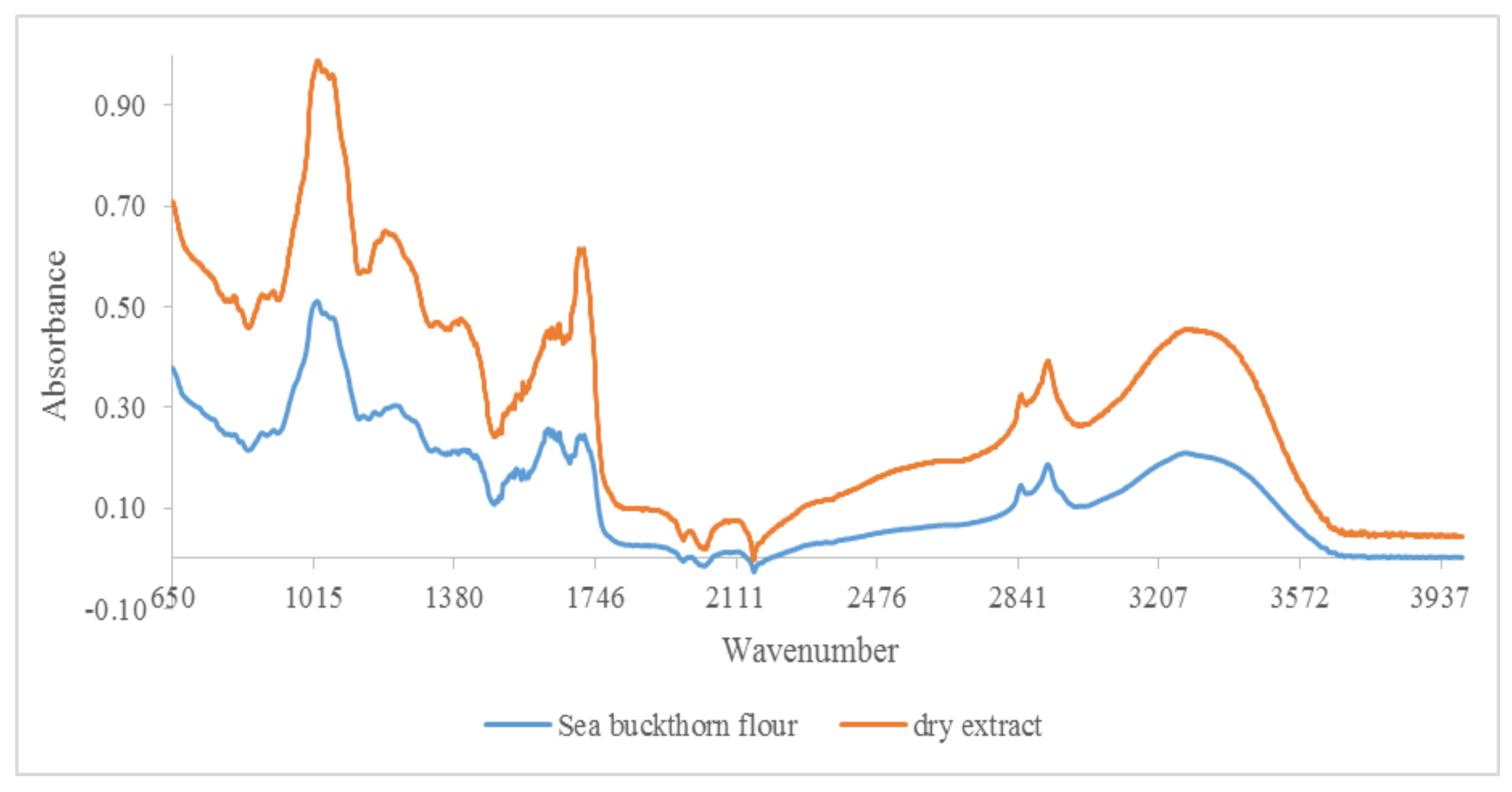
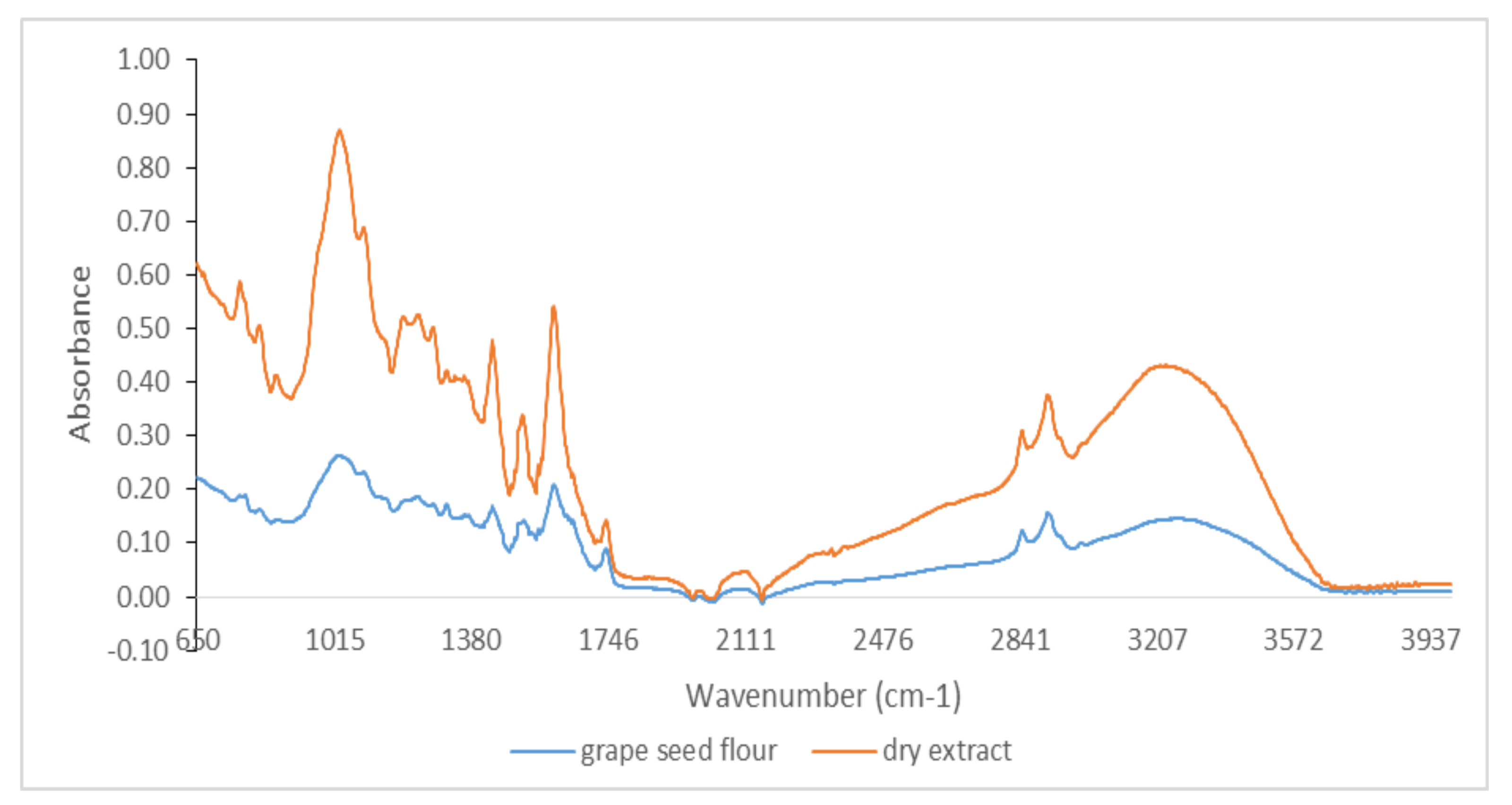
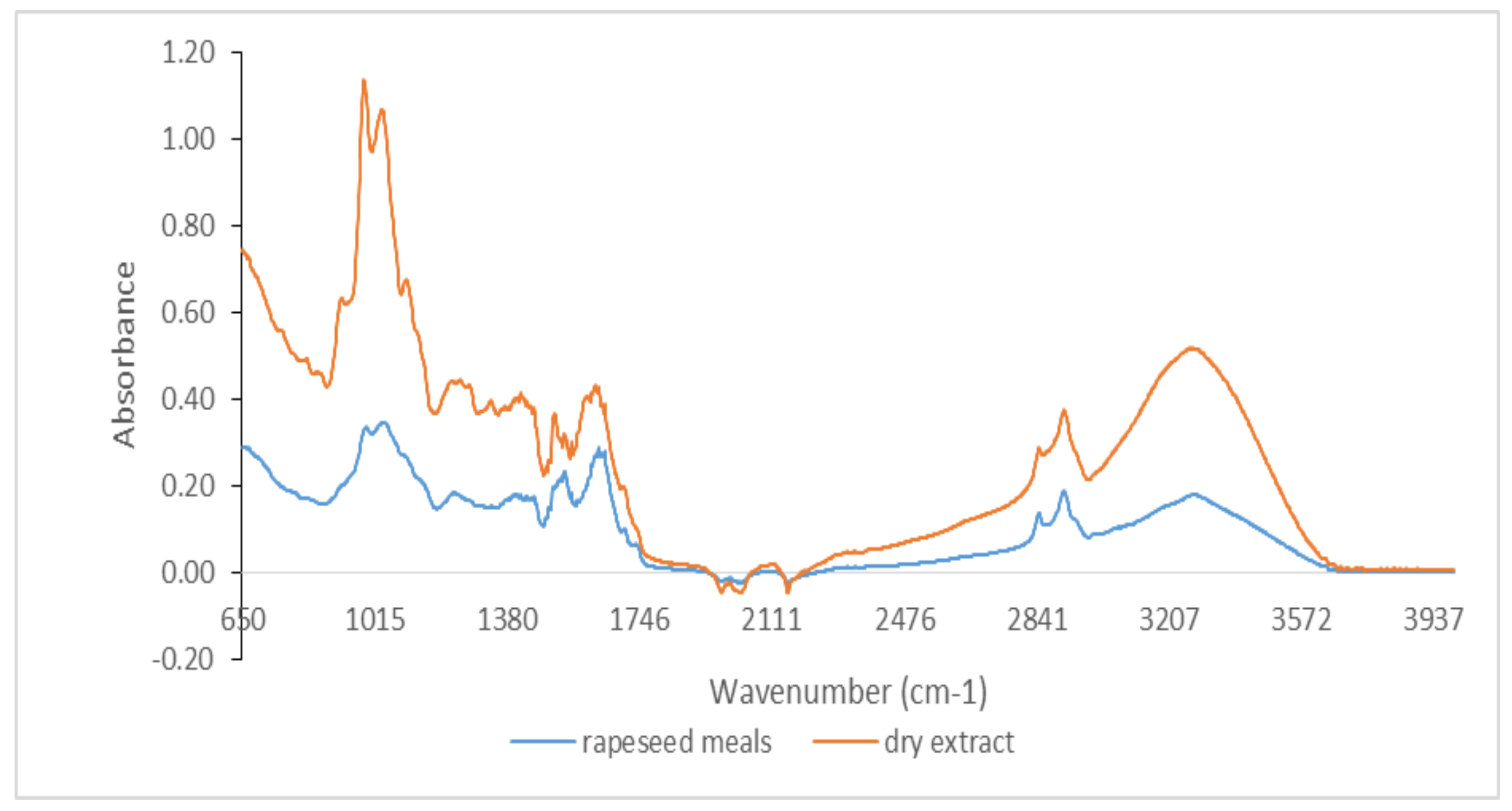
3.1. Qualitative Analysis by ATR-FTIR
3.2. Amount of Phenolic Compounds and Flavonoid Compounds
3.3. Comparison of the Antioxidant Activity of Selected byproducts
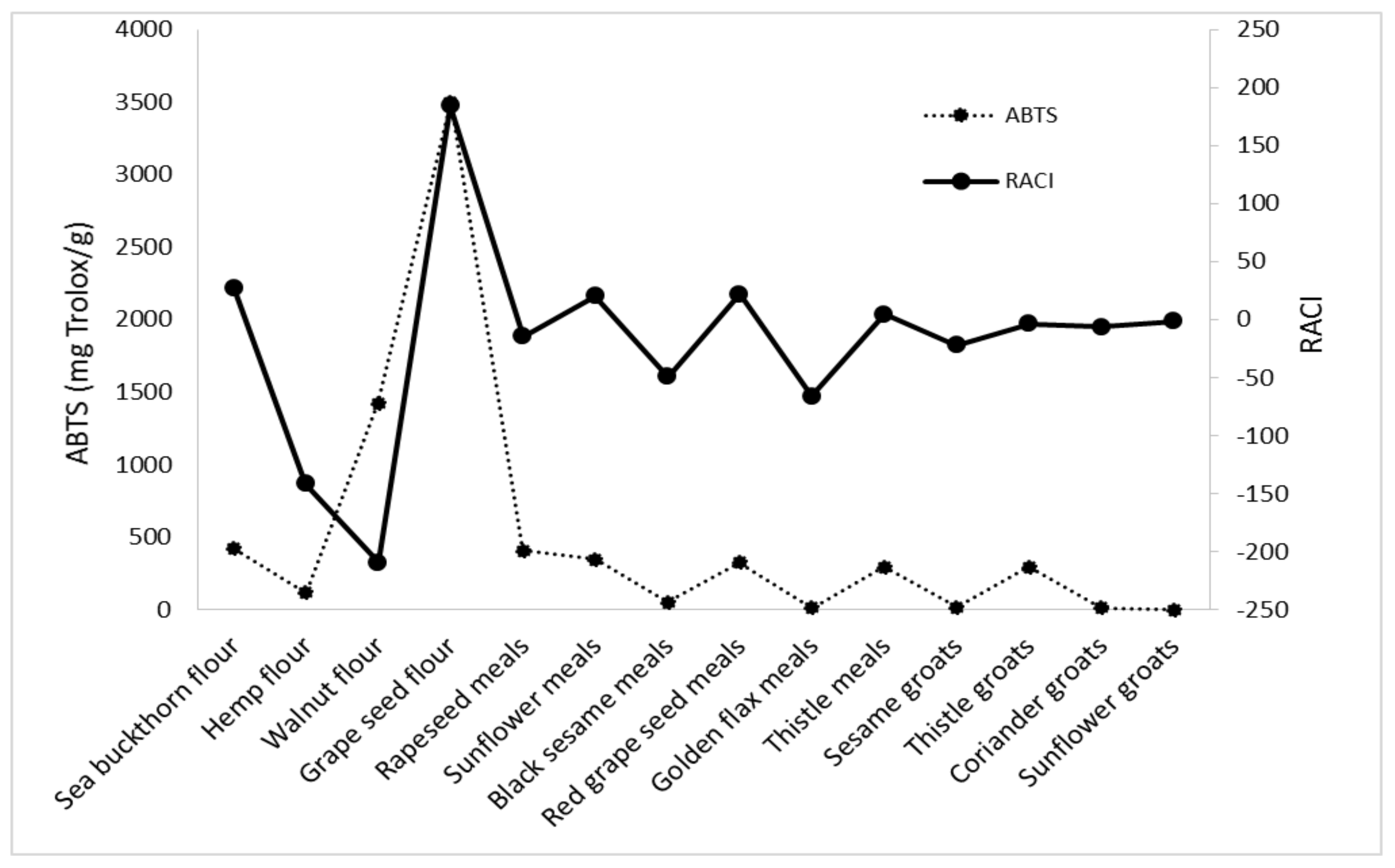
3.4. Development of the Relative Antioxidant Capacity Index RACI
3.5. Determination of Antioxidant Capacity Using the PHOTOCHEM Device in an ACL System
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Padureanu, S.; Deseatnicova, O.; Turculet, N.; Boestean, O.; Niculaua, M. Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules 2020, 25, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krejkarova, J.; Strakova, E.; Suchy, P.; Herzig, I.; Karaskova, K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities—A review. Acta Vet. Brno 2015, 84, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Rusu, I.E.; Marc (Vlaic), R.A.; Muresan, C.C.; Mursan, A.E.; Muresan, V.; Pop, C.R.; Chis, M.S.; Man, S.M.; Filip, M.R.; Onica, B.-M. Hemp (Cannabis sativa L.) Flour-Based Wheat Bread as Fortified Bakery Product. Plants 2021, 10, 1558. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [Green Version]
- Almoraie, N.M. The Effect of Walnut Flour on the Physical and Sensory Characteristics of Wheat Bread. Int. J. Food Sci. 2019, 2019, 5676205. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of Grape Seed Flour on the Antioxidant Profile, Textural and Sensory Properties of Waffles Textural and Sensory Properties of Waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Saleh, A.A.; El-Awady, A.; Amber, K.; Eid, Y.Z.; Alzawqari, M.H.; Selim, S.; Soliman, M.M.; Shukry, M. Effects of Sunflower Meal Supplementation as a Complementary Protein Source in the Laying Hen’s Diet on Productive Performance, Egg Quality, and Nutrient Digestibility. Sustainability 2021, 13, 3557. [Google Scholar] [CrossRef]
- Grasso, S.; Pintado, T.; Pérez-Jiménez, J.; Ruiz-Capillas, C.; Herrero, A.M. Characterisation of Muffins with Upcycled Sunflower Flour. Foods 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Amarowicz, R.; Szłyk, E. Antioxidant capacity of rapeseed meal and rapeseed oilsenriched with meal extract. Eur. J. Lipid Sci. Technol. 2010, 112, 750–760. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Bloedon, L.; Szapary, P. Flaxseed and cardiovascular risk. Nutr. Rev. 2004, 62, 18–27. [Google Scholar] [CrossRef]
- Pouzo, L.B.; Descalzo, A.M.; Zaritzky, N.E.; Rossetti, L.; Pavan, E. Antioxidant status, lipid and color stability of aged beef from grazing steers supplemented with corn grain and increasing levels of flaxseed. Meat Sci. 2016, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Aarabi, A.; Rahbaran, S. Effect of flaxseed flour on rheological properties, staling and total phenol of Iranian toast. J. Cereal Sci. 2017, 76, 173–178. [Google Scholar] [CrossRef]
- Lucini, L.; Pellizzoni, M.; Ruzickova, G.; Molinari, G.P. Phytochemical constituents and in vitro radical scavenging activity of different aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef]
- Křen, V.; Walterová, D. Silybin and silymarin—New effects and applications. Biomed. Pap. 2005, 149, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Kreps, F.; Vrbiková, L.; Schmidt, Š. Industrial Rapeseed and Sunflower Meal as Source of Antioxidants. Int. J. Eng. Res. Appl. 2004, 4, 45–54. [Google Scholar]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Shen, S.S.; Callaghan, D.; Juzwik, C.; Xiong, H.Q.; Huang, P.L.; Zhang, W.D. ABCG2 reduces ROS-mediated toxicity and inflammation: A potential role in Alzheimer’s disease. J. Neurochem. 2010, 114, 1590–1604. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Marks, S.C.; Crozier, A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J. Agric. Food Chem. 2007, 55, 3148–3157. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinas, I.C.; Oprea, E.; Geana, E.I.; Tutunaru, O.; Gradisteanu Pircalabioru, G.; Zgura, I.; Chifiriuc, M.C. Valorization of Gleditsia triacanthos Invasive Plant Cellulose Microfibers and Phenolic Compounds for Obtaining Multi-Functional Wound Dressings with Antimicrobial and Antioxidant Properties. Int. J. Mol. Sci. 2021, 22, 33. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe, I.; Marinas, I.C.; Geană, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef] [PubMed]
- Singleton, L.V.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Culetu, A.; Fernandez-Gomez, B.; Ullate, M.; Del Castillo, M.D.; Andlauer, W. Effect of theanine and polyphenols enriched fractions from decaffeinated tea dust on the formation of Maillard reaction products and sensory attributes of breads. Food Chem. 2016, 197, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.E.; Ozyürek, M.; Güçlü, K.; Apak, R. Determination of antioxidants by a novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) assay with post-column detection. Anal. Chim. Acta 2010, 674, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Popov, I.N.; Lewin, G. Photochemiluminescent detection of antiradical activity; IV: Testing of lipid-soluble antioxidant. J. Biochem. Biophys. Methods 1996, 31, 1–8. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Nicoli, S.F.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the polysaccharide-rich grape cell wall changes during winemaking using combined high-throughput and fractionation methods. Carbohydr. Polym. 2015, 133, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahyono, T.; Astuti, D.A.; Wiryawan, K.G.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in The Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Bosso, A.; Cassino, C.; Motta, S.; Panero, L.; Tsolakis, C.; Guaita, M. Polyphenolic Composition and In Vitro Antioxidant Activity of Red Grape Seeds as Byproducts of Short and Medium-Long Fermentative Macerations. Foods 2020, 9, 1451. [Google Scholar] [CrossRef]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Rajesh Kumar, S.; Thomas, S.; Kalarikkal, N. Grape seed extract-soluplus dispersion and its antioxidant activity. Drug Dev. Ind. Pharm. 2020, 46, 1219–1229. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef]
- Babiker, E.E.; Uslu, N.; Juhaimi, F.A.; Ahmed, I.A.M.; Ghafoor, K.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 2021, 139, 110537. [Google Scholar] [CrossRef]
- Ertaş, N.; Aslan, M. Antioxidant and physicochemical properties of cookies containing raw and roasted hemo flour. Acta Sci. Pol. Technol. Aliment. 2020, 19, 177–184. [Google Scholar] [PubMed]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, Ł.; Tabaszewska, M.; Wywrocka-Gurgul, A. Hemp flour as a valuable component for enriching physicochemical and antioxidant properties of wheat bread. LWT Food Sci. Technol. 2019, 102, 164–172. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F. Identifcation and quantifcation of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Burbano, J.J.; Correa, M.J. Composition and Physicochemical Characterization of Walnut Flour, a By-product of Oil Extraction. Plant Foods Hum. Nutr. 2021, 76, 233–239. [Google Scholar] [CrossRef]
- Santos, J.; Alvarez-Ortí, M.; Sena-Moreno, E. Efect of roasting conditions on the composition and antioxidant properties of defatted walnut four. J. Sci. Food Agric. 2018, 98, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Vuorela, S.; Meyer, A.S.; Heinonen, M. Impact of Isolation Method on the Antioxidant Activity of Rapeseed Meal Phenolics. J. Agric. Food Chem. 2004, 52, 8202–8207. [Google Scholar] [CrossRef]
- Yang, S.C.; Arasu, M.V.; Chun, J.H.; Jang, Y.S.; Lee, Y.H.; Kim, I.H.; Lee, K.T.; Hong, S.T.; Kim, S.J. Identification and Determination of Phenolic Compounds in Rapeseed Mealsm(Brassica napus L.). J. Agric. Chem. Environ. 2015, 4, 14–23. [Google Scholar]
- Žilić, S.; Maksimović Dragišić, J.; Maksimović, V.; Maksimović, M.; Basić, Z.; Crevar, M.; Stanković, G. The content of antioxidants in sunflower seed and kernel. Helia 2010, 33, 75–84. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre characteristics and antioxidant activity of sesame seed coats (Testae). Int. J. Food Prop. 2012, 15, 25–37. [Google Scholar] [CrossRef]
- Barthet, V.J.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant activity of flaxseed meal components. Can. J. Plant Sci. 2014, 94, 593602. [Google Scholar] [CrossRef]
- Stancheva, I.; Georgiev, G.; Geneva, M.; Ivanova, A.; Dolezal, M.; Tumova, L. Influence of foliar fertilization and growth regulator on milk thistle seed yield and quality. J. Plant Nutr. 2010, 33, 818–830. [Google Scholar] [CrossRef]
- Nilova, L.; Malyutenkova, S. The possibility of using powdered sea-buckthorn in the development of bakery products with antioxidant properties. Agron. Res. 2018, 16, 1444–1456. [Google Scholar]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can agronomic practices and cold-pressing extraction parameters affect phenols and polyphenols content in hempseed oils? Ind. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Khalil, A.A.; Khan, M.R.; Shabbir, M.A. In vitro antioxidant activity and punicalagin content quantification of pomegranate peel obtained as agro-waste after juice extraction. Pak. J. Agric. Sci. 2018, 55, 197–201. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Tița, O.; Lengyel, E.; Stegarus, D.I.; Savescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tița, M.A.; Rața, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye, C., Jr.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, C884–C890. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, I.; Cosmulescu, S.; Nour, V. Phenolic Profile and Antioxidant Capacity of Walnut Extract as Inflluenced by the Extraction Method and Solvent. Int. J. Food Eng. 2017, 13, 20150284. [Google Scholar] [CrossRef]
- Bakkalbasi, E.; Meral, R.; Dogan, I.S. Bioactive compounds, physical and sensory properties of cake made with walnut press-cake. J Food Qual. 2015, 38, 422–430. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Sabir, M.A.; Sosulski, F.W.; Kernan, J.A. Phenolic constituents in sunflower flour. J. Agric. Food Chem. 1974, 22, 572–574. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Grasso, S.; Omoarukhe, E.; Wen, X.; Papoutsis, K.; Methven, L. The Use of Upcycled Defatted Sunflower Seed Flour as a Functional Ingredient in Biscuits. Foods 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziedzic, S.Z.; Hudson, B.J.F. Polyhydroxy chalcones and flavanones as antioxidants for edible oils. Food Chem. 1983, 12, 205–212. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Birasuren, B.; Kim, N.Y.; Jeon, H.L.; Kim, M.R. Evaluation of the antioxidant capacity and phenolic content of Agriophyllum pungens seed extracts from Mongolia. Prev. Nutr. Food Sci. 2013, 18, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chainbreaking antioxidant activity in food. Food Chem. 2005, 92, 235. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamini, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy Hig. Med. Dosw. 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Arranz, S.; Perez-Jimenez, J.; Saura-Calixto, F. Antioxidant capacity of walnut (Juglans regia L.): Contribution of oil and defatted matter. Eur. Food Res. Technol. 2008, 227, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szalkiewicz, M. Composition of Phenolic Acids in Sea Buckthorn (Hippophae rhamnoides L.) Berries. JAOCS 2005, 82, 175–179. [Google Scholar] [CrossRef]
- Gougoulias, N.; Mashev, N. Evaluation of polyphenols antioxidant activity of grape seeds (V. vinifera). Oxid. Commun. 2008, 31, 88–97. [Google Scholar]
- Gvozdenac, S.M.; Prvulović, D.M.; Radovanović, M.N.; Ovuka, J.S.; Miklič, V.J.; Ačanski, J.M.; Tanasković, S.T.; Vukajlović, F.N. Life history of Plodia interpunctella Hübner on sunflower seeds: Effects of seed qualitative traits and the initial seed damage. J. Stored Prod. Res. 2018, 79, 89–97. [Google Scholar] [CrossRef]
- Bogoeva, A.L.; Durakova, A.G. Sorption characteristics of full-fatted grape seeds flour of Bulgarian origin. J. Agric. Food Res. 2020, 2, 100026. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci. Tech. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An integral approach to evaluate Food Antioxidant Capacity. J. Food Sci. 2007, 72, 159–165. [Google Scholar] [CrossRef]
- Senila, L.; Neag, E.; Cadar, O.; Haydee Kovacs, M.; Becze, A.; Senila, M. Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Appl. Sci. 2020, 10, 1589. [Google Scholar] [CrossRef] [Green Version]
- Sielicka, M.; Małecka, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Prevc, T.; Šegatin, N.; Ulrih, N.P.; Cigic, B. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta 2013, 109, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]





| Sample Name | Total Phenolic Content | Total Flavonoid Content |
|---|---|---|
| (Mg GAE/g) | (Mg QE/g) | |
| Sea buckthorn flour | 11.52 ± 0.50 | 9.28 ± 0.06 |
| Hemp flour | 4.68 ± 0.13 | 4.12 ± 0.07 |
| Walnut flour | 13.75 ± 0.24 | 7.57 ± 0.09 |
| Grape seed flour | 74.85 ± 0.43 | 51.24 ± 1.46 |
| Rapeseed meals | 11.24 ± 0.65 | 9.46 ± 0.50 |
| Sunflower meals | 11.70 ± 0.35 | 7.92 ± 0.14 |
| Black sesame meals | 3.88 ± 0.20 | nd |
| Red grape seed meals | 6.84 ± 0.67 | nd |
| Golden flax meals | 3.80 ± 0.28 | nd |
| Thistle meals | 7.97 ± 0.48 | nd |
| Sesame groats | 4.31 ± 0.32 | 1.41 ± 0.25 |
| Thistle groats | 8.39 ± 0.19 | 4.99 ± 0.37 |
| Coriander groats | 1.73 ± 0.09 | nd |
| Sunflower groats | 1.54 ± 0.03 | nd |
| Sample Name | DPPH | ABTS | FRAP | CUPRAC |
|---|---|---|---|---|
| Sea buckthorn flour | 394.17 ± 1.50 | 419.46 ± 2.45 | 547.45 ± 13.14 | 503.43 ± 14.52 |
| Hemp flour | 139.59 ± 1.17 | 113.73 ± 13.47 | 285.81 ± 17.23 | 231.94 ± 18.74 |
| Walnut flour | 1257.49 ± 3.85 | 1423.98 ± 24.57 | 913.44 ± 15.19 | 1202.75 ± 23.99 |
| Grape seed flour | 7182.53 ± 6.12 | 3500.52 ± 66.45 | 4716.75 ± 131.88 | 5936.76 ± 96.42 |
| Rapeseed meals | 647.29 ± 1.36 | 406.55 ± 6.61 | 1034.92 ± 39.63 | 478.43 ± 30.88 |
| Sunflower meals | 628.58 ± 3.85 | 347.01 ± 20.97 | 1350.86 ± 72.20 | 510.49 ± 35.22 |
| Black sesame meals | 17.73 ± 1.04 | 48.81 ± 2.68 | 42.69 ± 2.75 | 63.31 ± 1.09 |
| Red grape seed meals | 200.77 ± 1.08 | 322.76 ± 27.81 | 119.92 ± 8.06 | 119.99 ± 5.54 |
| Golden flax meals | 9.25 ± 0.68 | 12.13 ± 0.61 | 61.54 ± 4.98 | 75.50 ± 8.02 |
| Thistle meals | 85.58 ± 3.29 | 292.81 ± 13.59 | 84.89 ± 6.18 | 125.75 ± 4.55 |
| Sesame groats | 7.58 ± 1.30 | 15.77 ± 5.05 | 66.21 ± 3.45 | 62.45 ± 2.38 |
| Thistle groats | 22.74 ± 1.74 | 293.14 ± 34.32 | 105.31 ± 12.41 | 112.54 ± 2.92 |
| Coriander groats | 17.64 ± 0.61 | 9.37 ± 2.22 | 26.47 ± 2.78 | 67.53 ± 0.95 |
| Sunflower groats | 55.06 ± 2.64 | nd | 34.46 ± 1.51 | 70.47 ± 2.96 |
| DPPH | ABTS | FRAP | CUPRAC | |
|---|---|---|---|---|
| TPC | 0.9927 | 0.9660 | 0.9752 | 0.9920 |
| TFC | 0.9811 | 0.9477 | 0.9825 | 0.9815 |
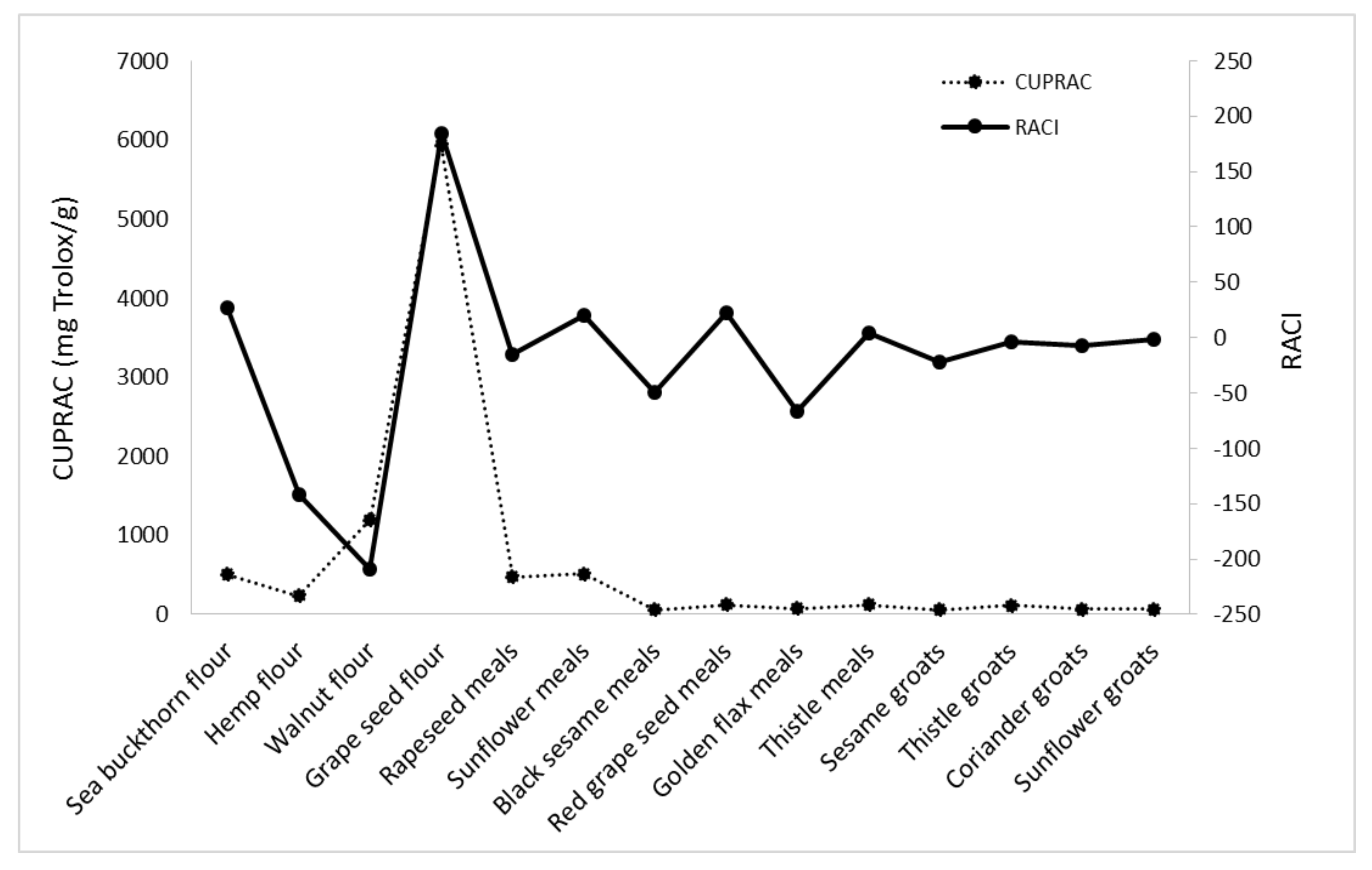
| Sample Name | DPPH | ABTS | FRAP | CUPRAC | RACI |
|---|---|---|---|---|---|
| Sea buckthorn flour | 43.15 | 36.74 | 16.59 | 11.98 | 27.12 |
| Hemp flour | −475.64 | −43.23 | −23.81 | −24.77 | −141.86 |
| Walnut flour | −521.89 | −75.00 | −154.93 | −86.04 | −209.46 |
| Grape seed flour | 685.39 | 7.71 | 13.11 | 30.58 | 184.20 |
| Rapeseed meal | −20.75 | −40.69 | 9.07 | −6.38 | −14.69 |
| Sunflower meal | 65.56 | −1.39 | 13.50 | 3.81 | 20.37 |
| Black sesame meal | −95.45 | −25.44 | −27.02 | −49.25 | −49.29 |
| Red grape seed meal | 79.20 | 7.46 | 0.58 | 0.86 | 22.03 |
| Golden flax meal | −123.80 | −133.28 | −6.40 | −2.24 | −66.43 |
| Thistle meal | −2.14 | 14.73 | −1.25 | 7.28 | 4.65 |
| Sesame groats | −60.11 | −13.85 | −5.65 | −9.78 | −22.35 |
| Thistle groats | −33.97 | 6.16 | 1.89 | 10.51 | −3.85 |
| Coriander groats | −36.89 | −13.86 | −4.92 | 28.83 | −6.71 |
| Sunflower groats | 0.66 | 0.00 | −12.50 | 5.79 | −1.51 |
| Method | DPPH | ABTS | FRAP | CUPRAC |
|---|---|---|---|---|
| ACL | 0.9952 | 0.9735 | 0.9874 | 0.9930 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. https://doi.org/10.3390/foods11030253
Multescu M, Marinas IC, Susman IE, Belc N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods. 2022; 11(3):253. https://doi.org/10.3390/foods11030253
Chicago/Turabian StyleMultescu, Mihaela, Ioana Cristina Marinas, Iulia Elena Susman, and Nastasia Belc. 2022. "Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants" Foods 11, no. 3: 253. https://doi.org/10.3390/foods11030253
APA StyleMultescu, M., Marinas, I. C., Susman, I. E., & Belc, N. (2022). Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods, 11(3), 253. https://doi.org/10.3390/foods11030253






