1H and 15N NMR Analyses on Heparin, Heparan Sulfates and Related Monosaccharides Concerning the Chemical Exchange Regime of the N-Sulfo-Glucosamine Sulfamate Proton
Abstract
:1. Introduction
2. Results and Discussion
2.1. Recognition of GlcNAc-Linked Uronic Acid Types in Heparin and Heparan Sulfates via 1H-15N HSQC Spectra
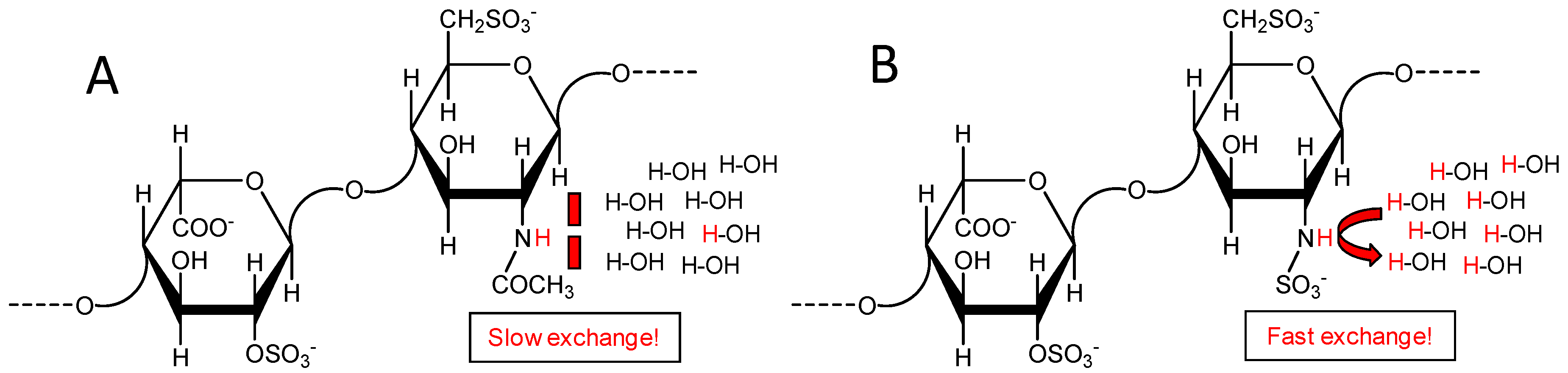
2.2. Fast Exchange Nature of the Sulfamate Proton in GlcNS from Heparin and Heparan Makes Difficult Its Signal Observation through 1H-15N HSQC Spectra at Regular Experimental Conditions
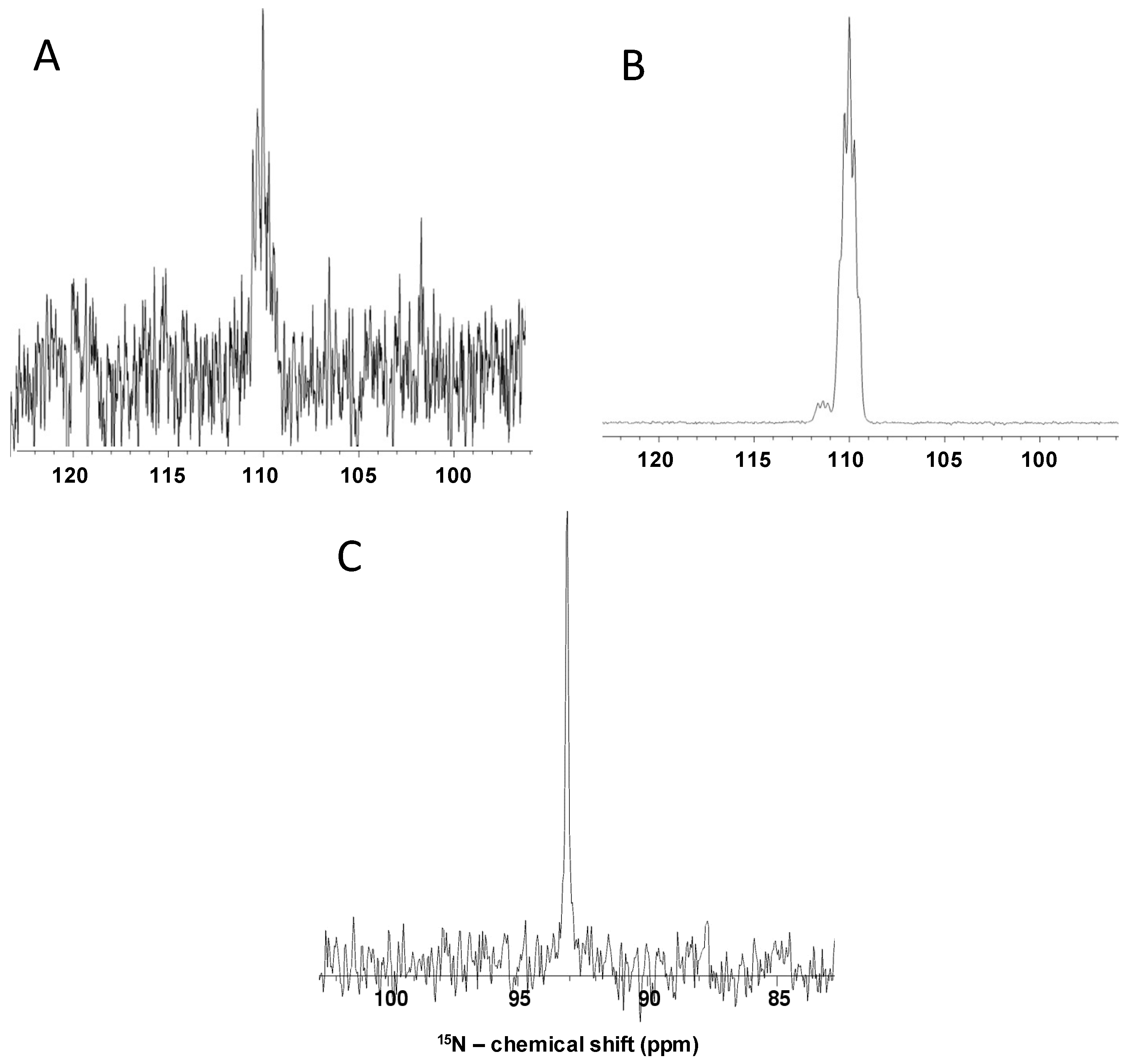
2.3. Non-polarized and Hyperpolarized 1D 15N and 1H Direct-observe of 15N-Gln, GlcNS and Other Monosaccharides
2.4. Kinetic of the α-β Mutarotation Monitored through Anomeric 1H Resonances
2.5. Reducing the Fast Exchange Rates of the GlcNHSO3− to Enable Proper NMR Detection
2.6. Fast Chemical Exchange of GlcNHSO3− and Validation of the Low-Temperature-and-Proton-Rich-Solution Condition for NMR Detection as Seen by 1H Coupling
3. Materials and Methods
3.1. General Materials
3.2. NMR Experiments, Instrumentation and Related Reagents
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of interest
References
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Rabestein, D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Advances in glycosaminoglycanomics by 15N-NMR spectroscopy. Anal. Bioanal. Chem. 2013, 405, 3035–3048. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Sharp, J.S.; Li, X.; Wang, L.; Prestegard, J.H. Characterization of glycosaminoglycans by 15N NMR spectroscopy and in vivo isotopic labeling. Anal. Chem. 2010, 82, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. NMR chemical shifts in structural biology of glycosaminoglycans. Anal. Chem. 2014, 86, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, D.J.; Young, R.P.; Beni, S.; Beecher, C.N.; Mueller, L.J.; Larive, C.K. Sulfamate proton solvent exchange in heparin oligosaccharides: Evidence for a persistent hydrogen bond in the antithrombin-binding pentasaccharide Arixtra. Glycobiology 2012, 22, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Beecher, C.N.; Young, R.P.; Langeslay, D.J.; Mueller, L.J.; Larive, C.K. Hydroxyl-proton hydrogen bonding in the heparin oligosaccharide Arixtra in aqueous solution. J. Phys. Chem. B 2014, 118, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Mobli, M.; Nilsson, M.; Almond, A. The structural plasticity of heparan sulfate NA-domains and hence their role in mediating multivalent interactions is confirmed by high-accuracy 15N-NMR relaxation studies. Glycoconj. J. 2008, 25, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Almond, A.; DeAngelis, P.L.; Blundell, C.D. Dynamics of hyaluronan oligosaccharides revealed by 15N relaxation. J. Am. Chem. Soc. 2005, 127, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.D.; Deangelis, P.L.; Almond, A. Hyaluronan: the absence of amide-carboxylate hydrogen bonds and the chain conformation in aqueous solution are incompatible with stable secondary and tertiary structure models. Biochem. J. 2006, 396, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Almond, A.; Deangelis, P.L.; Blundell, C.D. Hyaluronan: the local solution conformation determined by NMR and computer modeling is close to a contracted left-handed 4-fold helix. J. Mol. Biol. 2006, 358, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Mobli, M.; Almond, A. N-Acetylated amino sugars: the dependence of NMR 3J(HNH2)-couplings on conformation, dynamics and solvent. Org. Biomol. Chem. 2007, 5, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.D.; Almond, A. Temperature dependencies of amide 1H- and 15N-chemical shifts in hyaluronan oligosaccharides. Magn. Reson. Chem. 2007, 45, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Blundell, C.D.; DeAngelis, P.L.; Day, A.J.; Almond, A. Use of 15N-NMR to resolve molecular details in isotopically-enriched carbohydrates: sequence-specific observations in hyaluronan oligomers up to decasaccharides. Glycobiology 2004, 14, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; McCallum, S.A.; Xie, J.; Nieto, L.; Corzana, F.; Jiménez-Barbero, J.; Chen, M.; Liu, J.; Linhardt, R.J. Solution structures of chemoenzymatically synthesized heparin and its precursors. J. Am. Chem. Soc. 2008, 130, 12998–13007. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, B.M.; Shakeri, J.; Roberts, I.S.; Almond, A. A 3D-structural model of unsulfated chondroitin from high-field NMR: 4-Sulfation has little effect on backbone conformation. Carbohydr. Res. 2010, 345, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Limtiaco, J.F.; Langeslay, D.J.; Beni, S.; Larive, C.K. Getting to know the nitrogen next door: HNMBC measurements of amino sugars. J. Magn. Reson. 2011, 209, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, D.J.; Beni, S.; Larive, C.K. Detection of the 1H and 15N NMR resonances of sulfamate groups in aqueous solution: A new tool for heparin and heparan sulfate characterization. Anal. Chem. 2011, 83, 8006–8010. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, D.J.; Beni, S.; Larive, C.K. A closer look at the nitrogen next door: 1H-15N NMR methods for glycosaminoglycan structural characterization. J. Magn. Reson. 2012, 216, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Langeslay, D.J.; Beecher, C.N.; Naggi, A.; Guerrini, M.; Torri, G.; Larive, C.K. Characterizing the microstructure of heparin and heparan sulfate using N-sulfoglucosamine 1H and 15N NMR chemical shift analysis. Anal. Chem. 2013, 85, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Beecher, C.N.; Larive, C.K. Methods for measuring exchangeable protons in glycosaminoglycans. Methods Mol. Biol. 2015, 1229, 173–187. [Google Scholar] [PubMed]
- Beecher, C.N.; Larive, C.K. 1H and 15N NMR characterization of the amine groups of heparan sulfate related glucosamine monosaccharides in aqueous solution. Anal. Chem. 2015, 87, 6842–6848. [Google Scholar] [CrossRef] [PubMed]
- Beecher, C.N.; Manighalam, M.S.; Nwachuku, A.F.; Larive, C.K. Screening enoxaparin tetrasaccharide SEC fractions for 3-O-sulfo-N-sulfoglucosamine residues using [1H,15N] HSQC NMR. Anal. Bioanal. Chem. 2016, 408, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Kimata, K.; Lindahl, U. Proteoglycans and sulfated glycosaminoglycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2009; Chapter 16; pp. 243–244. [Google Scholar]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavão, M.S. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef] [PubMed]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomin, V.H. 1H and 15N NMR Analyses on Heparin, Heparan Sulfates and Related Monosaccharides Concerning the Chemical Exchange Regime of the N-Sulfo-Glucosamine Sulfamate Proton. Pharmaceuticals 2016, 9, 58. https://doi.org/10.3390/ph9030058
Pomin VH. 1H and 15N NMR Analyses on Heparin, Heparan Sulfates and Related Monosaccharides Concerning the Chemical Exchange Regime of the N-Sulfo-Glucosamine Sulfamate Proton. Pharmaceuticals. 2016; 9(3):58. https://doi.org/10.3390/ph9030058
Chicago/Turabian StylePomin, Vitor H. 2016. "1H and 15N NMR Analyses on Heparin, Heparan Sulfates and Related Monosaccharides Concerning the Chemical Exchange Regime of the N-Sulfo-Glucosamine Sulfamate Proton" Pharmaceuticals 9, no. 3: 58. https://doi.org/10.3390/ph9030058
APA StylePomin, V. H. (2016). 1H and 15N NMR Analyses on Heparin, Heparan Sulfates and Related Monosaccharides Concerning the Chemical Exchange Regime of the N-Sulfo-Glucosamine Sulfamate Proton. Pharmaceuticals, 9(3), 58. https://doi.org/10.3390/ph9030058





