Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections
Abstract
:1. Introduction
2. Results
2.1. Susceptibility of Tetracycline-Resistant E. faecalis to GS
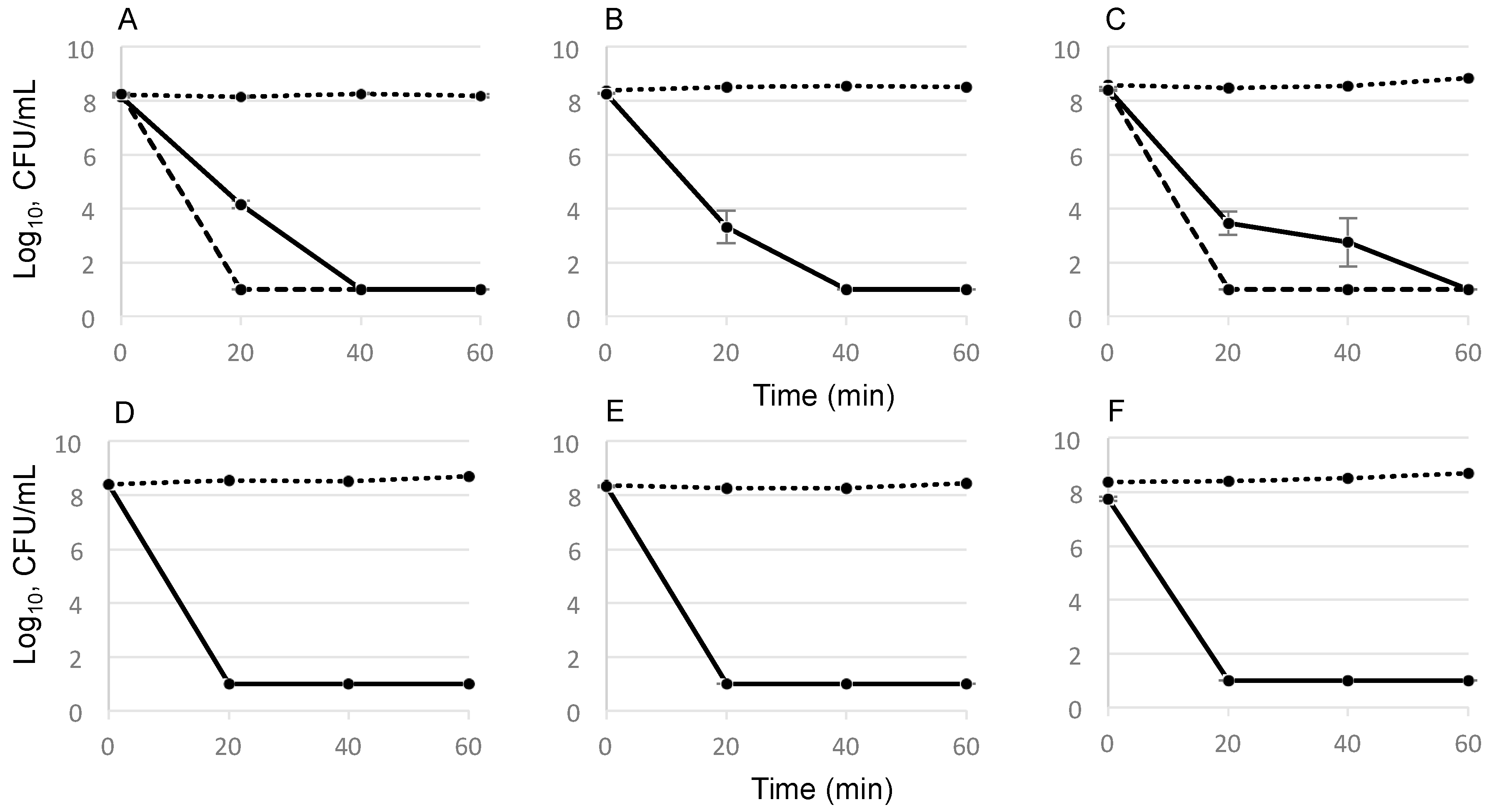
2.2. Time-Dependent Killing Effect of GS
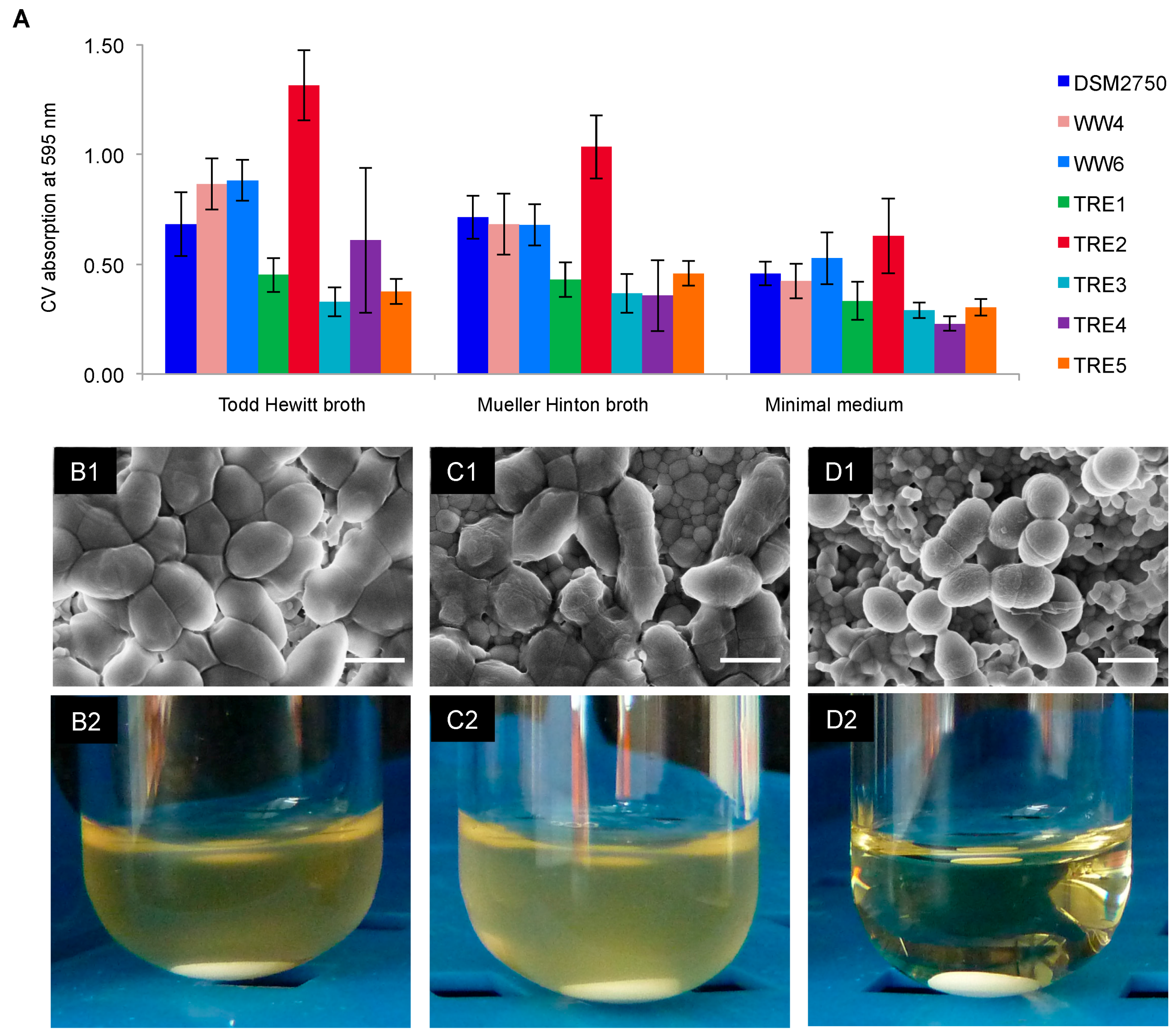
2.3. Biofilm-Killing Effect of GS
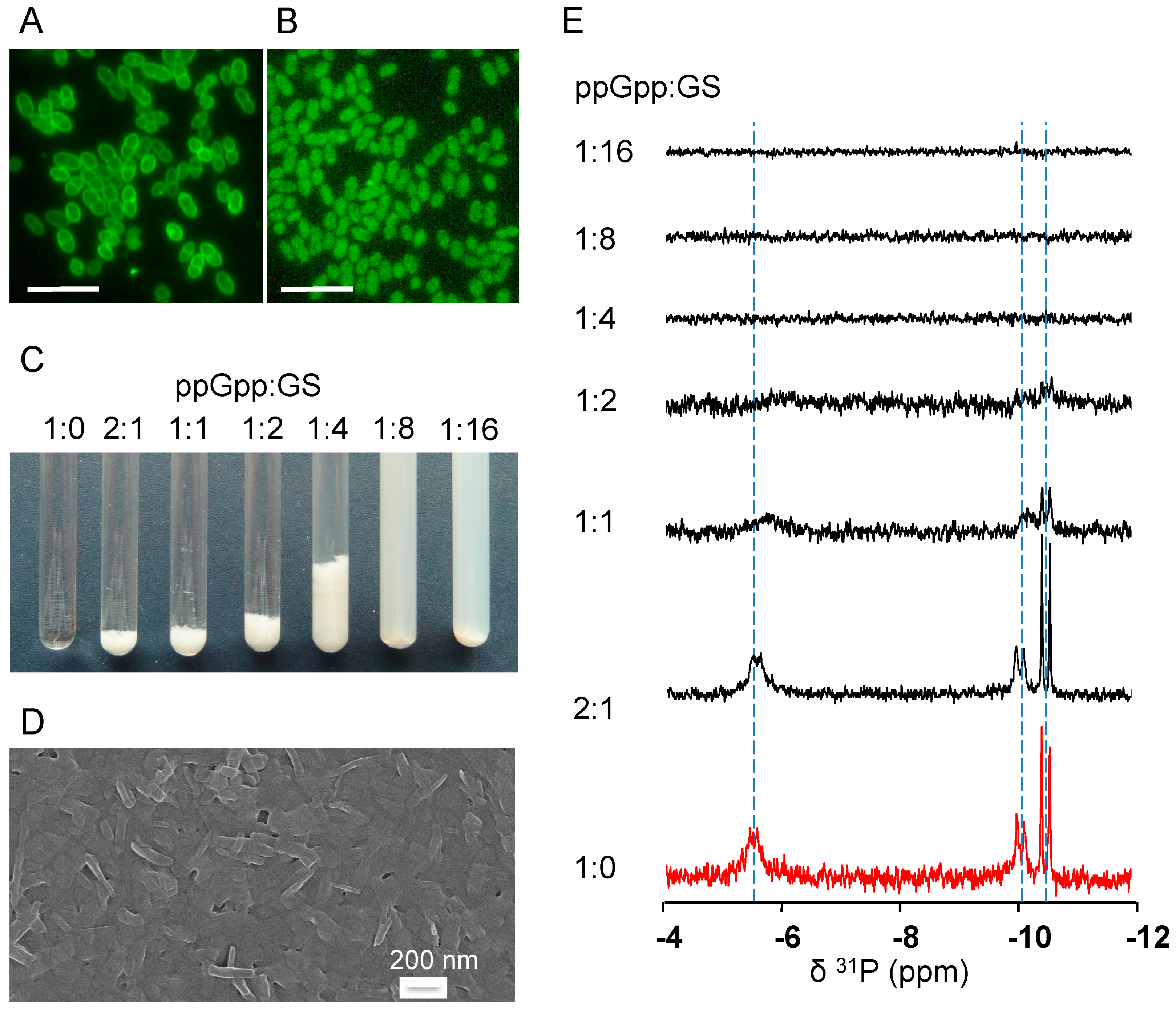
2.4. GS Penetration into the Bacterial Cells and Binding Affinity to Nucleotides
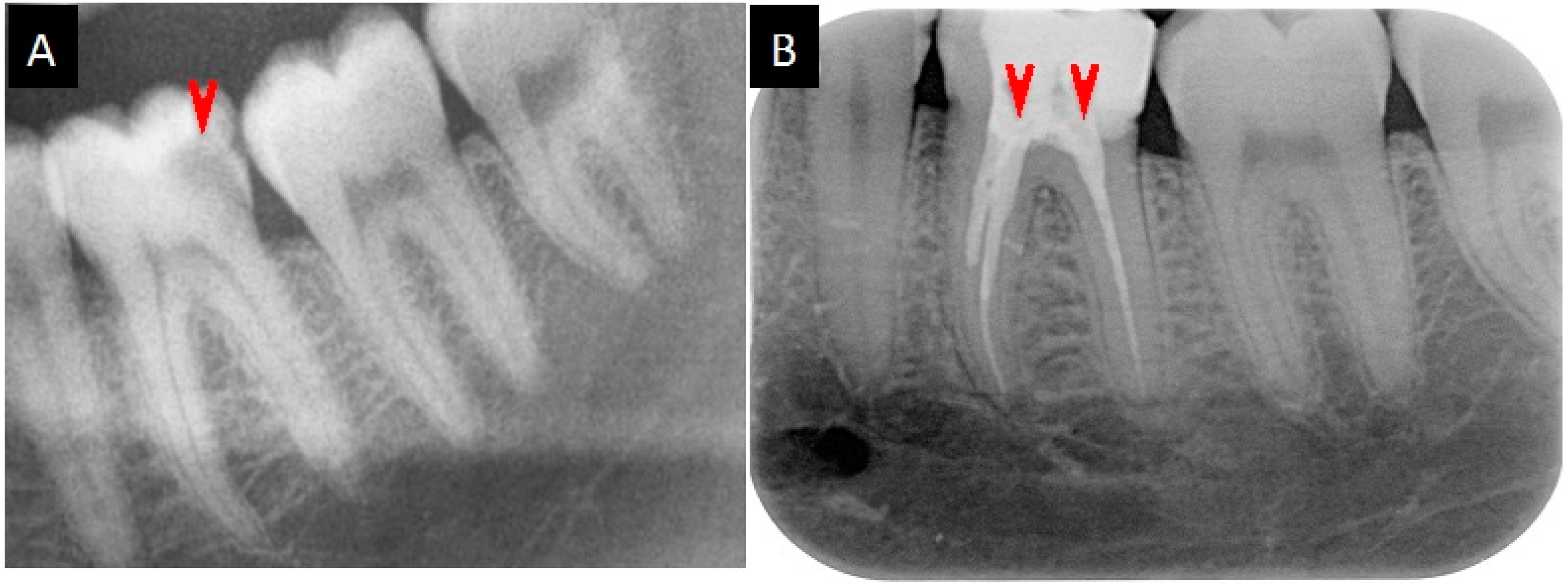
2.5. Medication Reports
3. Discussion
4. Materials and Methods
4.1. E. faecalis Strains, Antibiotics and the Determination of the Minimum Inhibitory Concentrations
4.2. Determination of the Minimum Bactericidal Concentration (MBC)
4.3. Determination of the Minimum Biofilm Inhibitory Concentration (MBIC)
4.4. Determination of the Biofilm-Forming Capacity of the E. faecalis Strains
4.5. Determination of GS Killing Activity
4.6. Biofilms on HAD: Scanning Electron Microscopy and Re-Growth
4.7. Fluorescence Microscopy
4.8. 31P-NMR Spectroscopy of GS with Nucleotides in An Aqueous Environment
4.9. Medication of the Root Canal Infections
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GS | gramicidin S |
| PMB | polymyxin B |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| MBIC90 | minimum biofilm inhibitory concentration, 90% inhibition |
| MBBC | minimal biofilm bactericidal concentration |
| TRE | tetracycline resistant enterococci |
| CFU/mL | colony forming units per mL |
| OD | optical density |
| SEM | scanning electron microscopy |
| CFSE | 5(6)-carboxy-fluorescein-N-hydroxysuccinimide ester |
| HAD | hydroxyapatite disc |
| NMR | nuclear magnet resonance analysis |
| MH | Mueller Hinton broth |
| TH | Todd Hewitt broth |
| BHI | brain heart infusion broth |
| MM | minimal medium |
| SPB | sodium phosphate buffer |
References
- Tronstad, L. Recent development in endodontic research. Eur. J. Oral Sci. 1992, 100, 52–59. [Google Scholar] [CrossRef]
- Figdor, D.; Sundqvist, G. A big role for the very small—understanding the endodontic microbial flora. Aust. Dent. J. 2007, 52, S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Livermore, D.M.; Nikaido, H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: Resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob. Agents Chemother. 1994, 38, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Zhang, C.F.; Chu, C.H.; Zhu, X.F. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int. J. Oral Sci. 2012, 4, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Portenier, I.; Waltimo, T.M.T.; Haapasalo, M. Enterococcus faecalis—The root canal survivor and "star" in post-treatment disease. Endod. Topics 2003, 6, 135–159. [Google Scholar] [CrossRef]
- Vidana, R.; Sullivan, A.; Billstrom, H.; Ahlquist, M.; Lund, B. Enterococcus faecalis infection in root canals—Host-derived or exogenous source? Lett. Appl. Microbiol. 2011, 52, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Molander, A.; Warfvinge, J.; Reit, C.; Kvist, T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J. Endod. 2007, 33, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Wang, J.; Jiang, W.; Zhu, C.; Liang, J. Assessment of dentinal tubule invasion capacity of Enterococcus faecalis under stress conditions ex vivo. Int. Endontic. J. 2015, 48, 362–372. [Google Scholar] [CrossRef] [PubMed]
- De Paz, L.C. Redefining the persistent infection in root canals: Possible role of biofilm communities. J. Endod. 2007, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ng, Y.L.; Gulabivala, K.; Moles, D.R.; Spratt, D.A. Susceptibilties of two Enterococcus faecalis phenotypes to root canal medications. J. Endod. 2005, 31, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Dunavant, T.R.; Regan, J.D.; Glickman, G.N.; Solomon, E.S.; Honeyman, A.L. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J. Endod. 2006, 32, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Sydney, G.B.; Figueiredo, J.A.; Estrela, C.R. Antibacterial efficacy of intracanal medicaments on bacterial biofilm: A critical review. J. Appl. Oral Sci. 2009, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.L.; Willems, R.J.; Leavis, H.L. Optimizing future treatment of enterococcal infections: Attacking the biofilm? Trends Microbiol. 2012, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lins, R.X.; de Oliveira Andrade, A.; Junior, R.H.; Lewis, M.A.O.; Wilson, M.J.; Fidel, R.A.S. Antimicrobial resistance and virulence traits of Enterococcus faecalis from primary endodontic infections. J. Dent. 2013, 41, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fourth Informational Supplement. Wayne, PA, USA, 2014; 76–79. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Babii, O.; Afonin, S.; Berditsch, M.; Reibetaer, S.; Mykhailiuk, P.K.; Kubyshkin, V.S.; Steinbrecher, T.; Ulrich, A.S.; Komarov, I.V. Controlling biological activity with light: Diarylethene-containing cyclic peptidomimetics. Angew. Chem. Int. Ed. Engl. 2014, 53, 3392–3395. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nunez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.M.; Chan, S.I. Complexation and phase transfer of nucleotides by gramicidin S. Biochemistry 1983, 22, 4280–4291. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.M.; Chan, S.I. Complexation and phase transfer of nucleic acids by gramicidin S. Biochemistry 1984, 23, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the treatment of infected wounds. Nature 1944, 154, 703. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S Origin and mode of action. Lancet 1944, 244, 715–716. [Google Scholar]
- Hoyer, K.M.; Mahlert, C.; Marahiel, M.A. The iterative gramicidin s thioesterase catalyzes peptide ligation and cyclization. Chem. Biol. 2007, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Katsu, T.; Ninomiya, C.; Kuroko, M.; Kobayashi, H.; Hirota, T.; Fujita, Y. Action mechanism of amphipathic peptides gramicidin S and melittin on erythrocyte membrane. Biochim. Biophys. Acta 1988, 939, 57–63. [Google Scholar] [CrossRef]
- Berditsch, M.; Afonin, S.; Ulrich, A.S. The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation. Appl. Environ. Microbiol. 2007, 73, 6620–6628. [Google Scholar] [CrossRef] [PubMed]
- Polin, A.N.; Egorov, N.S. Structural and functional characteristics of gramicidin S in connection with its antibiotic activity. Antibiot. Khimioter. 2003, 48, 29–32. [Google Scholar] [PubMed]
- Salgado, J.; Grage, S.L.; Kondejewski, L.H.; Hodges, R.S.; McElhaney, R.N.; Ulrich, A.S. Membrane-bound structure and alignment of the antimicrobial β-sheet peptide gramicidin S derived from angular and distance constraints by solid state 19F-NMR. J. Biomol. NMR 2001, 21, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rozek, A.; Hancock, R.E. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 2001, 276, 35714–35722. [Google Scholar] [CrossRef] [PubMed]
- Mogi, T.; Kita, K. Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell. Mol. Life Sci. 2009, 66, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nunez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S; Lewis, K.; Bertram, R. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. J. Mol. Microbiol. Biotechnol. 2012, 22, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Wade, W.G. A comparison of enterococcal genotypes from the human mouth and cheese. Int. Endontic. J. 2010, 43, 352–353. [Google Scholar] [CrossRef]
- Razavi, A.; Gmur, R.; Imfeld, T.; Zehnder, M. Recovery of Enterococcus faecalis from cheese in the oral cavity of healthy subjects. Oral Microbiol. Immunol. 2007, 22, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Berditsch, M.; Jager, T.; Strempel, N.; Schwartz, T.; Overhage, J.; Ulrich, A.S. Synergistic effect of membrane-active peptides polymyxin B and gramicidin S on multidrug-resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Sergiev, P.G. Clinical use of Gramicidin S. Lancet 1944, 244, 717–718. [Google Scholar] [CrossRef]
- Lapage, G. Gramicidin-S. Nature 1945, 155, 246–246. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Yip, J.W.; Chang, J.W.; Zhang, C.F.; Samaranayake, L.P. Effect of culture media and nutrients on biofilm growth kinetics of laboratory and clinical strains of Enterococcus faecalis. Arch. Oral Biol. 2013, 58, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.A.; Saunders, W.P. Root canal treatment and general health: A review of the literature. Int. Endontic. J. 2000, 33, 1–18. [Google Scholar] [CrossRef]
- Amsterdam, D. Susceptibility testing of antimicrobials in liquid media. Antibiot. Laboratory Med. 1996, 4, 61–143. [Google Scholar]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
| E. faecalis Strain | Resistance/Susceptibility (μg/mL) to | Antimicrobial Activity of GS (μg/mL) | |||
|---|---|---|---|---|---|
| Tetracycline | Demeclocycline | ||||
| MIC a | MIC | MIC | MBC | MBIC90 | |
| DSM 2570 | 16 | 8 | 8 | 8 | 4–8 |
| TRE1 | 16 | 16 | 8 | 16 | - |
| TRE2 | 16 | 32 | 16 | 16 | 8–16 |
| TRE4 | 16 | 8 | 8 | 8 | - |
| TRE5 | 16 | 16 | 8 | 16 | - |
| WW4 | 64 | 16 | 8 | 16 | - |
| WW6 | <1 | <1 | 8 | 16 | 8–16 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berditsch, M.; Lux, H.; Babii, O.; Afonin, S.; Ulrich, A.S. Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections. Pharmaceuticals 2016, 9, 56. https://doi.org/10.3390/ph9030056
Berditsch M, Lux H, Babii O, Afonin S, Ulrich AS. Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections. Pharmaceuticals. 2016; 9(3):56. https://doi.org/10.3390/ph9030056
Chicago/Turabian StyleBerditsch, Marina, Hannah Lux, Oleg Babii, Sergii Afonin, and Anne S. Ulrich. 2016. "Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections" Pharmaceuticals 9, no. 3: 56. https://doi.org/10.3390/ph9030056
APA StyleBerditsch, M., Lux, H., Babii, O., Afonin, S., & Ulrich, A. S. (2016). Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections. Pharmaceuticals, 9(3), 56. https://doi.org/10.3390/ph9030056






