Antimicrobial Peptides from Plants
Abstract
:1. Introduction
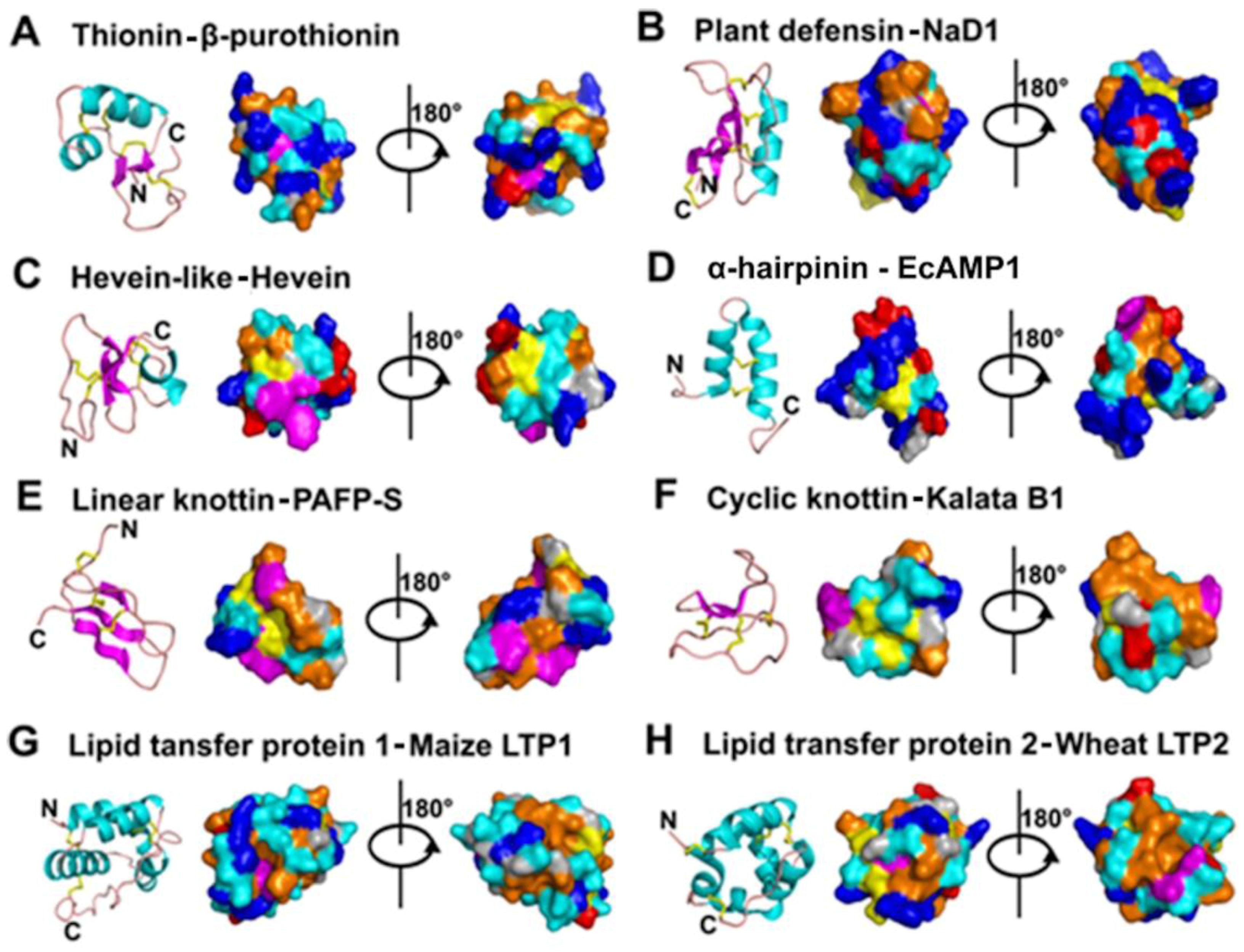
2. Classification and Characteristics
- Mostly characterized as moderate-size (MW of 2–6 kDa), basic, CRPs with two to six intra-molecular disulfide bonds.
- Members within a family are classified based on Cys motif, sequence similarity and are conserved in secondary and tertiary structure.
- One or two additional disulfide bonds are found in members of thionins, defensins, and hevein-like peptides. These additional bonds bolster structural stability without affecting the general scaffold. Because the varying number of Cys residues can create confusion, we refer to AMPs within a family based on the number of Cys when necessary throughout this review (e.g., 6C-thionins contain six Cys and 8C-thionins have eight Cys).
- In addition to being antimicrobial, AMPs also display “peptide promiscuity”, which refers to the multiple functions displayed by a single peptide.
- All are ribosomally derived and bioprocessed from precursors, which often contain three domains: N- and C-terminal pro-domains and a mature AMP domain. Mature sequences are often hypervariable and display more variation than the conserved terminal domains in the preproprotein to give sequence diversity for adaptation.
- Because of cross-bracing by multiple disulfide bonds, most CRP-AMPs with a molecular weight (MW) of 2–6 kDa are structurally compact with high thermal, chemical, and enzymatic stability.
| Peptide | S-S No. | Representative Member | Structural Motif | ||
|---|---|---|---|---|---|
| Name | AA No. | Disulfide Motif | |||
| 6C-Thionin | 3 | Crambin | 46 |  | Gamma (Г) fold β1-α1-α2-β2-coil motif |
| 8C-Thionin | 4 | β-Purothionin | 45 | ||
| 8C-Defensin | 4 | NaD1 | 47 | CSαβ motif β1-coil-α-β2-β3 | |
| 10C-Defensin | 5 | PhD1 | 47 | ||
| 6C-Hevein | 3 | Ac-AMP1 | 29 | Gly & Cys rich Central β strands & (short helical) side coils | |
| 8C-Hevein | 4 | Hevein | 43 | ||
| 10C-Hevein | 5 | EAFP1 | 41 | ||
| Knottin | 3 | PAFP-S | 38 | Cystine knot Short β strand & coil | |
| Cyclic Knottin | 3 | Kalata B1 | 29 | ||
| α-Hairpinin | 2 | Ec-AMP1 | 37 | α1-turn-α2 | |
| LTP | 4 | Maize LTP1 | 93 | Hydrophobic cavity α1-α2-α3-α4-coil | |
| LTP | 4 | Wheat LTP2 | 67 | ||
| Snakin * | 6 | Snakin-1 | 63 | α -helices | |
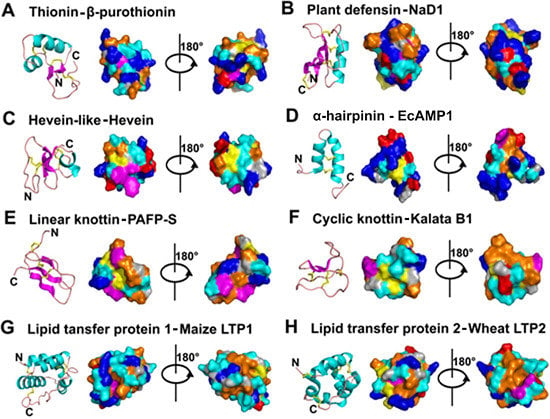
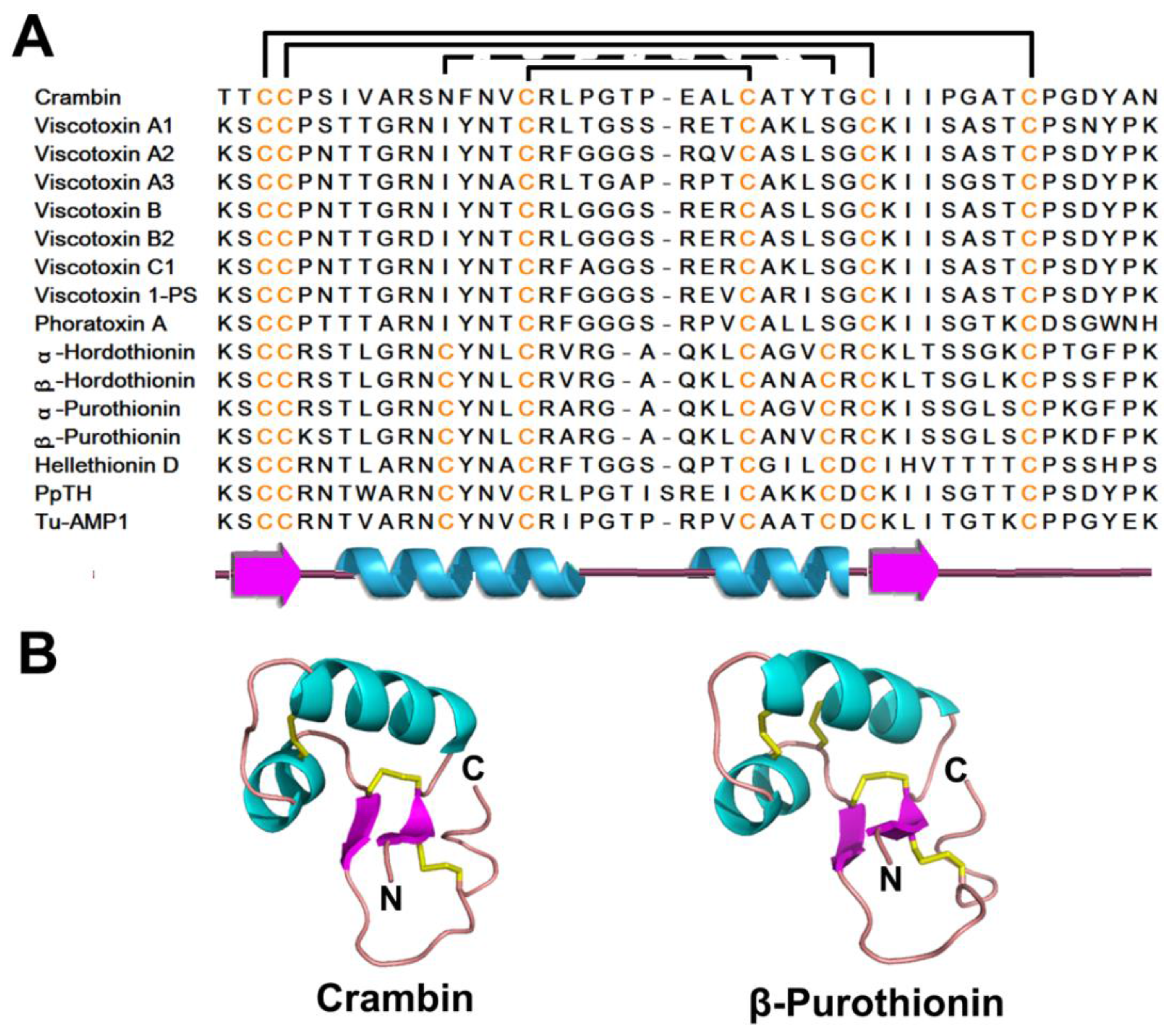
2.1. Thionins
2.1.1. Occurrences, Distribution, and Biosynthesis
2.1.2. Structure
2.1.3. Structure-Function Relationship
2.1.4. Mechanism of Action
2.2. Plant Defensins
2.2.1. Occurrences, Distribution, and Biosynthesis
2.2.2. Structures

2.2.3. Structure-Function Relationship
2.2.4. Mechanism of Action
2.3. Hevein-Like Peptides
2.3.1. Occurrences, Distribution and Biosynthesis
2.3.2. Structure
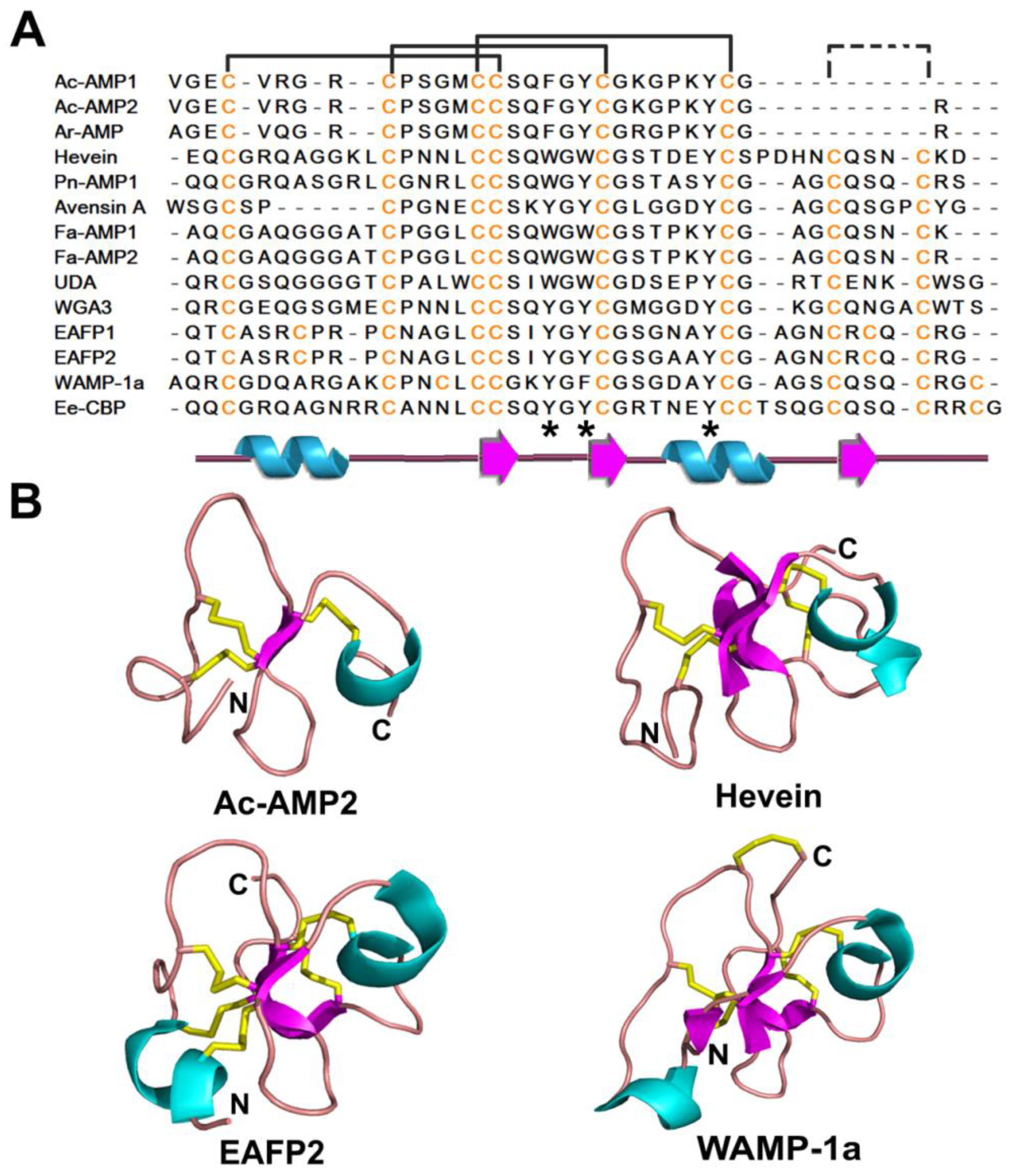
2.3.3. Structure-Function Study
2.3.4. Mechanism of Action
2.4. Knottin-Type Peptides
2.4.1. Occurrences, Distribution, and Biosynthesis
2.4.2. Structure
2.4.3. Structure-Function Relationship
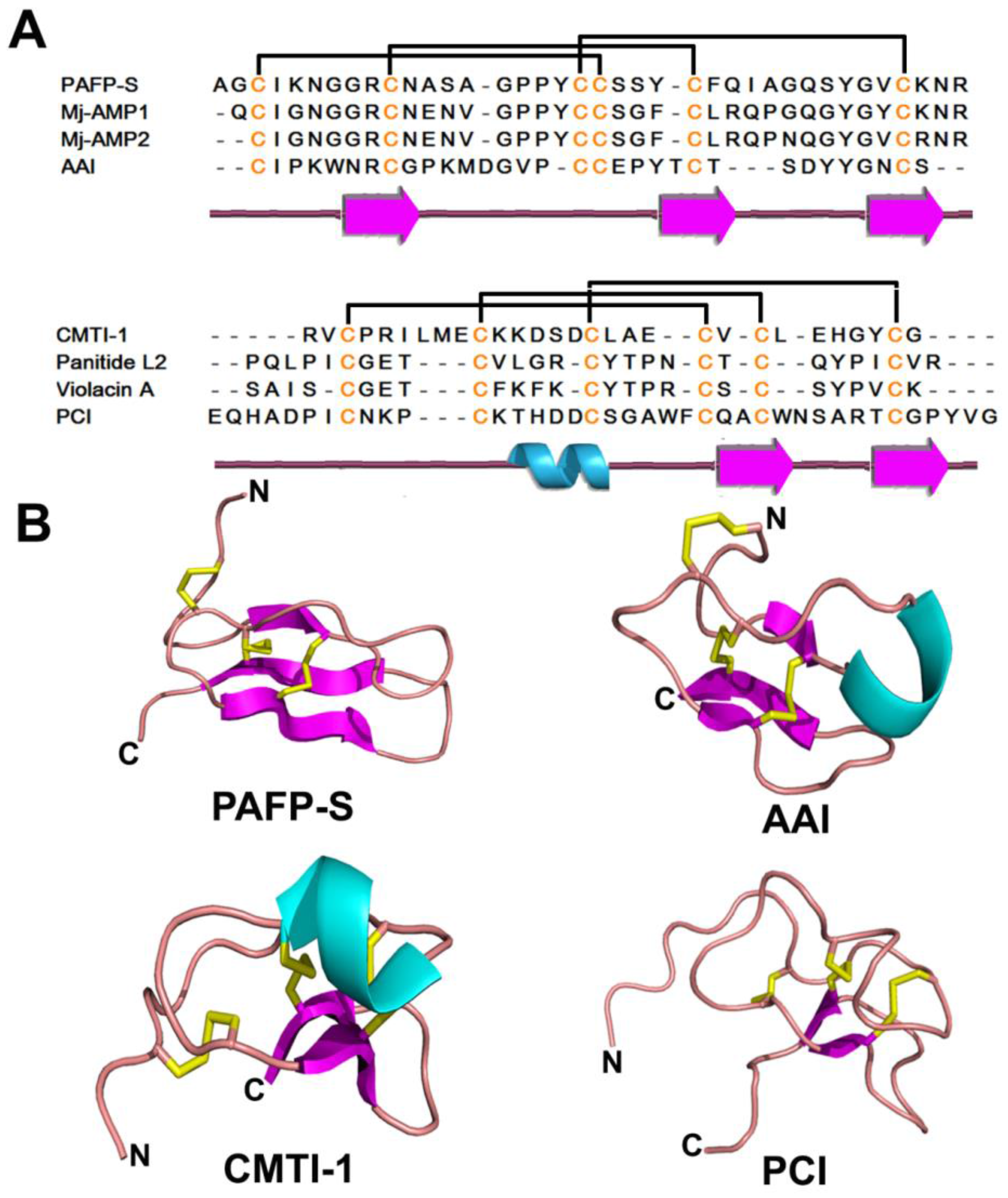
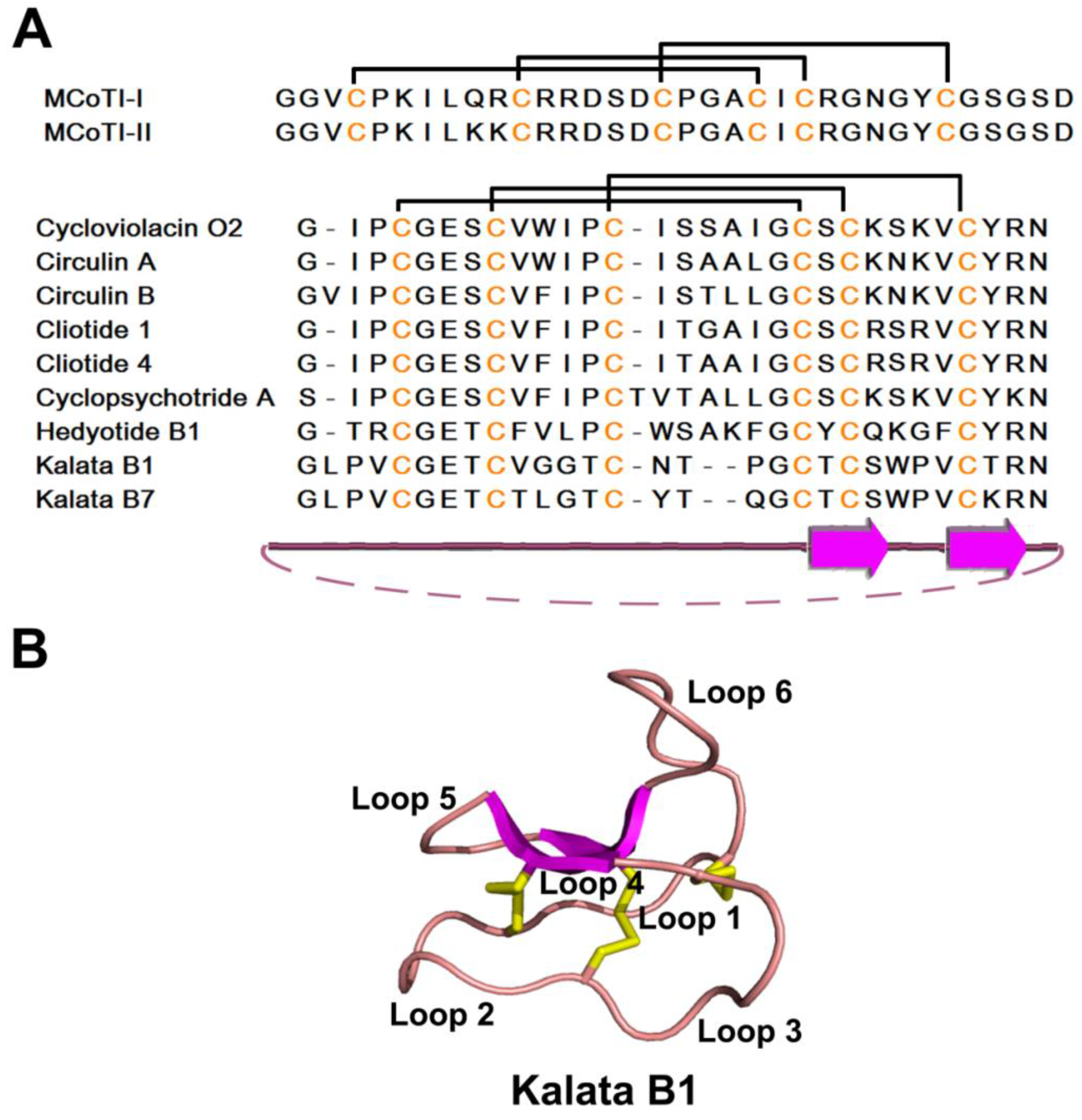
2.4.4. Mechanism of Action
2.4.5. Knottin Scaffold in Pharmaceutical Engineering
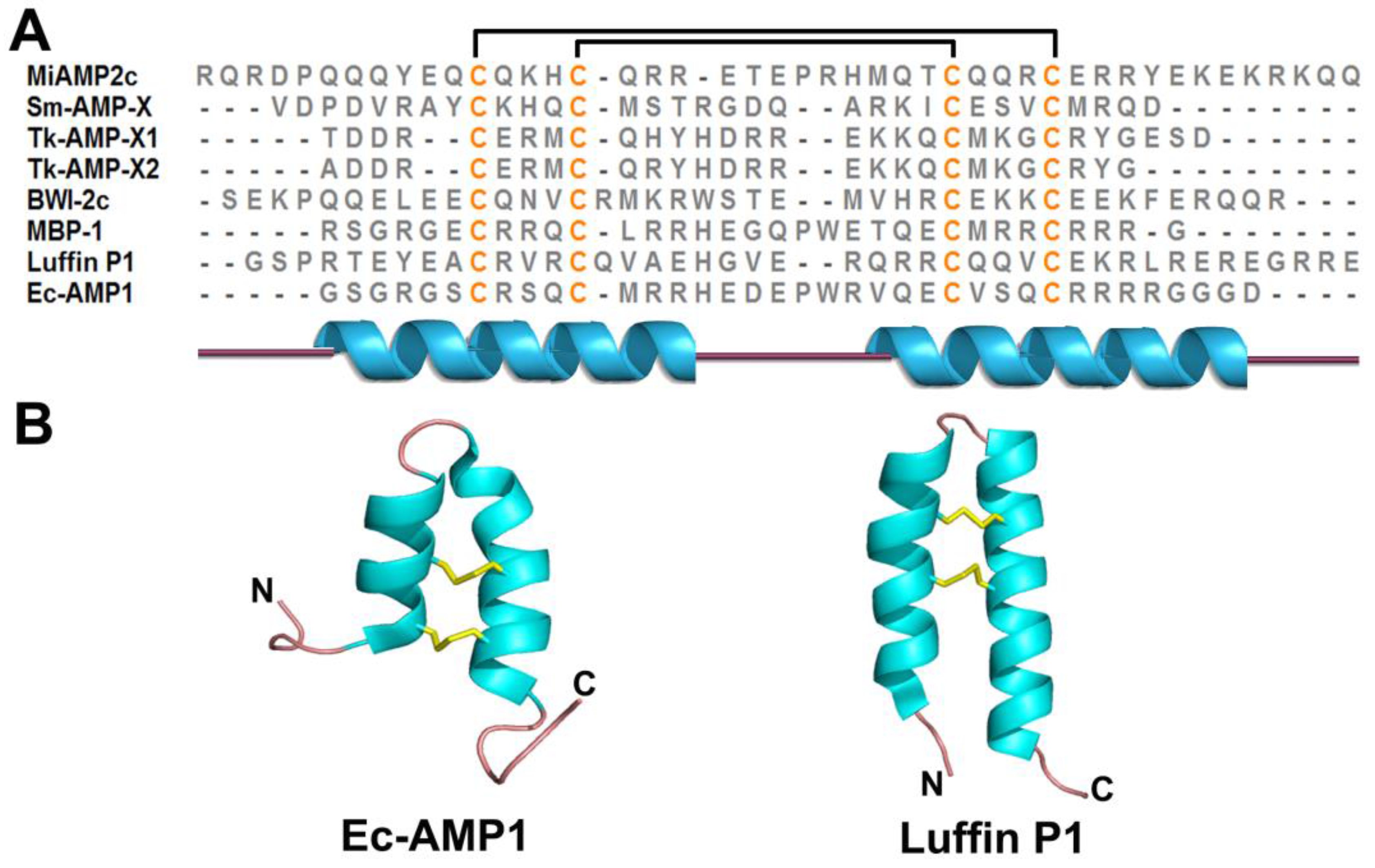
2.5. α-Hairpinin Family
2.6. Lipid Transfer Proteins
2.6.1. Occurrences, Distribution, and Biosynthesis
2.6.2. Structure
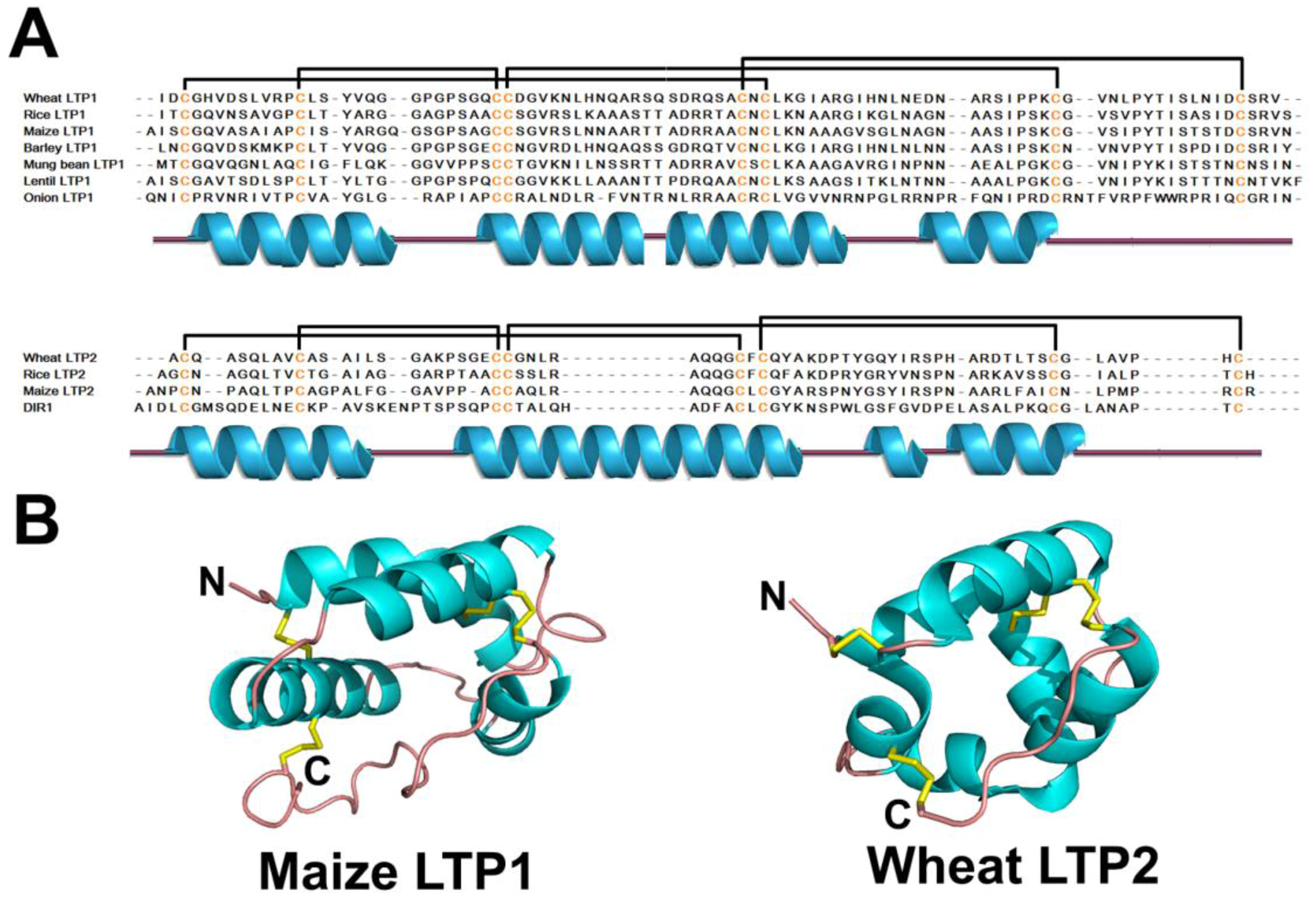
2.6.3. Structure-Function Relationship
2.6.4. Mechanism of Action
2.7. Snakins
2.8. Other Plant CRP-AMPs
2.9. Non-CRP Plant AMPs
2.10. Mechanism of AMP Action
3. Conclusions and Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant “pathogenesis-related” proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 543195. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Usha, K.; Singh, B. Pathogenesis related (pr) proteins in plant defense mechanism. Sci. Against Microb. Pathog. 2011, 2, 1043–1054. [Google Scholar]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (pr) proteins: A focus on pr peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Egorov, T.A.; Odintsova, T.I.; Pukhalsky, V.A.; Grishin, E.V. Diversity of wheat anti-microbial peptides. Peptides 2005, 26, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.G. Antimicrobial peptides. Mol. Plant Microbe Interact. 1995, 8, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Lopez, M.; Sanchez-Hidalgo, M.; Cebrian, R.; Maqueda, M. Discovering the bacterial circular proteins: Bacteriocins, cyanobactins, and pilins. J. Biol. Chem. 2012, 287, 27007–27013. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wong, C.T. Chemical synthesis of circular proteins. J. Biol. Chem. 2012, 287, 27020–27025. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Ben Hamida, J.; Vergoten, G.; Fliss, I. Phytamp: A database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009, 37, D963–D968. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, K.A.; Moskal, W.A., Jr.; Wu, H.C.; Underwood, B.A.; Graham, M.A.; Town, C.D.; VandenBosch, K.A. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant. J. 2007, 51, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmedo, F.; Molina, A.; Alamillo, J.M.; Rodriguez-Palenzuela, P. Plant defense peptides. Biopolymers 1998, 47, 479–491. [Google Scholar] [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Gozdzicka-Jozefiak, A. Plant antimicrobial peptides. Folia Microbiol. (Praha) 2013, 59, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Padovan, L.; Scocchi, M.; Tossi, A. Structural aspects of plant antimicrobial peptides. Curr. Protein Pept. Sci. 2010, 11, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Anderson, M.A. Defensins--components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.N.; Madhuprakash, J.; Sarma, P.V.; Purushotham, P.; Suma, K.; Manjeet, K.; Rambabu, S.; Gueddari, N.E.; Moerschbacher, B.M.; Podile, A.R. Biotechnological approaches for field applications of chitooligosaccharides (cos) to induce innate immunity in plants. Crit. Rev. Biotechnol. 2013, 35, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Mander, L.N.; Liu, H.-W. Comprehensive Natural Products ii Chemistry and Biology; Elsevier Science: Oxford, England, 2010; p. 1. [Google Scholar]
- Tavares, L.S.; Santos Mde, O.; Viccini, L.F.; Moreira, J.S.; Miller, R.N.; Franco, O.L. Biotechnological potential of antimicrobial peptides from flowers. Peptides 2008, 29, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J.; Cleveland, T.E.; Wedge, D.E. Plant-derived antifungal proteins and peptides. Can. J. Microbiol. 2005, 51, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.W.; Cemazar, M.; Anderson, M.A.; Craik, D.J. Insecticidal plant cyclotides and related cystine knot toxins. Toxicon 2007, 49, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J. Host-defense activities of cyclotides. Toxins (Basel) 2012, 4, 139–156. [Google Scholar] [CrossRef] [PubMed]
- van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 2004, 25, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Li, X.; Wang, Z. Apd2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G.S. Apd: The antimicrobial peptide database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef] [PubMed]
- Piotto, S.P.; Sessa, L.; Concilio, S.; Iannelli, P. Yadamp: Yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents 2012, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.S.; Gabere, M.N.; Pretorius, A.; Adam, S.; Christoffels, A.; Lehvaslaiho, M.; Archer, J.A.C.; Bajic, V.B. Dampd: A manually curated antimicrobial peptide database. Nucleic Acids Res. 2012, 40, D1108–D1112. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Caleya, R.; Gonzalez-Pascual, B.; Garcia-Olmedo, F.; Carbonero, P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl. Microbiol. 1972, 23, 998–1000. [Google Scholar] [PubMed]
- Ebrahimnesbat, F.; Behnke, S.; Kleinhofs, A.; Apel, K. Cultivar-related differences in the distribution of cell-wall-bound thionins in compatible and incompatible interactions between barley and powdery mildew. Planta 1989, 179, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Wang, Y.D.; Shaw, K.P.; Vernon, L.P. Cellular responses to pyrularia thionin are mediated by Ca2+ influx and phospholipase a2 activation and are inhibited by thionin tyrosine iodination. Proc. Natl. Acad. Sci. USA 1989, 86, 5849–5853. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Klassen, L.W.; Jones, B.L.; Speirs, R.D.; Kammer, A.E. Toxicity of purothionin and its homologues to the tobacco hornworm, manduca sexta (l.) (lepidoptera:Sphingidae). Toxicol. Appl. Pharmacol. 1979, 48, 179–183. [Google Scholar] [CrossRef]
- Balls, A.K.; Hale, W.S.; Harris, T.H. A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem. 1942, 19, 951–961. [Google Scholar]
- Ponz, F.; Paz-Ares, J.; Hernandez-Lucas, C.; Carbonero, P.; Garcia-Olmedo, F. Synthesis and processing of thionin precursors in developing endosperm from barley (hordeum vulgare l.). EMBO J. 1983, 2, 1035–1040. [Google Scholar] [PubMed]
- Steinmuller, K.; Batschauer, A.; Apel, K. Tissue-specific and light-dependent changes of chromatin organization in barley (hordeum vulgare). Eur. J. Biochem. 1986, 158, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gausing, K. Thionin genes specifically expressed in barley leaves. Planta 1987, 171, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Epple, P.; Apel, K.; Bohlmann, H. An arabidopsis-thaliana thionin gene is inducible via a signal-transduction pathway different from that for pathogenesis-related proteins. Plant. Physiol. 1995, 109, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, H.; Clausen, S.; Behnke, S.; Giese, H.; Hiller, C.; Reimann-Philipp, U.; Schrader, G.; Barkholt, V.; Apel, K. Leaf-specific thionins of barley-a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defence mechanism of plants. EMBO J. 1988, 7, 1559–1565. [Google Scholar] [PubMed]
- Andresen, I.; Becker, W.; Schluter, K.; Burges, J.; Parthier, B.; Apel, K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (hordeum vulgare). Plant. Mol. Biol. 1992, 19, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Metraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-induced systemic activation of a plant defensin gene in arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996, 8, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Castagnaro, A.; Marana, C.; Carbonero, P.; Garciaolmedo, F. Extreme divergence of a novel wheat thionin generated by a mutational burst specifically affecting the mature protein domain of the precursor. J. Mol. Biol. 1992, 224, 1003–1009. [Google Scholar] [CrossRef]
- Stec, B.; Markman, O.; Rao, U.; Heffron, G.; Henderson, S.; Vernon, L.P.; Brumfeld, V.; Teeter, M.M. Proposal for molecular mechanism of thionins deduced from physico-chemical studies of plant toxins. J. Pept. Res. 2004, 64, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Stec, B.; Teeter, M.M. Refinement of purothionins reveals solute particles important for lattice formation and toxicity. Part 1: Alpha1-purothionin revisited. Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Stec, B.; Rao, U.; Teeter, M.M. Refinement of purothionins reveals solute particles important for lattice formation and toxicity. Part 2: Structure of beta-purothionin at 1.7 a resolution. Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, A.G.; Kerek, F.; Moroder, L.; Renner, C. Structural characterization of hellethionins from helleborus purpurascens. Biochemistry 2003, 42, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Yamano, A.; Heo, N.H.; Teeter, M.M. Crystal structure of ser-22/ile-25 form crambin confirms solvent, side chain substate correlations. J. Biol. Chem. 1997, 272, 9597–9600. [Google Scholar] [PubMed]
- Yamano, A.; Teeter, M.M. Correlated disorder of the pure pro22/leu25 form of crambin at 150 k refined to 1.05-a resolution. J. Biol. Chem. 1994, 269, 13956–13965. [Google Scholar] [PubMed]
- Hendrickson, W.A.; Teeter, M.M. Structure of the hydrophobic protein crambin determined directly from the anomalous scattering of sulfur. Nature 1981, 290, 107–113. [Google Scholar] [CrossRef]
- Ahn, H.C.; Juranic, N.; Macura, S.; Markley, J.L. Three-dimensional structure of the water-insoluble protein crambin in dodecylphosphocholine micelles and its minimal solvent-exposed surface. J. Am. Chem. Soc. 2006, 128, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Teeter, M.; Weckert, E.; Lamzin, V.S. Crystal structure of small protein crambin at 0.48 a resolution. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 67, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hanson, B.L.; Fisher, S.Z.; Langan, P.; Kovalevsky, A.Y. Direct observation of hydrogen atom dynamics and interactions by ultrahigh resolution neutron protein crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 15301–15306. [Google Scholar] [CrossRef] [PubMed]
- Jelsch, C.; Teeter, M.M.; Lamzin, V.; Pichon-Pesme, V.; Blessing, R.H.; Lecomte, C. Accurate protein crystallography at ultra-high resolution: Valence electron distribution in crambin. Proc. Natl. Acad. Sci. USA 2000, 97, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Debreczeni, J.E.; Sevvana, M.; Gruene, T.; Kahle, B.; Zeeck, A.; Sheldrick, G.M. Structures of viscotoxins a1 and b2 from european mistletoe solved using native data alone. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S.; Fogolari, F.; Catalano, M.; Zetta, L.; Schaller, G.; Urech, K.; Giannattasio, M.; Ragona, L.; Molinari, H. Nmr solution structure of viscotoxin c1 from viscum album species coloratum ohwi: Toward a structure-function analysis of viscotoxins. Biochemistry 2003, 42, 12503–12510. [Google Scholar] [CrossRef] [PubMed]
- Coulon, A.; Mosbah, A.; Lopez, A.; Sautereau, A.M.; Schaller, G.; Urech, K.; Rouge, P.; Darbon, H. Comparative membrane interaction study of viscotoxins a3, a2 and b from mistletoe (viscum album) and connections with their structures. Biochem. J. 2003, 374, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S.; Ugolini, R.; Fogolari, F.; Schaller, G.; Urech, K.; Giannattasio, M.; Ragona, L.; Molinari, H. Nmr structural determination of viscotoxin a3 from viscum album l. Biochem. J. 2000, 350 (Pt. 2), 569–577. [Google Scholar] [CrossRef] [PubMed]
- Debreczeni, J.E.; Girmann, B.; Zeeck, A.; Kratzner, R.; Sheldrick, G.M. Structure of viscotoxin a3: Disulfide location from weak sad data. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Clore, G.M.; Sukumaran, D.K.; Nilges, M.; Gronenborn, A.M. 3-dimensional structure of phoratoxin in solution - combined use of nuclear-magnetic-resonance, distance geometry, and restrained molecular-dynamics. Biochemistry 1987, 26, 1732–1745. [Google Scholar] [CrossRef]
- Mellstra, S.T.; Samuelss, G. Phoratoxin, a toxic protein from mistletoe phoradendron-tomentosum subsp. Macrophyllum (loranthaceae) - improvements in isolation procedure and further studies on properties. Eur. J. Biochem. 1973, 32, 143–147. [Google Scholar] [CrossRef]
- Johnson, K.A.; Kim, E.; Teeter, M.M.; Suh, S.W.; Stec, B. Crystal structure of alpha-hordothionin at 1.9 angstrom resolution. FEBS Lett. 2005, 579, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Park, K.H.; Yoo, H.J.; Cha, H.; Suh, S.W.; Thomas, F.; Moon, T.S.; Kim, S.M. Determination of the three-dimensional structure of hordothionin-alpha by nuclear magnetic resonance. Biochem. J. 1996, 313 (Pt. 3), 885–892. [Google Scholar] [CrossRef] [PubMed]
- Vila-Perello, M.; Andreu, D. Characterization and structural role of disulfide bonds in a highly knotted thionin from pyrularia pubera. Biopolymers 2005, 80, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Vila-Perello, M.; Sanchez-Vallet, A.; Garcia-Olmedo, F.; Molina, A.; Andreu, D. Structural dissection of a highly knotted peptide reveals minimal motif with antimicrobial activity. J. Biol. Chem. 2005, 280, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of novel antimicrobial peptides, tu-amp 1 and tu-amp 2, from bulbs of tulip (tulipa gesneriana l.). Biosci. Biotechnol. Biochem. 2004, 68, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Florack, D.E.; Stiekema, W.J. Thionins: Properties, possible biological roles and mechanisms of action. Plant Mol. Biol. 1994, 26, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Osorio e Castro, V.R.; Vernon, L.P. Hemolytic activity of thionin from pyrularia pubera nuts and snake venom toxins of naja naja species: Pyrularia thionin and snake venom cardiotoxin compete for the same membrane site. Toxicon 1989, 27, 511–517. [Google Scholar] [CrossRef]
- Hughes, P.; Dennis, E.; Whitecross, M.; Llewellyn, D.; Gage, P. The cytotoxic plant protein, beta-purothionin, forms ion channels in lipid membranes. J. Biol. Chem. 2000, 275, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.; Vazquez, D.; Hernandez-Lucas, C.; Carbonero, P.; Garcia-Olmedo, F. Thionins: Plant peptides that modify membrane permeability in cultured mammalian cells. Eur. J. Biochem. 1981, 116, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Murata, Y.; Nakanishi, T.; Yoshizumi, H.; Hayashida, H.; Ohtsuki, Y.; Toyoshima, K.; Hakura, A. Similarity, in molecular-structure and function, between the plant toxin purothionin and the mammalian pore-forming proteins. Mol. Biol. Evol. 1992, 9, 707–715. [Google Scholar] [PubMed]
- Richard, J.A.; Kelly, I.; Marion, D.; Pezolet, M.; Auger, M. Interaction between beta-purothionin and dimyristoylphosphatidylglycerol: A p-31-nmr and infrared spectroscopic study. Biophysic. J. 2002, 83, 2074–2083. [Google Scholar] [CrossRef]
- Richard, J.A.; Kelly, I.; Marion, D.; Auger, M.; Pezolet, M. Structure of beta-purothionin in membranes: A two-dimensional infrared correlation spectroscopy study. Biochemistry 2005, 44, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Ozaki, Y.; Matsubara, H.; Yoshizumi, H. Studies on purothionin by chemical modifications. J. Biochem. 1982, 91, 257–263. [Google Scholar] [PubMed]
- Fracki, W.S.; Li, D.; Owen, N.; Perry, C.; Naisbitt, G.H.; Vernon, L.P. Role of tyr and trp in membrane responses of pyrularia thionin determined by optical and nmr spectra following tyr iodination and trp modification. Toxicon 1992, 30, 1427–1440. [Google Scholar] [CrossRef]
- Rao, A.G.; Hassan, M.; Hempel, J. Validation of the structure-function properties of alpha-hordothionin and derivatives through protein modeling. Protein Eng. 1993, 6, 117–117. [Google Scholar]
- Pelegrini, P.B.; Franco, O.L. Plant gamma-thionins: Novel insights on the mechanism of action of a multi-functional class of defense proteins. Int. J. Biochem. Cell. Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.G.; Hakimi, S.M.; Mittanck, C.A.; Wu, Y.; Woerner, B.M.; Stark, D.M.; Shah, D.M.; Liang, J.; Rommens, C.M. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 2000, 18, 1307–1310. [Google Scholar] [PubMed]
- Terras, F.R.; Schoofs, H.M.; De Bolle, M.F.; Van Leuven, F.; Rees, S.B.; Vanderleyden, J.; Cammue, B.P.; Broekaert, W.F. Analysis of two novel classes of plant antifungal proteins from radish (raphanus sativus l.) seeds. J. Biol. Chem. 1992, 267, 15301–15309. [Google Scholar] [PubMed]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J.; et al. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell. 1995, 7, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Fant, F.; Vranken, W.F.; Borremans, F.A. The three-dimensional solution structure of aesculus hippocastanum antimicrobial protein 1 determined by 1h nuclear magnetic resonance. Proteins 1999, 37, 388–403. [Google Scholar] [CrossRef]
- Fant, F.; Vranken, W.; Broekaert, W.; Borremans, F. Determination of the three-dimensional solution structure of raphanus sativus antifungal protein 1 by 1h nmr. J. Mol. Biol. 1998, 279, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of a novel type of antimicrobial peptides, fa-amp1 and fa-amp2, from seeds of buckwheat (Fagopyrum esculentum Moench.). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, N. Antimicrobial peptides with unusual amino acid compositions and unusual structures. Curr. Med. Chem. 2006, 13, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Bloch, C., Jr.; Richardson, M. A new family of small (5 kda) protein inhibitors of insect alpha-amylases from seeds or sorghum (Sorghum bicolar (L.) Moench.) have sequence homologies with wheat gamma-purothionins. FEBS Lett. 1991, 279, 101–104. [Google Scholar] [CrossRef]
- Wijaya, R.; Neumann, G.M.; Condron, R.; Hughes, A.B.; Polya, G.M. Defense proteins from seed of cassia fistula include a lipid transfer protein homologue and a protease inhibitory plant defensin. Plant Sci. 2000, 159, 243–255. [Google Scholar] [CrossRef]
- Colilla, F.J.; Rocher, A.; Mendez, E. Gamma-purothionins: Amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett. 1990, 270, 191–194. [Google Scholar] [CrossRef]
- Mendez, E.; Moreno, A.; Colilla, F.; Pelaez, F.; Limas, G.G.; Mendez, R.; Soriano, F.; Salinas, M.; de Haro, C. Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, gamma-hordothionin, from barley endosperm. Eur. J. Biochem. 1990, 194, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Bruix, M.; Jimenez, M.A.; Santoro, J.; Gonzalez, C.; Colilla, F.J.; Mendez, E.; Rico, M. Solution structure of gamma 1-h and gamma 1-p thionins from barley and wheat endosperm determined by 1h-nmr: A structural motif common to toxic arthropod proteins. Biochemistry 1993, 32, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.R.G.; Cammue, B.P.A.; Osborn, R.W. Plant defensins - novel antimicrobial peptides as components of the host-defense system. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.L.; Murad, A.M.; Leite, J.R.; Mendes, P.A.; Prates, M.V.; Bloch, C., Jr. Identification of a cowpea gamma-thionin with bactericidal activity. FEBS J. 2006, 273, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Segura, A.; Garcia-Olmedo, F. Pseudothionin-st1, a potato peptide active against potato pathogens. Eur. J. Biochem. 1994, 223, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Hadwiger, L.A. The fusarium solani-induced expression of a pea gene family encoding high cysteine content proteins. Mol. Plant Microbe Interact. 1991, 4, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Kang, Y.H.; Chun, H.J.; Koo, J.C.; Cheong, Y.H.; Kim, C.Y.; Kim, M.C.; Chung, W.S.; Kim, J.C.; Yoo, J.H.; et al. Characterization of a stamen-specific cdna encoding a novel plant defensin in chinese cabbage. Plant Mol. Biol. 2002, 50, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.B.; Gasser, C.S. Nature and regulation of pistil-expressed genes in tomato. Plant Mol. Biol. 1995, 28, 691–711. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Brugliera, F.; Anderson, M.A. Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 2003, 131, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lonneborg, A. Isolation and characterization of a cdna encoding a plant defensin-like protein from roots of norway spruce. Plant Mol. Biol. 1996, 31, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Shramova, E.I.; Tagaev, A.A.; Ovchinnikova, T.V. A novel defensin from the lentil lens culinaris seeds. Biochem. Biophys. Res. Commun. 2008, 371, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Bontems, F.; Roumestand, C.; Boyot, P.; Gilquin, B.; Doljansky, Y.; Menez, A.; Toma, F. Three-dimensional structure of natural charybdotoxin in aqueous solution by 1h-nmr. Charybdotoxin possesses a structural motif found in other scorpion toxins. Eur. J. Biochem. 1991, 196, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cornet, B.; Bonmatin, J.M.; Hetru, C.; Hoffmann, J.A.; Ptak, M.; Vovelle, F. Refined three-dimensional solution structure of insect defensin A. Structure 1995, 3, 435–448. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Cammue, B.P.A.; DeBolle, M.F.C.; Thevissen, K.; DeSamblanx, G.W.; Osborn, R.W. Antimicrobial peptides from plants. Crit. Rev. Plant. Sci. 1997, 16, 297–323. [Google Scholar] [CrossRef]
- Thomma, B.P.; Cammue, B.P.; Thevissen, K. Plant defensins. Planta 2002, 216, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.A.; Dentino, A.R. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 2002, 206, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Carvalho Ade, O.; Gomes, V.M. Plant defensins and defensin-like peptides - biological activities and biotechnological applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Cheng, K.C.; Tsai, P.H.; Liu, C.C.; Lee, T.R.; Lyu, P.C. Alanine substitutions of noncysteine residues in the cysteine-stabilized alphabeta motif. Protein Sci. 2009, 18, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Schirra, H.J.; Scanlon, M.J.; Anderson, M.A.; Craik, D.J. The three-dimensional solution structure of nad1, a new floral defensin from nicotiana alata and its application to a homology model of the crop defense protein alfafp. J. Mol. Biol. 2003, 325, 175–188. [Google Scholar] [CrossRef]
- Chen, K.C.; Lin, C.Y.; Kuan, C.C.; Sung, H.Y.; Chen, C.S. A novel defensin encoded by a mungbean cdna exhibits insecticidal activity against bruchid. J. Agric. Food Chem. 2002, 50, 7258–7263. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.J.; Schirra, H.J.; Lay, F.T.; Anderson, M.A.; Craik, D.J. Structure of petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds. Biochemistry 2003, 42, 8214–8222. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.; Rocher, A.; Calero, M.; Girbes, T.; Citores, L.; Soriano, F. Primary structure of omega-hordothionin, a member of a novel family of thionins from barley endosperm, and its inhibition of protein synthesis in eukaryotic and prokaryotic cell-free systems. Eur. J. Biochem. 1996, 239, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, Z.; Wang, J.; Wu, F.; Gong, W. Purification, characterization and preliminary crystallographic studies of a novel plant defensin from pachyrrhizus erosus seeds. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, M.; Zhou, Z.; Gong, W. Ultra-high resolution crystal structure of a dimeric defensin spe10. FEBS Lett. 2011, 585, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Osborn, R.W.; De Samblanx, G.W.; Thevissen, K.; Goderis, I.; Torrekens, S.; Van Leuven, F.; Attenborough, S.; Rees, S.B.; Broekaert, W.F. Isolation and characterisation of plant defensins from seeds of asteraceae, fabaceae, hippocastanaceae and saxifragaceae. FEBS Lett. 1995, 368, 257–262. [Google Scholar] [CrossRef]
- Almeida, M.S.; Cabral, K.M.; Kurtenbach, E.; Almeida, F.C.; Valente, A.P. Solution structure of pisum sativum defensin 1 by high resolution nmr: Plant defensins, identical backbone with different mechanisms of action. J. Mol. Biol. 2002, 315, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lewis, K. Fabatins: New antimicrobial plant peptides. FEMS Microbiol. Lett. 1997, 149, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Garciaolmedo, F.; Carbonero, P.; Hernandezlucas, C.; Pazares, J.; Ponz, F.; Vicente, O.; Sierra, J.M. Inhibition of eukaryotic cell-free protein-synthesis by thionins from wheat endosperm. Biochim. Biophys. Acta 1983, 740, 52–56. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Lay, F.T.; Murad, A.M.; Anderson, M.A.; Franco, O.L. Novel insights on the mechanism of action of alpha-amylase inhibitors from the plant defensin family. Proteins 2008, 73, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, I.S.; Carvalho Ade, O.; de Souza-Filho, G.A.; do Nascimento, V.V.; Machado, O.L.; Gomes, V.M. Purification of a defensin isolated from vigna unguiculata seeds, its functional expression in escherichia coli, and assessment of its insect alpha-amylase inhibitory activity. Protein Expr. Purif. 2010, 71, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Ade, O.; Gomes, V.M. Plant defensins--prospects for the biological functions and biotechnological properties. Peptides 2009, 30, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Kushmerick, C.; de Souza Castro, M.; Santos Cruz, J.; Bloch, C., Jr.; Beirao, P.S. Functional and structural features of gamma-zeathionins, a new class of sodium channel blockers. FEBS Lett. 1998, 440, 302–306. [Google Scholar] [CrossRef]
- Melo, F.R.; Rigden, D.J.; Franco, O.L.; Mello, L.V.; Ary, M.B.; Grossi de Sa, M.F.; Bloch, C., Jr. Inhibition of trypsin by cowpea thionin: Characterization, molecular modeling, and docking. Proteins 2002, 48, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Lai, H.C.; Chang, Y.S.; Sheu, M.J.; Lu, T.L.; Huang, S.S.; Lin, Y.H. Antimicrobial, dehydroascorbate reductase, and monodehydroascorbate reductase activities of defensin from sweet potato [ipomoea batatas (l.) lam. 'Tainong 57'] storage roots. J. Agric. Food Chem. 2008, 56, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gallie, D.R. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006, 142, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Osborn, R.W.; Acland, D.P.; Broekaert, W.F. Specific binding sites for an antifungal plant defensin from dahlia (dahlia merckii) on fungal cells are required for antifungal activity. Mol. Plant Microbe Interact. 2000, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Warnecke, D.C.; Francois, I.E.; Leipelt, M.; Heinz, E.; Ott, C.; Zahringer, U.; Thomma, B.P.; Ferket, K.K.; Cammue, B.P. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2004, 279, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Ghazi, A.; De Samblanx, G.W.; Brownlee, C.; Osborn, R.W.; Broekaert, W.F. Fungal membrane responses induced by plant defensins and thionins. J. Biol. Chem. 1996, 271, 15018–15025. [Google Scholar] [PubMed]
- Thevissen, K.; Terras, F.R.; Broekaert, W.F. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl. Environ. Microbiol. 1999, 65, 5451–5458. [Google Scholar] [PubMed]
- Van der Weerden, N.L.; Hancock, R.E.; Anderson, M.A. Permeabilization of fungal hyphae by the plant defensin nad1 occurs through a cell wall-dependent process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Lay, F.T.; Anderson, M.A. The plant defensin, nad1, enters the cytoplasm of fusarium oxysporum hyphae. J. Biol. Chem. 2008, 283, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.M.; Bleackley, M.R.; Wiltshire, J.L.; Anderson, M.A.; Traven, A.; van der Weerden, N.L. Identification and mechanism of action of the plant defensin nad1 as a new member of the antifungal drug arsenal against candida albicans. Antimicrob. Agents Chemother. 2013, 57, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, B.; Cammue, B.P.A.; Thevissen, K. Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biol. Rev. 2013, 26, 109–120. [Google Scholar] [CrossRef]
- Gachomo, E.W.; Jimenez-Lopez, J.C.; Kayode, A.P.; Baba-Moussa, L.; Kotchoni, S.O. Structural characterization of plant defensin protein superfamily. Mol. Biol. Rep. 2012, 39, 4461–4469. [Google Scholar] [CrossRef] [PubMed]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159–163. [Google Scholar] [CrossRef]
- Jimenez-Barbero, J.; Javier Canada, F.; Asensio, J.L.; Aboitiz, N.; Vidal, P.; Canales, A.; Groves, P.; Gabius, H.J.; Siebert, H.C. Hevein domains: An attractive model to study carbohydrate-protein interactions at atomic resolution. Adv. Carbohydr. Chem. Biochem. 2006, 60, 303–354. [Google Scholar] [PubMed]
- Kini, S.G.; Nguyen, P.Q.; Weissbach, S.; Mallagaray, A.; Shin, J.; Yoon, H.S.; Tam, J.P. Studies on the chitin binding property of novel cysteine-rich peptides from alternanthera sessilis. Biochemistry 2015, 54, 6639–6649. [Google Scholar] [CrossRef] [PubMed]
- Archer, B.L. The proteins of hevea brasiliensis latex. 4. Isolation and characterization of crystalline hevein. Biochem. J. 1960, 75, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Van Parijs, J.; Broekaert, W.F.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 183, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Perales, A.; Collada, C.; Blanco, C.; Sanchez-Monge, R.; Carrillo, T.; Aragoncillo, C.; Salcedo, G. Cross-reactions in the latex-fruit syndrome: A relevant role of chitinases but not of complex asparagine-linked glycans. J. Allergy Clin. Immunol. 1999, 104, 681–687. [Google Scholar] [CrossRef]
- Blanco, C.; Diaz-Perales, A.; Collada, C.; Sanchez-Monge, R.; Aragoncillo, C.; Castillo, R.; Ortega, N.; Alvarez, M.; Carrillo, T.; Salcedo, G. Class i chitinases as potential panallergens involved in the latex-fruit syndrome. J. Allergy Clin. Immunol. 1999, 103, 507–513. [Google Scholar] [CrossRef]
- Gidrol, X.; Chrestin, H.; Tan, H.L.; Kush, A. Hevein, a lectin-like protein from hevea brasiliensis (rubber tree) is involved in the coagulation of latex. J. Biol. Chem. 1994, 269, 9278–9283. [Google Scholar] [PubMed]
- Peumans, W.J.; Van Damme, E.J. Plant lectins: Specific tools for the identification, isolation, and characterization of o-linked glycans. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 209–258. [Google Scholar] [PubMed]
- Asensio, J.L.; Siebert, H.C.; von Der Lieth, C.W.; Laynez, J.; Bruix, M.; Soedjanaamadja, U.M.; Beintema, J.J.; Canada, F.J.; Gabius, H.J.; Jimenez-Barbero, J. Nmr investigations of protein-carbohydrate interactions: Studies on the relevance of trp/tyr variations in lectin binding sites as deduced from titration microcalorimetry and nmr studies on hevein domains. Determination of the nmr structure of the complex between pseudohevein and n,n',n"-triacetylchitotriose. Proteins 2000, 40, 218–236. [Google Scholar] [PubMed]
- Lipkin, A.; Anisimova, V.; Nikonorova, A.; Babakov, A.; Krause, E.; Bienert, M.; Grishin, E.; Egorov, T. An antimicrobial peptide ar-amp from amaranth (Amaranthus retroflexus L.) seeds. Phytochemistry 2005, 66, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Rogozhin, E.A.; Utkina, L.L.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding hevein-like defense peptides in wheat: Distribution, evolution, and role in stress response. Biochimie 2012, 94, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, K.P.; Rouge, P.; Proost, P.; Coosemans, J.; Krouglova, T.; Engelborghs, Y.; Peumans, W.J.; Van Damme, E.J. Synergistic antifungal activity of two chitin-binding proteins from spindle tree (Euonymus europaeus L.). Planta 2004, 219, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.L.; Canada, F.J.; Bruix, M.; Gonzalez, C.; Khiar, N.; Rodriguez-Romero, A.; Jimenez-Barbero, J. Nmr investigations of protein-carbohydrate interactions: Refined three-dimensional structure of the complex between hevein and methyl beta-chitobioside. Glycobiology 1998, 8, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Harata, K.; Muraki, M. Crystal structures of urtica dioica agglutinin and its complex with tri-n-acetylchitotriose. J. Mol. Biol. 2000, 297, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Marien, W.; Terras, F.R.; De Bolle, M.F.; Proost, P.; Van Damme, J.; Dillen, L.; Claeys, M.; Rees, S.B.; Vanderleyden, J.; et al. Antimicrobial peptides from amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 1992, 31, 4308–4314. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.C.; Maes, D.; Loris, R.; Pepermans, H.A.; Wyns, L.; Willem, R.; Verheyden, P. H nmr study of the solution structure of ac-amp2, a sugar binding antimicrobial protein isolated from amaranthus caudatus. J. Mol. Biol. 1996, 258, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, N.; Vila-Perello, M.; Groves, P.; Asensio, J.L.; Andreu, D.; Canada, F.J.; Jimenez-Barbero, J. Nmr and modeling studies of protein-carbohydrate interactions: Synthesis, three-dimensional structure, and recognition properties of a minimum hevein domain with binding affinity for chitooligosaccharides. ChemBioChem 2004, 5, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.C.; Lee, S.Y.; Chun, H.J.; Cheong, Y.H.; Choi, J.S.; Kawabata, S.; Miyagi, M.; Tsunasawa, S.; Ha, K.S.; Bae, D.W.; et al. Two hevein homologs isolated from the seed of pharbitis nil l. Exhibit potent antifungal activity. Biochim. Biophys. Acta 1998, 1382, 80–90. [Google Scholar] [CrossRef]
- Lee, O.S.; Lee, B.; Park, N.; Koo, J.C.; Kim, Y.H.; Prasad, D.T.; Karigar, C.; Chun, H.J.; Jeong, B.R.; Kim, D.H.; et al. Pn-amps, the hevein-like proteins from pharbitis nil confers disease resistance against phytopathogenic fungi in tomato, lycopersicum esculentum. Phytochemistry 2003, 62, 1073–1079. [Google Scholar] [CrossRef]
- Koo, J.C.; Chun, H.J.; Park, H.C.; Kim, M.C.; Koo, Y.D.; Koo, S.C.; Ok, H.M.; Park, S.J.; Lee, S.H.; Yun, D.J.; et al. Over-expression of a seed specific hevein-like antimicrobial peptide from pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol. Biol. 2002, 50, 441–452. [Google Scholar] [PubMed]
- Li, S.S.; Claeson, P. Cys/gly-rich proteins with a putative single chitin-binding domain from oat (Avena sativa) seeds. Phytochemistry 2003, 63, 249–255. [Google Scholar] [CrossRef]
- Huang, R.H.; Xiang, Y.; Liu, X.Z.; Zhang, Y.; Hu, Z.; Wang, D.C. Two novel antifungal peptides distinct with a five-disulfide motif from the bark of eucommia ulmoides oliv. FEBS Lett. 2002, 521, 87–90. [Google Scholar] [CrossRef]
- Huang, R.H.; Xiang, Y.; Tu, G.Z.; Zhang, Y.; Wang, D.C. Solution structure of eucommia antifungal peptide: A novel structural model distinct with a five-disulfide motif. Biochemistry 2004, 43, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, R.H.; Liu, X.Z.; Zhang, Y.; Wang, D.C. Crystal structure of a novel antifungal protein distinct with five disulfide bridges from eucommia ulmoides oliver at an atomic resolution. J. Struct. Biol 2004, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Vassilevski, A.A.; Slavokhotova, A.A.; Musolyamov, A.K.; Finkina, E.I.; Khadeeva, N.V.; Rogozhin, E.A.; Korostyleva, T.V.; Pukhalsky, V.A.; Grishin, E.V.; et al. A novel antifungal hevein-type peptide from triticum kiharae seeds with a unique 10-cysteine motif. FEBS J. 2009, 276, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Vassilevski, A.A.; Slavokhotova, A.A.; Odintsova, T.I.; Grishin, E.V.; Egorov, T.A.; Arseniev, A.S. Solution structure of a defense peptide from wheat with a 10-cysteine motif. Biochem. Biophys. Res. Commun. 2011, 411, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, K.P.; Proost, P.; Van Damme, J.; Coosemans, J.; Van Damme, E.J.; Peumans, W.J. Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.). FEBS Lett. 2002, 530, 181–185. [Google Scholar] [CrossRef]
- Chavez, M.I.; Vila-Perello, M.; Canada, F.J.; Andreu, D.; Jimenez-Barbero, J. Effect of a serine-to-aspartate replacement on the recognition of chitin oligosaccharides by truncated hevein. A 3d view by using nmr. Carbohydr. Res. 2010, 345, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.F.; Asensio, J.L.; Garcia, J.L.; Laynez, J.; Bruix, M.; Wright, C.; Siebert, H.C.; Gabius, H.J.; Canada, F.J.; Jimenez-Barbero, J. Nmr investigations of protein-carbohydrate interactions binding studies and refined three-dimensional solution structure of the complex between the b domain of wheat germ agglutinin and n,n',n"-triacetylchitotriose. Eur. J. Biochem. 2000, 267, 3965–3978. [Google Scholar] [CrossRef] [PubMed]
- Muraki, M. The importance of ch/pi interactions to the function of carbohydrate binding proteins. Protein Pept. Lett. 2002, 9, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.I.; Andreu, C.; Vidal, P.; Aboitiz, N.; Freire, F.; Groves, P.; Asensio, J.L.; Asensio, G.; Muraki, M.; Canada, F.J.; et al. On the importance of carbohydrate-aromatic interactions for the molecular recognition of oligosaccharides by proteins: Nmr studies of the structure and binding affinity of acamp2-like peptides with non-natural naphthyl and fluoroaromatic residues. Chemistry 2005, 11, 7060–7074. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Naumann, T.A.; Price, N.P.; Rogozhin, E.A.; Andreev, Y.A.; Vassilevski, A.A.; Odintsova, T.I. Novel mode of action of plant defense peptides - hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J. 2014, 281, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Pallaghy, P.K.; Nielsen, K.J.; Craik, D.J.; Norton, R.S. A common structural motif incorporating a cystine knot and a triple-stranded beta-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994, 3, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.L. Peptide promiscuity: An evolutionary concept for plant defense. FEBS Lett. 2011, 585, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Lipscomb, W.N. Refined crystal structure of the potato inhibitor complex of carboxypeptidase a at 2.5 a resolution. J. Mol. Biol. 1982, 160, 475–498. [Google Scholar] [CrossRef]
- McDonald, N.Q.; Hendrickson, W.A. A structural superfamily of growth factors containing a cystine knot motif. Cell 1993, 73, 421–424. [Google Scholar] [CrossRef]
- Craik, D.J.; Daly, N.L.; Bond, T.; Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 1999, 294, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.W.; Elliott, A.G.; Ireland, D.C.; Delprete, P.G.; Dessein, S.; Goransson, U.; Trabi, M.; Wang, C.K.; Kinghorn, A.B.; Robbrecht, E.; et al. Distribution and evolution of circular miniproteins in flowering plants. Plant Cell. 2008, 20, 2471–2483. [Google Scholar] [CrossRef] [PubMed]
- Poth, A.G.; Mylne, J.S.; Grassl, J.; Lyons, R.E.; Millar, A.H.; Colgrave, M.L.; Craik, D.J. Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (solanaceae). J. Biol. Chem. 2012, 287, 27033–27046. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.; Zhang, S.; Nguyen, N.T.; Nguyen, P.Q.; Chiu, M.S.; Hardjojo, A.; Tam, J.P. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the fabaceae family. J. Biol. Chem. 2011, 286, 24275–24287. [Google Scholar] [CrossRef] [PubMed]
- Poth, A.G.; Colgrave, M.L.; Lyons, R.E.; Daly, N.L.; Craik, D.J. Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc. Natl. Acad. Sci. USA 2011, 108, 10127–10132. [Google Scholar] [CrossRef] [PubMed]
- Mylne, J.S.; Chan, L.Y.; Chanson, A.H.; Daly, N.L.; Schaefer, H.; Bailey, T.L.; Nguyencong, P.; Cascales, L.; Craik, D.J. Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell. 2012, 24, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.; Lian, Y.; Pang, E.W.; Nguyen, P.Q.; Tran, T.D.; Tam, J.P. Discovery of linear cyclotides in monocot plant panicum laxum of poaceae family provides new insights into evolution and distribution of cyclotides in plants. J. Biol. Chem. 2012, 288, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.C.; Colgrave, M.L.; Nguyencong, P.; Daly, N.L.; Craik, D.J. Discovery and characterization of a linear cyclotide from viola odorata: Implications for the processing of circular proteins. J. Mol. Biol. 2006, 357, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.W.; Cemazar, M.; Clark, R.J.; Horibe, T.; Renda, R.F.; Anderson, M.A.; Craik, D.J. A novel plant protein-disulfide isomerase involved in the oxidative folding of cystine knot defense proteins. J. Biol. Chem. 2007, 282, 20435–20446. [Google Scholar] [CrossRef] [PubMed]
- Saska, I.; Gillon, A.D.; Hatsugai, N.; Dietzgen, R.G.; Hara-Nishimura, I.; Anderson, M.A.; Craik, D.J. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J. Biol. Chem. 2007, 282, 29721–29728. [Google Scholar] [CrossRef] [PubMed]
- Conlan, B.F.; Gillon, A.D.; Craik, D.J.; Anderson, M.A. Circular proteins and mechanisms of cyclization. Biopolymers 2010, 94, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.T.; Wang, S.J.; Qiu, Y.B.; Hemu, X.; Lian, Y.L.; Tam, J.P. Butelase 1 is an asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 2014, 10, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Nguyen, G.K.; Tam, J.P.; Liu, C.F. Butelase-mediated synthesis of protein thioesters and its application for tandem chemoenzymatic ligation. Chem. Commun. (Camb) 2015. [Google Scholar] [CrossRef] [PubMed]
- Chagolla-Lopez, A.; Blanco-Labra, A.; Patthy, A.; Sanchez, R.; Pongor, S. A novel alpha-amylase inhibitor from amaranth (Amaranthus hypocondriacus) seeds. J. Biol. Chem. 1994, 269, 23675–23680. [Google Scholar] [PubMed]
- Svensson, B.; Fukuda, K.; Nielsen, P.K.; Bonsager, B.C. Proteinaceous alpha-amylase inhibitors. Biochim. Biophys. Acta 2004, 1696, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.C.; Enassar, M.; Willem, R.; Wieruzeski, J.M.; Lippens, G.; Wodak, S.J. Solution structure of the main alpha-amylase inhibitor from amaranth seeds. Eur. J. Biochem. 2001, 268, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.T.; Wang, S.J.; Kumar, A.; Yap, L.J.; Luu, T.T.; Lescar, J.; Tam, J.P. Discovery and characterization of pseudocyclic cystine-knot alpha-amylase inhibitors with high resistance to heat and proteolytic degradation. FEBS J. 2014, 281, 4351–4366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Luu, T.T.; Bai, Y.; Nguyen, G.K.; Pervushin, K.; Tam, J.P. Allotides: Proline-rich cystine knot alpha-amylase inhibitors from allamanda cathartica. J. Nat. Prod. 2015, 78, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Le-Nguyen, D.; Heitz, A.; Chiche, L.; el Hajji, M.; Castro, B. Characterization and 2d nmr study of the stable [9–21, 15–27] 2 disulfide intermediate in the folding of the 3 disulfide trypsin inhibitor eeti ii. Protein Sci. 1993, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Heitz, A.; Le-Nguyen, D.; Chiche, L. Min-21 and min-23, the smallest peptides that fold like a cystine-stabilized beta-sheet motif: Design, solution structure, and thermal stability. Biochemistry 1999, 38, 10615–10625. [Google Scholar] [CrossRef] [PubMed]
- Kolmar, H. Biological diversity and therapeutic potential of natural and engineered cystine knot miniproteins. Curr. Opin. Pharmacol. 2009, 9, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Craik, D.J. Thermal, chemical, and enzymatic stability of the cyclotide kalata b1: The importance of the cyclic cystine knot. Biochemistry 2004, 43, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- Heitz, A.; Avrutina, O.; Le-Nguyen, D.; Diederichsen, U.; Hernandez, J.F.; Gracy, J.; Kolmar, H.; Chiche, L. Knottin cyclization: Impact on structure and dynamics. BMC Struct. Biol. 2008, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.C.; Colgrave, M.L.; Craik, D.J. A novel suite of cyclotides from viola odorata: Sequence variation and the implications for structure, function and stability. Biochem. J. 2006, 400, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Kafedjiiski, K.; Kolmar, H.; Bernkop-Schnurch, A. Evaluation and improvement of the properties of the novel cystine-knot microprotein mcoeeti for oral administration. Int. J. Pharm. 2007, 332, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Werle, M.; Schmitz, T.; Huang, H.L.; Wentzel, A.; Kolmar, H.; Bernkop-Schnurch, A. The potential of cystine-knot microproteins as novel pharmacophoric scaffolds in oral peptide drug delivery. J. Drug Target. 2006, 14, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.H.; Liu, W.; Dai, J.X.; Wang, J.F.; Hu, Z.; Zhang, Y.; Wang, D.C. Solution structure of pafp-s: A new knottin-type antifungal peptide from the seeds of phytolacca americana. Biochemistry 2001, 40, 10973–10978. [Google Scholar] [CrossRef] [PubMed]
- Cammue, B.P.A.; Debolle, M.F.C.; Terras, F.R.G.; Proost, P.; Vandamme, J.; Rees, S.B.; Vanderleyden, J.; Broekaert, W.F. Isolation and characterization of a novel class of plant antimicrobial peptides from mirabilis-jalapa l seeds. J. Biol. Chem. 1992, 267, 2228–2233. [Google Scholar] [PubMed]
- Hwang, J.S.; Lee, J.; Hwang, B.; Nam, S.H.; Yun, E.Y.; Kim, S.R.; Lee, D.G. Isolation and characterization of psacotheasin, a novel knottin-type antimicrobial peptide, from psacothea hilaris. J. Microbiol. Biotechnol. 2010, 20, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Konarev, A.V.; Anisimova, I.N.; Gavrilova, V.A.; Vachrusheva, T.E.; Konechnaya, G.Y.; Lewis, M.; Shewry, P.R. Serine proteinase inhibitors in the compositae: Distribution, polymorphism and properties. Phytochemistry 2002, 59, 279–291. [Google Scholar] [CrossRef]
- Bode, W.; Greyling, H.J.; Huber, R.; Otlewski, J.; Wilusz, T. The refined 2.0 a x-ray crystal structure of the complex formed between bovine beta-trypsin and cmti-i, a trypsin inhibitor from squash seeds (Cucurbita maxima). Topological similarity of the squash seed inhibitors with the carboxypeptidase a inhibitor from potatoes. FEBS Lett. 1989, 242, 285–292. [Google Scholar] [PubMed]
- Polanowski, A.; Wilusz, T.; Nienartowicz, B.; Cieslar, E.; Slominska, A.; Nowak, K. Isolation and partial amino acid sequence of the trypsin inhibitor from the seeds of cucurbita maxima. Acta Biochim. Pol. 1980, 27, 371–382. [Google Scholar] [PubMed]
- Korsinczky, M.L.; Schirra, H.J.; Craik, D.J. Sunflower trypsin inhibitor-1. Curr. Protein Pept. Sci. 2004, 5, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Hass, G.M.; Ryan, C.A. Carboxypeptidase inhibitor from ripened tomatoes - purification and properties. Phytochemistry 1980, 19, 1329–1333. [Google Scholar] [CrossRef]
- Arolas, J.L.; Lorenzo, J.; Rovira, A.; Vendrell, J.; Aviles, F.X.; Ventura, S. Secondary binding site of the potato carboxypeptidase inhibitor. Contribution to its structure, folding, and biological properties. Biochemistry 2004, 43, 7973–7982. [Google Scholar] [CrossRef] [PubMed]
- Marino-Buslje, C.; Venhudova, G.; Molina, M.A.; Oliva, B.; Jorba, X.; Canals, F.; Aviles, F.X.; Querol, E. Contribution of c-tail residues of potato carboxypeptidase inhibitor to the binding to carboxypeptidase a a mutagenesis analysis. Eur. J. Biochem. 2000, 267, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J. Plant cyclotides: Circular, knotted peptide toxins. Toxicon 2001, 39, 1809–1813. [Google Scholar] [CrossRef]
- Gould, A.; Ji, Y.; Aboye, T.L.; Camarero, J.A. Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr. Pharm. Des. 2011, 17, 4294–4307. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Rosengren, K.J.; Craik, D.J. Discovery, structure and biological activities of cyclotides. Adv. Drug Deliv. Rev. 2009, 61, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Gran, L. On the effect of a polypeptide isolated from "kalata-kalata" (oldenlandia affinis dc) on the oestrogen dominated uterus. Acta Pharmacol. Toxicol. (Copenh) 1973, 33, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, K.J.; Daly, N.L.; Plan, M.R.; Waine, C.; Craik, D.J. Twists, knots, and rings in proteins. Structural definition of the cyclotide framework. J. Biol. Chem. 2003, 278, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A.; Yang, J.L.; Chiu, K.W. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. USA 1999, 96, 8913–8918. [Google Scholar] [CrossRef] [PubMed]
- Gran, L.; Sletten, K.; Skjeldal, L. Cyclic peptides from oldenlandia affinis dc. Molecular and biological properties. Chem. Biodivers. 2008, 5, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Pranting, M.; Loov, C.; Burman, R.; Goransson, U.; Andersson, D.I. The cyclotide cycloviolacin o2 from viola odorata has potent bactericidal activity against gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Taichi, M.; Nishio, H.; Nishiuchi, Y.; Tam, J.P. Optimal oxidative folding of the novel antimicrobial cyclotide from hedyotis biflora requires high alcohol concentrations. Biochemistry 2011, 50, 7275–7283. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.P.; Heitz, A.; Trinh Hong, T.; Pham, T.T.; Le Nguyen, D. Squash trypsin inhibitors from momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Chiche, L.; Heitz, A.; Gelly, J.C.; Gracy, J.; Chau, P.T.; Ha, P.T.; Hernandez, J.F.; Le-Nguyen, D. Squash inhibitors: From structural motifs to macrocyclic knottins. Curr. Protein Pept. Sci. 2004, 5, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Heitz, A.; Hernandez, J.F.; Gagnon, J.; Hong, T.T.; Pham, T.T.; Nguyen, T.M.; Le-Nguyen, D.; Chiche, L. Solution structure of the squash trypsin inhibitor mcoti-ii. A new family for cyclic knottins. Biochemistry 2001, 40, 7973–7983. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.T.; Craik, D.J. Cyclotides as templates in drug design. Drug Discov. Today 2010, 15, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.O.; Nadezhdin, K.D.; Sobol, V.A.; Sobol, A.G.; Skjeldal, L.; Arseniev, A.S. Conformation and mode of membrane interaction in cyclotides. Spatial structure of kalata b1 bound to a dodecylphosphocholine micelle. FEBS J. 2006, 273, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Colgrave, M.L.; Ireland, D.C.; Kaas, Q.; Craik, D.J. Despite a conserved cystine knot motif, different cyclotides have different membrane binding modes. Biophys. J. 2009, 97, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.T.; Rowlands, D.K.; Wong, C.-H.; Lo, T.W.C.; Nguyen, G.K.T.; Li, H.-Y.; Tam, J.P. Orally active peptidic bradykinin b1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew. Chem. Int. Ed. 2012, 51, 5620–5624. [Google Scholar] [CrossRef] [PubMed]
- Hemu, X.; Taichi, M.; Qiu, Y.; Liu, D.-X.; Tam, J.P. Biomimetic synthesis of cyclic peptides using novel thioester surrogates. Pept. Sci. 2013, 100, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Hemu, X.; Liu, D.X.; Tam, J.P. Selective bi-directional amide bond cleavage of N-methylcysteinyl peptide. Eur. J. Org. Chem. 2014, 2014, 4370–4380. [Google Scholar] [CrossRef]
- Taichi, M.; Hemu, X.; Qiu, Y.; Tam, J.P. A thioethylalkylamido (tea) thioester surrogate in the synthesis of a cyclic peptide via a tandem acyl shift. Org. Lett. 2013, 15, 2620–2623. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A. Synthesis of large cyclic cystine-knot peptide by orthogonal coupling strategy using unprotected peptide precursor. Tetrahedron Lett. 1997, 38, 5599–5602. [Google Scholar] [CrossRef]
- Tam, J.P.; Lu, Y.A. A biomimetic strategy in the synthesis and fragmentation of cyclic protein. Protein Sci. 1998, 7, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Love, S.; Alewood, P.F.; Craik, D.J. Chemical synthesis and folding pathways of large cyclic polypeptide: Studies of the cystine knot polypeptide kalata b1. Biochemistry 1999, 38, 10606–10614. [Google Scholar] [CrossRef] [PubMed]
- Thongyoo, P.; Roque-Rosell, N.; Leatherbarrow, R.J.; Tate, E.W. Chemical and biomimetic total syntheses of natural and engineered mcoti cyclotides. Org. Biomol. Chem. 2008, 6, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Thongyoo, P.; Jaulent, A.M.; Tate, E.W.; Leatherbarrow, R.J. Immobilized protease-assisted synthesis of engineered cysteine-knot microproteins. ChemBioChem 2007, 8, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.H.; Tran, A.T.; Camarero, J.A. Biosynthesis of the cyclotide kalata b1 by using protein splicing. Angew. Chem. Int. Ed. 2006, 45, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.; Wang, W.; Puttamadappa, S.; Shekhtman, A.; Camarero, J.A. Biosynthesis and biological screening of a genetically encoded library based on the cyclotide mcoti-i. ChemBioChem 2009, 10, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A.; Yu, Q.T. Thia zip reaction for synthesis of large cyclic peptides: Mechanisms and applications. J. Am. Chem. Soc. 1999, 121, 4316–4324. [Google Scholar] [CrossRef]
- Duvick, J.P.; Rood, T.; Rao, A.G.; Marshak, D.R. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J. Biol. Chem. 1992, 267, 18814–18820. [Google Scholar] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7s globulin protein in macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Nolde, S.B.; Vassilevski, A.A.; Rogozhin, E.A.; Barinov, N.A.; Balashova, T.A.; Samsonova, O.V.; Baranov, Y.V.; Feofanov, A.V.; Egorov, T.A.; Arseniev, A.S.; et al. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide ecamp1 from seeds of barnyard grass (Echinochloa crus-galli). J. Biol. Chem. 2011, 286, 25145–25153. [Google Scholar] [CrossRef] [PubMed]
- Utkina, L.L.; Andreev, Y.A.; Rogozhin, E.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Oparin, P.B.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding 4-cys antimicrobial peptides in wheat triticum kiharae dorof. Et migush.: Multimodular structural organization, instraspecific variability, distribution and role in defence. FEBS J. 2013, 280, 3594–3608. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Oparin, P.B.; Berkut, A.A.; Vassilevski, A.A.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Novel antifungal alpha-hairpinin peptide from stellaria media seeds: Structure, biosynthesis, gene structure and evolution. Plant. Mol. Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Conners, R.; Konarev, A.V.; Forsyth, J.; Lovegrove, A.; Marsh, J.; Joseph-Horne, T.; Shewry, P.; Brady, R.L. An unusual helix-turn-helix protease inhibitory motif in a novel trypsin inhibitor from seeds of veronica (veronica hederifolia l.). J. Biol. Chem. 2007, 282, 27760–27768. [Google Scholar] [CrossRef] [PubMed]
- Oparin, P.B.; Mineev, K.S.; Dunaevsky, Y.E.; Arseniev, A.S.; Belozersky, M.A.; Grishin, E.V.; Egorov, T.A.; Vassilevski, A.A. Buckwheat trypsin inhibitor with helical hairpin structure belongs to a new family of plant defence peptides. Biochem. J. 2012, 446, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.M.; Yang, Y.; Sze, K.H.; Zhang, X.; Zheng, Y.T.; Shaw, P.C. Structural characterization and anti-hiv-1 activities of arginine/glutamate-rich polypeptide luffin p1 from the seeds of sponge gourd (Luffa cylindrica). J. Struct. Biol. 2011, 174, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kader, J.C. Lipid-transfer proteins in plants. Annu Rev. Plant. Physiol Plant. Mol. Biol 1996, 47, 627–654. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Ade, O.; Gomes, V.M. Role of plant lipid transfer proteins in plant cell physiology-a concise review. Peptides 2007, 28, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.G.; Goderis, I.J.; Vanleuven, F.; Vanderleyden, J.; Cammue, B.P.A.; Broekaert, W.F. Invitro antifungal activity of a radish (Raphanus-sativus L.) seed protein homologous to nonspecific lipid transfer proteins. Plant. Physiol. 1992, 100, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Segura, A.; Garcia-Olmedo, F. Lipid transfer proteins (nsltps) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993, 316, 119–122. [Google Scholar] [CrossRef]
- Segura, A.; Moreno, M.; Garcia-Olmedo, F. Purification and antipathogenic activity of lipid transfer proteins (ltps) from the leaves of arabidopsis and spinach. FEBS Lett. 1993, 332, 243–246. [Google Scholar] [CrossRef]
- Cammue, B.P.; Thevissen, K.; Hendriks, M.; Eggermont, K.; Goderis, I.J.; Proost, P.; Van Damme, J.; Osborn, R.W.; Guerbette, F.; Kader, J.C.; et al. A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol. 1995, 109, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Arondel, V.V.; Vergnolle, C.; Cantrel, C.; Kader, J. Lipid transfer proteins are encoded by a small multigene family in arabidopsis thaliana. Plant Sci. 2000, 157, 1–12. [Google Scholar] [CrossRef]
- Douliez, J.P.; Pato, C.; Rabesona, H.; Molle, D.; Marion, D. Disulfide bond assignment, lipid transfer activity and secondary structure of a 7-kda plant lipid transfer protein, ltp2. Eur. J. Biochem. 2001, 268, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.L.; de Lamotte, F.; Gautier, M.F.; Delsuc, M.A. Refined solution structure of a liganded type 2 wheat nonspecific lipid transfer protein. J. Biol. Chem. 2003, 278, 14249–14256. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Liu, Y.J.; Cheng, C.S.; Lyu, P.C. Solution structure of plant nonspecific lipid transfer protein-2 from rice (Oryza sativa). J. Biol. Chem. 2002, 277, 35267–35273. [Google Scholar] [CrossRef] [PubMed]
- Gomar, J.; Sodano, P.; Sy, D.; Shin, D.H.; Lee, J.Y.; Suh, S.W.; Marion, D.; Vovelle, F.; Ptak, M. Comparison of solution and crystal structures of maize nonspecific lipid transfer protein: A model for a potential in vivo lipid carrier protein. Proteins 1998, 31, 160–171. [Google Scholar] [CrossRef]
- Tassin, S.; Broekaert, W.F.; Marion, D.; Acland, D.P.; Ptak, M.; Vovelle, F.; Sodano, P. Solution structure of ace-amp1, a potent antimicrobial protein extracted from onion seeds. Structural analogies with plant nonspecific lipid transfer proteins. Biochemistry 1998, 37, 3623–3637. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Min, K.; Cha, H.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. Rice non-specific lipid transfer protein: The 1.6 a crystal structure in the unliganded state reveals a small hydrophobic cavity. J. Mol. Biol. 1998, 276, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Han, G.W.; Lee, J.Y.; Song, H.K.; Chang, C.; Min, K.; Moon, J.; Shin, D.H.; Kopka, M.L.; Sawaya, M.R.; Yuan, H.S.; et al. Structural basis of non-specific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution x-ray crystallography. J. Mol. Biol. 2001, 308, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Cheng, P.T.; Peng, P.; Lyu, P.C.; Sun, Y.J. Lipid binding in rice nonspecific lipid transfer protein-1 complexes from Oryza sativa. Protein Sci. 2004, 13, 2304–2315. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, J.; Sodano, P.; Suh, S.W.; Lee, J.Y.; Ptak, M.; Vovelle, F. Solution structure of a lipid transfer protein extracted from rice seeds. Comparison with homologous proteins. Eur. J. Biochem. 1999, 259, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Gincel, E.; Simorre, J.P.; Caille, A.; Marion, D.; Ptak, M.; Vovelle, F. Three-dimensional structure in solution of a wheat lipid-transfer protein from multidimensional 1h-nmr data. A new folding for lipid carriers. Eur. J. Biochem. 1994, 226, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Charvolin, D.; Douliez, J.P.; Marion, D.; Cohen-Addad, C.; Pebay-Peyroula, E. The crystal structure of a wheat nonspecific lipid transfer protein (ns-ltp1) complexed with two molecules of phospholipid at 2.1 a resolution. Eur. J. Biochem. 1999, 264, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Lerche, M.H.; Kragelund, B.B.; Bech, L.M.; Poulsen, F.M. Barley lipid-transfer protein complexed with palmitoyl coa: The structure reveals a hydrophobic binding site that can expand to fit both large and small lipid-like ligands. Structure 1997, 5, 291–306. [Google Scholar] [CrossRef]
- Lerche, M.H.; Poulsen, F.M. Solution structure of barley lipid transfer protein complexed with palmitate. Two different binding modes of palmitate in the homologous maize and barley nonspecific lipid transfer proteins. Protein Sci. 1998, 7, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Gomar, J.; Petit, M.C.; Sodano, P.; Sy, D.; Marion, D.; Kader, J.C.; Vovelle, F.; Ptak, M. Solution structure and lipid binding of a nonspecific lipid transfer protein extracted from maize seeds. Protein Sci. 1996, 5, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Gerhardt, I.R.; Orru, S.; Pucci, P.; Bloch, C., Jr. Purification and characterization of a small (7.3 kda) putative lipid transfer protein from maize seeds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 794, 109–114. [Google Scholar] [CrossRef]
- Gizatullina, A.K.; Finkina, E.I.; Mineev, K.S.; Melnikova, D.N.; Bogdanov, I.V.; Telezhinskaya, I.N.; Balandin, S.V.; Shenkarev, Z.O.; Arseniev, A.S.; Ovchinnikova, T.V. Recombinant production and solution structure of lipid transfer protein from lentil lens culinaris. Biochem. Biophys. Res. Commun. 2013, 439, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.F.; Liu, Y.N.; Hsu, S.T.; Samuel, D.; Cheng, C.S.; Bonvin, A.M.; Lyu, P.C. Characterization and structural analyses of nonspecific lipid transfer protein 1 from mung bean. Biochemistry 2005, 44, 5703–5712. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.; Landon, C.; Industri, B.; Marais, A.; Marion, D.; Ponchet, M.; Vovelle, F. Solution structure of a tobacco lipid transfer protein exhibiting new biophysical and biological features. Proteins 2005, 59, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Chen, M.N.; Lai, Y.T.; Chen, T.; Lin, K.F.; Liu, Y.J.; Lyu, P.C. Mutagenesis study of rice nonspecific lipid transfer protein 2 reveals residues that contribute to structure and ligand binding. Proteins 2008, 70, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Samuel, D.; Liu, Y.J.; Shyu, J.C.; Lai, S.M.; Lin, K.F.; Lyu, P.C. Binding mechanism of nonspecific lipid transfer proteins and their role in plant defense. Biochemistry 2004, 43, 13628–13636. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Xia, L.; Wong, J.H.; Ng, T.B.; Ye, X.; Wang, S.; Shi, X. Lipid transfer proteins from brassica campestris and mung bean surpass mung bean chitinase in exploitability. J. Pept. Sci. 2007, 13, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Garcia-Olmedo, F. Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein ltp2. Plant J. 1997, 12, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Lascombe, M.B.; Bakan, B.; Buhot, N.; Marion, D.; Blein, J.P.; Larue, V.; Lamb, C.; Prange, T. The structure of “defective in induced resistance” protein of arabidopsis thaliana, dir1, reveals a new type of lipid transfer protein. Protein Sci. 2008, 17, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K. The biochemistry and biology of extracellular plant lipid-transfer proteins (ltps). Protein Sci. 2008, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kader, J.C. Proteins and intracellular exchange of lipids.1. Stimulation of phospholipid exchange between mitochondria and microsomal fractions by proteins isolated from potato-tuber. Biochem. Biophys. Acta 1975, 380, 31–44. [Google Scholar] [CrossRef]
- Kader, J.C.; Julienne, M.; Vergnolle, C. Purification and characterization of a spinach-leaf protein capable of transferring phospholipids from liposomes to mitochondria or chloroplasts. Eur. J. Biochem. 1984, 139, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Thoma, S.; Kaneko, Y.; Somerville, C. A non-specific lipid transfer protein from arabidopsis is a cell wall protein. Plant J. 1993, 3, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Pyee, J.; Yu, H.S.; Kolattukudy, P.E. Identification of a lipid transfer protein as the major protein in the surface wax of broccoli (Brassica oleracea) leaves. Archiv. Biochem. Biophys. 1994, 311, 460–468. [Google Scholar] [CrossRef]
- Regente, M.C.; Giudici, A.M.; Villalain, J.; de la Canal, L. The cytotoxic properties of a plant lipid transfer protein involve membrane permeabilization of target cells. Lett. Appl. Microbiol. 2005, 40, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Moreno, M.; Madueno, F.; Molina, A.; Garcia-Olmedo, F. Snakin-1, a peptide from potato that is active against plant pathogens. Mol. Plant Microbe Interact. 1999, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; Lopez, G.; Garcia-Olmedo, F.; Molina, A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Franco, O.L. Theoretical structural insights into the snakin/gasa family. Peptides 2013, 44, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Bindschedler, L.V.; Whitelegge, J.P.; Millar, D.J.; Bolwell, G.P. A two component chitin-binding protein from french bean -- association of a proline-rich protein with a cysteine-rich polypeptide. FEBS Lett. 2006, 580, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, F.; Zare-Zardini, H.; Ebrahimi, L. Investigation of the antimicrobial activities of snakin-z, a new cationic peptide derived from zizyphus jujuba fruits. Nat. Prod. Res. 2013, 27, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Porto, W.F.; Dey, P.; Maiti, M.K.; Ghosh, A.K.; Franco, O.L. The attack of the phytopathogens and the trumpet solo: Identification of a novel plant antifungal peptide with distinct fold and disulfide bond pattern. Biochimie 2013, 2013, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Tailor, R.H.; Acland, D.P.; Attenborough, S.; Cammue, B.P.; Evans, I.J.; Osborn, R.W.; Ray, J.A.; Rees, S.B.; Broekaert, W.F. A novel family of small cysteine-rich antimicrobial peptides from seed of impatiens balsamina is derived from a single precursor protein. J. Biol. Chem. 1997, 272, 24480–24487. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Francois, I.E.; Sijtsma, L.; van Amerongen, A.; Schaaper, W.M.; Meloen, R.; Posthuma-Trumpie, T.; Broekaert, W.F.; Cammue, B.P. Antifungal activity of synthetic peptides derived from impatiens balsamina antimicrobial peptides ib-amp1 and ib-amp4. Peptides 2005, 26, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.U.; Osborn, R.; Rees, S.; Thornton, J.M. Structural studies of impatiens balsamina antimicrobial protein (ib-amp1). Biochemistry 1998, 37, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bang, J.K.; Kim, H.J.; Kim, J.K.; Kim, Y.; Shin, S.Y. Antimicrobial specificity and mechanism of action of disulfide-removed linear analogs of the plant-derived cys-rich antimicrobial peptide ib-amp1. Peptides 2009, 30, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Amino acid sequence and antimicrobial activity of chitin-binding peptides, pp-amp 1 and pp-amp 2, from japanese bamboo shoots (phyllostachys pubescens). Biosci. Biotechnol. Biochem. 2005, 69, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, A.A.; Rogozhin, E.A.; Odintsova, T.I.; Khadeeva, N.V.; Grishin, E.V.; Egorov Ts, A. Discovery of novel antimicrobial peptides with unusual cysteine motifs in dandelion taraxacum officinale wigg. Flowers. Peptides 2012, 36, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, A.A.; Rogozhin, E.A.; Andreev, Y.A.; Odintsova, T.I.; Kozlov, S.A.; Grishin, E.V.; Egorov, T.A. A novel cysteine-rich antifungal peptide toamp4 from taraxacum officinale wigg. Flowers. Plant Physiol. Biochem. 2013, 70, 93–99. [Google Scholar] [CrossRef] [PubMed]
- McManus, A.M.; Nielsen, K.J.; Marcus, J.P.; Harrison, S.J.; Green, J.L.; Manners, J.M.; Craik, D.J. Miamp1, a novel protein from macadamia integrifolia adopts a greek key beta-barrel fold unique amongst plant antimicrobial proteins. J. Mol. Biol. 1999, 293, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.N.; Porto, W.F.; Migliolo, L.; Mandal, S.M.; Gomes, D.G.; Holanda, H.H.; Silva, R.S.; Dias, S.C.; Costa, M.P.; Costa, C.R.; et al. Cn-amp1: A new promiscuous peptide with potential for microbial infections treatment. Biopolymers 2012, 98, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Migliolo, L.; Das, S.; Mandal, M.; Franco, O.L.; Hazra, T.K. Identification and characterization of a bactericidal and proapoptotic peptide from cycas revoluta seeds with DNA binding properties. J. Cell. Biochem. 2012, 113, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, P.B.; Murad, A.M.; Silva, L.P.; Dos Santos, R.C.; Costa, F.T.; Tagliari, P.D.; Bloch, C., Jr.; Noronha, E.F.; Miller, R.N.; Franco, O.L. Identification of a novel storage glycine-rich peptide from guava (psidium guajava) seeds with activity against gram-negative bacteria. Peptides 2008, 29, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.S.; Rettore, J.V.; Freitas, R.M.; Porto, W.F.; Duque, A.P.; Singulani Jde, L.; Silva, O.N.; Detoni Mde, L.; Vasconcelos, E.G.; Dias, S.C.; et al. Antimicrobial activity of recombinant pg-amp1, a glycine-rich peptide from guava seeds. Peptides 2012, 37, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Park, C.B.; Hong, S.S.; Lee, H.S.; Lee, S.Y.; Kim, S.C. Characterization and cdna cloning of two glycine- and histidine-rich antimicrobial peptides from the roots of shepherd's purse, capsella bursa-pastoris. Plant. Mol. Biol. 2000, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaeian, M. Antimicrobial peptides: Modes of mechanism, modulation of defense responses. Plant Signal. Behav. 2011, 6, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Pelegrini, P.; Del Sarto, R.P.; Silva, O.N.; Franco, O.L.; Grossi-de-Sa, M.F. Antibacterial peptides from plants: What they are and how they probably work. Biochem. Res. Int. 2011, 2011, 250349. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Aoki, M.; Yano, Y.; Matsuzaki, K. Interaction of antimicrobial peptide magainin 2 with gangliosides as a target for human cell binding. Biochemistry 2012, 51, 10229–10235. [Google Scholar] [CrossRef] [PubMed]
- Sarika; Iquebal, M.A.; Rai, A. Biotic stress resistance in agriculture through antimicrobial peptides. Peptides 2012, 36, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E. Antimicrobial peptides and plant disease control. FEMS Microbiol. Lett. 2007, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, K.; Camarero, J.A. Cyclotides, a promising molecular scaffold for peptide-based therapeutics. Biopolymers 2010, 94, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lehrer, R.I.; Tam, J.P. Engineered salt-insensitive alpha-defensins with end-to-end circularized structures. J. Biol. Chem. 2000, 275, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.; Gunasekera, S.; Henriques, S.T.; Worth, N.F.; Le, S.J.; Clark, R.J.; Campbell, J.H.; Craik, D.J.; Daly, N.L. Engineering pro-angiogenic peptides using stable, disulfide-rich cyclic scaffolds. Blood 2011, 118, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, R.; Daly, N.L.; Wulff, B.S.; Andresen, T.L.; Conde-Frieboes, K.W.; Craik, D.J. Design, synthesis, structural and functional characterization of novel melanocortin agonists based on the cyclotide kalata b1. J. Biol. Chem. 2012, 287, 40493–40501. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.A.; Shah, D.; Abbas, D.; Madkour, M. Stable integration and expression of a plant defensin in tomato confers resistance to fusarium wilt. GM Crops 2010, 1, 344–350. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711-757. https://doi.org/10.3390/ph8040711
Tam JP, Wang S, Wong KH, Tan WL. Antimicrobial Peptides from Plants. Pharmaceuticals. 2015; 8(4):711-757. https://doi.org/10.3390/ph8040711
Chicago/Turabian StyleTam, James P., Shujing Wang, Ka H. Wong, and Wei Liang Tan. 2015. "Antimicrobial Peptides from Plants" Pharmaceuticals 8, no. 4: 711-757. https://doi.org/10.3390/ph8040711
APA StyleTam, J. P., Wang, S., Wong, K. H., & Tan, W. L. (2015). Antimicrobial Peptides from Plants. Pharmaceuticals, 8(4), 711-757. https://doi.org/10.3390/ph8040711